Abstract
Electrophilic nitration of halo-substituted benzo[c]cinnolines and benzenoids has been achieved regioselectively. The nitro group entry was always ortho to the halo group or/and the aromatic ring. This regioselective electrophilic ortho-nitration was accomplished in mixed acid/mild temperature conditions. Regioselectivity ortho to the halo/ring group(s) was observed with or without proximal steric hindrance. Chlorides and bromides worked equally well in directing these high-yielding ortho-selective reactions.
Graphical abstract
Introduction
The importance of electrophilic aromatic nitration as an industrial process cannot be overstated.1 Nitroaromatics continue to play an indispensable role as intermediates in synthetic applications ranging from pharmaceuticals to explosives. As a classical reaction, the electrophilic aromatic (EAr) nitration has been extensively studied and reviewed over the past two decades.2 the mechanism of this reaction, first proposed by Ingold and Hughes has, since, been modified by several authors casting doubts on both the proposed mechanism and its reported regioselectivity. In fact, the latter is still a subject of animated discussion as it has proved intriguing and difficult to predict. Attempts to rationalize the results have only led to more disagreement.3–8 notwithstanding the well documented, yet challenging work by Olah and coworkers on nitration of highly deactivated aromatics in superacids.9–10 a simpler route to nitroaryl halides using classical conditions, and with view to regioselectivity was a starter.11–12
Especially as we became intrigued when a routine mixed acids nitration of 2,3-dichloronitrobenzene afforded 2,3-dichIoro-1,4-dinitrobenzene selectively. Our facile synthesis of halogenated benzo[c]cinnolines (BCs) has provided the stage to test this theory.13–14 Benzo[c]cinnoline (BC) and its derivatives are interesting because of their biological activities, their potential as ligands for metals, and also for their highly stable molecular conformations with different symmetries. BCs are being increasingly investigated as highly organized materials.15–18 We have envisioned building up annulated BCs starting from a regioselective ortho-nitration of dihalobenzo[c]cinnolines. Upon further investigation, we can report on numerous examples of ortho-, also known as ipso-nitration at work in a general manner with both aryl chlorides and bromides without being affected by steric hindrance nearby.
Results and Discussion
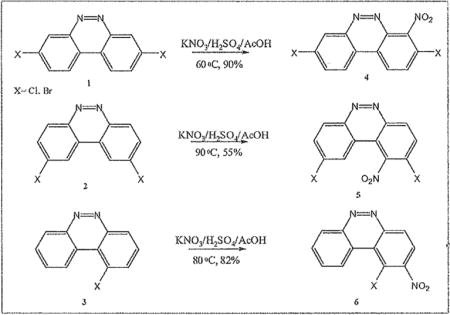
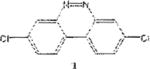
We have been investigating the electrophilic aromatic nitration of nitroaryl halides, and benzo[c]cinnolines in order to prepare substrates for our HSBM Ullmann coupling reactions.19 Benzo[c]cinnoline 0 and dichlorinated benzo[c]cinnolines 1–4 were previously prepared in our laboratory from simple chlorinated nitrobenzenes as outlined in the scheme below.
Aryl halides have been shown to undergo nitration with ortho/para selectivity owing to the halogen’s lone pair.20 Nitration of diazo-deactivated aryl halides promised to inform on the regioselectivity of the reaction with respect to activation at ortho relative to the halo group, deactivation meta relative to the electron-withdrawing diazo group, and on the synergy of the different electronic as well as steric effects. Starting from previously synthesized dihalobenzo[c]cmno lines 1–4 below, electrophilic aromatic nitration afforded the dihalonitrobenzo[c]cinnolines 6–8 using classical conditions (see scheme 2). A solution of Potassium nitrate dissolved in sulfuric acid was, first, diluted in acetic acid. Then the solid dihalobenzo[c]cinnoline was added to the solution which was gradually warmed with a noticeable change of color, and brown fume formation. The same procedure was utilized for nitration of parent benzo[c]cinnoline 0 and the benzenoids 10 and 12 as further evidence of a general method.
Scheme 2.
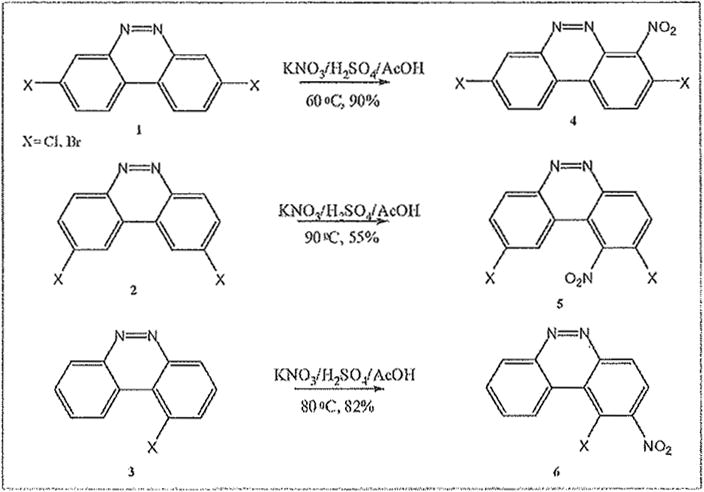
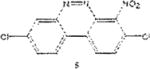
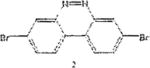
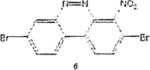
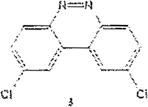
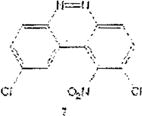
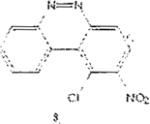
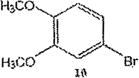
Table 1 below summarizes our results for the syntheses of nitrated benzo[c]cinnolines 5–8 and nitroaryl halides 11, and 13 from the benzo[c]cinnolines 0–4 and the aryl halides 10, 12 respectively. Nitration of benzo[c]cinnoline 0 and its halo derivatives 1–4 with a slight excess (2.2 molar equivalents) of potassium nitrate in a moderately warm mixed acid solution was regioselective in every case. Using these conditions, nitration of 3,8-dichlorobenzo[c]cinnoline 1 afforded 3,8-dichloro-4-nitro-benzo[c]cinnoline 5 in quantitative yield. Similar result was obtained when we switched to the brominated compound 2, which afforded 3,8-dibromo-4nitrobenzo[c]cinnoline 6 in equally quantitative yield. Using a large excess of nitrating reagent (4.0 molar equivalent) confirmed previous results. Seemingly, nitration occurred at ortho position to both the halo group and the cinnoline ring, regardless of the congestion at that position. Meta substituted 2,9-dichlorobenzo[c]cinnoline 3 was, similarly, nitrated ortho to both the halo group and the ring albeit the most sterically hindered position, to afford 2,9-dichloro-lnitrobenzo[c]cinnoline 7 in modest yield, due probably to the mentioned steric congestion.
Table 1.
| Entry | Arly Halide | Nitroarly Hhailde | % Yield* |
|---|---|---|---|
| 1 |
|
|
90 |
| 2 |
|
|
90 |
| 3 |
|
|
55 |
| 4 |
|
|
82 |
| 5 |
|
|
80 |
| 6 |
|
|
95 |
| 7 |
|
|
85 |
isolated yields
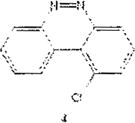
In order to rule out the “deactivating” influence of the diazo group, previously described 1chlorobenzo[c]cinnoline 4 was also nitrated in good yield regioselectively at ortho, or adjacent to the chloro group to afford 1-chloro-2-nitrobenzo[c]cinnoline 8.
Heating the reaction mixture moderately achieved ortho selectivity as a general principle in these systems. This ortho-selectivity has been explained as a 1,2-rearrangement of the NO2 group, which occurs subsequent to the formation of the Wheland’s ipso σ-complex21 during electrophilic aromatic nitration (Scheme 3).
Scheme 3.
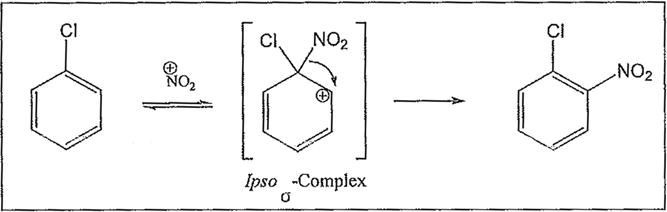
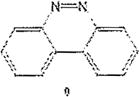
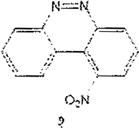
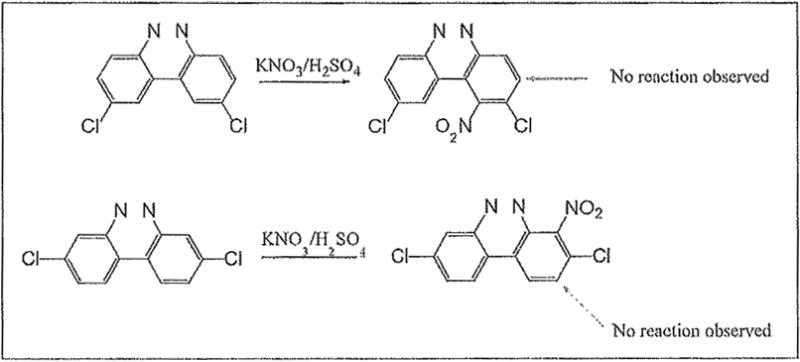
Examples of the occurrence of ortho-selectivity during nitration are numerous, usually in conjunction with greater para-selectivity yield of products.22 Lower temperatures, and shorter reaction time are usually associated with ortho selectivity, as suggested by Tanaka as well.23 By applying these kinetically favorable conditions, we noticed a trend of nitration adjacent to chloro groups, and adjacent to both the chloro and the cinnoline ring, despite existing steric hindrance. This is in agreement with observation by others on the synergy of these effects.24 It is noteworthy to point out that in the chlorinated benzo[c]cinnoline series (entries 1–4), the combined electronic effects of the halo substituent and the adjacent cinnoline ring directed orthoselectivity exclusively to the most electronically favored yet sterically hindered ortho positions instead of the sterically unhindered ortho position to the halo group in a demonstration of the synergetic electronic effects from both substituents (Scheme 4). Further evidence of this regioselectivity was provided when parent benzo[c]cinnoline 0 was nitrated with an excess of potassium nitrate, as shown in entry 5, resulting in a unique nitration product, the somewhat congested 1-nitrobenzo[c]cinnoline 9. Interestingly, the formation of 9 rather than 4nitrobenzo[c]cinnoline establishes the proximity of the aromatic ring, rather than the diazo moiety in the adjacent cinnoline, as the main factor in stabilizing the positive charge.
Scheme 4.
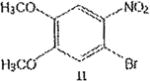
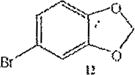
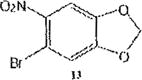
The trend seems to be general with other halides such as bromides. To demonstrate the general nature of the reaction, we conducted a similar nitration of the previously synthesized 3,8dibromobenzo[c]cinnoline 2 to afford 4-nitro-3,8-dibromobenzo[c]cinnoline 6 (entry 2). In the benzenoid series (entries 6, and 7), we used 3,4dimethoxybromobenzene 10 and 3,4methylenedioxybromobenzene 12 to selectively direct nitration at ortho position to the bromo group in either compound to afford nitrated aryl halides 11 and 13 respectively.
We have observed a trend consisting in regioselective nitration of deactivated aryl halides and halobenzo[c]cinnolines at ortho position relative to the adjacent halo group, the aromatic ring or both despite noted steric hindrance. This regioselective nitration can be rationalized by the formation of an ipso σ-complex which is stabilized by either an adjacent halo group’s lone pair or a ring’s π system.25
Experimental Section
Commercially available reagents were ACS grade and used without further purification. NMR spectra were recorded on a Varian 400 MHz (CDC13 as solvent). GC-MS (Direct Probe) and ESI-MS were recorded at the University of South Carolina.
General Procedure
A 50mL Erlenmeyer flask, equipped with a magnetic bar was clamped and placed on a hot plate/stirrer, and then charged with concentrated sulfuric acid (10–20mL), and potassium nitrate (1.1 or 2.2 molar equivalents of the chlorinated aromatic compound, see table) while stirring. The mixture was diluted with acetic acid (40mL) before being warmed, and the heat was adjusted to 90 °C to dissolve the solid. The compound (1 mol) was then quickly added in portions while the temperature was kept near 90 °C. Gradually the mixture became colored, darkening over several minutes before becoming clear after a noticeable brown gas above the liquid has receded. In some instances, the mixture became dark-red/brown immediately after adding the compound. After 1h, the reaction mixture was cooled to room temperature, and poured into a beaker containing crushed ice. The precipitate was collected with suction (Buchner) and dried to provide a crude compound which was recrystallized (commonly with ethanol or/and a mixture of ethanol/acetone) or/and chromatographed in silica gel using a 4:1 hexane/ethyl acetate eluent.
Synthesis of 3,8-dichloro-4-nitrobenzo[c]cinnoline 5
3,8-Dichioro-benzo[c]cinnoline 1 (1.0 molar equivalent) was added into an oven-dried 100mL round-bottomed flask, and stirred in a hot (90 °C) mixture of KNO3/H2SO4/AcOH for 1h. The crude precipitate was vacuum filtered, dried and recrystailized in hot ethanol to afford 3,8-dichloro-4-nitrobenzo[c]cinnoline 5 as brown crystals. M.p. 1870–190 °C.
1H NMR (400 MHz, CDCl3, TMS): δ(ppm) 7.94(d, 1H), 7.97(d, 1H), 8.46(d, 1H), 8.56(d, 1H), 8.79(s, 1H) GCMS: Calculated 293.55, Found: 293.
Synthesis of 3,8-dibromo-4-nitrobenzo[c]cinnoline 6
3,8-Dibromo-benzo[c]cinnoiine[*] 2 (1.0 molar equivalent) was added into an oven-dried 100mL round-bottomed flask, and stirred in a hot (90 °C) mixture of KNO3/H2SO4 for 1h. The crude precipitate was vacuum filtered, dried and recrystailized in hot ethanol to afford 3,8-dibromo-4-nitrobenzo[c]cinnoline 6 as brown crystals. M.p. 207–209 °C.
Recrystailized in hot ethanol to afford 2,9-dichloro-l-nitrobenzo[c]cinnoline 7 as brown crystals. M.p. 156–159 °C.
2H NMR (400 MHz, CDCl3, TMS): δ(ppm) 8.00(d, 1H), 8.06(d, 1H), 8.12(s, 1H), 8.80(d, 1H), 8.88(d, 1H) GCMS: Calculated 293.55, Found: 293.
Synthesis of 1-chloro-2-mtrobenzo[c]ciimoime 8
1-Chloro-benzo[c]cinnoline[*] 4 (1.0 molar equivalent) was added into an oven-dried l00mL round-bottomed flask, and stirred in a warm (90 °C) mixture of KNO3/H2SO4 for 1h. The crude precipitate was vacuum filtered, dried and recrystailized in hot ethanol to afford 1-chloro-2-nitrobenzo[c]cinnoline 8 as yellow crystals. M.p. 150–155 °C.
1H NMR (400 MHz, CDCl3, TMS): δ(ppm) 7.30(s, 2H), 7.59(d, 2H), 8.22(d, 2H)
1H NMR (400 MHz, CDCl3, TMS): δ(ppm) 7.90(d, 1H), 7.99(d, 1H), 8.40(d, 1H), 8.6(d, 1H), 8.9(s, 1H)
Synthesis of 2,9-dichioro-nitrobenzo[c]cihnoiine 7
2,9-Dichloro-benzo[c]cinnoline[*] 3 (1.0 molar equivalent) was added into an oven-dried 100mL round-bottomed flask, and stirred in a warm (90 °C) mixture of KNO3/H2SO4 for 1h. The crude precipitate was vacuum filtered, dried and 2H NMR (400 MHz, CDCl3, TMS): δ(ppm) 3.9(s, 3H), 4.0(s, 3H), 7.11(s, 1H), 7.59(s, 1H)
GCMS: Caicuiated 260.96, Found: 261.
Synthesis of 1,2-methylenedioxy-4-bromo-5-nitro-benzene 13
1,2-methylenedioxy-4bromobenzene 12 (1.0 molar equivalent) was added into an oven-dried 100mL round-bottomed flask, and stirred in a warm (90 °C) mixture of KNO3/H2SO4 for 1h. The crude precipitate was 13C NMR (400MHz,CDCl3,TMS) δ(ppm) 118.5, 119.2, 125.1, 128.0, 129.2, 130.3, 131.0, 131.5, 131.7, 134.2, 146.1, 146.5.
GCMS: Calculated 260.35, Found: 214 (NO2 flight: 46) = 260.
Synthesis of 1-nitrobenzo[c]tinnoline 9
Parent benzo[c]cinnoline 0 (1.0 molar equivalent) was added into an oven-dried 100mL round-bottomed flask, and stirred in a warm (90 °C) mixture of KNO3/H2SO4 for 1h. The crude precipitate was vacuum filtered, dried and recrystallized in hot ethanol to afford l-nitrobenzo[c]cinnoline 9 as yellow crystals. M.p. 137–140 °C.
1H NMR (400 MHz, CDCl3, TMS): δ(ppm) 7.30(s, 2H), 7.59(d, 2H), 8.22(d, 2H)
13C NMR (400 MHz, CDCl3,TMS) δ(ppm) 114.0, 116.2, 123.1, 126.0, 128.2, 131.3, 133.0, 133.5, 134.2, 136.1, 145.0, 146.2.
GCMS: Calculated 225.55, Found: 225.
Synthesis of 1-bromo-2-nitro-4,5-dimethoxybenzene 11
1-bromo-3,4-dimethoxybenzene 10 (1.0 molar equivalent) was added into an oven-dried 100mL round-bottomed flask, and stirred in a warm (90 °C) mixture of KNO3/H2SO4 for 1h. The crude precipitate was vacuum filtered, dried and recrystallized in hot ethanol to afford 1-bromo-2-nitro-4,5-dimethoxybenzene 11 as yellow crystals. M.p. 117–120 °C.
Vacuum filtered, dried and recrystallized in hot ethanol to afford l,2-methylenedioxy-4-bromo5-nitro-benzene 13 as yellow crystals. M.p. 142–144 °C.
1H NMR (400 MHz, CDCl3, TMS): δ(ppm) 6.17(s, 2H), 7.12(s, 1H), 7.45(s, 1H)
13C NMR (400 MHz, CDCl3, TMS) δ(ppm) 104.0, 107.2, 109.1, 115.0, 144.2, 147.3, 152.0 GCMS: Calculated 246.55, Found: 167 (Br = 79) 146.
Supplementary Material
Scheme 1.
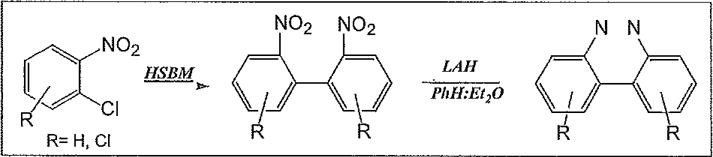
Synthesis of Benzo[c]cinnoline and Chlorinated Benzo[c]cinnolines in two steps.
References
- 1.Yan G, Yang M. Org Biomol Chem. 2013;11:2554. doi: 10.1039/c3ob27354g. and references therein. [DOI] [PubMed] [Google Scholar]
- 2.Hermann H, Gebauer J, Konieczny P. In: Nitration, Recent Laboratory and Industrial Developments. Albright LF, Carr RVC, Schmitt RJ, editors. American Chemical Society; Washington, DC: 1995. p. 234. [Google Scholar]
- 3.Ingold CK. Structure and Mechanism in Organic Chemistry. 2nd. Cornell Univ Press; Ithaca: 1986. [Google Scholar]
- 4.Olah GA, Malhotra R, Narang SC. Nitration. VCH; New York: 1989. [Google Scholar]
- 5.Esteves PM, de Carneiro JM, Cardoso SP, Barbosa AGH, Laali KK, Rasul G, Prakash GKS, Olah GA. J Am Chem Soc. 2003;125:4836–4849. doi: 10.1021/ja021307w. [DOI] [PubMed] [Google Scholar]
- 6.Koleva G, Galabov B, Wu JI, Schaefer HF, III, Schleyer PVR. J Am Chem Soc. 2009;131:14722–14727. doi: 10.1021/ja902194y. [DOI] [PubMed] [Google Scholar]
- 7.Aridoss G, Laali KK. J Org Chem. 2011;76:8088–8094. doi: 10.1021/jo201374a. [DOI] [PubMed] [Google Scholar]
- 8.Tran NG, Kalyvas H, Skodje KM, Hayashi T, Mo€enne-Loccoz P, Callan PE, Shearer J, Kirschenbaum LJ, Kim E. J Am Chem Soc. 2011;133:1184. doi: 10.1021/ja108313u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olah GA, Narang SC, Olah JA. Proc Natl Acad Sci USA. 1981;78:3298. doi: 10.1073/pnas.78.6.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olah GA, Orlinkov A, Oxyzoglou AB, Prakash GKS. J Org Chem. 1995;60:7348–7350. [Google Scholar]
- 11.Olah GA, Laali KK, Sandford G. Proc Natl Acad Sci USA. 1992;89:6670–6672. doi: 10.1073/pnas.89.15.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schofield K. Aromatic Nitrations. Cambridge University Press; Cambridge: 1980. [Google Scholar]; (b) Olah GA, Malhorta R, Narang SC. Nitration: Methods and Mechanisms. VCH; New York: 1989. [Google Scholar]
- 13.Epps AK, Jackson S, Horne TM, Mandouma G. Curr Green Chem. Manuscript Submitted. [Google Scholar]
- 14.Sands B, Nembhard T, Mandouma G. Green Chemistry. Manuscript under Review. [Google Scholar]
- 15.Sienkowska MJ, Farrar JM, Zhang F, Kusuma S, Heiney PA, Kaszynski P. J Mater Chem. 2007;17:1399–1411. [Google Scholar]
- 16.Singh SK, Ruchelman AL, Li T-K, Liu A, Liu LF, LaVoie EJ. J Med Chem. 2003;46:2254–2257. doi: 10.1021/jm020498a. [DOI] [PubMed] [Google Scholar]
- 17.Hansen T, Itoua S, Kamounah FS, Christensen JB, Bjørnholm T, Schaumburg K, Bechgaard K, Wilkes S B. J Mater Chem. 1999;9:1107. [Google Scholar]
- 18.De Feyter S, Abdel-Mottaleb MMS, Schuurmans N, Verkuijl BJV, van Esch JH, Feringa BL, De Schryver FC. Chem Eur J. 2004;10:1124. doi: 10.1002/chem.200305424. [DOI] [PubMed] [Google Scholar]
- 19.Epps A, Barbas J, Mandouma G. Synthesis of Substituted 2,2′-Dinitrobiphenyls by a Novel Solvent-Free High Yielding Ullmann Coupling Biarylation. IJIER. 2014;2:133–149. [PMC free article] [PubMed] [Google Scholar]
- 20.Freire de Queiroz J, Walkimar de M Carneiro J, Sabino AA, Sparrapan R, Eberlin MN, Esteves PM. J Org Chem. 2006;71:6192. doi: 10.1021/jo0609475. [DOI] [PubMed] [Google Scholar]
- 21.Wheland GW. The Theory of Resonance. Wiley; New York: 1944. [Google Scholar]
- 22.Manglik AK, Moodie RB, Schofield K, Dedeoglu E, Dutly A, Rys P. J Chem Soc Perkin Trans. 1981;2:1358. [Google Scholar]
- 23.Tanaka M, Muro E, Ando H, Xu Q, Fujiwara M, Souma Y, Yamaguchi Y. J Org Chem. 2000;65:2972–2978. doi: 10.1021/jo991538u. [DOI] [PubMed] [Google Scholar]
- 24.Esteves PM, Carneiro JWDM, Cardoso SP, Barbosa AGH, Laali KK, Rasul G, Prakash GKS, Olah GA. J Am Chem Soc. 2003;125:4836. doi: 10.1021/ja021307w. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Liu Z, Li H, Fang G, Badru-Deen B, Tuemay Abadi B, Bi X, Qun Liu Q. Organic Letters. 2011;13:6536–6539. doi: 10.1021/ol2028288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


