Abstract
Background
Women with a history of venous thromboembolism (VTE) have an increased recurrence risk during pregnancy. Low molecular weight heparin (LMWH) reduces this risk, but is costly, burdensome, and may increase risk of bleeding. The decision to start thromboprophylaxis during pregnancy is sensitive to women's values and preferences. Our objective was to compare women's choices using a holistic approach in which they were presented all of the relevant information (direct-choice) versus a personalized decision analysis in which a mathematical model incorporated their preferences and VTE risk to make a treatment recommendation.
Methods
Multicenter, international study. Structured interviews were on women with a history of VTE who were pregnant, planning, or considering pregnancy. Women indicated their willingness to receive thromboprophylaxis based on scenarios using personalized estimates of VTE recurrence and bleeding risks. We also obtained women's values for health outcomes using a visual analog scale. We performed individualized decision analyses for each participant and compared model recommendations to decisions made when presented with the direct-choice exercise.
Results
Of the 123 women in the study, the decision model recommended LMWH for 51 women and recommended against LMWH for 72 women. 12% (6/51) of women for whom the decision model recommended thromboprophylaxis chose not to take LMWH; 72% (52/72) of women for whom the decision model recommended against thromboprophylaxis chose LMWH.
Conclusions
We observed a high degree of discordance between decisions in the direct-choice exercise and decision model recommendations. Although which approach best captures individuals’ true values remains uncertain, personalized decision support tools presenting results based on personalized risks and values may improve decision making.
Keywords: Decision making, Decision support techniques, Venous thromboembolism, Heparin, Pregnancy
Introduction
Venous thromboembolism (VTE) complicates 0.5 to 2.2 per 1,000 deliveries [1,2]. Although absolute rates are low, pregnancy-associated VTE is an important cause of maternal morbidity and mortality [1–3].
The most important individual risk factor for pregnancy-associated VTE is a prior history of thrombosis [4]. The absolute risk of recurrent VTE during pregnancy remains controversial [5–10]. However, the risk of pregnancy-associated recurrent VTE may be lower in women without a history of thrombophilia whose prior thrombosis was associated with a transient risk factor such as acute trauma, surgery, or prolonged immobilization; compared with those whose prior event was unprovoked or associated with pregnancy or hormonal contraception [1].
Thromboprophylaxis during pregnancy is problematic for several reasons. Anticoagulation may increase bleeding risk during labor [1]. Vitamin K antagonists cross the placenta and have the potential to cause teratogenicity as well as pregnancy loss, fetal bleeding, and neuro-developmental deficits [1,11]. Oral direct thrombin and Xa inhibitors cross the placenta and may be associated with reproductive toxicity [1]. Unfractionated heparin and low molecular weight heparin (LMWH) do not cross the placenta and are safe for the fetus. However, both are inconvenient and burdensome to use due to parenteral administration. Further, unfractionated heparin and to a lesser extent LMWH may cause thrombocytopenia, osteoporosis and symptomatic fracture when given for longer than 1 month [12–16].
No rigorously designed study has been performed to assess women's thromboprophylaxis options during pregnancy. Thus, the optimal strategy for pregnant women with prior VTE remains unclear. The 9th American College of Chest Physicians Antithrombotic Guidelines suggests antepartum surveillance without thromboprophylaxis, followed by post-partum anticoagulants for 6 weeks for lower risk women. For women at moderate to high risk of recurrence the guidelines suggest antepartum prophylaxis with LMWH, in addition to postpartum prophylaxis [1]. However the strength of both recommendations is weak, thus the right decision is sensitive to women's underlying values and preferences.
Given the uncertainties, trade-offs, and weak recommendations, optimal care is likely to involve a shared decision-making approach. There are several potential approaches to explore “patient-specific” values and preferences and subsequent decision-making [17]. We have focused on: (1) a holistic direct-choice procedure and (2) utility elicitation from individual patients followed by “patient-specific” decision analysis.
In the “direct-choice” exercise, participants are presented with relevant health states and their probabilities under different management strategies. An alternative approach to decision-making asks patients to provide their values and preferences for health outcomes. With the help of a decision analytic model using best estimates of the probabilities of events (e.g., DVT, PE, or major bleeding) and patient's personal values for health states, the effectiveness of each strategy can be calculated and expressed as quality adjusted life years (QALYs).
The relative merits of the direct-choice and decision analytic approaches are open to question as few studies have addressed this issue [18]. Thus, our objective was to compare women's choices regarding thromboprophylaxis during pregnancy using these two methods.
Methods
Setting
Between the years 2011 and 2013, we performed a multicenter, cross-sectional study at seven centers in six countries (Canada, USA, Brazil, Finland, Norway and Spain), using a structured interview design. We have previously published a detailed description of our study protocol. [19]
Study Population
We included women between the ages of 18 and 45 years, with a history of VTE who were pregnant, planning, or considering pregnancy. We excluded women who were currently receiving thromboprophylaxis or full-dose anticoagulation, have undergone surgical sterilization, have a partner who has had a vasectomy, or were unwilling or unable to provide informed consent. Women were identified prospectively as they were referred for counseling. Ethics committees at all participating institutions approved the study and all patients provided written informed consent.
Outcomes and Statistical Analyses
The main outcomes of this study were patients’ values and preferences for 5 health states most relevant to this clinical question (described below), their choices regarding thromboprophylaxis, and the results of patient-specific decision analyses that used each patient's own health state utilities and VTE risk during pregnancy. Results were reported as means and standard deviations. Subgroup analyses were performed using two-tailed t-tests of independent samples to explore whether there were statistically significant differences in results among groups (e.g., willing or not willing to receive thromboprophylaxis). Results of the patient-specific decision analyses were reported as quality-adjusted life years projected for each of the two strategies considered and the gain (or loss) resulting from thromboprophylaxis during pregnancy was calculated (see details below). Subgroup analyses were performed to see if there were significant differences in the gain or loss projected by the decision model for thromboprophylaxis among women whose choices were concordant or discordant with decision model recommendations.
The patient-specific decision analysis component of this study was added after the parent study exploring direct choice was already underway. The parent study was powered to address the question, “how many episodes of VTE must be prevented to make prophylactic treatment with LMWH acceptable?” Previous research from our group in patients with atrial fibrillation [20], and from other groups studying non-pregnant women with prior VTE [21], suggested that moderately precise estimates of patient preferences can be obtained with sample sizes of approximately 100 participants.
Patients provided standard demographic information, including age, current pregnancy status, and details of their past VTE (PE or DVT, presence of precipitating risk factors, and experience with LMWH). We classified women as being at low or high risk of recurrence. We defined low risk as the absence of known thrombophilia or history of VTE associated with a major transient risk factor within the prior 8 weeks; and higher risk as prior unprovoked VTE, VTE associated with a minor transient risk factor (including pregnancy and hormonal contraception), or known thrombophilia. We estimated the risk of antepartum recurrence to be between 0 and 5% for low risk women, and between 5 and 10% for those at high risk. We used indirect evidence to estimate that prophylactic LMWH reduces the risk of antepartum recurrence by approximately 70% [22].
Direct-choice Exercises
We determined women's willingness to receive daily injections of LMWH through direct-choice exercises. Study personnel used scripts to present each woman with patient-specific information on a decision board that included the probabilities of VTE during pregnancy given the characteristics of her prior VTE. (Figures available in study protocol and Appendix Fig. 3 [19]).
To ensure understanding, we presented the risk of recurrence with and without LMWH prophylaxis in three different ways: table, bar chart and pictograph. To aid in decision-making we provided detailed descriptions of relevant outcomes and LMWH use during pregnancy (see Appendix). We stressed that there were no fetal risks associated with antepartum use of LMWH. We advised women to contemplate their prior VTE experience(s) along with their previous experience receiving prophylactic LMWH (if received for longer than 2 weeks during pregnancy) when making a decision. We then asked participants whether they would be willing to take LMWH during their pregnancy (for those who are pregnant) or whether they would be willing to do so in a future pregnancy.
Utility Assessment
We assessed patient's values for health states using visual analog scales (VAS) that we described to women as feeling thermometers (FT) [23]. Women chose the score on the thermometer that represented their value for each health state considered. The FT is anchored at death (value of 0) and full health (value of 100). Health states included: (1) pregnancy with LMWH prophylaxis using the description provided (see Appendix) or their previous experience (for those with two weeks or more of prophylactic LMWH during pregnancy), (2) pregnancy with their own most recent VTE experience, (3) pregnancy-related DVT, (4) pregnancy-related PE, and (5) an obstetrical bleed.
Decision Analytic Model
We updated a previously developed Markov state transition decision model examining two strategies: antepartum prophylaxis with low molecular weight heparin; and expectant management during the antepartum period without prophylaxis (Appendix Fig. 1) [24]. We used a life-time modeling horizon along with a 6-week cycle length to model both antepartum events and future lifetime events. Model parameters (see Table 1) were updated based on a review of the English-language literature. We used a cumulative risk of antepartum VTE recurrence of 2.5% for low risk and 7.5% for high risk women. Prophylaxis involved administration of subcutaneous LMWH once daily starting on average at a gestational age of 10 weeks, and until delivery. Expectant management involved no prophylactic anticoagulation and no care beyond that provided during routine prenatal visits, unless clinical VTE developed. Management of patients in the postpartum period and beyond was identical for both strategies. Postpartum care included administration of prophylactic warfarin for 6 weeks postpartum [1]. We also modeled a risk of remote VTE recurrence following pregnancy. This risk was 1.0%/year for low risk women, and 2.9% per year for high-risk women.
Table 1.
Data Used in the Decision Analysis.
| Variable | Baseline Value | Plausible Range | References |
|---|---|---|---|
| Probability of recurrent venous thromboembolism (weeks 10-40)† | [8,10,37] | ||
| Low-risk women | 2.5% | 0-5% | |
| High-risk women | 7.5% | 5 - 10% | |
| Short-term probability of second venous thromboembolism following first recurrence while receiving anticoagulant therapy‡ | [38] | ||
| 0-6 weeks | 4% | ||
| 6-12 weeks | 0.6% | ||
| 12-24 weeks | 0.5% | ||
| Long-term rate of recurrent venous thromboembolism (ie, after first 24 weeks) | 0-5.8%/year | [38-43] | |
| Overall risk | 2%/year | ||
| Low risk (relative risk 0.5) | 1%/year | ||
| High risk (relative risk 1.43) | 2.9%/year | ||
| Probability of major obstetrical bleed without prophylactic LMWH during weeks 10-40¥ | 1.3% | [44] | |
| Relative risk of major obstetrical bleed with prophylactic LMWH | 1.57 | [44] | |
| Probability of major obstetrical bleed on prophylactic LMWH during weeks 10-40¥ | 2.0% | 0-2.0% | [44,45] |
| Rate of bleeding on treatment doses of warfarin | 2.0%/year | 0-5.3% | [42,43,45-47] |
| Probability of pulmonary embolism/deep venous thrombosis given venous thromboembolism | 25%/75% | [42,47] | |
| Probability of death from | |||
| Deep venous thrombosis | 3% | 0-10% | [48-53] |
| Pulmonary embolism | 21% | 10-30% | [48-52,54-56] |
| Major hemorrhage | 13.4% | 9.4-17.4% | [57] |
| Probability of long-term morbidity from major hemorrhage | 8.7% | 5-20% | [57-59] |
| Efficacy of prophylactic LMWH | 64% | 33-80% | [60-62] |
| Efficacy of inferior vena caval filter for preventing pulmonary embolism | 90% | 50-100% | [63-65] |
For modeling purposes, these 30-week cumulative probabilities were converted to 6-week transition probabilities. The risks of recurrence and hemorrhage were assumed to be constant from cycle to cycle during pregnancy.
These represent 6-week cumulative probabilities.
Includes major antepartum hemorrhage, post-partum hemorrhage, and wound hematoma.
We used a standard computer program (Decision Maker, Boston, Massachusetts) to build the decision analytic model and analyze results. We used Decision Maker's remote control function to run a script file containing the required information for each patient (patient age at the time of interview, cumulative antepartum risk of VTE recurrence [high vs low risk] and patient-specific utilities for the relevant health states) through a decision analytic model that estimates the quality-adjusted life expectancy for each strategy. For each patient, the strategy with greatest expected utility in QALYs represented the decision model recommended strategy. We compared results of the direct-choice exercises with the optimal strategy recommended by the decision analytic model, using each patient's own utilities for health outcomes and estimated risk of those outcomes.
Results
Recruitment and Characteristics of Participants
We included 123 women who completed the interview from seven centers in six countries [Canada, USA, Brazil, Finland, Norway and Spain] (see Table 2).
Table 2.
Baseline Characteristics.
| Characteristic | |
|---|---|
| Age (years; mean, SD) | 33.94 (6.2) |
| Region | |
| North America (2 sites) | 53 (43.1%) |
| Spain (1 site) | 24 (19.5%) |
| Brazil (2 sites) | 33 (26.8%) |
| Scandinavia (2 sites) | 13 (10.6%) |
| Education level | |
| Did not complete high school | 17 (13.8%) |
| Completed high school | 23 (18.7%) |
| Some post-secondary or higher | 83 (67.5%) |
| Pregnancy status | |
| Pregnant & Planning | 56 (45.5%) |
| Neither | 67 (54.5%) |
| Previous VTE experience | |
| Severe (PE or DVT with residual symptoms) | 85 (69.1%) |
| Non-severe (DVT without residual symptoms) | 38 (30.9%) |
| Previous experience with LMWH | |
| Use > = 2 weeks during pregnancy | 31 (25.2%) |
| Never or < 2 weeks | 92 (74.8 %) |
| Date of last event | |
| In the last year | 18 (14.6%) |
| 1 to 3 years ago | 35 (28.4%) |
| More than 3 years ago | 70 (56.9%) |
| Risk of recurrence | |
| High | 88 (71.5%) |
| Low | 35 (28.5%) |
VTE: venous thromboembolism, PE: pulmonary embolism, DVT: deep venous thrombosis. Low risk of recurrence: prior VTE associated with a major transient risk factor within 8 weeks prior to event (i.e. leg casting, major surgery [spinal or general anesthetic 30 minutes], significant medical illness with hospitalization for ≥ 3 days, immobilization for ≥ 3 days, active malignancy) and no known thrombophilia. High risk of recurrence: prior unprovoked VTE or VTE associated with minor transient risk factor within 8 weeks prior to event (i.e. pregnancy, hormonal contraception, or air travel > 6 hours).
Direct-choice Exercise
The majority of women 76% (93/123) were willing to take LMWH prophylaxis. In particular, 82% (72/88) of women at high risk and 60% (21/35) of women at low risk were willing to take LMWH injections throughout the antepartum period.
Health Values
Patient values for health states varied widely (see Table 3). We omitted data from 4 patients due to inconsistent results suggesting they did not understand scoring for the VAS. VAS ratings from women who were and were not willing to take LMWH were not significantly different.
Table 3.
Patient Values for Health States.
| Health State | VAS Among all patients (n = 123) Average (SD) | VAS Among patients willing to take LMWH (n = 93) Average (SD) | VAS Among patients not willing to take LMWH (n = 20) Average (SD) | p-value† |
|---|---|---|---|---|
| Pregnancy with PE | 32.73 (21.97) | 34.87 (21.34) | 36.85 (23.30) | 0.60 |
| Pregnancy with DVT | 46.42 (22.20) | 47.46 (21.83) | 49.65 (20.93) | 0.68 |
| Previous VTE Experience | 45.23 (24.97) | 45.57 (25.59) | 45.40 (23.30) | 0.98 |
| Obstetrical Bleed | 34.25 (23.07) | 35.84 (23.57) | 36.25 (19.09) | 0.93 |
| Pregnancy while Receiving LMWH | 80.15 (16.45) | 81.55 (15.22) | 78.70 (17.33) | 0.50 |
Two-tailed t-test of independent samples.
Patient-specific Decision Analyses
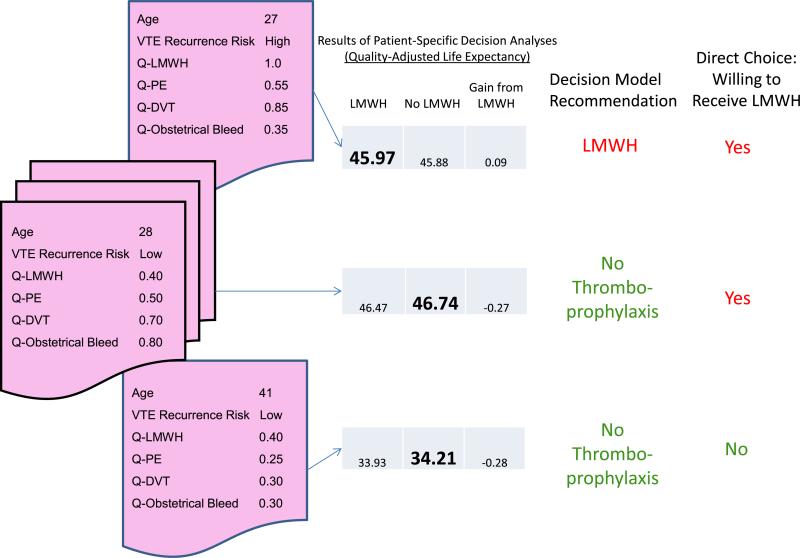
For illustrative purposes, we show results of patient-specific decision analyses for 3 participants in Fig. 1. For one woman, use of LMWH results in a gain in QALYs and her decision in the direct-choice exercise is consistent with this result. The other two examples show women for whom both their values and risk of recurrence lead to a recommendation against the use of thromboprophylaxis. In one case the woman's decision in the direct-choice exercise is consistent with the decision model recommendation, while in the other example it is not.
Fig. 1.
Sample Cases of Patient-Specific Decision Analyses. Results of patient-specific decision analyses are shown for three different women in our study. On the left side of the figure, the patient-specific information used to inform the decision model is shown for each woman. For instance, the woman at the top left of the figure is a 27-year old who is at high risk of VTE recurrence. Quality of life for health states is shown on a zero to one scale, where 1.0 represents perfect health and zero represents equivalence to being dead. This woman believes that pregnancy while receiving daily injections of LMWH presents no decrement in quality of life. She values pregnancy with either a pulmonary embolism, deep venous thrombosis, or major obstetrical bleed at 0.55, 0.85, and 0.35, respectively. Running these data through the decision model results in a quality-adjusted life expectancy of 45.97 QALYs for LMWH, 45.88 QALYs for No LMWH, and a gain of 0.89 QALYs for thromboprophylaxis. The other two cases demonstrate women for whom the personalized decision analysis calculates a loss of quality-adjusted life expectancy for thromboprophylaxis. In the middle example, the woman was willing to receive LMWH in the direct-choice exercise, while in the bottom example the woman was not willing to accept thromboprophylaxis.
The decision model recommended LMWH for 51 women and recommended against LMWH for 72 women (see Table 4). Among women for whom LMWH was recommended, gain in quality-adjusted life expectancy ranged from 0.001 to 0.089 QALYs, (average gain 0.038 QALYs; ~2 weeks). Women for whom LMWH was not recommended had a loss that ranged between 0.003 and 0.351, with an average loss of 0.09 QALYs (~5 weeks). There was a non-significant trend towards a larger gain in QALYs among women for whom the decision model and direct-choice experiment both resulted in the choice of thromboprophylaxis (p = 0.66). Similarly, there was a non-significant trend towards a greater loss in QALYs among women for whom the decision model and direct-choice experiment both resulted in the choice not to receive LMWH (p = 0.28).
Table 4.
Comparison of Patient-Specific Decision Model Recommendation and Direct-choice Exercise.
| Decision Model Recommendation | Direct-choice Exercise | Number of Patients | Average Gain or Loss in QALYs |
|---|---|---|---|
| LMWH | Total - | 51 | 0.038 |
| LMWH | 41 | 0.038† | |
| No LMWH | 6 | 0.031† | |
| Unsure | 4 | 0.044 | |
| No LMWH | Total - | 72 | −0.087 |
| LMWH | 52 | −0.076‡ | |
| No LMWH | 13 | −0.105‡ | |
| Unsure | 7 | −0.137 |
p-value 0.66 (two-tailed t-test of independent samples).
p-value 0.28 (two-tailed t-test of independent samples).
LMWH was favored by the decision model in 40 out of 88 women (46%) at high risk of VTE recurrence and in 11 out of 35 women (31%) at low risk, while no thromboprophylaxis with LMWH was favored in 48/88 high risk women (55%) and 24/35 low risk women (69%) (Appendix Table 1). Among 6 patients for whom the decision model recommended LMWH and the patient chose no LMWH in the direct-choice exercise, 3 were at high risk of VTE recurrence. Among 52 patients for whom the decision model recommended against LMWH and the patient chose LMWH in the direct-choice exercise, 14 were at low risk of VTE recurrence. Of 88 women at higher risk, 72 (82%) decided to use thromboprophylaxis with LMWH, as did 21/35 (60%) of low risk women.
Appendix Table 2 reports patient values for health states and VTE recurrence risk among discordant sets of women for whom the decision model recommended a different strategy from that selected in the direct-choice exercise. This table highlights seeming inconsistencies between values for health states or VTE recurrence risk and decisions in the direct-choice exercises. For instance, the average quality of life for pregnancy while receiving LMWH was rated 0.95 among women for whom the decision model recommended LMWH but in the direct-choice exercise they opted for no thromboprophylaxis. Similarly, the average quality of life for pregnancy while receiving LMWH was rated 0.72 among women chose LMWH in the direct-choice exercise, but for whom the decision model recommended against LMWH. Another apparent inconsistency was that 57% of women chose No LMWH despite being at high risk of VTE recurrence, and 27% of women chose to receive LMWH despite being at low risk for VTE recurrence.
Discussion
Our objective was to compare women's choices regarding thromboprophylaxis during pregnancy using direct-choice and a personalized decision analysis. We found a high degree of discordance between the direct-choice and decision model recommendations. A greater proportion of discordant decisions occurred among women for whom the decision model recommended against thromboprophylaxis. Of most concern, many women who were at low risk of VTE recurrence and who had health state values that led the decision model to recommend against thromboprophylaxis (e.g., low quality of life for pregnancy while receiving LMWH injections), chose to accept this therapy. Our study has a number of strengths. It is a multicenter international study that included women from six countries in Europe, South America and North America. We designed a rigorous study with a published protocol. [19] Numeric estimates were based on a thorough review of the literature; the multiple presentations of information included visual aids to ensure optimal understanding in the direct-choice exercise, presentation of a range of risks in the direct-choice to capture the uncertainty regarding recurrence, and a carefully structured interview protocol with training of all interviewers. Limitations include exclusive use of VAS ratings to capture patient preferences rather than an approach, such as the standard gamble that meets econometric assumptions. Although the total number of patients in our study was modest, the incidence of VTE in pregnancy is low, between 1 in 500 and 1 in 2,000 pregnancies (absolute incidence; 0.025 to 0.1 percent), making patient recruitment a challenge [25]. That being said, among studies enrolling pregnant women with a prior history of VTE, this is one of the largest. [10,26–28]
As shown in an example of a decision board from the direct choice exercises (see Appendix Fig. 3), women at high risk for VTE were told their cumulative risk of VTE during pregnancy was between 5 and 10 in 100. Low risk women were given a range between 0 and 5 in 100. In order to be consistent with the information we presented to women in the direct choice exercises, we used mid-range estimates for the cumulative probability of VTE in the decision analyses, 7.5% and 5% respectively for high and low risk patients. More recent reviews of the probability of VTE in low risk women suggest the cumulative probability over the course of pregnancy may be as low as 1% [29]. The impact of overestimating the risk of VTE in the low risk women would be to increase the gain afforded by prophylaxis with LMWH and possibly the number of low risk women for whom prophylaxis was recommended. Even using what may have been a somewhat high estimate in the low risk group, the decision model still recommended no prophylaxis in 24 out of 35 low risk women. Fourteen of those 24 low risk women still choice to take prophylactic LMWH (see Appendix Table 1). If we had used a lower estimate for VTE in this group our results showing discordance between patient choice and the personalized decision analyses would have been even more dramatic.
A number of approaches are available for eliciting health state evaluations [23]. The standard gamble is most consistent with utility theory and is generally preferred by health economists [30,31]. Although the visual analog scale (VAS) is theoretically less satisfactory, it is easier to understand, takes less time to administer and has superior psychometric measurement properties [32]. Furthermore, the standard gamble, which generally requires assessing what risk of death a patient is willing to accept in order to be free of a health state with decreased quality of life, is difficult to use in the valuation of temporary health states, such as an episode of VTE or an obstetrical bleed [33]. Since there is no risk involved in the VAS assessments, health values determined in this manner tend to be lower than those assessed by other techniques such as the standard gamble or the time tradeoff. It is hard to estimate the impact of using the VAS in place of other utility assessment methods on our results, as VAS assessments were used for all health states, including VTE, major obstetrical bleeds, and pregnancy while receiving LMWH shots. Thus, lower health state values won't predictably bias our results one way or the other.
There are many possible explanations for the discrepancies we observed. It could be that patients have difficulty reconciling information regarding multiple competing risks of events with differential health impact and consequences. Women may have focused on the upper boundary of the risk (5% in low risk women and 10% in high risk, rather than the average used in the decision model), and thus the risk reduction, associated with LMWH treatment. Women may have overestimated the significance of risk magnitude, particularly for lower event rates. Some women may not tolerate even a minimal risk of VTE. For instance, some women at low risk for VTE, who rated quality of life while receiving thromboprophylaxis as being very low, still chose to accept daily injections with LMWH because they “wanted to make sure they didn't get a blood clot.” [34] For other women, concern about the impact of either treatment on their baby, and particularly concern about VTE, influenced their decision. Finally, the VAS ratings may not provide an accurate representation of the utilities that women were implicitly applying when they made their direct-choice decisions.
One might conjecture that by relieving the patient of the computational task, but allowing them to “weigh in” on what they know best, their own values and preferences for health outcomes, an adequately detailed decision model should give the “right” answer for them. However there is no gold standard for this type of research.
In a recent review examining the use of values clarification methods in patient decision aids, there was no consensus regarding the optimal approach. Many questions remain, such as - does a values clarification exercise (VCE) actually improve shared decision-making? Should a VCE precede a visit with the health care provider, be used during the visit, or follow the visit? [17].
It may be that the most informed decision is the best decision. There is no consensus on how best to inform a patient making a decision. For example, the manner in which information about health outcomes and risks is presented may be more important than the magnitude of those risks in affecting decision-making [35,36]. Although we did not explore this in the current study, presenting women with feedback on how their own risk and preferences influenced the decision model's recommendation may help them to further clarify their values, and identify misunderstandings about the data and inconsistencies in their decision-making logic. How to best integrate this step into the clinical visit remains an open question. Our next steps and future plans are to design and conduct a clinical trial to explore the incremental impact of providing feedback from a personalized decision model in addition to the simple presentation of personalized data, on decision quality and patient understanding. We believe this is likely to enhance insight and understanding of this important, but so far under-investigated, issue.
Supplementary Material
Acknowledgments
Funding Sources
This study is funded by the Physicians’ Services Incorporated Foundation. Additional support for this study came from the Pfizer Educational Group, the Informed Medical Decisions Foundation, and NIH/NCATS Grant Number 8UL1TR000077-05.
PAC is funded by a Miguel Servet contract by the Instituto de Salud Carlos III (CP09/00137). SE is supported by a Canadian Institutes of Health Research (CIHR) Doctoral Award. KAOT was supported by unrestricted grants from the Finnish Medical Foundation and the Finnish Cultural Foundation. LCL received support from FAPESP (Number of Process: 2013/051658). SDM is supported by a CIHR New Investigator Salary Award. SMB holds the Eli Lilly Canada/May Cohen Chair in Women's Health at McMaster University.
The funding sources had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.thromres.2015.05.020.
Authorship
All authors had access to the data and played a role in writing this manuscript.
Additional Conflict of Interest Disclosures
None of the authors has disclosed additional conflicts of interests.
References
- 1.Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis. Chest. 9th ed. Vol. 141. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines; 2012. pp. e691S–e736S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am. J. Obstet. Gynecol. 2006;194:1311–1315. doi: 10.1016/j.ajog.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 3.McColl MD, Ellison J, Greer IA, Tait RC, Walker ID. Prevalence of the post-thrombotic syndrome in young women with previous venous thromboembolism. Br. J. Haematol. 2000;108:272–274. doi: 10.1046/j.1365-2141.2000.01877.x. [DOI] [PubMed] [Google Scholar]
- 4.Pabinger I, Grafenhofer H, Kyrle PA, Quehenberger P, Mannhalter C, Lechner K, Kaider A. Temporary increase in the risk for recurrence during pregnancy in women with a history of venous thromboembolism. Blood. 2002;100:1060–1062. doi: 10.1182/blood-2002-01-0149. [DOI] [PubMed] [Google Scholar]
- 5.Brill-Edwards P, Ginsberg J, Burrows R. Heparin prophylaxis during pregnancy. Am. J. Obstet. Gynecol. 1990;162:870–871. doi: 10.1016/0002-9378(90)91037-d. [DOI] [PubMed] [Google Scholar]
- 6.Tengborn L, Bergqvist D, Matzsch T, Bergqvist A, Hedner U. Recurrent thromboembolism in pregnancy and puerperium. Is there a need for thromboprophylaxis? Am. J. Obstet. Gynecol. 1989;160:90–94. doi: 10.1016/0002-9378(89)90095-1. [DOI] [PubMed] [Google Scholar]
- 7.Badaracco MA, Vessey MP. Recurrence of venous thromboembolic disease and use of oral contraceptives. Br. Med. J. 1974;1:215–217. doi: 10.1136/bmj.1.5901.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Stefano V, Martinelli I, Rossi E, Battaglioli T, Za T, Mannuccio Mannucci P, Leone G. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br. J. Haematol. 2006;135:386–391. doi: 10.1111/j.1365-2141.2006.06317.x. [DOI] [PubMed] [Google Scholar]
- 9.de Swiet M, Floyd E, Letsky E. Low risk of recurrent thromboembolism in pregnancy. Br. J. Hosp. Med. 1987;38:264. [PubMed] [Google Scholar]
- 10.Brill-Edwards P, Ginsberg JS, Gent M, Hirsh J, Burrows R, Kearon C, Geerts W, Kovacs M, Weitz JI, Robinson KS, Whittom R, Couture G. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of Clot in This Pregnancy Study Group. N. Engl. J. Med. 2000;343:1439–1444. doi: 10.1056/NEJM200011163432002. [DOI] [PubMed] [Google Scholar]
- 11.Ginsberg JS, Hirsh J, Turner DC, Levine MN, Burrows R. Risks to the fetus of anticoagulant therapy during pregnancy. Thromb. Haemost. 1989;61:197–203. [PubMed] [Google Scholar]
- 12.Dahlman T, Lindvall N, Hellgren M. Osteopenia in pregnancy during long-term heparin treatment: a radiological study post partum. Br. J. Obstet. Gynaecol. 1990;97:221–228. doi: 10.1111/j.1471-0528.1990.tb01785.x. [DOI] [PubMed] [Google Scholar]
- 13.Hall JG, Pauli RM, Wilson KM. Maternal and fetal sequelae of anticoagulation during pregnancy. Am. J. Med. 1980;68:122–140. doi: 10.1016/0002-9343(80)90181-3. [DOI] [PubMed] [Google Scholar]
- 14.Hellgren M, Hahn L, Dahlman T. Thromboprophylaxis during pregnancy. Am. J. Obstet. Gynecol. 1990;162:1338–1339. doi: 10.1016/0002-9378(90)90050-h. [DOI] [PubMed] [Google Scholar]
- 15.Sanson BJ, Lensing AW, Prins MH, Ginsberg JS, Barkagan ZS, Lavenne-Pardonge E, Brenner B, Dulitzky M, Nielsen JD, Boda Z, Turi S, Mac Gillavry MR, Hamulyak K, Theunissen IM, Hunt BJ, Buller HR. Safety of low-molecular-weight heparin in pregnancy: a systematic review. Thromb. Haemost. 1999;81:668–672. [PubMed] [Google Scholar]
- 16.Sorensen HT, Johnsen SP, Larsen H, Pedersen L, Nielsen GL, Moller M. Birth outcomes in pregnant women treated with low-molecular-weight heparin. Acta Obstet. Gynecol. Scand. 2000;79:655–659. [PubMed] [Google Scholar]
- 17.Fagerlin A, Pignone M, Abhyankar P, Col N, Feldman-Stewart D, Gavaruzzi T, Kryworuchko J, Levin CA, Pieterse AH, Reyna V, Stiggelbout A, Scherer LD, Wills C, Witteman HO. Clarifying values: an updated review. BMC Med. Inform. Decis. Mak. 2013;13(Suppl. 2):S8. doi: 10.1186/1472-6947-13-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Man-Son-Hing M, Gage BF, Montgomery AA, Howitt A, Thomson R, Devereaux PJ, Protheroe J, Fahey T, Armstrong D, Laupacis A. Preference-based anti-thrombotic therapy in atrial fibrillation: implications for clinical decision making. Med. Decis. Mak. 2005;25:548–559. doi: 10.1177/0272989X05280558. [DOI] [PubMed] [Google Scholar]
- 19.Alonso-Coello P, Ebrahim S, Guyatt GH, Tikkinen KA, Eckman MH, Neumann I, McDonald SD, Akl EA, Bates SM. Evaluating patient values and preferences for thromboprophylaxis decision making during pregnancy: A study protocol. BMC Pregnancy Childbirth. 2012;12:40. doi: 10.1186/1471-2393-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonso-Coello P, Montori VM, Sola I, Schunemann HJ, Devereaux P, Charles C, Roura M, Diaz MG, Souto JC, Alonso R, Oliver S, Ruiz R, Coll-Vinent B, Diez AI, Gich I, Guyatt G. Values and preferences in oral anticoagulation in patients with atrial fibrillation, physicians' and patients' perspectives: protocol for a two-phase study. BMC Health Serv. Res. 2008;8:221. doi: 10.1186/1472-6963-8-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacLean S, Mulla S, Akl EA, Jankowski M, Vandvik PO, Ebrahim S, McLeod S, Bhatnagar N, Guyatt GH. Chest. 9th ed. Vol. 141. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines; 2012. Patient values and preferences in decision making for anti-thrombotic therapy: a systematic review: Antithrombotic Therapy and Prevention of Thrombosis; pp. e1S–e23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, Balekian AA, Klein RC, Le H, Schulman S, Murad MH. Chest. 9th ed. Vol. 141. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines; 2012. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis; pp. e195S–e226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green C, Brazier J, Deverill M. Valuing health-related quality of life. A review of health state valuation techniques. Pharmacoeconomics. 2000;17:151–165. doi: 10.2165/00019053-200017020-00004. [DOI] [PubMed] [Google Scholar]
- 24.Johnston JA, Brill-Edwards P, Ginsberg JS, Pauker SG, Eckman MH. Cost-effectiveness of prophylactic low molecular weight heparin in pregnant women with a prior history of venous thromboembolism. Am. J. Med. 2005;118:503–514. doi: 10.1016/j.amjmed.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N. Engl. J. Med. 2008;359:2025–2033. doi: 10.1056/NEJMra0707993. [DOI] [PubMed] [Google Scholar]
- 26.Bauersachs RM, Dudenhausen J, Faridi A, Fischer T, Fung S, Geisen U, Harenberg J, Herchenhan E, Keller F, Kemkes-Matthes B, Schinzel H, Spannagl M, Thaler CJ. Risk stratification and heparin prophylaxis to prevent venous thromboembolism in pregnant women. Thromb. Haemost. 2007;98:1237–1245. doi: 10.1160/th07-05-0329. [DOI] [PubMed] [Google Scholar]
- 27.Dargaud Y, Rugeri L, Vergnes MC, Arnuti B, Miranda P, Negrier C, Bestion A, Desmurs-Clavel H, Ninet J, Gaucherand P, Rudigoz RC, Berland M, Champion F, Trzeciak MC. A risk score for the management of pregnant women with increased risk of venous thromboembolism: a multicentre prospective study. Br. J. Haematol. 2009;145:825–835. doi: 10.1111/j.1365-2141.2009.07698.x. [DOI] [PubMed] [Google Scholar]
- 28.MacLean S, Mulla S, Akl EA, Jankowski M, Vandvik PO, Ebrahim S, McLeod S, Bhatnagar N, Guyatt GH. Chest. 9th ed. Vol. 141. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines; 2012. Patient values and preferences in decision making for anti-thrombotic therapy: a systematic review: Antithrombotic Therapy and Prevention of Thrombosis; pp. e1S–e23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodger M. Pregnancy and venous thromboembolism: 'TIPPS' for risk stratification. Hematol. Am. Soc. Hematol. Educ Program. 2014:387–392. doi: 10.1182/asheducation-2014.1.387. [DOI] [PubMed] [Google Scholar]
- 30.Torrance GW. Measurement of health state utilities for economic appraisal. J. Health Econ. 1986;5:1–30. doi: 10.1016/0167-6296(86)90020-2. [DOI] [PubMed] [Google Scholar]
- 31.Von Neumann J, Morgenstern O. Theory of games and economic behavior. Princeton University Press; Princeton, NJ: 1944. [Google Scholar]
- 32.Schunemann HJ, Norman G, Puhan MA, Stahl E, Griffith L, Heels-Ansdell D, Montori VM, Wiklund I, Goldstein R, Mador MJ, Guyatt GH. Application of generaliz-ability theory confirmed lower reliability of the standard gamble than the feeling thermometer. J. Clin. Epidemiol. 2007;60:1256–1262. doi: 10.1016/j.jclinepi.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Wright DR, Wittenberg E, Swan JS, Miksad RA, Prosser LA. Methods for measuring temporary health States for cost-utility analyses. Pharmacoeconomics. 2009;27:713–723. doi: 10.2165/11317060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Bates SM, Alonso-Coello P, Eckman MH, Tikkinen KA, Ebrahim S, Lopes LC, McDonald SD, Neumann I, Zhang Y, Zhou Q, Jacobson AF, Akl E, Santamaria A, Annichino-Bizzacchi JM, Bitar W, Guyatt GH. Women Values and Preferences and Health State Valuations for Thromboprophylaxis during Pregnancy. 2014 doi: 10.1016/j.thromres.2015.12.015. Vol under review. [DOI] [PubMed] [Google Scholar]
- 35.Akl EA, Oxman AD, Herrin J, Vist GE, Terrenato I, Sperati F, Costiniuk C, Blank D, Schunemann H. Framing of health information messages. Cochrane Database Syst. Rev. 2011:CD006777. doi: 10.1002/14651858.CD006777.pub2. [DOI] [PubMed] [Google Scholar]
- 36.Akl EA, Oxman AD, Herrin J, Vist GE, Terrenato I, Sperati F, Costiniuk C, Blank D, Schunemann H. Using alternative statistical formats for presenting risks and risk reductions. Cochrane Database Syst. Rev. 2011:CD006776. doi: 10.1002/14651858.CD006776.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pabinger I, Grafenhofer H, Kaider A, Kyrle PA, Quehenberger P, Mannhalter C, Lechner K. Risk of pregnancy-associated recurrent venous thromboembolism in women with a history of venous thrombosis. J. Thromb. Haemost. 2005;3:949–954. doi: 10.1111/j.1538-7836.2005.01307.x. [DOI] [PubMed] [Google Scholar]
- 38.Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E, Prins MH. The long-term clinical course of acute deep venous thrombosis(see comments) Ann. Intern. Med. 1996;125:1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 39.Simioni P, Prandoni P, Lensing AW, Scudeller A, Sardella C, Prins MH, Villalta S, Dazzi F, Girolami A. The risk of recurrent venous thromboembolism in patients with an Arg506- - N Gln mutation in the gene for factor V (factor V Leiden) N. Engl. J. Med. 1997;336:399–403. doi: 10.1056/NEJM199702063360602. [DOI] [PubMed] [Google Scholar]
- 40.Douketis JD, Foster GA, Crowther MA, Prins MH, Ginsberg JS. Clinical risk factors and timing of recurrent venous thromboembolism during the initial 3 months of anticoagulant therapy. Arch. Intern. Med. 2000;160:3431–3436. doi: 10.1001/archinte.160.22.3431. [DOI] [PubMed] [Google Scholar]
- 41.Gould MK, Dembitzer AD, Doyle RL, Hastie TJ, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A meta-analysis of randomized, controlled trials. Ann. Intern. Med. 1999;130:800–809. doi: 10.7326/0003-4819-130-10-199905180-00003. [DOI] [PubMed] [Google Scholar]
- 42.Kearon C, Gent M, Hirsh J, Weitz J, Kovacs MJ, Anderson DR, Turpie AG, Green D, Ginsberg JS, Wells P, MacKinnon B, Julian JA. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N. Engl. J. Med. 1999;340:901–907. doi: 10.1056/NEJM199903253401201. [DOI] [PubMed] [Google Scholar]
- 43.Schulman S, Granqvist S, Holmstrom M, Carlsson A, Lindmarker P, Nicol P, Eklund SG, Nordlander S, Larfars G, Leijd B, Linder O, Loogna E. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. The Duration of Anticoagulation Trial Study Group. N. Engl. J. Med. 1997;336:393–398. doi: 10.1056/NEJM199702063360601. [DOI] [PubMed] [Google Scholar]
- 44.Neumann I, Akl EA, Vandvik PO, Agoritsas T, Alonso-Coello P, Rind DM, Santesso N, Alexander PE, Mustafa RA, Prasad K, Bates SM, Schunemann HJ, Guyatt G. Guyatt G, Renni EM, M.O., Cook DJ, editors. How to use a patient management recommendation: clinical practice guidelines and decision analyses. Users' Guides to the Medical Literature - A Manual for Evidence-Based Clinical PracticeMcGraw Hill Education. (3rd ed.) 2015:531–545. [Google Scholar]
- 45.Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood. 2005;106:401–407. doi: 10.1182/blood-2005-02-0626. [DOI] [PubMed] [Google Scholar]
- 46.Palareti G, Leali N, Coccheri S, Poggi M, Manotti C, D'Angelo A, Pengo V, Erba N, Moia M, Ciavarella N, Devoto G, Berrettini M, Musolesi S. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996;348:423–428. doi: 10.1016/s0140-6736(96)01109-9. [DOI] [PubMed] [Google Scholar]
- 47.Gherman RB, Goodwin TM, Leung B, Byrne JD, Hethumumi R, Montoro M. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet. Gynecol. 1999;94:730–734. doi: 10.1016/s0029-7844(99)00426-3. [DOI] [PubMed] [Google Scholar]
- 48.Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI, Schwartz JS, Thompson BT, Popovich J, Jr., Hobbins TE, Spera MA, Alavi A, Terrin ML. The clinical course of pulmonary embolism. N. Engl. J. Med. 1992;326:1240–1245. doi: 10.1056/NEJM199205073261902. [DOI] [PubMed] [Google Scholar]
- 49.Douketis JD, Kearon C, Bates S, Duku EK, Ginsberg JS. Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. JAMA. 1998;279:458–462. doi: 10.1001/jama.279.6.458. [DOI] [PubMed] [Google Scholar]
- 50.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., III Predictors of survival after deep vein thrombosis and pulmonary embolism: a population-based, cohort study [In Process Citation] Arch. Intern. Med. 1999;159:445–453. doi: 10.1001/archinte.159.5.445. [DOI] [PubMed] [Google Scholar]
- 51.Kniffin WD, Jr., Baron JA, Barrett J, Birkmeyer JD, Anderson FA., Jr. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch. Intern. Med. 1994;154:861–866. [PubMed] [Google Scholar]
- 52.Siddique RM, Siddique MI, Connors AF, Jr., Rimm AA. Thirty-day case-fatality rates for pulmonary embolism in the elderly. Arch. Intern. Med. 1996;156:2343–2347. [PubMed] [Google Scholar]
- 53.Alpert JS, Dalen JE. Epidemiology and natural history of venous thromboembolism. Prog. Cardiovasc. Dis. 1994;36:417–422. doi: 10.1016/s0033-0620(94)80050-2. [DOI] [PubMed] [Google Scholar]
- 54.Jeffries WS, Bochner F. Thromboembolism and its management in pregnancy. Med. J. Aust. 1991;155:253–258. doi: 10.5694/j.1326-5377.1991.tb142235.x. [DOI] [PubMed] [Google Scholar]
- 55.Tawes RL, Jr., Kennedy PA, Harris EJ, Brown WH, Scribner RG, Sydorak GR, Beare JP. Management of deep venous thrombosis and pulmonary embolism during pregnancy. Am. J. Surg. 1982;144:141–145. doi: 10.1016/0002-9610(82)90615-8. [DOI] [PubMed] [Google Scholar]
- 56.Rutherford SE, Phelan JP. Deep venous thrombosis and pulmonary embolism in pregnancy. Obstet. Gynecol. Clin. N. Am. 1991;18:345–370. [PubMed] [Google Scholar]
- 57.Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann. Intern. Med. 2003;139:893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- 58.Risks of long-term anticoagulant therapy in elderly patients after myocardial infarction: Second report of the Sixty Plus Reinfarction Study Research Group. Lancet. 1982;1:64–68. [PubMed] [Google Scholar]
- 59.Bleeding during antithrombotic therapy in patients with atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators. Arch. Intern. Med. 1996;156:409–416. [PubMed] [Google Scholar]
- 60.Gates S, Brocklehurst P, Ayers S, Bowler U. Thromboprophylaxis and pregnancy: two randomized controlled pilot trials that used low-molecular-weight heparin. Am. J. Obstet. Gynecol. 2004;191:1296–1303. doi: 10.1016/j.ajog.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 61.Tooher R, Gates S, Dowswell T, Davis LJ. Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period. Cochrane Database Syst. Rev. 2010:CD001689. doi: 10.1002/14651858.CD001689.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hull RD, Pineo GF, Stein PD, Mah AF, MacIsaac SM, Dahl OE, Butcher M, Brant RF, Ghali WA, Bergqvist D, Raskob GE. Extended out-of-hospital low-molecular-weight heparin prophylaxis against deep venous thrombosis in patients after elective hip arthroplasty: a systematic review. Ann. Intern. Med. 2001;135:858–869. doi: 10.7326/0003-4819-135-10-200111200-00006. [DOI] [PubMed] [Google Scholar]
- 63.Greenfield LJ, Proctor MC. Twenty-year clinical experience with the Greenfield filter. Cardiovasc. Surg. 1995;3:199–205. doi: 10.1016/0967-2109(95)90895-c. [DOI] [PubMed] [Google Scholar]
- 64.Greenfield LJ, Proctor MC. The percutaneous greenfield filter: outcomes and practice patterns. J. Vasc. Surg. 2000;32:888–893. doi: 10.1067/mva.2000.110346. [DOI] [PubMed] [Google Scholar]
- 65.Kanter B, Moser KM. The Greenfield vena cava filter. Chest. 1988;93:170–175. doi: 10.1378/chest.93.1.170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.