Abstract
With the scale-up of effective antiretroviral therapy in resource-limited settings, many HIV-infected children are now able to survive into adulthood. To achieve this potential, children must navigate normative developmental processes and challenges while living with an unusually complex, stigmatizing, potentially fatal chronic illness and meeting the demands of treatment.
Yet many of these children, especially preadolescents, do not know they are HIV-infected. Despite compelling evidence supporting the merits of informing children of their HIV status, there has been little emphasis on equipping the child’s caregiver with information and skills to promote disclosure, particularly, when the caregiver faces a variety of sociocultural barriers and is reluctant to do so. In this study, we present the background, process and methods for a first of its kind collaboration that is examining the efficacy of an intervention developed to facilitate the engagement of caregivers in the process of disclosure in a manner suitable to the sociocultural context and developmental age and needs of the child in Ghana. We also report preliminary data that supported the design of the intervention approach and currently available domains of the data system. Finally, we discuss challenges and implications for future research.
Keywords: clinical trial, disclosure, Ghana, HIV, intervention, paediatric
Introduction
In the era of expanded access to antiretroviral therapy (ART) in resource-limited settings, the landscape of paediatric HIV care has dramatically changed. Millions of children infected with HIV at birth are now living longer and with better qualityof life [1,2]. Yet, these successes pose new challenges, as children with perinatal HIV infection survive into adolescence and adulthood. One challenging, but critical aspect of care not being adequately addressed is disclosure of an HIV diagnosis to the infected child in a timely, age-appropriate manner [3].
Disclosing an HIV diagnosis to the infected child has been recommended by leaders in the field for over a decade [4,5]. This recommendation is consistent with child rights and research that shows disclosure may confer several benefits, importantly, improved adherence to therapy, clinical outcomes and psychological adjustment and lower risk of transmitting HIV when the child becomes sexually active [5–8]. However, in sub-Saharan Africa, which is home to 91% of the world’s estimated 3.34 million children infected with HIV, disclosure recommendations have generally not been implemented [1,3,9]. It is estimated that in some regions, as many as 80% of these children have not been informed of their HIV status [10–12].
Guidelines published by the WHO, the American Academy of Pediatrics and others conceptualize disclosure as a process and recommend that disclosure should be initiated by the healthcare provider or caregiver incrementally in a manner tailored to the child’s cognitive and emotional development [4,5,13–18]. Although available guidelines provide guidance on appropriate ways to disclose an HIV diagnosis to children of different levels of maturity, there is a paucity of data on how to aid the initiation of disclosure when healthcare providers and caregivers are persistently reluctant to do so. Our preliminary research has furthered our understanding of the problem and informed the development of a paediatric disclosure intervention that is designed to change the behaviour of ‘nondisclosers’ in Ghana (where the rates of nondisclosure are high), as well as guide the process of disclosure in a socioculturally appropriate manner once initiated. This article provides an overview of the problem in Ghana and the design and progress of the ongoing trial (ClinicalTrials.gov Identifier: NCT01701635) that is testing the intervention in Ghana.
HIV and paediatric disclosure in Ghana
The HIV epidemic in Ghana is characterized by a relatively low, but stable prevalence estimated at 1.3% nationally in 2013 [19]. Among the 224 488 persons estimated to be living with HIV in Ghana, 34 557 are children [19]. A National Strategic Plan for HIV and AIDS (NSP) was developed for 2011–2015 with an overall goal of virtual elimination of mother-to-child transmission (MTCT) of HIV [19]. An MTCT programme was deployed to all regions in the country [20]. In 2013, HIV prevalence amongst pregnant women attending antenatal clinics was 1.9%, a drop from 2.1% in 2012 [19]. With ongoing improvements to the ART programme, it is estimated that 69% of children with advanced HIV now receive ART services [19].
Although Ghana has made impressive gains through the implementation of the multisectorial national strategies, the full range of care needed for children already living with HIV is not being achieved. Disclosure of HIV status is a complex and crucial component in the continuum of HIV. With greater access to therapy, a particularly important advantage of disclosure is the potential for better treatment adherence [21,22]. Nevertheless, in research conducted in Ghana to date, the prevalence of disclosure to children is estimated at only 21–53% and 35–45% of children taking ART have been found to have suboptimal adherence [10,23]. Although complete disclosure of HIV status has been associated with improved adherence to ART, partial and nondisclosure can strain the relationship between the caregiver and the child and prompt defiance and nonadherence by the child [22,23]. These findings underscore the need for protocols to facilitate disclosure.
Preliminary research and guiding framework
Building on the findings from an earlier study [10], we interviewed a sample of providers and caregivers in Ghana to identify factors influencing paediatric disclosure and to inform the development of a culturally appropriate and acceptable disclosure intervention. Participants were recruited from the Korle-Bu Teaching Hospital in Accra, Ghana, and interviewed in separate focus groups by an experienced moderator using open-ended questions. Interview data were audio taped, transcribed verbatim and systematically analysed by the investigators using inductive techniques.
Similar to findings from other studies conducted in sub-Saharan Africa, disclosure was found to be a controversial and difficult issue among the healthcare providers and caregivers interviewed in Ghana. The caregivers (n =7; mean age 38) described several factors as barriers to disclosing an HIV diagnosis to children, including parental concern about upsetting the child, or the child being unable to cope with the information, fear of the child telling others of the diagnosis and being stigmatized, fear of extreme anger by the child at the parent’s responsibility for transmission and the parent’s desire for the child to live without the burden of knowledge of the diagnosis. Nondisclosure was viewed as justifiable given these considerations. Yet, at the same time, the caregivers perceived the importance of children being informed of their diagnosis to facilitate cooperation with necessary self-care/treatment and to avoid transmission to others. Caregivers were especially reluctant to disclose to younger children and perceived that they lacked the necessary skills to disclose and believed that it should be handled by the healthcare provider who they presumed had the requisite skills (e.g. communication, discretion and knowledge about HIV to answer child’s questions). The providers (n =10) also identified advantages to disclosing an HIV diagnosis to the child (e.g. better cooperation and adherence to the treatment regimen, prevention of transmission), but described a number of barriers to a clinic-based approach. Prominent among these was the lack of time, the current clinic culture (including limited attention given to the topic generally) and concerns about potential detriment to the child if disclosure was not handled competently. Providers also expressed discomfort about their skill level and thought it was important for the caregivers to handle the disclosure.
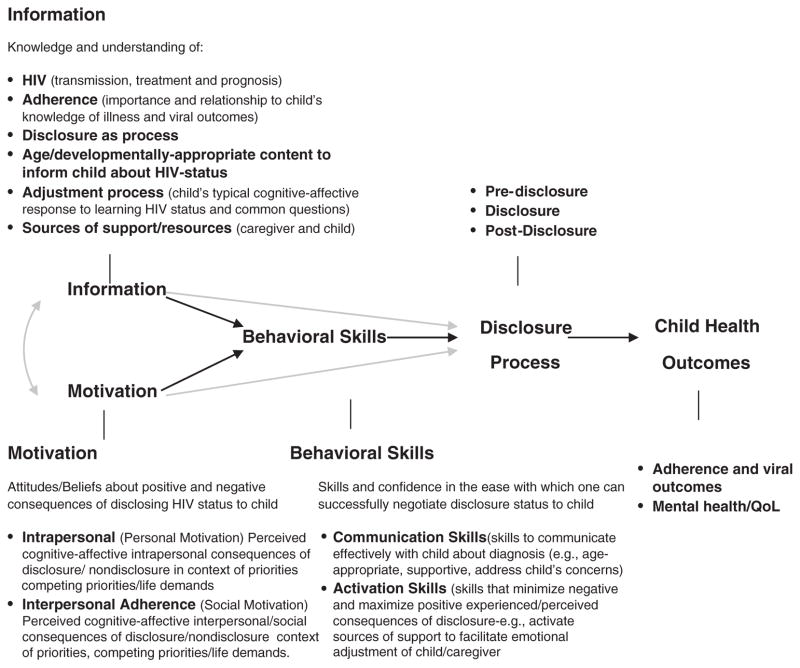
Drawing from our preliminary data, as well as extant empiric and theoretic knowledge, we developed a structured, culturally relevant HIV paediatric disclosure intervention that could be delivered as an integral component of routine HIV healthcare in Ghana. The intervention is termed ‘Sankofa’, a traditional Ghanaian concept that is literally translated to mean ‘It is not wrong to go back for that which you have forgotten’. The content of the intervention is guided by an HIV paediatric disclosure model that incorporates bioecological systems theory [24] and core elements of the Information-Motivation-Behavioural Skills (IMB) model of Health Behavior Change and other applications of it [25–28] and it is designed to facilitate the initiation as well as the process of disclosure over time. The IMB model postulates that health-related information, motivation and behavioural skills are important determinants of whether or not a health behaviour is performed; a person who is well informed, motivated to act and has the skills and confidence to take action is more likely to initiate and maintain behaviours that produce positive outcomes. Hence, health interventions should be focused on dispersing effective health information that is relevant to the target health behaviour and specific to a population, increasing personal motivation and social support and skill-training to increase self-efficacy for performing a behaviour. With the integration of bio ecological systems theory [24] and current recommendations [5], the IMB-based disclosure model situates the core IMB variables within the cognitive-affective, socio-cultural environment, developmental age of the HIV-infected child and the relationships that form the caregiver’s and child’s environment in which disclosure occurs (Fig. 1).
Fig. 1.
Guiding conceptual framework.
SANKOFA protocol
Study aims and design
We are currently conducting a trial that compares the SANKOFA disclosure intervention as well as usual care (n =378) with usual care alone (control; n=378). The primary outcome is the rate of caregiver disclosure at 1-year follow-up. In addition, we are examining factors predictive of caregiver disclosure and the effect of disclosure on ART adherence and health outcomes. Data are collected at baseline and longitudinal outcomes evaluated every 6 months for 3 years postentry (Fig. 2).
Fig. 2.
Schema of study design.
Study sites and sample
Caregiver–child dyads (N =756) are enrolled from two tertiary HIV clinics in Accra and Kumasi, Ghana with a similar socioeconomic structure and healthcare infrastructure. The clinics take care of over 3500 children including those with confirmed HIV diagnosis. The sites were selected because they have the patient population and infrastructure (e.g. laboratory facilities, experienced clinical investigators) that support successful conduct of the study. Ethical clearances were obtained from institutional review boards of each hospital, the Ghana Health Services and the Yale University Human Investigation Committee.
All HIV-infected children receiving care at the two clinics ages 7–18 years and who were started on ART within 12 months of study enrolment and who do not know their HIV diagnosis (based on caregiver account and medical records confirmation) are eligible to participate in the study. Children with congenital or developmental disorders, conditions (e.g. sickle cell, diabetes) that require frequent clinic visits or hospitalizations, or those with an AIDS-defining illness or end-stage AIDS are excluded.
The study was designed to achieve 90% power (alpha =0.05) at detecting a hazard ratio of 1.3 (equivalent to a higher detectable proportion disclosing in the intervention group of 30% when the proportion disclosing in the control group is 21%). The total sample size of 756 (N =378 per group) takes into account the duration of enrolment (3 years), with a minimum follow-up of 1 year for the primary outcome of interest, and an expected attrition rate of 20% during the study period.
Study entry
During regular clinic visits, the study is introduced to caregivers who have not disclosed their child’s HIV status. If the caregiver is interested in learning more about the study, the designated site project staff member screens, fully explains the study and obtains written consent from the caregiver and assent from the child. Baseline data are then collected. Data collection interviews are conducted by a trained interviewer mainly in English, the official language in Ghana and the language of instruction at schools. To avoid inadvertent disclosure of a child’s status, children are assented and interviewed in the presence of their caregivers, while the caregivers are interviewed without their children present. Children assent to provide general information about their health and well being.
Randomization
The study sites were randomized by a coin flip to either receive the disclosure intervention (intervention arm) or to continue their current disclosure practice (control arm). Site randomization was used to avoid cross-contamination within the same clinic where elements of the disclosure intervention administered to the intervention group might filter into the control group [29,30]. The sample is stratified by age groups (7–11 and ≥11 years of age). Enrolment into the two age group strata (7–11 and ≥11 years of age) was implemented after the random allocation of the two sites to either the intervention or control arm; once an age stratum is filled at each site, no additional participants will be enrolled into that stratum. This is done to ensure that there is a sufficient representation of children in each age group at each site.
Description of the study arms
Experimental group – SANKOFA paediatric disclosure intervention as well as usual care
The experimental group receives the theory-guided Sankofa intervention as well as usual care. As summarized in Tables 1 and 2, the intervention is family-centred and uses an adherence and disclosure specialist (ADDS) to deliver the intervention. The ADDS is familiar with the sociocultural norms of the community and, in keeping with the guiding model, is trained to target modifiable information, motivation and behavioural skills of caregivers to facilitate their engagement in the process of disclosure in a manner suitable to the developmental age and needs of the child [3,8,18].
Table 1.
Summary of key elements SANKOFA paediatric disclosure intervention.
| An Adherence and Disclosure | A clinician is selected from the clinic’s personnel for assumption of the specialty role. The specialist approach:
|
| Information, Motivation and Behavioral Skills of care-givers are targeted | The ADDS uses therapeutic communication to target modifiable information, motivation and behavioral skills of caregivers to facilitate their engagement in the process of disclosure suitable to the age and needs of the child.
|
| Content is contextualized to socio-cultural environment and age of the HIV-infected child | How the child is informed of his or her HIV diagnosis is tailored to child’s stage of developmental understanding and sociocultural context.
|
| Individualized and process-oriented | Disclosure is understood as a process that moves through the phases of pre-disclosure, disclosure, and post-disclosure and is recursive. |
Table 2.
Phases of the intervention process.
| Individualized process over time | |
| Phase 1. Pre-Disclosure | The ADDS meets with the caregiver during the child’s routine clinic visits and builds rapport showing sensitivity and respect for the intense feelings the caregiver may have about disclosure. (M, C) The ADDS determines whether and when the caregiver is considering disclosing (P). If the caregiver is ready to disclose, the ADDS begins Phase 2; If the caregiver is not ready to disclose, the ADDS continues to meet with the caregiver during the child’s routine clinic visits to provide an opportunity for the caregiver to discuss thoughts and concerns that are barriers to disclosure and to offer relevant information and skills building. The ADDS:
|
| Phase 2. Disclosure |
|
| Phase 3. Post-Disclosure | The ADDS makes a follow up call or meets with the caregiver within a few days following the initial disclosure. The ADDS continues to have follow up meetings with the caregiver and child during routine clinic appointments or more frequently if indicated to:
|
Note: Key theory guided elements/principles - ↑Information (I), ↑Motivation (M), ↑Skills (S), Contextualized to socio-cultural environment and age of child (C), Process-oriented (P).
Standardization of intervention delivery in accordance with the principles of the disclosure intervention is achieved by use of a manualized intervention protocol. The ADDS received training until mastery of the protocol was demonstrated. As part of the content validation, the ADDS maintains a log of each contact that details date, length of visit, whether full intervention protocol was completed, content of visit discussion and difficulties encountered and strategies used to manage them. Participant exposure to the intervention is quantified both in terms of the number of contacts and time spent in each contact.
Control group – usual care
Participants in the control arm receive usual clinic care wherein the provider assesses the caregiver’s disclosure readiness, but typically does not discuss disclosure unless impelled by queries of the caregiver or child. To prevent the rate of disclosure at the intervention site being confounded by the time/attention of the ADDS, an attention control ADDS is employed. This individual meets with the caregivers and provides general health information (e.g. medication adherence) and answers questions the caregivers may have. Given that our disclosure intervention has not demonstrated feasibility and efficacy, the trial design meets equipoise. If the intervention is successful, we plan to collaborate with the National AIDS Control Programme to scale it up nationally.
Data collection
Data are collected at baseline and follow-up with standardized instruments (summarized in Table 3) [31–41] that were selected in accordance with our guiding conceptual model and prior studies [16,42]. The primary outcome is the proportion of caregiver disclosure of paediatric HIV at 1-year follow-up. Other outcomes of interest are information, motivation and behavioural skills of the participants, health outcomes (medication adherence, viral and immunologic markers, mental health parameters) and fidelity and acceptability of the disclosure intervention.
Table 3.
Summary of study measures.
| Concept | Measure |
|---|---|
| Social support (caregiver) | Social Provisions Questionnaire (SPS) [31] |
| HIV knowledge (caregiver) | HIV knowledge (HIV-KQ-18) [32] |
| Illness perceptions (caregiver) | Illness perceptions (Brief IPQ) [33] |
| Perceived stigma (caregiver) | HIV stigma scale [34] |
| Depressive symptoms (caregiver and child) | Beck Depression Inventory (BDI) [35,36] Child Depression Inventory (CDI) [37] |
| Child disclosure status | Project specific (caregiver self-report and child’s physician report) |
| Adherence to antiretroviral medications (child) | ACTG adherence questionnaire [38,39] Time to ART Refill [40] |
| Child’s behaviour | Child Behavior Checklist (CBCL) [41] |
| Child’s health status | Medical history and physical examination, CD4+ cell count, HIV1-RNA |
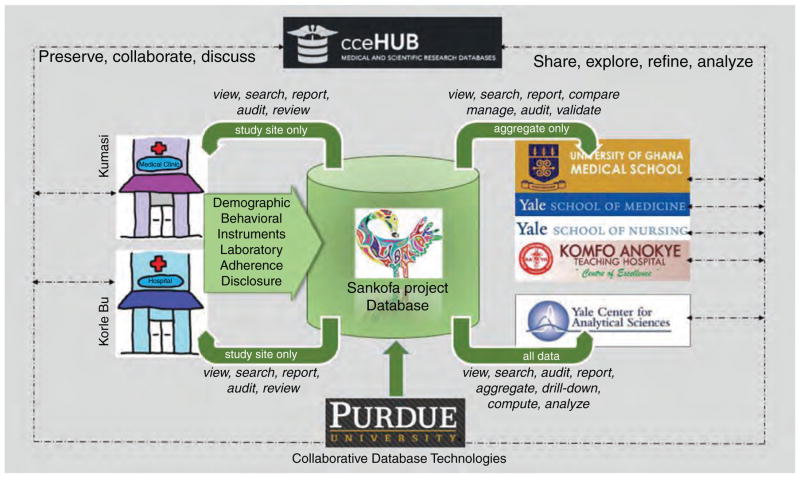
A web-accessible and interactive database system, developed at Purdue University, is used for the acquisition, storage and exploration of and other study data. The system is built on top of the HUBzero cyber infrastructure (http://hubzero.org), which uses web-based data technology components to create a secure and private (compliant with HIPPA regulations), customized database. The interactive nature of the database is illustrated in Fig. 3 and described fully elsewhere [43].
Fig. 3.
WebDatabase design and organization.
Analyses
Statistical analyses will be conducted on an intention-to-treat sample. Where appropriate, hypotheses will be tested by independent statistical analyses using mixed models procedures to test for the effect of group assignment (intervention vs. control), time and the interaction between assignment and time. Analysis will be carried out to account for different impact on different age groups. Possible correlation between individuals within each of the two sites will be assessed during analysis by taking into account the intracluster correlation coefficient. Interval-censored survival analysis techniques will be used to analyse the primary outcome. In addition, the proportion of participants with disclosure will be compared between the two study arms using Chi-square methods.
Progress to date
A team of bi-national (Ghanaian, U.S./Ghanaian and U.S.), multidisciplinary investigators with complementary individual and collective expertise were assembled for development and execution of the trial. The investigators at the participating institutions have worked together closely on implementation of the project. The project was launched with an intensive, 3-day face-to-face team meeting held at a location off-site in Ghana. The meeting focused on training in all aspects of the study procedures and building collaborative team relationships and patterns of open communication, an important consideration among team members with different disciplinary backgrounds, culturally informed assumptions and patterns of communication that could be constrained by hierarchical norms. This was followed by weekly Skype calls with the U.S. and Ghanaian investigators and project coordinators, monthly Skype calls with the full team and intermittent Skype and e-mail communication carried out on an ‘as needed’ basis. In addition, one of the U.S. investigators meets with the Ghanaian investigators in Ghana at least quarterly and follow-up face-to-face meetings are held with the full team annually.
Critical to the success of the project to date have been the two dedicated on-site project coordinators (one full time at each site), development of a close working relationship among team members and regular, open communication that has facilitated prompt action when needed and monitoring of project data in ‘real-time’ with use of the web-accessible database system.
Participant baseline characteristics
Nearly one-half of the target caregiver–child dyads have been enrolled (from January 2013 to July 2014). Sixty-six percent (N =298) of the caregiver/child dyads screened (N =451) have been enrolled to the study. Refusal of the caregiver or child has accounted for 15% of caregiver/ child dyads who were not enrolled to the study. Enrolment and characteristics of the participants have been comparable at the two sites (see Table 4). The mean age of the caregivers is 41 years, roughly 60% are HIV positive and 80% are female. The children are 50% female with a mean age of 10 years. No adverse events have been reported to date, even among participants who have elected to disclose while enrolled in the study.
Table 4.
Caregiver–child dyad demographic characteristics at enrolment.
| Intervention arm Disclosure intervention (N =131) | Control arm Usual care (N =167) | |
|---|---|---|
| Caregiver’s age (mean) | 42.0 (43.2, 10.8) | 40.0 (42.1, 10.0) |
| Caregiver’s sex | ||
| Male | 22 (16.8) | 36 (21.6) |
| Female | 109 (83.2) | 131 (78.4) |
| Caregiver’s HIV status | ||
| Negative or not sure | 53 (40.8) | 65 (39.6) |
| Positive | 77 (59.2) | 99 (60.4) |
| Caregiver’s employment status | ||
| Unemployed | 18 (13.8) | 22 (13.3) |
| Self-employed | 103 (79.2) | 127 (76.5) |
| Private or government sector | 9 (6.9) | 17 (10.2) |
| Child’s age (mean) | 10.0 (10.8, 2.5) | 10.0 (9.7, 1.9) |
| Child’s sex | ||
| Male | 51 (41.5) | 92 (55.1) |
| Female | 72 (58.5) | 75 (44.9) |
| Attending school | ||
| No | 1 (0.8) | 0 (0.0) |
| Yes | 121 (99.2) | 167 (100.0) |
| HIV transmission | ||
| MTC | 92 (97.9) | 144 (91.1) |
| Other | 2 (2.1) | 14 (8.9) |
Unexpected challenges
Among the challenges encountered thus far are factors that have had an unexpected influence on the participating clinic operations including a lengthy physician strike and irregularity of paediatric antiretroviral medications available for distribution at the participating sites. Shortages of CD4+ reagents and viral load tests kits have also been challenging as has the unexpected change in some of the children’s caregivers during the course of the study follow-up.
It was anticipated that some participants, given low levels of education, would have difficulty completing the self-report study questionnaires independently. This is handled by collecting the data orally in face-to-face interviews. However, a related, unanticipated issue has been the difficulty some participants have rendering responses in scales with a graded response format (e.g. 1–7 Likert-type scales). In order to facilitate an understanding of the meaning of the numeric gradations, the interviewers have developed a culturally familiar, pictorial aid, such as food baskets filled to different levels, to signify amount of agreement with the questionnaire statement.
Discussion
Despite compelling evidence supporting the merits of informing children of their HIV status, disclosure of HIV to infected children is lagging. Little emphasis has been placed on equipping the child’s caregiver with information and skills to promote disclosure, particularly, when the caregiver faces a variety of sociocultural barriers and is reluctant to do so. The purpose of this ongoing project is to provide information on the efficacy of a structured disclosure intervention that can be integrated into usual care in resource-limited settings to facilitate the engagement of caregivers in the process of disclosure in a socioculturally, developmentally appropriate manner.
The project builds on our preliminary and ongoing work in Ghana and the complementary expertise of the team; together, they provide a socioculturally contextualized understanding of the problem and inform the content and structure of the disclosure intervention that is being tested. Our preliminary work shows a variety of sociocultural contextual barriers and deficient skills drive the persistent reluctance of caregivers and healthcare providers to inform children of their diagnosis. We propose that several key factors may be modified and the process of disclosure promoted with an intervention approach that is grounded in behavioural and bioecological systems theory.
The project is progressing well with nearly half of the target caregiver–child dyads enrolled to date. Success may be attributed to a number of factors, including the strength of the working relationships between the bi-national team, the web-accessible database system and the interest and engagement of participants in the project.
Implementation has not been without challenges. One unanticipated issue of note to date is our use of standardized Likert-type self-report measures in a population with lower numeric literacy than the population in which the measures were originally developed and tested. The literature is replete with information on methods for the cross-cultural adaptation of self-report questionnaires for use in a new country, culture and/or language in order to reach equivalence between the original source and target setting [44–46]. However, little attention has been given to methods to reduce error associated with numerical literacy [47–50]. In this study, we elected to retain the established response anchors of the standardized measures, but use a pictorial aid to enhance comprehension. We acknowledge the potential limitations of this approach and think that it is an important area for further study.
Randomization by site is advantageous in that it allows us to control for contamination across individuals within the study sites. However, this design introduces the potential for baseline differences between treatment and control groups and correlation between individuals within each site. When comparing differences in outcomes achieved, we must account for the fact that two participants sampled from a single site are more likely to be similar (in terms of outcomes) than two participants sampled from different sites. Compared with trial designs in which individuals are randomized, the SANKOFA trial design introduces greater complexity as nonindependence must be assessed and corrected analytically as indicated.
Generalizability of the study may be limited by a few factors. The sites selected for conduct of the study offer the advantage of an infrastructure to support conduct of the study with rigor. It is acknowledged that the level of clinical research expertise available at these sites may not be representative of other HIV treatment centres in Ghana, but resources important for the delivery of the intervention are likely to be available at most sites. The intervention is delivered by a clinic staff member and designated as the site ‘disclosure specialist’. This approach was selected because it was thought to be both practical and a potentially effective, sustainable model. The clinic staff member is knowledgeable of local sociocultural norms and the challenges of living with HIV. In the role as ‘disclosure specialist’, the clinic staff member becomes the ‘point person’ and agent for change over time. It is possible that this specialist approach could limit general-izability of the intervention to other sites with more limited resources. This warrants further examination. Findings from this study should provide some insight, as we are assessing the total amount of time the specialist spends in delivery of the intervention and other aspects of feasibility.
In conclusion, the low prevalence of disclosure underscores the need for a systematic and a staged approach in disclosing HIV status to infected children in resource-limited countries. Findings from this study will show whether a culturally relevant, standardized disclosure intervention that can be integrated into routine clinical paediatric HIV care can improve the welfare of children and their caregivers in Ghana. Results from this project will also contribute to a better understanding of factors and processes driving paediatric HIV disclosure as well as methods for use in future studies.
Acknowledgments
We thank the Sankofa Project caregiver and child dyads for their participation. We are grateful to the staff at Pediatric AIDS Clinics at Korle-Bu and Komfo Anokye Teaching Hospitals and the Ghana–Yale Partnership for Global Health for their support. We would also like to acknowledge the full roster of study team members by site: Elijah Paintsil, Nancy Reynolds, Tassos Kyriakides, Xiangyu Cong and Yram Foli, Yale University, USA; Lorna Renner, Margaret Lartey, Angela Ofori-Atta, Jonas Kusah Tetteh, Joyceline Assimeng, Obedia Akweley Seaneke, Dramani Yakubu, and Kevin Bonsu, Korle-Bu Teaching Hospital, Accra, Ghana; Sampson Antwi, Kofi Aikins Amissah, Anthony Enimil, Amina Alhassan and Irene Pokuaa Ofori, Komfo Anokye Teaching Hospital, Kumasi, Ghana; Ann Christine Catlin, Sumudinie Fernando, Ruwan Egoda Gamage, Ruchith Fernando and Sudheera Fernando, Purdue University, USA.
This study was made possible by a grant from NIH/ NICHD (R01HD074253). The content of the study is solely the responsibility of the authors and does not necessarily represent the official view of NIH.
Nancy Reynolds did the conception and design of the work, analysis and interpretation of data, drafting manuscript.
Angela Ofori-Atta and Tassos Kyriakides did the conception and design of the work, analysis and interpretation of data, revising manuscript for important intellectual content.
Margaret Lartey and Sampson Antwi did the conception and design of the work, interpretation of data, revising manuscript for important intellectual content.
Anthony Enimil did the acquisition and interpretation of data, revising manuscript for important intellectual content.
Ann Christine Catlin did the conception and design of the work, drafting and revising manuscript for important intellectual content.
Sumudinie Fernando did the conception and design of the work, revising manuscript for important intellectual content.
Elijah Paintsil did the conception and design of the work, analysis and interpretation of data, drafting and revising manuscript for important intellectual content.
All authors gave final approval of this version and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding for this study was provided by National Institutes of Health (NIH), Eunice Kennedy Shriver National Institute of Child Health & Human Development (R01 HD074252).
ClinicalTrials.gov Identifier: NCT01701635.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.UNAIDS. Global report. [Accessed 15 March 2015];UNAIDS report on the global AIDS epidemic. 2013 http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
- 2.WHO. [Accessed 15 March 2015];Global update on the health sector response to HIV. 2014 http://apps.who.int/iris/bitstream/10665/128196/1/WHO_HIV_2014.15_eng.pdf?ua=1.
- 3.Vreeman RC, Gramelspacher AM, Gisore PO, Scanlon ML, Nyandiko WM. Disclosure of HIV status to children in resource-limited settings: a systematic review. J Int AIDS Soc. 2013;16:1–14. doi: 10.7448/IAS.16.1.18466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Disclosure of illness status to children and adolescents with HIV infection. American Academy of Pediatrics Committee on Pediatrics AIDS. Pediatrics. 1999;103:164–166. doi: 10.1542/peds.103.1.164. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Guideline on HIV disclosure counselling for children up to 12 years of age. Geneva, Switzerland: World Health Organization; 2011. [PubMed] [Google Scholar]
- 6.Ayres JR, Paiva V, Franca I, Jr, Gravato N, Lacerda R, Della Negra M, et al. Vulnerability, human rights, and comprehensive healthcare needs of young people living with HIV/AIDS. Am J Public Health. 2006;96:1001–1006. doi: 10.2105/AJPH.2004.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United Nations General Assembly. UN Convention on the Rights of the Child. UN Office of the High Commissioner for Human Rights; Nov 20, 1989. [Accessed March 15, 2015]. http://www.ohchr.org/en/professionalinterest/pages/crc.aspx. [Google Scholar]
- 8.Gerson AC, Joyner M, Fosarelli P, Butz A, Wissow L, Lee S, et al. Disclosure of HIV diagnosis to children: when, where, why, and how. J Pediatr Healthcare. 2001;15:161–167. doi: 10.1067/mph.2001.114835. [DOI] [PubMed] [Google Scholar]
- 9.Wiener L, Mellins CA, Marhefka S, Battles HB. Disclosure of an HIV diagnosis to children: history, current research, and future directions. J Dev Behav Pediatr. 2007;28:155–166. doi: 10.1097/01.DBP.0000267570.87564.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallem S, Renner L, Ghebremichael M, Paintsil E. Prevalence and pattern of disclosure of HIV status in HIV-infected children in Ghana. AIDS Behav. 2011;15:1121–1127. doi: 10.1007/s10461-010-9741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinzon-Iregui MC, Beck-Sague CM, Malow RM. Disclosure of their HIV status to infected children: a review of the literature. J Trop Pediatr. 2013;59:84–89. doi: 10.1093/tropej/fms052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vreeman RC, Scanlon ML, Mwangi A, Turissini M, Ayaya SO, Tenge C, et al. A cross-sectional study of disclosure of HIV status to children and adolescents in western Kenya. PLoS One. 2014;9:e86616. doi: 10.1371/journal.pone.0086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heeren GA. Changing methods of disclosure. Literature review of disclosure to children with terminal illnesses, including HIV. Innovation (Abingdon) 2011;24:199–208. doi: 10.1080/13511610.2011.553506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abadia-Barrero CE, Larusso MD. The disclosure model versus a developmental illness experience model for children and adolescents living with HIV/AIDS in Sao Paulo, Brazil. AIDS Patient Care STDS. 2006;20:36–43. doi: 10.1089/apc.2006.20.36. [DOI] [PubMed] [Google Scholar]
- 15.Jemmott JB, 3rd, Heeren GA, Sidloyi L, Marange CS, Tyler JC, Ngwane Z. Caregivers’ intentions to disclose HIV diagnosis to children living with HIV in South Africa: a theory-based approach. AIDS Behav. 2014;18:1027–1036. doi: 10.1007/s10461-013-0672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberdorfer P, Puthanakit T, Louthrenoo O, Charnsil C, Sirisanthana V, Sirisanthana T. Disclosure of HIV/AIDS diagnosis to HIV-infected children in Thailand. J Paediatr Child Health. 2006;42:283–288. doi: 10.1111/j.1440-1754.2006.00855.x. [DOI] [PubMed] [Google Scholar]
- 17.Lesch A, Swartz L, Kagee A, Moodley K, Kafaar Z, Myer L, et al. Paediatric HIV/AIDS disclosure: towards a developmental and process-oriented approach. AIDS Care. 2007;19:811–816. doi: 10.1080/09540120601129301. [DOI] [PubMed] [Google Scholar]
- 18.Cantrell K, Patel N, Mandrell B, Grissom S. Pediatric HIV disclosure: a process-oriented framework. AIDS Educ Prev. 2013;25:302–314. doi: 10.1521/aeap.2013.25.4.302. [DOI] [PubMed] [Google Scholar]
- 19.Ghana AIDS Commission (GAC) Country AIDS Response Progress Report – Ghana. Accra, Ghana: GAC; Mar 31, 2014. [Google Scholar]
- 20.Ghana AIDS Commission (GAC) National strategic plan for HIV, 2011–2015. Accra, Ghana: GAC; 2011. [Google Scholar]
- 21.Arrive E, Dicko F, Amghar H, Aka AE, Dior H, Bouah B, et al. HIV status disclosure and retention in care in HIV-infected adolescents on antiretroviral therapy (ART) in West Africa. PLoS One. 2012;7:e33690. doi: 10.1371/journal.pone.0033690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bikaako-Kajura W, Luyirika E, Purcell DW, Downing J, Kaharuza F, Mermin J, et al. Disclosure of HIV status and adherence to daily drug regimens among HIV-infected children in Uganda. AIDS Behav. 2006;10:S85–93. doi: 10.1007/s10461-006-9141-3. [DOI] [PubMed] [Google Scholar]
- 23.Kenu E, Obo-Akwa A, Nuamah GB, Brefo A, Sam M, Lartey M. Knowledge and disclosure of HIV status among adolescents and young adults attending an adolescent HIV clinic in Accra, Ghana. BMC Research Notes. 2014;7:1–6. doi: 10.1186/1756-0500-7-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bronfenbrenner U, Ceci SJ. Nature-nurture reconceptualized in developmental perspective: a bioecological model. Psychol Rev. 1994;101:568–586. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- 25.Fisher JD, Fisher WA, Williams SS, Malloy TE. Empirical tests of an information-motivation-behavioral skills model of AIDS-preventive behavior with gay men and heterosexual university students. Health Psychol. 1994;13:238–250. doi: 10.1037//0278-6133.13.3.238. [DOI] [PubMed] [Google Scholar]
- 26.Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25:462–473. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- 27.Fisher JD, Amico KR, Fisher WA, Harman JJ. The information-motivation-behavioral skills model of antiretroviral adherence and its applications. Curr HIV/AIDS Rep. 2008;5:193–203. doi: 10.1007/s11904-008-0028-y. [DOI] [PubMed] [Google Scholar]
- 28.Amico RK. A situated-Information Motivation Behavioral Skills Model of Care Initiation and Maintenance (sIMB-CIM): an IMB model based approach to understanding and intervening in engagement in care for chronic medical conditions. J Health Psychol. 2011;16:1071–1081. doi: 10.1177/1359105311398727. [DOI] [PubMed] [Google Scholar]
- 29.Levin KA. Study design I. Evid Based Dent. 2005;6:78–79. doi: 10.1038/sj.ebd.6400355. [DOI] [PubMed] [Google Scholar]
- 30.Levin KA. Study design II. Issues of chance, bias, confounding and contamination. Evid Based Dent. 2005;6:102–103. doi: 10.1038/sj.ebd.6400356. [DOI] [PubMed] [Google Scholar]
- 31.Cutrona CE, Russell DW. The provisions of social relationships and adaptation to stress. Advances in Personal Relationships. 1987;1:37–67. [Google Scholar]
- 32.Carey MP, Schroder KE. Development and psychometric evaluation of the brief HIV Knowledge Questionnaire. AIDS Educ Prev. 2002;14:172–182. doi: 10.1521/aeap.14.2.172.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60:631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 34.Berger BE, Ferrans CE, Lashley FR. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Res Nurs Health. 2001;24:518–529. doi: 10.1002/nur.10011. [DOI] [PubMed] [Google Scholar]
- 35.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7:151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 36.Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol. 1984;40:1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs M. Children’s depression inventory. Manual. North Tonawanda, NY: Multi-Health Systems; 1992. [Google Scholar]
- 38.Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds NR, Sun J, Nagaraja HN, Gifford AL, Wu AW, Chesney MA. Optimizing measurement of self-reported adherence with the ACTG Adherence Questionnaire: a cross-protocol analysis. J Acquir Immune Defic Syndr. 2007;46:402–409. doi: 10.1097/qai.0b013e318158a44f. [DOI] [PubMed] [Google Scholar]
- 40.Grossberg R, Zhang Y, Gross R. A time-to-prescription-refill measure of antiretroviral adherence predicted changes in viral load in HIV. J Clin Epidemiol. 2004;57:1107–1110. doi: 10.1016/j.jclinepi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Achenbach T. Manual for the child behavior checklist. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 42.Vaz L, Corneli A, Dulyx J, Rennie S, Omba S, Kitetele F, et al. The process of HIV status disclosure to HIV-positive youth in Kinshasa, Democratic Republic of the Congo. AIDS Care. 2008;20:842–852. doi: 10.1080/09540120701742276. [DOI] [PubMed] [Google Scholar]
- 43.Catlin AC, Fernando S, Gamage R, Renner L, Antwi S, Tettey JK, et al. Sankofa Pediatric HIV Disclosure Intervention Cyber Data Management: building capacity in a resource-limited setting and ensuring data quality. AIDS Care. doi: 10.1080/09540121.2015.1023246. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993;46:1417–1432. doi: 10.1016/0895-4356(93)90142-n. [DOI] [PubMed] [Google Scholar]
- 45.Hunt SM, Alonso J, Bucquet D, Niero M, Wiklund I, McKenna S. Cross-cultural adaptation of health measures. Health Policy. 1991;19:33–44. doi: 10.1016/0168-8510(91)90072-6. [DOI] [PubMed] [Google Scholar]
- 46.Perneger TV, Leplège A, Etter JF. Cross-cultural adaptation of a psychometric instrument: two methods compared. J Clin Epidemiol. 1999;52:1037–1046. doi: 10.1016/s0895-4356(99)00088-8. [DOI] [PubMed] [Google Scholar]
- 47.Mays MZ, Martz W, Castro KM, DeWalt DA, Pignone MP, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3:514–522. doi: 10.1370/afm.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rawson KA, Gunstad J, Hughes J, Spitznagel MB, Potter V, Waechter D, Rosneck J. The METER: a brief, self-administered measure of health literacy. J Gen Intern Med. 2009;25:67–71. doi: 10.1007/s11606-009-1158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.NIMH Collaborative HIV/STD Prevention Trial Group. The feasibility of audio computer-assisted self-interviewing in international settings. AIDS. 2007;21(Suppl 2):S49–S58. doi: 10.1097/01.aids.0000266457.11020.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown SM, Culverb JO, Osannc KE, MacDonald DJ, Sand S, Thorntonb AA, et al. Health literacy, numeracy, and interpretation of graphical breast cancer risk estimates. Patient Educ Couns. 2011;83:92–98. doi: 10.1016/j.pec.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]