Abstract
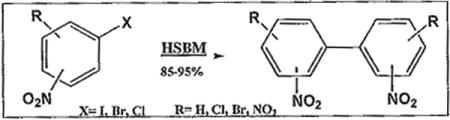
Solvent-free reaction using a high-speed ball milling technique has been applied to the classical Ullmann coupling reaction for the first time. Biarylation of 2-iodonitrobenzene was achieved in quantitative yield when performed in a custom-made copper vial through continuous shaking without additional copper or solvent. The product was solid, NMR ready and required no lengthy extraction for purification. This reaction was cleaner, and faster than solution phase coupling which requires longer reaction time in high boiling solvents, added copper catalyst, and lengthy extraction and purification steps. Gram quantities of the biaryl compound were synthesized in larger copper vials. This is a general method that can be used to effectively reduce industrial waste en route to sustainability.
Graphical abstract
Introduction
The importance of aryl-aryl bond formation cannot be overstated. Over the last few years, hundreds of articles addressing this issue have been published.1–3 The elaboration of the biaryl moiety has often been the key intermediate step towards many important drugs in medicinal chemistry. Modern organometallic reactions involving expensive metals such as nickel, palladium, and ruthenium are efficient in achieving this goal.4–6 However, the most cost effective method of biarylation is arguably the copper-mediated Ullmann coupling reaction of aromatic halides.7 However, the use of elevated temperature, and lower rates of coupling in case of deactivated aromatic halides are often cited as limitations of this reaction. Improved copper catalysts, and the introduction of solventless processes over the last few years have ushered a sort of renaissance for these copper-mediated biaryl forming reactions.8 Historically, it is important to note that the traditional 1901 Ullmann coupling reaction is a copper-mediated aryl-aryl bond formation while the 1903 Goldberg-modified Ullmann condensation reaction is a essentially a copper-mediated aryl-ether and aryl-amine bond formation.9 Reviews on Cu-mediated coupling reactions by Beletskaya, Ley, and Lemaire have been expansive on the recent modifications aimed at improving these reactions.10–12 Nevertheless, with the recent push in the scientific community over environmental issues, the need to drastically reduce polluting chemicals, especially non-biodegradable solvents and fuels, has led to the search for less aggressive methods.13 One such method involves solvent-free chemistry, which in fact is not new since solid phase chemistry has been known for centuries. It has always been neglected due to our trust to the old Aristotle belief that “no Coopora nisi Fluida” or “no reaction occurs in the absence of solvent.”14 Solvent free methods are being revisited, and being used to great effect.15 Of practical importance among them is high speed ball milling (HSBM) which consists in shaking a compound in a capped vial along with a ball. We have found High Speed Ball Milling (HSBM) to be efficient conditions for preparing biaryl compounds by the Ullmann reaction.16 HSBM is a solvent-less process of continuous grinding through shaking a compound in a vial (made of copper) using a ball (also made of copper), with no additional copper added. We used Parr model 2500 shaker instead of a traditional Spex mill. The Parr 2500 has a frequency of 5 m/s and its loading chamber that can accommodate several vials of variable sizes. Thus, several milling processes could be done at once, allowing for the synthesis of several biaryl compounds from different aryl halides. This method is “green” as it avoids a significant drawback of the traditional Ullmann coupling reaction which uses high boiling solvents such as dimethyl formamide, nitrobenzene, or pyridine. Very small amounts of inorganic biproducts are formed on the wall of the copper vessel which, characteristically, loses its shine. This seems to allow the organic product to be almost pure and ready for analysis by NMR without further purification, This method, completely, circumvents the need to use high boiling, often toxic, solvents or additional copper powder. It is also economical in reducing the requirement for work up, and lengthy purification of the product by chromatographic methods.
Results and Discussion
Biaryl synthesis, an important tool in medicinal chemistry, has been achieved through various synthetic methods.17 Palladium chemistry remains very popular for biarylation. However, due to the high cost of palladium catalysts, their incompatibility with a host of function groups, and the need to reduce the use of toxic solvents, alternative methods of aryl-aryl bond formation are being sought and evaluated. The use of high speed ball milling methods permits to circumvent both added catalyst and elevated temperatures.18–19 We have been exploring the feasibility of a solvent-less, catalyst-free Ullmann biarylation in an all copper vessel with an all copper ball as an alternative method to heating copper with a nitroaryl halide, which can be lead to detonation. Goj et al. reported a solvent-free synthesis of 2,2′-dinitrobiphenyl in modest yield by heating 2-iodonitrobenzene with copper in a vial20 Higher yields of a biaryl product were expected from coupling reactions conducted in a custom- made copper vial as the source of copper catalyst, together with a copper ball. Coupling of 2-iodonitrobenzene was performed, under these conditions, to afford the biaryl 2,2′-dinitrobiphenyl without using high boiling solvents, nor any additional copper heated at higher temperature. Building on the assumption that Ullmann coupling proceeds most rapidly with aryl halides bearing electron-withdrawing groups at ortho position, we selected nitrated aryl halides, some commercially available and the others were prepared in our laboratory.21 To start, we observed rapid dimerization of o-iodonitrobenzene 1 in a copper vial using a copper ball to afford 2,2′-dinitrobiphenyl 6, achieved in quantitative yield (97%) shaking overnight (entry 1). The scope of this reaction was broadened to incorporate several halogenated nitroaryls which, under similar HSBM conditions, yielded corresponding dihalo-substituted dinitrobiphenyls 7–10 (see table 1).
Table 1.
| Entry | Arene | Biaryl | Yield (%) |
|---|---|---|---|
| 1. |

|

|
97 |
| 2. |

|

|
88 |
| 3. |

|

|
95 |
| 4. |

|

|
85 |
| 5. |

|

|
82 |
The coupling of commercially available 2,3-dichloronitrobenzene 2 using the same HSBM conditions yielded the atropisomeric 2,2′dichloro-6,6′-dinitrobiphenyl 7 in 88%. Likewise, commercially available 2,4-dichloronitrobenzene 3, and 2,5-dichlorobenzene 4 were simultaneously loaded into the HSBM shaker to undergo biarylation in high yields. From nitroaryl halide 3, we obtained 4,4′-dichloro-2,2′dinitrobiphenyl 8 in 95% yield (entry 3) while the homocoupling of nitroaryl halide 4 afforded 5,5′dichloro-2,2′-dinitrobiphenyl 9 in 85% yield (entry 4). It seemed that activation by an electronwithdrawing nitro group at ortho position to the halo group was the overriding factor rather than steric hindrance as shown in entry 2. In order to probe this observation we prepared 2,3-dichloro-l,5dinitrobenzene 5 from 1,2-dichlorobenzene. HSBM biarylation of the tetrasubstituted aryl halide 5 afforded the corresponding highly substituted dimer 2,2′-dichloro-4,4′,6,6′-tetranitrobiphenyl 10 in very good yield as well. These results stand in stark contrast with those from solution phase Ullmann coupling reactions where significantly lower yields are observed despite stoichiometric catalyst payloads, higher temperature, and longer reaction times used. The effects of solubilization seem detrimental to an increase in the yields of biarylation. In contrast, one can speculate that coupling reactions taking place on the metal surface seem to happen faster, cleaner and abundantly. In traditional Ullmann reactions, the solubility of aryl halides or lack thereof affects the yields of biarylation in favor of the most soluble aryl halide.23 In HSBM conditions, it is the rate of collisions between molecules that seems to determine the yield of the product(s).24 Our results show that, in Ullmanntype reactions, the most important factor is activation by an electrowithdrawing group such as the nitro group ortho to the halo group, not solubility since couplingoccurs in a “solventless” phase, in many of the HSBM reactions,. The mechanism by which the HSBM biarylation reactions proceed is yet to be elucidated. Any mechanism would be intrinsically similar to what is believed to occur in the traditional Ullmann coupling reaction.25 The use of copper vials and copper balls assumes that the necessary catalyst for the reaction is incorporated in the vessel and ball. The inner walls of the copper vial constitute the reactive sites as suggested by the corrosion and loss of shine due to a deposition of copper halides. A single electron transfer (SET) mechanism leads to the formation of an aryl radical26 The formation of aryl radical is supported by the dimerization of highly hindered haloarenes forming substituted biphenyls.
Given that the aryl halide is the sole compound loaded into the all-copper reactor, solubilization of Cu(I)X species in melting aryl halide would rather be minimal. Oxidative addition leading up to the formation of Cu(II) species would be speculative.
Conclusion
The future of chemical manufacturing is closely linked to the development of “green” processes to reduce risk and safety concerns.13 Ullmann reactions can be used, using HSBM conditions to generate various biaryl compounds, as shown above, without generating unnecessary waste from expensive and toxic solvents and catalysts. The scope of this solvent-free process is being broadened to cross coupling condensation to include arylation with phenols and amines.
Experimental
All NMR spectra were recorded on a Varian Mercuiy 400MHz spectrometer. Deuterated NMR solvents were obtained from Cambridge Isotope Laboratories, Inc., Andover MA, and used without further purification. All products were confirmed by 1H, 13CNMR, and GCMS.
Starting materials were purchased from Sigma-Aldrich and used without further purification. Ball milling was carried out in a 2500 Parr Hydrogenator/Shaker. Ball bearings were purchased from Small Parts incorporated. Custom made vials were made at the machine shop of Furman University (Dr. Lon Knight) from parts purchased at Lowe’s distribution center, Greenville, SC.
Synthesis of 2,2′-dinitrobiphenyl 6
2-Iodonitrobenzene 1 (2.5g ~ 10mmol) was added to a copper vial charged with a copper ball-bearing and subjected to high speed ball milling procedure as described above to afford 2.02g (97% yield) bright yellow needle crystals of 2,2′-dinitrobiphenyl 3 after recrystallization in hot ethanol. Mp 114–116 °C.
1 H NMR(400 MHz, CDCl3, TMS): □□(ppm) 7.29(D, 2H), 7.60(T, 2H), 7.68(T, 2H), 8.22(D, 2H) 13 C NMR (400 MHz, CDC13, TMS): □□(ppm) 124.7, 129.1, 130.8, 133.3, 134.1, 147.1 GC-MS: m/z 198, calculated: 244; found: 198 (loss of NO2 as shown to be common for nitrated aromatic compounds by NIST).
Synthesis of 2,2′-dichloro-6,6′-dinitrobiphenyl 7
2,3-dichloronitrobenzene 2 (1.92g – 10mmol) was added to a copper vial charged with a copper ball-bearing and subjected to high speed ball milling procedure as described above to afford 1.7g of a pale yellow needle-like crystals of 2,2′-dichloro-6,6′dinitrobiphenyl 7 after recrystallization in hot ethanol. Mp 124–126 °C.
1 H NMR (400 MHz, CDCI3, TMS): □ (ppm) 7.60(T, 2H), 7.81(D, 2H), 8.22(D, 2H) 13 C NMR (400 MHz, CDCI3, TMS): □ (ppm) 123.8, 130.3, 130.8, 135.1, 135.3, 148.1 GC-MS: GC-MS: m/z 312, calculated: 312; found: 312.
Synthesis of 2,2′-dinitro-4,4′-dichlorobiphenyl 8
l,4-dichloro-2-nitrobenzene 3 (1.92g–10mmol) was added to a copper vial charged with a copper ball-bearing and subjected to high speed ball milling procedure as described above to afford 1.69g of crystals of 2,2′-dinitro-4,4′-dichlorobiphenyl 8 after recrystallization in hot ethanol. Mp 174–176 °C 1 H NMR (400 MHz, CDCl3, TMS): □ (ppm) 7.30(D, 2H), 7.72(D, 2H), 8.25(S, 2H) 13 C NMR (400 MHz, CDCl3, TMS): □ (ppm) 124.8, 131.3, 132.0, 134.8, 135.3, 147.8 GC-MS: m/z 312, calculated: 313; found: 312.
Synthesis of 2,2′-dinitro-5,5′-dichlorobiphenyl 9
2,4-dichloronitrobenzene 4 (1.92g–10mmol) was added to a copper vial charged with a copper ball-bearing and subjected to high speed ball milling procedure as described above to afford 1.60g crystals of 2,2′-dinitro-5,5′-dichlorobiphenyl 9 after recrystallization in hot ethanol. Mp 144–146 °C 1 H NMR (400 MHz, CDC13, TMS): □ (ppm) 7.30(S, 2H), 7.59(D, 2H), 8.22(D, 2H) 13 C NMR (400 MHz, CDC13, TMS): □ (ppm) 124.5, 130.5, 130.8, 133.8, 135.8, 147.5 GC-MS: m/z 312, calculated: 313; found: 312.
Synthesis of 2,2′-dichloro-4,4′,6,6′-tetranitrobiphenyl 10
1,2-dichloro-3,5-dinitrobenzene 5 (2.37g~10mmol) was added to a copper vial charged with a copper ball-bearing and subjected to high speed ball milling procedure as described above to afford yellow needle-like crystals of 2,2′-dichloro-4,4′,6,6′-tetranitrobiphenyl 10 after recrystallization in hot methanol. Mp 276 °C 1 H NMR (400 MHz, CDCl3, TMS): □ (ppm) 7.60(T, 2H), 7.81(D, 2H), 8.22(D, 2H) 13 C NMR (400 MHz, CDC13, TMS): □ (ppm) 119.0, 129.5, 135, 136.1, 148.0, 148.5 GC-MS: m/z 402, calculated: 403; found: 402.
Supplementary Material
References
- 1.a) Beletskaya IP, Cheprakov AV. Chem Rev. 2000;100:3009–3066. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]; b) Liu Y, Wang SS, Liu W, Wan QX, Wu HH, Gao GH. Curr Org Chem. 2009;13:1322–1346. [Google Scholar]; c) Moreno-Manas M, Pleixats R. Acc Chem Res. 2003;36:638–643. doi: 10.1021/ar020267y. [DOI] [PubMed] [Google Scholar]
- 2.Phan NTS, Van Der Sluys M, Jones CW. Adv Synth Catal. 2006;348:609–679. [Google Scholar]
- 3.Yin LX, Liebscher J. Chem Rev. 2007;107:133–173. doi: 10.1021/cr0505674. [DOI] [PubMed] [Google Scholar]
- 4.Miyaura N, Yanagi T, Suzuki A. Synth Commun. 1981;11:513. [Google Scholar]
- 5.Stille JK. Angew Chem, Int Ed Engl. 1986;98:504. [Google Scholar]
- 6.Negishi EI, Luo FT, Frisbee R, Matsushita H. Heterocycles. 1982;18:117. [Google Scholar]
- 7.Ullmann F, Bielecki J. Chem Ber. 1901;34:2174. [Google Scholar]
- 8.Ullmann F. Ber Dtsch Chem Ges. 1904;37:853–857. [Google Scholar]
- 9.Goldberg I. Ber Dtsch Chem Ges. 1906;39:1691–1696. [Google Scholar]
- 10.Beletskaya IP, Cheprakov AV. Coord Chem Rev. 2004;248:2337. [Google Scholar]
- 11.Thomas AW, Ley SV. Angew Chem Int Ed. 2003;42:5400–5449. doi: 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]
- 12.Hassan J, Sevingnon M, Gozzi C, Shulz E, Lemaire M. Chem Rev. 2002;102:1359–1469. doi: 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]
- 13.Anastas P, Warner J. Green Chemistry: Theory and Practice. 1998 [Google Scholar]
- 14.Toda F. Pure Appl Chem. 1996;68:285–290. [Google Scholar]
- 15.Tanaka K. Solvent-Free Organic Synthesis. Wiley-VCH; Cambridge: 2003. [Google Scholar]
- 16.Nelson TD, Crouch RD. Organic Reactions Vol. Vol. 63. Wiley; 2004. Chap.3. [Google Scholar]
- 17.de Meijere A, Diederich F. Metal-Catalyzed Cross-Coupling Reactions. 2nd. Vol. 1. Wiley-VCH; 2004. [Google Scholar]
- 18.a) Tanaka K, Toda F. Chem Rev. 2000;100:1025–1074. doi: 10.1021/cr940089p. [DOI] [PubMed] [Google Scholar]; b) Dong YW, Wang GW, Wang L. Tetrahedron. 2008;64:10148–10154. [Google Scholar]
- 19.a) Waddell DC, Mack J. Green Chem. 2009;11:79–82. [Google Scholar]; b) Waddell DC, Thiel I, Clark TD, Marcum ST, Mack J. Green Chem. 2010;12:209–211. [Google Scholar]; c) Mack J, Fulmer D, Stofel S, Santos N. Green Chem. 2007;9:1041–1043. [Google Scholar]
- 20.Gregor RW, Goj LA. J Chem Ed. 2011;88:331–333. [Google Scholar]
- 21.A.K. Epps, G.R. Mandouma, manuscript submitted for publication.
- 22.Forrest J. J Chem Soc. 1960;592 [Google Scholar]
- 23.Kornblum N, Kendall DL. J Am Chem Soc. 1952;74:5782. [Google Scholar]
- 24.Concas A, Lai N, Pisu M, Cao G. Chem Eng Set. 2006;61:3746–3760. [Google Scholar]
- 25.a) Rapson WS, Shuttleworth RG. Nature. 1941;147:675. [Google Scholar]; b) Bell F, Morgan WHD. J Chem Soc. 1954:1716. [Google Scholar]
- 26.a) Jones GO, Liu P, Houk KN, Buchwald SL. J Am Chem Soc. 2010;132:6205–6213. doi: 10.1021/ja100739h. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sperotto E, van Klink GPM, van Koten G, de Vries JG. Dalton Trans. 2010;39:10338–10351. doi: 10.1039/c0dt00674b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


