Abstract
The human brain possesses a remarkable ability to adapt in response to changing anatomical (e.g., aging) or environmental modifications. This form of neuroplasticity is important at all stages of life but is critical in neurological disorders such as amblyopia and stroke. This review focuses upon our new understanding of possible mechanisms underlying functional deficits evidenced after adult-onset stroke. We review the functional interactions between different brain regions that may contribute to motor disability after stroke and, based on this information, possible interventional approaches to motor stroke disability. New information now points to the involvement of non-primary motor areas and their interaction with the primary motor cortex as areas of interest. The emergence of this new information is likely to impact new efforts to develop more effective neurorehabilitative interventions using transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) that may be relevant to other neurological disorders such as amblyopia.
Keywords: rehabilitation, motor recovery, stroke
INTRODUCTION
The human brain continues to adjust throughout life and this form of neuroplasticity is particularly important in neurological disorders such as stroke. In addition to producing a significant socio-economic burden, stroke is still the leading cause of long-term motor disability among adults in the world (Cumberland Consensus Working Group, 2009; Feigin, Lawes, Bennett, Barker-Collo, & Parag, 2010). Even years after the initial stroke the human brain still retains the capacity to reorganize in response to interventions that can influence recovery of motor function (Cramer, 2008; Hodics, Cohen, & Cramer, 2006; Johansen-Berg et al., 2002a; Liepert, Bauder, Miltner, Taub, & Weiller, 2000; Taub, Uswatte, & Elbert, 2002; Ward & Cohen, 2004). Understanding and influencing this form of neuroplasticity is critical to finding towards better therapies for patients (Cramer, 2008; Fregni & Pascual-Leone, 2006; Hodics et al., 2006; Hummel & Cohen, 2006). This review focuses upon recent developments in clinical and system-level neuroscience that contributed to the understanding of the mechanisms of recovery of motor function after stroke and possible strategies to influence it.
Since the emergence of techniques able to probe the human brain in vivo, it has become clear that practice of a particular task is associated with dynamic changes in the neural networks as the subjects learn (Karni et al., 1998). After brain lesions like stroke, cortical areas that are remote from the structural damage, such as the primary motor cortex, can reorganize to facilitate motor performance as well as motor learning (Calautti & Baron, 2003; Cramer, 2008; Cramer et al., 1997; Grefkes et al., 2008; Loubinoux et al., 2007; Talelli, Greenwood, & Rothwell, 2006; Ward, Brown, Thompson, & Frackowiak, 2003a; Ward & Cohen, 2004). The available evidence suggests that cortical reorganization accompanies recovery of motor function after stroke. One form of cortical reorganization involves the modulation of interactions between the primary motor cortices in the ipsilesional hemisphere (same as the stroke), and the contralesional hemisphere (opposite to the stroke; Calautti et al., 2007; Duque et al., 2005; Grefkes et al., 2008; Murase, Duque, Mazzocchio, & Cohen, 2004; Talelli et al., 2006; Tecchio et al., 2007; Ward et al., 2003a; Ward, Brown, Thompson, & Frackowiak, 2003b). The influence of abnormal interhemispheric interactions on normal and abnormal cognitive functions after brain lesions has been reported in the fields of language and spatial orientation (Kinsbourne, 1980; Koch et al., 2008; Oliveri et al., 2000).
In healthy subjects, performance of unilateral hand movements is associated with activation of predominantly contralateral motor areas, including the primary motor cortex (Blinkenberg et al., 1996). Performance of complex motor tasks when acquiring a novel motor skill entrains bihemispheric activity to a larger extent (Horenstein, Lowe, Koenig, & Phillips, 2008; Karni et al., 1998). In stroke patients, performance of simple hand movements using the weak hand leads to activation of a widespread bilateral motor network that includes both primary motor cortices (Calautti et al., 2007; Grefkes et al., 2008; Tecchio et al., 2007; Ward et al., 2003a,b), that is, both the ipsilesional and the contralesional hemisphere show active changes in BOLD signal (see Corbetta, this issue). However, direct comparison between neuroimaging studies involving healthy subjects and stroke patients are often methodologically difficult and certain caveats must be considered when interpreting the data. For instance, the magnitude of BOLD changes in cortical activity associated with performance of a task depends on the force, number of muscles activated and even attention paid to the particular movements—all factors that differ between stroke patients and healthy subjects performing the same task. Therefore, it is important to monitor these factors carefully and make sure that they are comparable when assessing differences in functional activation studies. For example, the extent to which subjects in both groups activated similar or different muscles (EMG monitoring) or moved different body parts in the same or homologous body parts (accelerometer monitoring) has largely been overlooked but may partly explain the difference between the cortical activation patterns of stroke patients compared to control subjects (see, Sehm, Perez, Xu, Hidler, & Cohen, 2009 for discussion).
Despite these issues, performance of longitudinal studies has started to provide new information on the functional neuroanatomy of recovery of function after stroke. Different authors reported that better functional recovery is associated with increased fMRI activity in the ipsilesional primary motor cortex (Calautti et al., 2007; Gerloff et al., 2006; Ward et al., 2003a; Ward & Cohen, 2004), a finding consistent with transcranial magnetic stimulation (TMS) studies (Turton, Wroe, Trepte, Fraser, & Lemon, 1996; Werhahn, Mortensen, Kaelin-Lang, Boroojerdi, & Cohen, 2002). In other words, the general picture that emerged from these early studies is that the greater the activity in the ipsilesional primary motor cortex with movements of the paretic hand, the better the recovery of motor performance (Calautti et al., 2007; Grefkes et al., 2008; Johansen-Berg et al., 2002a; Tecchio et al., 2007; Ward et al., 2003a,b).
It is not clear why involvement of the contralesional primary motor cortex during movements of the paretic hand should be associated with poor recovery. Using different techniques, it has been demonstrated that the contralesional primary motor cortex exerts a persistent inhibitory drive over the ipsilesional primary motor cortex in the process of generation of voluntary movements by the paretic hand. Importantly, the magnitude of this inhibition correlates with motor impairment after stroke (Duque et al., 2005; Grefkes et al., 2008; Harris-Love, Perez, Chen, & Cohen, 2007; Murase et al., 2004). It remains to be determined if this effect occurs through direct interactions between interhemispheric inhibition across the primary motor cortices or via intracortical inhibitory circuits (GABAergic) within the ipsilesional primary motor cortex (Hummel et al., 2009; Perez & Cohen, 2008). It also remains to be determined the extent to which this abnormality applies to a wide range of tasks.
It stands to reason that interventions capable of normalizing this activity-dependent hemispheric imbalance between the motor cortices could improve motor function. In other words, promoting cortical reorganization that leads to activation and interhemispheric inhibitory interactions that resembles those observed in healthy subjects may contribute to the process of recovery of motor function. It is on this foundation that a simple model of post-stroke interactions between the primary motor cortices was proposed years ago (Ward & Cohen, 2004). It was suggested that facilitating activity in the ipsilesional primary motor cortex or down regulating activity in the contralesional primary motor cortex in association with motor training could facilitate functional recovery after stroke (Hummel & Cohen, 2006). Various techniques have been proposed to accomplish these goals (Tab. 1).
Table 1.
Overview of Transcranial Magnetic Stimulation (TMS), Transcranial Direct Current Stimulation (TDCS) and Somatosensory Stimulation
| TMS |
| TMS was first used in humans in 1986 |
| A high voltage capacitor is discharged through insulated wires to produce a rapidly changing magnetic field |
| The magnetic field passes through the skull unchanged and when it collapses it induces an electrical current in underlying brain tissue |
| A number of different coil shapes exist that influence the magnetic field and therefore the characteristic of the stimulation |
| The activated neurons include excitatory and inhibitory neurons |
| Depending upon the coil used, the area stimulated can be relatively focal (mm) |
| The frequency and intensity of the stimulation govern whether the overall effect is inhibitory or excitatory |
| Sham TMS coils exist though it is currently impossible to blind the experimenter |
| Current TMS equipment is not designed to be portable |
| For further details see Dimyan and Cohen (2010) and Hummel and Cohen (2006) |
| tDCS |
| tDCS was first used in humans in the 1960’s |
| Two electrodes (cathode and anode) are placed over the scalp. A number of different configurations or montages have been developed |
| A weak electrical current (1–2 mA) is applied to the electrodes that alter the excitability of the underlying neurons |
| The Cathode and Anode have different effects on the underlying neurons |
| Although different electrodes exist the area stimulated is generally diffuse (cm) |
| The subject only feels “tingling” under the electrodes while the electric current is increased which then disappears once the electrical current is stable (30 s)—this characteristic means that unlike TMS, TDCS can be easily double blinded |
| tDCS equipment is small and portable |
| For further details see Schlaug, Renga, & Nair (2008) |
| Somatosensory stimulation |
| Peripheral nerve stimulation was first used in humans in the late 1960’s |
| Electrodes are placed over the peripheral nerve. A number of different protocols exist but essentially trains of electrical pulses are delivered along the nerve |
| The effects of somatosensory stimulation are thought to be modulated cortically |
| It is difficult to double blind the intervention |
| The equipment is small and portable |
| For further details see Celnik et al. (2007), Conforto et al. (2007), Cramer (2008), Floel et al. (2008), Wu et al. (2006) |
By in large this early model of recovery of motor function was supported by the evidence (see also Corbetta, this issue). Methods capable of increasing cortical excitability or activity within the ipsilesional primary motor cortex, including transcranial magnetic (TMS) and direct current (tDCS) stimulation (Di Lazzaro et al., 2008; Khedr, Ahmed, Fathy, & Rothwell, 2005; Talelli, Greenwood, & Rothwell, 2007; Hummel et al., 2005, 2006; Hummel & Cohen, 2006) and somatosensory stimulation (Celnik, Hummel, Harris-Love, Wolk, & Cohen, 2007; Conforto, Cohen, Santos, Scaff, & Marie, 2007; Cramer, 2008; Floel et al., 2008; Wu, Seo, & Cohen, 2006) have been tested in both healthy subjects and patients with stroke. In stroke patients, the overall purpose as stated above was to document the ability of these techniques to facilitate excitability within the ipsilesional or decrease excitability in the contralesional primary motor cortices to modify motor function when applied alone or in combination with motor training after stroke. Proof of principle studies have been implemented in various laboratories at this point and several reviews are available proving that both approaches lead to some level of improvement in motor function after stroke in small clinical trials. It should be kept in mind that results of multicenter well-controlled clinical trials are not available yet (see Fregni & Pascual-Leone, 2006 for review and Tab. 1). Of interest is that application of tDCS montages that engage both motor cortices have been proposed: placement of anodal tDCS electrodes over the one primary motor cortex (M1) and cathodal tDCS over the opposite M1 provides beneficial effects that may go beyond those elicited by only anodal or cathodal tDCS alone (Vines, Cerruti, & Schlaug, 2008).
An additional strategy utilized to facilitate activity in ipsilesional M1 or downregulate it in contralesional M1 has been modulation of somatosensory input originated in the paretic or healthy hands. For example, it has been shown that either anesthesizing the non-affected hand of patients with chronic stroke (e.g., by a peripheral nerve block) or application of somatosensory stimulation to the paretic hand results in performance improvements in the paretic limb (Floel et al., 2004; Voller et al., 2006). Normalization of activity-dependent modulation of interhemispheric inhibitory interactions accompanies these functional improvements (Floel et al., 2008). Caveats similar to those described above apply to these investigations since most of them engaged small number of patients and well-controlled multicenter clinical trials are required.
As stated above, a further development in cortical manipulation was to combine strategies. For example, it appears that up-regulating excitability in the ipsilesional motor cortex while down regulating the contralesional motor cortex is capable of improving motor performance to a greater extent than either intervention alone (Vines et al., 2008). Another combination tested recently has been facilitating training effects by up regulating excitability in the ipsilesional primary motor cortex while stimulating the paretic hand with peripheral nerve stimulation (Celnik, Paik, Vandermeeren, Dimyan, & Cohen, 2009). One important piece of information emerging from these investigations is that in general none of these forms of stimulation (central or peripheral) can by themselves induce profound facilitatory effects on motor performance. In order to accomplish optimal effects, they require synchronous application, along Hebbian rules, with motor training protocols (Reis et al., 2009). These promising results have raised many questions, including the influence of specific genetic polimorphisms on the ability to learn a new skill or recover motor function in response to training protocols or to cortical stimulation (Fritsch et al., 2010).
Research in the last few years has made clear though that despite these exciting results, activity in the primary motor cortex and its relationship to functional recovery is amenable to modulation from many other cortical regions and is influenced by activity changes in more widespread bilateral motor networks (Gerloff et al., 2006; Lotze et al., 2006; Sharma, Baron, & Rowe, 2009a). Further more functionally important changes in cortical activity may be overlooked unless movement is broken down into separate cognitive processes (Sharma et al., 2009a,b; see Corbetta, this issue). For the remainder of the review we will discuss the additional areas that may impact recovery of motor function and its influences on motor output after stroke. Better understanding of the specific aspects of movement function to be modulated by either training or interventional protocols is important. In Figure 1, we divide movement into three broad categories and hence cortical networks; motor processes that precede movement (Figure 1A), movement itself (Figure 1B), and sensory feedback (Figure 1C). It is likely that a better understanding of how these different interventional approaches influence each of these stages will lead in the future to better designed rehabilitative interventions. For instance, it is plausible that multiple cortical areas need to be stimulated but at different stages of the motor process to optimally facilitate the recovery of motor performance after stroke.
FIGURE 1.
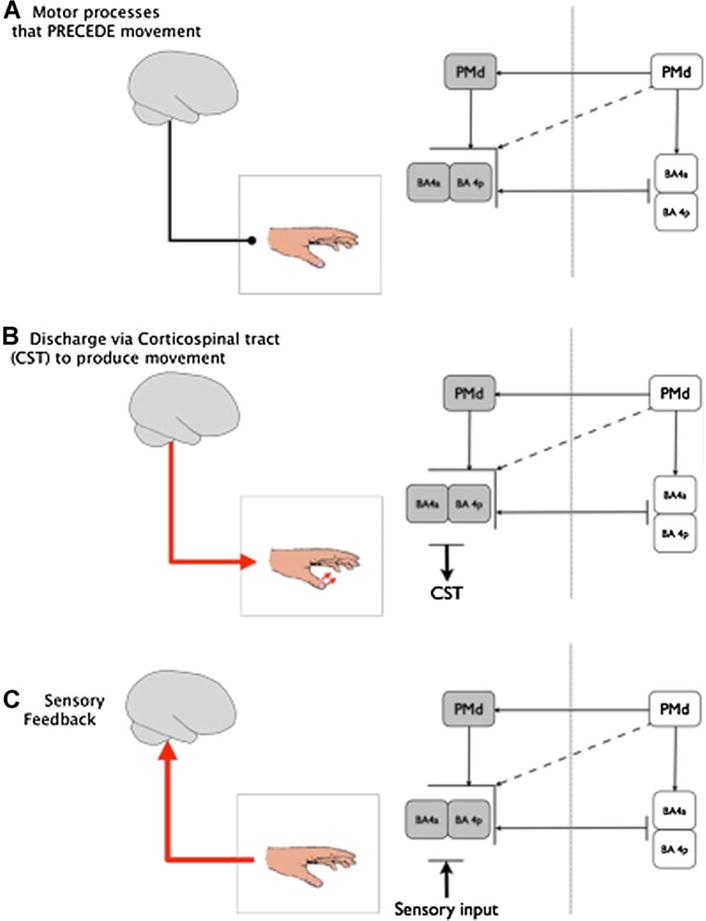
Movement has been divided into three broad cortical networks that reflect each stage of movement. It is likely that stroke has a differential effect on each of the networks and by understanding the interaction between and within these networks we can devise novel methods to improve motor function after stroke. The affected hemisphere is shaded in gray while the non-affected hemisphere is white. (A) Motor processes that precede movement such as motor planning. For example, this network can be accessed using motor imagery and action observation. (B) Discharge via the corticospinal tract to produce movement. Although all of these networks are present during physical movement this cortical network is dominant when combined with the other 2. (C) Sensory feedback or input. For example, this can be accessed using peripheral nerve stimulation.
Although the primary motor cortex is largely considered a single node, it actually consists of two distinct regions, an anterior and posterior component (labeled BA 4a and BA 4p respectively in humans (Geyer et al., 1996)). BA4a and BA4p have discrete characteristics including different cytoarchitecture and receptor density (Geyer et al., 1996) that suggest different but not exclusive functions. Of the two regions BA 4a is thought to be the phylogentically older of the two regions (Rathelot & Strick, 2009) and more “executive” in nature, that is, the output from BA4a is conducted via the corticospinal tract and spinal interneurons to produce physical movement. BA4p, the “new” motor cortex, contains cortico-motoneuronal cells that synapse directly onto the spinal motoneurons (Rathelot & Strick, 2009). These monosynaptic connections bypass the spinal interneurons and appear to be involved in sculpting highly skilled movements. In other words, BA4p is likely involved in “non-executive” functions required by complex movement (“non-executive” in this context refers to the notion that it is not involved in generating the actual physical movement shown in Figure 1A). This issue is presently under investigation and is relevant to the process of recovery of motor function after stroke because it may impact both specific cortical location, as well as timing of application of brain stimulation techniques. After stroke, the magnitude of fMRI activity in BA4p has been shown to correlate with the magnitude of recovery (Sharma et al., 2009a; Ward et al., 2003b). Indeed the degree of BA4p activity after sub-cortical stroke appears to predict the ability of patients to perform motor tasks 1 year later (Loubinoux et al., 2007). How does this relate to our understanding of recovery processes after stroke? Interestingly, somatosensory stimulation applied to the upper limb accesses area BA4p in healthy volunteers (Geyer et al., 1996). It is conceivable but as yet unconfirmed, that activity in BA4p and normal sensory feedback during movement (Figure 1C) may contribute to the improvements in motor function reported with somatosensory stimulation of the paretic hand (Conforto et al., 2007; Floel et al., 2008; Wu et al., 2006). If this were the case, it would be desirable to combine manipulation of somatosensory input from the paretic hand with cognitive training geared to facilitate activity in this area BA4p (i.e., to focus on Figure 1A,C)—an issue for future investigation.
In healthy volunteers thinking about movement, motor imagery, predominantly engages area BA4p relative to BA4a (Sharma, Jones, Carpenter, & Baron, 2008). There is already considerable interest in using motor imagery to access motor system in stroke patients and as an adjuvant form of rehabilitation (Celnik, Webster, Glasser, & Cohen, 2008; Sharma, Pomeroy, & Baron, 2006). The attraction of motor imagery is that it is not limited by the patient’s ability to execute motions required during physical training and so it can be utilized in patients that are unable to carry out customary motor training rehabilitative protocols. Furthermore, as it does not require physical movements motor imagery allows evaluation of neuroimaging changes in the motor system that may be confounded during motor execution (Sharma et al., 2009a,b; Figure 1A). An interesting example is that while fMRI activity appears normal in well-recovered subcortical stroke patients performing physical movements, motor imagery highlights abnormal hemispheric imbalances within area BA4p, the degree of which correlates with motor impairment (Sharma et al., 2009a). An important conclusion from these previous investigations is that future work should focus more on the different roles of different subregions of M1 on skill acquisition and functional recovery. Thus, motor imagery represents an exciting means to access the motor processes that precede physical movement (Figure 1A). Could area BA4p be targeted using tDCS and TMS? As shown in Table 1, tDCS alters neuronal activity in a wide cortical region and so is unlikely to be able to selectively stimulate BA4p. In principle, TMS could provide focal stimulation to BA4a and BA4p by use of a frameless sterotactic tools but as the precentral gyrus is extremely heterogeneous in humans (Rademacher et al., 2001) a method of identifying BA4p and BA4a in vivo is needed.
The dorsal premotor cortex is involved in action selection (O’Shea, Johansen-Berg, Trief, Gobel, & Rushworth, 2007; Figure 1A). After stroke, previous work identified increased fMRI activation in the contralesional dorsal premotor cortex (Gerloff et al., 2006; Johansen-Berg et al., 2002b), which appeared more prominent in patients with less recovery (Johansen-Berg et al., 2002b; Ward et al., 2006). In poorly recovered stroke patients, disruption of the contralesional dorsal premotor cortex by TMS impairs motor performance (Johansen-Berg et al., 2002b; Lotze et al., 2006), pointing to a cause–effect link between this fMRI activation and performance. In contrast, it is disruption of the ipsilesional dorsal premotor cortex in subjects that have made good recovery after stroke that impairs motor performance (Fridman et al., 2004). Perhaps, this suggests a differential role for ipsi and contralesional homologous regions in the process of functional recovery that is dependent upon the magnitude of remaining impairment. Unlike the primary motor cortex, however, the hemispheric balance of influences of the dorsal premotor cortex on the opposite primary motor cortex does not relate to recovery of motor function when measured with fMRI (Calautti et al., 2007). Importantly, the dorsal premotor cortex has the capacity to rapidly adapt to disruption, at least when tested with virtual lesion approaches in healthy subjects (O’Shea et al., 2007). It also has bilateral corticospinal projections although evidence from primates suggest that these are in areas that are less relevant for performance of distal hand movements (Kuypers & Brinkman, 1970).
Another important anatomical finding reported in both primates (Marconi, Genovesio, Giannetti, Molinari, & Caminiti, 2003) and humans (Boorman, O’Shea, Sebastian, Rushworth, & Johansen-Berg, 2007; Koch et al., 2007) is that there are distinct transcallosal connection between dorsal premotor cortex and the primary motor cortex (which is shown in Figure 1). Indeed acting via these transcallosal pathways the primary motor cortex can be modulated by applying TMS stimulation to the opposite dorsal premotor cortex (Baumer et al., 2006; Mochizuki, Huang, & Rothwell, 2004). The interaction between these regions appears to contribute more to the process of action selection than to the process of execution per se (O’Shea et al., 2007), supporting the idea that cognitive processes up stream from execution operate in parallel or serial manner with those described above that connect both primary motor cortices. This area of research has so far developed intensively in healthy subjects and it is possible that future interventional approaches involving TMS or tDCS will target this region in combination with training protocols in an attempt to ameliorate recovery of function after stroke.
In addition to the dorsal premotor cortex, and subregions of the primary motor cortex including BA4a and BA4p, there are other regions that may prove to be important in the process of functional recovery after stroke. They include parietal, temporal, and non-primary frontal areas that interact directly or indirectly with primary motor cortex (Reis et al., 2008). The contribution of activity in these areas and their connections with primary motor cortex after stroke have not been explored yet but early studies suggest they are important (Sharma et al., 2009a). Studies evaluating the effects of stimulation of nonprimary motor areas on motor function in stroke patients are under way.
SUMMARY AND IMPLICATION FOR AMBLYOPIA
We have reviewed information on various interventional tools available to modulate motor function and their possible impact as adjuvant strategies to facilitate recovery of function after adult stroke. Additionally, we discussed the possible neurophysiological mechanisms and brain regions involved in contributing to various aspects of motor performance. Understanding these interactions and how they can be modulated after stroke will allow more focused rehabilitation. It should be kept in mind that these approaches have so far been tested in small number of patients, mostly adults and that they do not represent the standard of care, awaiting results from multicenter well-controlled clinical trials.
Clearly more studies are needed in the field of stroke taking place at earlier stages in life. Data is available, for example, from children who underwent hemispherectomy to control intractable seizures, but this differs substantially from stroke. These children are mostly able to recover limb function but often have difficulties in recovering individuated finger movements. However, there is an expectation for windows of opportunity for cortical reorganization. This has been explored very well in the field of language, for example (Vargha-Khadem et al., 1997). What is the impact of the development in mechanistic understanding of functional recovery and development of interventional tools in adult stroke studies for the field of amblyopia? While speculative, two considerations may be of interest. First, it would be interesting to consider the combination of modulation of sensory input as implemented during customary treatment for amblyopia with cortical stimulation to facilitate selective activity in target cortical areas and improve cortical reorganization. Second, it is possible that purposeful modulation of interhemispheric inhibitory interactions between parietal and occipital areas of both hemispheres could be utilized in combination with customary treatments, as mechanistic understanding of changes in network organization after amblyopia increases.
Acknowledgments
Contract grant sponsor: Intramural Research Program of the NINDS, NIH
References
- Baumer T, Bock F, Koch G, Lange R, Rothwell JC, Siebner HR, et al. Magnetic stimulation of human premotor or motor cortex produces interhemispheric facilitation through distinct pathways. The Journal of Physiology. 2006;572(3):857–868. doi: 10.1113/jphysiol.2006.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinkenberg M, Bonde C, Holm S, Svarer C, Andersen J, Paulson OB, et al. Rate dependence of regional cerebral activation during performance of a repetitive motor task: A PET study. J Cerebral Blood Flow Metabolism. 1996;16(5):794–803. doi: 10.1097/00004647-199609000-00004. [DOI] [PubMed] [Google Scholar]
- Boorman ED, O’Shea J, Sebastian C, Rushworth MFS, Johansen-Berg H. Individual differences in white-matter microstructure reflect variation in functional connectivity during choice. Current Biology. 2007;17(16):1426–1431. doi: 10.1016/j.cub.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: A review. Stroke. 2003;34(6):1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- Calautti C, Naccarato M, Jones PS, Sharma N, Day DD, Carpenter AT, et al. The relationship between motor deficit and hemisphere activation balance after stroke: A 3T fMRI study. NeuroImage. 2007;34(1):322–331. doi: 10.1016/j.neuroimage.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Celnik P, Hummel F, Harris-Love M, Wolk R, Cohen LG. Somatosensory stimulation enhances the effects of training functional hand tasks in patients with chronic stroke. Archives of Physical Medicine and Rehabilitation. 2007;88(11):1369–1376. doi: 10.1016/j.apmr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Celnik P, Paik NJ, Vandermeeren Y, Dimyan M, Cohen LG. Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke. 2009 doi: 10.1161/STROKEAHA.108.540500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celnik P, Webster B, Glasser DM, Cohen LG. Effects of action observation on physical training after stroke. Stroke, STROKEAHA. 2008;107:508184. doi: 10.1161/STROKEAHA.107.508184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforto A, Cohen L, Santos R, Scaff M, Marie S. Effects of somatosensory stimulation on motor function in chronic cortico-subcortical strokes. Journal of Neurology. 2007;254(3):333–339. doi: 10.1007/s00415-006-0364-z. [DOI] [PubMed] [Google Scholar]
- Cramer S. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Annals of Neurology. 2008;63(3):272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- Cramer S. Repairing the human brain after stroke. II. Restorative therapies. Annals of Neurology. 2008;63(5):549–560. doi: 10.1002/ana.21412. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, et al. A Functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28(12):2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- Cumberland Consensus Working Group. Cheeran B, Cohen L, Dobkin B, Ford G, Greenwood R, et al. The future of restorative neurosciences in stroke: Driving the translational research pipeline from basic science to rehabilitation of people after stroke. Neurorehabilitation and Neural Repair. 2009;23(2):97–107. doi: 10.1177/1545968308326636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Capone F, Ranieri F, et al. Modulating cortical excitability in acute stroke: A repetitive TMS study. Clinical Neurophysiology. 2008;119(3):715–723. doi: 10.1016/j.clinph.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Dimyan MA, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of functional recovery mechanisms after stroke. Neurorehabilitation and Neural Repair. 2010;24(2):125–135. doi: 10.1177/1545968309345270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. NeuroImage. 2005;28(4):940–946. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Lawes CMM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. The Lancet Neurology. 2010 doi: 10.1016/S1474-4422(09)70025-0. in press, corrected proof. [DOI] [PubMed] [Google Scholar]
- Floel A, Hummel F, Duque J, Knecht S, Cohen LG. Influence of somatosensory input on interhemispheric interactions in patients with chronic stroke. Neurorehabilitation and Neural Repair. 2008;22(5):477–485. doi: 10.1177/1545968308316388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floel A, Nagorsen U, Werhahn KJ, Ravindran S, Birbaumer N, Knecht S, et al. Influence of somatosensory input on motor function in patients with chronic stroke. Annals of Neurology. 2004;56(2):206–212. doi: 10.1002/ana.20170. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Hand motor recovery after stroke: tuning the orchestra to improve hand motor function. Cognitive and Behavioral Neurology. 2006;19(1):21–33. doi: 10.1097/00146965-200603000-00003. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127(4):747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron. 2010;66(2):198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129(3):791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, et al. Two different areas within the primary motor cortex of man. Nature. 1996;382(6594):805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak D, Eickhoff S, Dafotakis M, Jutta K, Karbe H, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Annals of Neurology. 2008;63(2):236–246. doi: 10.1002/ana.21228. [DOI] [PubMed] [Google Scholar]
- Harris-Love ML, Perez MA, Chen R, Cohen LG. Interhemispheric inhibition in distal and proximal arm representations in the primary motor cortex. Journal of Neurophysiology. 2007;97(3):2511–2515. doi: 10.1152/jn.01331.2006. [DOI] [PubMed] [Google Scholar]
- Hodics T, Cohen LG, Cramer SC. Functional imaging of intervention effects in stroke motor rehabilitation. Archives of Physical Medicine and Rehabilitation. 2006;87(12 Supplement 1):36–42. doi: 10.1016/j.apmr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Horenstein C, Lowe MJ, Koenig KA, Phillips MD. Comparison of unilateral and bilateral complex finger tapping-related activation in premotor and primary motor cortex. Human Brain Mapping. 2008;30(4):1397–1412. doi: 10.1002/hbm.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128(3):490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Non-invasive brain stimulation: A new strategy to improve neurorehabilitation after stroke? The Lancet Neurology. 2006;5(8):708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- Hummel F, Steven B, Hoppe J, Heise K, Thomalla G, Cohen L, et al. Deficient short intracortical inhibition (SICI) during movement preparation after chronic stroke. Neurology. 2009 doi: 10.1212/WNL.0b013e3181a609c5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F, Voller B, Celnik P, Floel A, Giraux P, Gerloff C, et al. Effects of brain polarization on reaction times and pinch force in chronic stroke. BMC Neuroscience. 2006;7(73) doi: 10.1186/1471-2202-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002a;125(12):2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MFS, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. PNAS. 2002b;99(22):14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, et al. The acquisition of skilled motor performance: Fast and slow experience-driven changes in primary motor cortex. Proceedings of the National Academy of Sciences. 1998;95(3):861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65(3):466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. Dichotic imbalance due to isolated hemisphere occlusion or directional rivalry? Brain and Language. 1980;11(1):221–224. doi: 10.1016/0093-934x(80)90123-6. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, Mochizuki H, Marconi B, Caltagirone C, Rothwell JC. Interactions between pairs of transcranial magnetic stimuli over the human left dorsal premotor cortex differ from those seen in primary motor cortex. The Journal of Physiology Online. 2007;578(2):551–562. doi: 10.1113/jphysiol.2006.123562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Cheeran B, Ruge D, Gerfo EL, Salerno S, et al. Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain. 2008;131(12):3147–3155. doi: 10.1093/brain/awn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers HGJM, Brinkman J. Precentral projections to different parts of the spinal intermediate zone in the rhesus monkey. Brain Research. 1970;24(1):29–48. doi: 10.1016/0006-8993(70)90272-6. [DOI] [PubMed] [Google Scholar]
- Liepert J, Bauder H, Miltner WHR, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31(6):1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. Journal of Neuroscience. 2006;26(22):6096–6102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubinoux I, Dechaumont-Palacin S, Castel-Lacanal E, De Boissezon X, Marque P, Pariente J, et al. Prognostic value of fMRI in recovery of hand function in subcortical stroke patients. Cerebral Cortex. 2007;17(12):2980–2987. doi: 10.1093/cercor/bhm023. [DOI] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Giannetti S, Molinari M, Caminiti R. Callosal connections of dorso-lateral premotor cortex. European Journal of Neuroscience. 2003;18(4):775–788. doi: 10.1046/j.1460-9568.2003.02807.x. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Huang YZ, Rothwell JC. Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. The Journal of Physiology Online. 2004;561(1):331–338. doi: 10.1113/jphysiol.2004.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen L. Influence of interhemispheric interactions on motor function in chronic stroke. Annals of Neurology. 2004;55(3):400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Rossini PM, Filippi MM, Traversa R, Cicinelli P, Palmieri MG, et al. Time-dependent activation of parieto-frontal networks for directing attention to tactile space. A study with paired transcranial magnetic stimulation pulses in right-brain-damaged patients with extinction. Brain. 2000;123(Pt 9):1939–1947. doi: 10.1093/brain/123.9.1939. [DOI] [PubMed] [Google Scholar]
- O’Shea J, Johansen-Berg H, Trief D, Gobel S, Rushworth MFS. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54(3):479–490. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. Journal of Neuroscience. 2008;28(22):5631–5640. doi: 10.1523/JNEUROSCI.0093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher J, Burgel U, Geyer S, Schormann T, Schleicher A, Freund HJ, et al. Variability and asymmetry in the human precentral motor system: A cytoarchitectonic and myeloarchitectonic brain mapping study. Brain. 2001;124(11):2232–2258. doi: 10.1093/brain/124.11.2232. [DOI] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proceedings of the National Academy of Sciences. 2009;106(3):918–923. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(5):1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. The Journal of Physiology Online. 2008;586(2):325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Archives of Neurology. 2008;65(12):1571–1576. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehm B, Perez M, Xu B, Hidler J, Cohen L. Functional neuroanatomy of mirroring during a unimanual force generation task. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhp075. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Baron JC, Rowe JB. Motor imagery after stroke: Relating outcome to motor network connectivity. Annals of Neurology. 2009 doi: 10.1002/ana.21810. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Jones PS, Carpenter TA, Baron JC. Mapping the involvement of BA 4a and 4p during motor imagery. NeuroImage. 2008;41(1):92–99. doi: 10.1016/j.neuroimage.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Sharma N, Pomeroy VM, Baron JC. Motor imagery: A backdoor to the motor system after stroke? Stroke. 2006;37(7):1941–1952. doi: 10.1161/01.STR.0000226902.43357.fc. [DOI] [PubMed] [Google Scholar]
- Sharma N, Simmons L, Jones PS, Day DD, Carpenter AT, Warburton EA, et al. Motor imagery after sub-cortical stroke: An fMRI study. Stroke. 2009;40(4):1315–1324. doi: 10.1161/STROKEAHA.108.525766. [DOI] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: Neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clinical Neurophysiology. 2006;117(8):1641–1659. doi: 10.1016/j.clinph.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. Exploring theta burst stimulation as an intervention to improve motor recovery in chronic stroke. Clinical Neurophysiology. 2007;118(2):333–342. doi: 10.1016/j.clinph.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Taub E, Uswatte G, Elbert T. New treatments in neurorehabiliation founded on basic research. Nature Reviews Neuroscience. 2002;3(3):228–236. doi: 10.1038/nrn754. [DOI] [PubMed] [Google Scholar]
- Tecchio F, Zappasodi F, Tombini M, Caulo M, Vernieri F, Rossini PM. Interhemispheric asymmetry of primary hand representation and recovery after stroke: A MEG study. NeuroImage. 2007;36(4):1057–1064. doi: 10.1016/j.neuroimage.2007.02.058. [DOI] [PubMed] [Google Scholar]
- Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalography and Clinical Neurophysiology. 1996;101(4):316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Carr LJ, Isaacs E, Brett E, Adams C, Mishkin M. Onset of speech after left hemispherectomy in a nine-year-old boy. Brain. 1997;120(Pt 1):159–182. doi: 10.1093/brain/120.1.159. [DOI] [PubMed] [Google Scholar]
- Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects’ non-dominant hand compared to unihemisphere stimulation. BMC Neuroscience. 2008;9:103. doi: 10.1186/1471-2202-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller B, Konrad A, Werhahn J, Ravindran S, Wu C, Cohen L. Contralateral hand anesthesia transiently improves poststroke sensory deficits. Annals of Neurology. 2006;59(2):385–388. doi: 10.1002/ana.20689. [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of motor recovery after stroke: A longitudinal fMRI study. Brain. 2003a;126(11):2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of outcome after stroke: A cross-sectional fMRI study. Brain. 2003b;126(6):1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Archives of Neurology. 2004;61(12):1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OBC, Lee L, Thompson AJ, Greenwood RJ, et al. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129(3):809–819. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Mortensen J, Kaelin-Lang A, Boroojerdi B, Cohen LG. Cortical excitability changes induced by deafferentation of the contralateral hemisphere. Brain. 2002;125(6):1402–1413. doi: 10.1093/brain/awf140. [DOI] [PubMed] [Google Scholar]
- Wu CW, Seo HJ, Cohen LG. Influence of electric somatosensory stimulation on paretic-hand function in chronic stroke. Archives of Physical Medicine and Rehabilitation. 2006;87(3):351–357. doi: 10.1016/j.apmr.2005.11.019. [DOI] [PubMed] [Google Scholar]


