Abstract
A direct C–H functionalization approach to produce aryl(azaaryl)methylamines from azaarylmethylamines without directing groups is described. Under conditions where the azaarylmethylamines’ C–H is reversibly deprotonated, a Pd(OAc)2/NIXANTPHOS-based catalyst couples the resulting carbanions with various aryl halides to provide aryl(azaaryl)methylamines. This umpolung strategy directly provides tertiary amines without protecting or activating groups.
Pyridine and quinoline derivatives are important heterocycles that are present in a broad range of biologically active natural products and pharmaceutically relevant compounds.1 Aryl(azaaryl)methylamines are prominent in biologically active small molecules, exhibiting antitumor,2 anti-virus,3 anti-HIV,4 and antihistamine activities5 (Figure 1). Furthermore, recent statistical studies indicate that the prominence of heteroaromatic rings in marketed oral drugs is increasing.6
Figure 1.

Selected pharmacologically active compounds containing aryl(azaaryl)methylamines.
Conventional approaches to the synthesis of 1,1-diarylmethylamine derivatives7 include substitution reactions with amine nucleophiles, addition of aryl or azaaryl organometallic reagents to imines, and reduction of imines.8 Despite the popularity of these methods, they involve prefunctionalized electrophiles or nucleophiles. Transition-metal-catalyzed cross-coupling reactions also represent an attractive approach.7d The direct C–H functionalization of amino alkyl moieties (R’CH2NR2), however, is challenging9. Therefore, activated substrates, such as Boc-protected amines, ketimines, and (η6-benzylamine)Cr(CO)3 complexes, have been employed to facilitate deprotonation of C–H bonds adjacent to nitrogen (Figure 2).10 The activating groups necessary in Figure 2 to increase reactivity are often subsequently transformed into the desired functional groups or excised from the product entirely, decreasing the synthetic efficiency.
Figure 2.
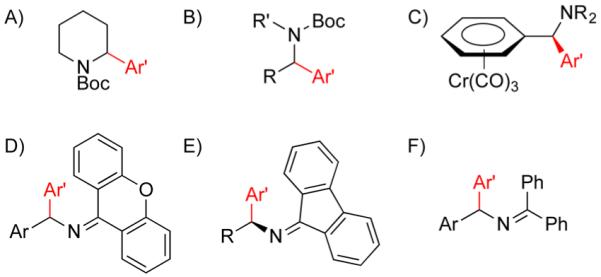
Representative synthetic intermediates with directing groups (A, B) and activating groups (C–F) employed in metal-catalyzed C–H functionalization adjacent to nitrogen.
Considering the importance of aryl(azaaryl)methylamines, the development of in situ deprotonation/arylation strategies for their synthesis that do not employ activating groups would be valuable. Based on our experience in deprotonative cross-coupling reactions10c–f,11 with weakly acidic substrates, we hypothesized that the reversible deprotonation of (aminomethyl)pyridines in the presence of a palladium catalyst should be possible.
Herein, we report a practical method to prepare aryl(azaaryl)methylamines from (aminomethyl)pyridines and aryl halides using a Pd–NIXANTPHOS-based catalyst (Scheme 1). This approach affords single-step access to aryl(azaaryl)methylamines from simple starting materials. Notably, to the best of our knowledge, the direct functionalization of aminomethyl azaarenes has not been successfully achieved prior to this work.
Scheme 1.

Palladium-Catalyzed Benzylic C–H Arylation with Azaarylmethylamines
We anticipated two challenges to advancing a successful deprotonative cross-coupling process (DCCP) with (aminomethyl)pyridine derivatives: (1) introduction of conditions for the in situ deprotonation of the weakly acidic C–H’s of the substrate and (2) identification of a catalyst capable of promoting coupling of the resulting organolithium, -sodium, or -potassium derivatives with aryl halides. In the latter case, we have found that palladium complexes of van Leeuwen’s NIXANTPHOS ligand12 outperform other Pd–phosphine complexes in this class of reactions. As such, it has become our “go to” ligand for new substrate classes. Concerning the deprotonation step, the pKa of the sp3-hybridized C–H’s of 2-(aminomethyl)pyridines are unknown, although that of 2-methylpyridine has been determined to be 34 in THF.13 The high pKa value of these substrates suggests that alkoxide and silylamide bases are good starting points. Additionally, palladium-catalyzed benzylic arylations of pyridine derivatives have become a powerful method to construct aryl(azaaryl)methane derivatives.14
We initially tested the reactions of 2-(morphorinomethyl)pyridine (1a) with 1-bromo-4-tert-butylbenzene using Pd-(OAc)2 (5 mol %) and NIXANTPHOS (7.5 mol %) in THF at 65 °C. Six bases [LiO-t-Bu, NaO-t-Bu, KO-t-Bu, LiN-(SiMe3)2, NaN(SiMe3)2, and KN(SiMe3)2] were employed, and the results are displayed in entries 1–6 of Table 1.
Table 1. Selected Optimization of Pd-Catalyzed C(sp3)–H Arylation of 1a with 2a.

| entry | base | 1a/2a/base | solventa | yieldb (%) |
|---|---|---|---|---|
| 1 | LiO-t-Bu | 1:1.5:3 | THF | 0 |
| 2 | NaO-t-Bu | 1:1.5:3 | THF | 0 |
| 3 | KO-t-Bu | 1:1.5:3 | THF | 0 |
| 4 | LiN(SiMe3)2 | 1:1.5:3 | THF | 87 |
| 5 | NaN(SiMe3)2 | 1:1.5:3 | THF | 80 |
| 6 | KN(SiMe3)2 | 1:1.5:3 | THF | 83 |
| 7 | LiN(SiMe3)2 | 1:1.5:3 | DME | 82 |
| 8 | LiN(SiMe3)2 | 1:1.5:3 | CPME | 85 |
| 9 | LiN(SiMe3)2 | 1:1.5:3 | 1,4-dioxane | 90 |
| 10 | LiN(SiMe3)2 | 1:1.5:2 | 1,4-dioxane | 92 |
| 11 | LiN(SiMe3)2 | 1:1.2:2 | 1,4-dioxane | 98 (92)c |
| 12 | LiN(SiMe3)2 | 1:1:1.5 | 1,4-dioxane | 73d |
| 13 | LiN(SiMe3)2 | 1:1.2:2 | 1,4-dioxane | 64e |
Tetrahydrofuran (THF), 1,2-dimethoxyethane (DME), cyclopentyl methyl ether (CPME), and 1,4-dioxane (dioxane).
Yields were determined by 1H NMR analysis of unpurified reaction mixtures with internal standard CH2Br2.
Isolated yield.
14% of 1a remained.
2.5 mol % of Pd(OAc)2 and 3.75 mol % of NIXANTPHOS.
Although MO-t-Bu bases [M = Li, Na, K] did not generate the desired product 3a (entries 1–3), MN(SiMe3)2 bases (M = Li, Na, K) were all very promising, affording 80–87% assay yield (AY) of the cross-coupling product 3a (entries 4–6). The most promising result was obtained with LiN(SiMe3)2 (87% AY as determined by 1H NMR, entry 4). We next screened other ethereal solvents (DME, CPME, 1,4-dioxane) (entries 7–9). Of these, 1,4-dioxane led to 90% AY of the desired product (entry 9). Examination of the stoichiometry indicated use of a 1:1.2:2 ratio of pyridylmethylamine (1a)/1-bromo-4-tert-butylbenzene (2a)/LiN(SiMe3)2 rendered 98% AY of 3a, leading to 92% isolated yield (entry 11). Use of a stoichiometric amount of 1-bromo-4-tert-butylbenzene (2a) or lower catalyst loading [2.5 mol % Pd(OAc)2 and 3.75 mol % of NIXANTPHOS] resulted in incomplete conversion at 65 °C after 12 h (entries 12–13). The optimized conditions (entry 11, Table 1) were then used to define the substrate scope.
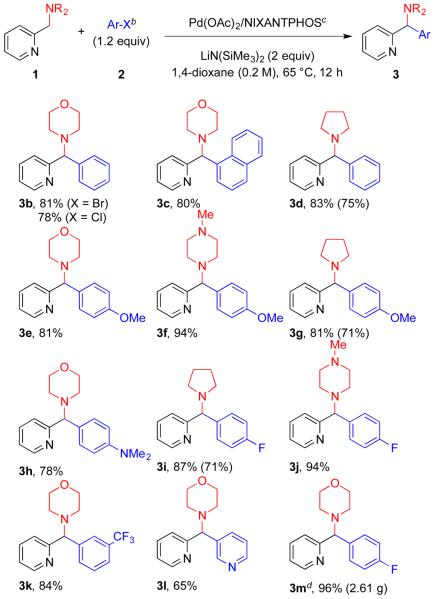
The scope of the arylation was initially explored with 2-(cyclic amino)methylpyridines (1) using aryl bromides (Scheme 2). Morpholine, pyrrolidine, and N-methylpiperazine were coupled with aryl bromides bearing various substituents, generating aryl(2-pyridyl)methylamines in good to excellent isolated yields (65–98%). The arylated products 3b–d were obtained in 78–83% yield with bromobenzene and 1-bromonaphthalene. Under the same reaction conditions, chlorobenzene was also a suitable coupling partner, providing 3b in 78% yield. Aryl bromides bearing electron-donating 4-NMe2 and 4-OMe resulted in coupling products 3e–h in 71–94% yield. Aryl bromides with electron-withdrawing 4-F and 3-CF3 groups were well tolerated and provided aryl(2-pyridyl)methylamines 3i–k in 71–94% yield. Heterocyclic 3-bromopyridine was also a suitable coupling partner to generate dipyridylmethyl morpholine 3l in 65% yield. The reactions with 1 mol % of catalyst generally occurred in good yields (71–75% yield, 3d, 3g, and 3i). To illustrate the scalability of our method, we conducted coupling of 2-(morphorinomethyl)pyridine (1a, 10 mmol) with 1-bromo-4-fluorobenzene using 4 mol % of catalyst. The coupling product 3m was isolated in 96% yield (2.61 g).
Scheme 2. Pd-Catalyzed C(sp3)–H Arylation of 2-Pyridylmethylamines 1 with Aryl Bromides 2a.
aIsolated yields. bX = Br was used unless noted. c5 mol % of Pd(OAc)2 and 7.5 mol % of NIXANTPHOS and the yields in parentheses with 1 mol % of Pd(OAc)2 and 1.5 mol % of NIXANTPHOS. d4 mol % of Pd(OAc)2 and 6 mol % of NIXANTPHOS.
Next, we turned our attention to acyclic amines. The optimized arylation conditions employed with cyclic amines were easily transferable to N,N-dimethyl- or -diethylamine derivatives with a variety of aryl bromides. Thus, aryl bromides with neutral (4-t-Bu, 3-Me), electron-donating (4-OMe, 4-NMe2), or electron-withdrawing (4-F, 4-Cl, 3-OMe, 3-CF3) substituents coupled with 2-(N,N-dialkylaminomethyl)pyridine, providing the cross-coupling products 4a–o in 60–98% yield (Scheme 3). The yields with N,N-dimethylamine derivatives are slightly higher than those with cyclic amines. Several relatively challenging substrates (1-bromo-4-trifluoromethylbenzene and 5-bromobenzofuran) were also competent coupling partners, albeit in reduced yields (4i,j, 60–66% yield). While 1-bromonaphthylene furnished coupling product 4k in 69% yield, the sterically more hindered 2-bromotoluene failed to provide the desired product (<2% yield). 2-(3-Bromophenyl)-1,3-dioxolane generated acetal-protected aldehyde product 4l in 60% yield. When the same conditions were applied to 2-(N,N-diethylaminomethyl)pyridine, the desired products 4m–o were isolated in 66–69% yield. Sterically more hindered 2-(N-benzyl-N-methylaminomethyl)pyridine did not react (<2% yield). Similar results were observed in related reactions by Baudoin and our groups with bulkier substituents on nitrogen.11o,15
Scheme 3. Benzylic Cross-Coupling of 2-(Dialkylaminomethyl)pyridine 1 with Aryl Bromides 2a.

aIsolated yields.
The above results led us to wonder if this method would be limited to 2-pyridyl derivatives because it requires a directed metalation for the deprotonation. To answer this question, other heterocyclic substrates were tested. Although the pKa’s of 4-pyridylmethylamines are not reported, 4-methylpyridine has a pKa of 32.2 in THF.13 As shown in Scheme 4, 4-pyridylmethylamine derivatives were excellent substrates, providing coupling products in 55–92% yield (5a–h) with 1 mol % catalyst loading. These results indicate that chelation of the base to the 2-pyridyl group is not necessary for the reversible deprotonation step of this DCCP.
Scheme 4. Benzylic Cross-Coupling of Azaarene Derivatives.

aIsolated yields. bX = Br unless noted. c1 mol % of Pd(OAc)2 and 1.5 mol % of NIXANTPHOS and the yields in parentheses with 5 mol % of Pd(OAc)2 and 7.5 mol % of NIXANTPHOS. d5 mol % of Pd(OAc)2 and 10 mol % of NIXANTPHOS. eThe reaction was conducted in THF at 110 °C with KN(SiMe3)2 instead of LiN(SiMe3)2.
Additional heterocycles were also amenable to the DCCP. Under the conditions used for 2-pyridylamines, a 2-(aminomethyl)quinoline derivative underwent a direct arylation reaction in 52% yield (5i). The least acidic substrate tested in this study was the 3-pyridylmethylamine derivative. The pKa of 3-methylpyridine is 37.7 in THF.13 After extensive optimization of bases, solvents, and temperature, we were able to achieve coupling in the presence of 2 equiv of KN(SiMe3)2 in THF at 110 °C, resulting in arylation product 5j in 65% yield.
In summary, we report the first direct palladium-catalyzed benzylic C(sp3)–H arylation of cyclic and acyclic aminomethyl-substituted azaarenes with aryl bromides. A variety of aryl(azaaryl)methylamines were prepared in good to excellent yields from readily accessible tertiary aminomethyl azaarenes. It is noteworthy that addition and removal of directing groups was not necessary with these substrates, facilitating their streamlined synthesis. This new method enables the rapid preparation of druglike molecules using straightforward techniques that are ideal for applications in medicinal chemistry.16
Supplementary Material
ACKNOWLEDGMENTS
Financial support for this work was provided by NIH/NIGMS (GM 104349). J.J. thanks CONACyT (México) for fellowships. F.G. thanks the China Scholarship Counsel for fellowships.
Footnotes
- Spectroscopic characterization data and procedures for preparation of all new compounds (PDF)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1) (a).Henry GD. Tetrahedron. 2004;60:6043. [Google Scholar]; (b) Michael JP. Nat. Prod. Rep. 2005;22:627. doi: 10.1039/b413750g. [DOI] [PubMed] [Google Scholar]; (c) Laird T. Org. Process Res. Dev. 2006;10:851. [Google Scholar]; (d) Joule JA, Mills K. Heterocyclic Chemistry. 5th ed John Wiley & Sons; Chichester, U.K.: 2010. [Google Scholar]; (e) Alexander F, Pozharskii AS, Katritzky AR. Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry, Biochemistry and Applications. 2nd ed John Wiley & Sons; Chichester, U.K.: 2011. [Google Scholar]; (f) Baumann M, Baxendale IR. Beilstein J. Org. Chem. 2013;9:2265. doi: 10.3762/bjoc.9.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Liu M, Bryant MS, Chen J, Lee S, Yaremko B, Li Z, Dell J, Lipari P, Malkowski M, Prioli N, Rossman RR, Korfmacher WA, Nomeir AA, Lin CC, Mallams AK, Doll RJ, Catino JJ, Girijavallabhan VM, Kirschmeier P, Bishop WR. Cancer Chemother. Pharmacol. 1998;43:50. doi: 10.1007/s002800050862. [DOI] [PubMed] [Google Scholar]
- (3).Chern J-H, Shia K-S, Hsu T-A, Tai C-L, Lee C-C, Lee Y-C, Chang C-S, Tseng S-N, Shih S-R. Bioorg. Med. Chem. Lett. 2004;14:2519. doi: 10.1016/j.bmcl.2004.02.092. [DOI] [PubMed] [Google Scholar]
- (4).Summa V, Petrocchi A, Matassa VG, Gardelli C, Muraglia E, Rowley M, Paz OG, Laufer R, Monteagudo E, Pace P. J. Med. Chem. 2006;49:6646. doi: 10.1021/jm060854f. [DOI] [PubMed] [Google Scholar]
- (5).Anagnostopulos H, Bartlett RR, Elben U, Stoll P. Eur. J. Med. Chem. 1989;24:227. [Google Scholar]
- (6).Ritchie TJ, Macdonald SJF, Young RJ, Pickett SD. Drug Discovery Today. 2011;16:164. doi: 10.1016/j.drudis.2010.11.014. [DOI] [PubMed] [Google Scholar]
- (7) (a).Doggrell SA, Liang LC. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1998;357:126. doi: 10.1007/pl00005146. [DOI] [PubMed] [Google Scholar]; (b) Plobeck N, Delorme D, Wei Z-Y, Yang H, Zhou F, Schwarz P, Gawell L, Gagnon H, Pelcman B, Schmidt R, Yue SY, Walpole C, Brown W, Zhou E, Labarre M, Payza K, St-Onge S, Kamassah A, Morin P-E, Projean D, Ducharme J, Roberts E. J. Med. Chem. 2000;43:3878. doi: 10.1021/jm000228x. [DOI] [PubMed] [Google Scholar]; (c) Ko Y, Malone DC, Armstrong EP. Pharmacotherapy. 2006;26:1694. doi: 10.1592/phco.26.12.1694. [DOI] [PubMed] [Google Scholar]; (d) Ameen D, Snape TJ. MedChemComm. 2013;4:893. [Google Scholar]
- (8) (a).Kobayashi S, Ishitani H. Chem. Rev. 1999;99:1069. doi: 10.1021/cr980414z. [DOI] [PubMed] [Google Scholar]; (b) Carey FA, Sundberg RJ. Advanced Organic Chemistry: Part B: Reaction and Synthesis. 5th ed Springer; New York: 2007. [Google Scholar]; (c) Kobayashi S, Mori Y, Fossey JS, Salter MM. Chem. Rev. 2011;111:2626. doi: 10.1021/cr100204f. [DOI] [PubMed] [Google Scholar]; (d) Smith MB, March J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. 7th ed John Wiley & Sons, Inc.; Hoboken: 2013. [Google Scholar]
- (9).Dastbaravardeh N, Schnuerch M, Mihovilovic MD. Org. Lett. 2012;14:1930. doi: 10.1021/ol300627p. [DOI] [PubMed] [Google Scholar]
- (10) (a).Niwa T, Yorimitsu H, Oshima K. Org. Lett. 2008;10:4689. doi: 10.1021/ol802070d. [DOI] [PubMed] [Google Scholar]; (b) Niwa T, Suehiro T, Yorimitsu H, Oshima K. Tetrahedron. 2009;65:5125. [Google Scholar]; (c) McGrew GI, Temaismithi J, Carroll PJ, Walsh PJ. Angew. Chem., Int. Ed. 2010;49:5541. doi: 10.1002/anie.201000957. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) McGrew GI, Stanciu C, Zhang J, Carroll PJ, Dreher SD, Walsh PJ. Angew. Chem., Int. Ed. 2012;51:11510. doi: 10.1002/anie.201201874. [DOI] [PubMed] [Google Scholar]; (e) Li M, Yücel B, Adrio J, Bellomo A, Walsh PJ. Chem. Sci. 2014;5:2383. doi: 10.1039/C3SC53526F. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Li M, Berritt S, Walsh PJ. Org. Lett. 2014;16:4312. doi: 10.1021/ol502043j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Fernandez-Salas JA, Marelli E, Nolan SP. Chem. Sci. 2015;6:4973. doi: 10.1039/c5sc01589h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11) (a).Zhang J, Bellomo A, Creamer AD, Dreher SD, Walsh PJ. J. Am. Chem. Soc. 2012;134:13765. doi: 10.1021/ja3047816. [DOI] [PubMed] [Google Scholar]; (b) Jia T, Bellomo A, El Baina K, Dreher SD, Walsh PJ. J. Am. Chem. Soc. 2013;135:3740. doi: 10.1021/ja4009776. [DOI] [PubMed] [Google Scholar]; (c) Montel S, Jia T, Walsh PJ. Org. Lett. 2014;16:130. doi: 10.1021/ol403124g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zheng B, Jia T, Walsh PJ. Org. Lett. 2013;15:1690. doi: 10.1021/ol400472v. [DOI] [PubMed] [Google Scholar]; (e) Zheng B, Jia TZ, Walsh PJ. Org. Lett. 2013;15:4190. doi: 10.1021/ol4019002. [DOI] [PubMed] [Google Scholar]; (f) Frensch G, Hussain N, Marques FA, Walsh PJ. Adv. Synth. Catal. 2014;356:2517. doi: 10.1002/adsc.201400679. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Gao F, Kim B-S, Walsh PJ. Chem. Commun. 2014;50:10661. doi: 10.1039/c4cc05307a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Hussain N, Frensch G, Zhang J, Walsh PJ. Angew. Chem., Int. Ed. 2014;53:3693. doi: 10.1002/anie.201309084. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Montel S, Raffier L, He Y, Walsh PJ. Org. Lett. 2014;16:1446. doi: 10.1021/ol5002413. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Yücel B, Walsh PJ. Adv. Synth. Catal. 2014;356:3659. doi: 10.1002/adsc.201400695. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Zheng B, Jia T, Walsh PJ. Adv. Synth. Catal. 2014;356:165. doi: 10.1002/adsc.201300851. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Zhang J, Bellomo A, Trongsiriwat N, Jia T, Carroll PJ, Dreher SD, Tudge MT, Yin H, Robinson JR, Schelter EJ, Walsh PJ. J. Am. Chem. Soc. 2014;136:6276. doi: 10.1021/ja411855d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Mao J, Eberle K, Zhang J, Rodríguez-Escrich C, Xi Z, Pericàs MA, Walsh PJ. Tetrahedron Lett. 2015;56:3604. doi: 10.1016/j.tetlet.2015.01.189. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Sha S-C, Zhang J, Walsh PJ. Org. Lett. 2015;17:410. doi: 10.1021/ol503545j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Hussain N, Kim B-S, Walsh PJ. Chem. - Eur. J. 2015;21:11010. doi: 10.1002/chem.201502017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12) (a).van der Veen LA, Keeven PH, Schoemaker GC, Reek JNH, Kamer PCJ, van Leeuwen PWNM, Lutz M, Spek AL. Organometallics. 2000;19:872. [Google Scholar]; (b) Kamer PCJ, van Leeuwen PWNM, Reek JNH. Acc. Chem. Res. 2001;34:895. doi: 10.1021/ar000060+. [DOI] [PubMed] [Google Scholar]
- (13).Fraser RR, Mansour TS, Savard S. J. Org. Chem. 1985;50:3232. [Google Scholar]
- (14) (a).Niwa T, Yorimitsu H, Oshima K. Angew. Chem., Int. Ed. 2007;46:2643. doi: 10.1002/anie.200604472. [DOI] [PubMed] [Google Scholar]; (b) Niwa T, Yorimitsu H, Oshima K. Org. Lett. 2007;9:2373. doi: 10.1021/ol0708119. [DOI] [PubMed] [Google Scholar]; (c) Hlavinka ML, Hagadorn JR. Organometallics. 2007;26:4105. [Google Scholar]; (d) Campeau L-C, Schipper DJ, Fagnou K. J. Am. Chem. Soc. 2008;130:3266. doi: 10.1021/ja710451s. [DOI] [PubMed] [Google Scholar]; (e) Mousseau JJ, Larivée A, Charette AB. Org. Lett. 2008;10:1641. doi: 10.1021/ol800396v. [DOI] [PubMed] [Google Scholar]; (f) Schipper DJ, Campeau L-C, Fagnou K. Tetrahedron. 2009;65:3155. [Google Scholar]; (g) Burton PM, Morris JA. Org. Lett. 2010;12:5359. doi: 10.1021/ol102276e. [DOI] [PubMed] [Google Scholar]; (h) Shang R, Yang Z-W, Wang Y, Zhang S-L, Liu L. J. Am. Chem. Soc. 2010;132:14391. doi: 10.1021/ja107103b. [DOI] [PubMed] [Google Scholar]; (i) Duez S, Steib AK, Manolikakes SM, Knochel P. Angew. Chem., Int. Ed. 2011;50:7686. doi: 10.1002/anie.201103074. [DOI] [PubMed] [Google Scholar]; (j) Song G, Su Y, Gong X, Han K, Li X. Org. Lett. 2011;13:1968. doi: 10.1021/ol200345a. [DOI] [PubMed] [Google Scholar]; (k) Zhao D, Zhu M-X, Wang Y, Shen Q, Li J-X. Org. Biomol. Chem. 2013;11:6246. doi: 10.1039/c3ob41488d. [DOI] [PubMed] [Google Scholar]
- (15).Millet A, Dailler D, Larini P, Baudoin O. Angew. Chem., Int. Ed. 2014;53:2678. doi: 10.1002/anie.201310904. [DOI] [PubMed] [Google Scholar]
- (16).From preliminary experiments screening 37 different Ni(phosphine)-based catalysts in the coupling of 1a with bromobenzene, it was found that the Ni(NIXANTPHOS)-based catalyst gave the highest product/internal standard ratio; see: Cao X, Sha S-C, Li M, Kim B-S, Morgan C, Huang R, Yang X, Walsh PJ. Chem. Sci. 2016 doi: 10.1039/c5sc03704b. DOI: 10.1039/C5SC03704B.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.