Abstract
The enteric serotonin transporter (SERT) plays a critical role in modulating serotonin availability and thus has been implicated in the pathogenesis of various intestinal disorders. To date, SERT expression and function in the human intestine have not been investigated. Current studies were designed to characterize the function, expression, distribution, and membrane localization of SERT in the native human intestine. Real-time PCR studies showed relatively higher SERT mRNA expression in the human small intestine compared with colon (ileum ≫ duodenum ≫ jejunum). Northern blot analysis revealed three mRNA hybridizing species encoding SERT (3.0, 4.9, and 6.8 kb) in the human ileum. Consistent with SERT mRNA expression, SERT immunostaining was mainly detected in the epithelial cells of human duodenal and ileal resected tissues. Notably, SERT expression was localized predominantly to the apical and intracellular compartments and was distributed throughout the crypt-villus axis. Immunoblotting studies detected a prominent protein band (~70 kDa) in the ileal apical plasma membrane vesicles (AMVs) isolated from mucosa obtained from organ-donor intestine. Functional studies showed that uptake of [3H]serotonin (150 nM) in human ileal AMVs was 1) significantly increased in the presence of both Na+ and Cl−; 2) inhibited (~50%) by the neuronal SERT inhibitor, fluoxetine (10 μM) and by unlabeled 5-HT; and 3) exhibited saturation kinetics indicating the presence of a carrier-mediated process. Our studies demonstrated differential expression of SERT across various regions of the human intestine and provide evidence for the existence of a functional SERT capable of removing intraluminal serotonin in human ileal epithelial cells.
Keywords: serotonin uptake, serotonin transporter expression
SEROTONIN (5-hydroxytryptamine, 5-HT) is a neurotransmitter and hormone that influences diverse physiological functions. About 90% of the whole body content of 5-HT is present in the gastrointestinal (GI) tract, where it plays a critical role in modulation of gut motility and fluid and electrolyte transport (10, 12, 28, 30). The majority of 5-HT in the GI tract is synthesized and stored in secretory granules of mucosal en-terochromaffin (EC) cells. EC cells continuously release small amounts of 5-HT in response to a number of chemical and mechanical stimuli (13). Because 5-HT exerts its effects via specific 5-HT receptor subtypes, a mechanism for 5-HT deactivation is necessary to prevent the receptors from desensitization. The enzymes that catabolize 5-HT, including monoamine oxidases and glucuronyl transferases, are localized intracellu-larly and thus require transport of 5-HT across plasma membranes (5, 31). However, 5-HT is a highly charged molecule at physiological pH (an acid dissociation constant of 10.0) and crosses lipid membranes poorly. The 5-HT transporter (SERT) accelerates transport of 5-HT across membranes for its internalization and is, therefore, a critical regulatory mechanism that terminates serotonergic signaling.
SERT belongs to a large superfamily of sodium/chloride-dependent transporters, which also include transporters for norepinephrine, dopamine, glycine, taurine, proline, and γ-aminobutyric acid (27). These transporters consist of ~600 amino acids with 12 transmembrane domains. SERT has recently generated considerable interest because it represents a pharmacological target of tricyclic antidepressants and selective serotonin reuptake inhibitors (SSRIs). Furthermore, alterations in serotonergic activity have been implicated in the pathophysiology of irritable bowel syndrome and inflammatory bowel diseases (6). Given the important role of SERT in modulating the 5-HT availability, it is of great importance to understand SERT function and expression in the human intestine.
SERT activity and expression have been extensively described in the central and peripheral nervous systems. A neuronal SERT has been cloned in rats (16), guinea pigs (5), mice (3), bovines (24), and humans (25). Functionally, this transporter is Na+ and Cl− dependent and is inhibited by SSRIs such as fluoxetine (20). SERT has also been characterized in specialized nonneuronal cells, including platelets (2, 26), pulmonary endothelium (19), and placental syncytiotrophoblasts (26). At the intestinal level, previous studies have shown that rat and guinea pig mucosal epithelial cells express SERT mRNA and display functional 5-HT uptake through a Na+/Cl−-dependent process (5, 29, 31). Recently, Martel et al. (21) demonstrated that human intestinal Caco-2 cells functionally express SERT at both apical and basolateral cell membranes. However, SERT function, expression along the longitudinal (duodenum to colon) and vertical (crypt to surface) axes, and polarized membrane distribution in native intestine have not been explored.
Our studies demonstrate that SERT is differentially expressed along the longitudinal axis of the intestine. SERT mRNA and protein are expressed at the highest level in the small intestine. SERT protein is predominantly localized to the apical and intracellular compartments and uniformly distributed along the vertical axis. Functional analysis demonstrated that the 5-HT uptake in ileal apical plasma membrane vesicles occurs through the previously characterized neuronal SERT, which may play an important role in the clearance of 5-HT from the small intestine lumen.
MATERIALS AND METHODS
Materials
[3H]5-HT (specific activity 10 Ci/mmol; radiochemical purity > 97%) was obtained from American Radiolabeled Chemicals (St. Louis, MO). Unlabeled 5-HT (creatinine sulfate) was obtained from Sigma (St. Louis, MO). All other chemicals and reagents were obtained from either Fisher Scientific (Fairlawn, NJ) or Sigma, unless otherwise stated, and were of the highest purity available.
Real Time Quantitative RT-PCR Analysis
Total RNA from human small intestine and colon was commercially obtained from Promega Corp (Madison, WI). Equal amounts of RNA from both small intestine and colonic samples were reverse transcribed and amplified in one step reaction by using Brilliant SYBR Green quantitative RT-PCR (QRT-PCR) Master Mix kit (Stratagene). Real-time QRT-PCR was performed by using Mx3000P (Stratagene). For assessing region-specific expression of SERT, human cDNAs from duodenum, ileum, jejunum, and colon were commercially obtained (from BioChain). Human SERT was amplified with gene-specific primers (sense primer: 5′-CAGCGTGTGAAGATGGA-GAAG-3′; antisense primer: 5′-TGGGATAGAGTGCCGTGTGT-3′). β-actin was amplified as an internal control by using gene-specific primers (sense primer: 5′-CATGTTTGAGACCTTCAACAC-3′; anti-sense primer: 5′-CCAGGAAGGAAGGCTGGAA-3′). Analysis of standard curve with the primers used in the present study indicated that threshold was in the linear range with the amount of template being used. After real-time PCR amplification, the machine was programmed to do a melt curve, in which the temperature was raised by a fraction of a degree and the change in fluorescence intensity was measured. The melting curve for all samples showed a single peak, indicating the amplification of only one amplicon for each primer set. Because the amplification efficiencies for both SERT and β-actin were approximately equal, the quantitation was expressed as a ratio of 2ΔCt:SERT to 2ΔCt:βactin, where ΔCt:SERT and Δ:βactin represent the difference between the threshold cycle of amplification for SERT and β-actin, respectively.
Northern Blot Analysis
Commercially available normal human multiple-tissue Northern blots (BioChain) each containing poly(A)+ RNA (3 μg/lane) from different human tissues, were used. A 491-bp fragment of human SERT was amplified by using human SERT gene-specific primers. The sequences of the primers used were forward, 5′-CATCACCT-GCTTCTTTGGATCCCTGGTCA-3′, and reverse, 5′-TGAGTGT-GTTACACAGCATTCAAGCGGATG-3′. The PCR fragment was gel purified and was radiolabeled with [32P]dCTP (3,000 Ci/mmol; ICN, Costa Mesa, CA) by random priming to a specific activity of 109 cpm/μg. Prehybridization and hybridization were performed at 68°C in ExpressHyb solution (Clontech) according to the manufacturer’s instructions, with a final probe concentration of 106 cpm/ml. The blots were washed for 40 min at room temperature in 2× SSC-0.05% SDS and for 20 min at 50°C in 0.1× SSC-0.1% SDS and were visualized by X-ray autoradiography.
Sample Collection for Immunofluorescence Staining
Intestinal resection specimens from patients undergoing surgery at The University of Chicago Medical Center were sampled at uninvolved surgical margins and were snap frozen as full-thickness sections in optimal cutting temperature media within 60 min of surgical resection. Histological analysis by an expert surgical pathologist verified that the samples obtained represented normal tissues. These were stored at −80°C until experimental use. All procedures were approved by The University of Chicago Institutional Review Board. Neutral buffered zinc (10%) formalin-fixed paraffin-embedded normal tissues from human intestinal resection specimens were selected and obtained from the archives of the pathology department at The University of Virginia at Charlottesville. All procedures were approved by the Institutional Review Board for Health Sciences Research at the University of Virginia.
SERT Antibody
The commercially available histochemical antibody for SERT (Immunostar) generated in a rabbit against a synthetic peptide sequence corresponding to amino acids 602–622 of rat neuronal SERT coupled to KLH (keyhole lympet hemocyanin) was used.
Immunofluorescence Tissue Staining in Optimal Cutting Temperature Sections
Frozen sections (4 μm) were fixed in 1% paraformaldehyde (prepared in PBS) for 2 min, washed three times for 5 min with PBS, permeabilized with 0.5% Igepal CA-630 in PBS for 5 min, and washed two more times in PBS. Sections were then blocked by incubation in PBS with 5% normal goat serum for 15 min before a 90-min incubation in primary SERT antisera. After five washes in PBS with 1% normal goat serum, sections were incubated with secondary antisera for 60 min. Fluorescent conjugates of phalloidin, to label F-actin, and Hoechst 33324, to label nuclei, were included with secondary antisera.
Immunofluorescence Staining in Paraffin-Embedded Sections
Immunofluorescent studies were carried out on 4-μm paraffin sections of human small intestine and colonic tissues mounted on (3-aminopropyl) triethoxysilane-treated slides. The slides were placed at 60°C for 30 min, deparaffinized in xylene for 20 min to remove the embedding media, and washed in absolute ethanol for 5 min. The slides were then gradually rehydrated gently in a series of alcohol washes, including 96, 85, and 50%, and were placed in distilled water for 5 min each. Antigen retrieval was used for tissue sections submerged in 0.1 M citrate buffer, pH 6.0 for 30 min at 100°C in a steamer. Slides were allowed to cool to room temperature and were rinsed in wash buffer (Tris-buffered saline containing 0.1% Triton X-100). The slides were then incubated for 1 h at room temperature in 5% normal goat serum in Tris-buffered saline to block nonspecific antibody binding. This was followed by incubation of the slides with primary antibody, diluted 1:100 (4°C, overnight). The primary antibodies used were anti-SERT, monoclonal anti-villin, and monoclonal anti-Na+-K+-ATPase. Tissues were then stained with anti-goat or anti-mouse fluorescein isothiocyanate antibody diluted at 1:100 (Molecular Probes) and Hoechst 33342 1:20,000 (Invitrogen) for 60 min. After final washes, slides were mounted with a coverslip by using Permount and were sealed with clear nail polish.
Image Acquisition
Cells were visualized by using confocal or fluorescence microscopy. Sections were imaged by using a Leica DM4000 epifluorescence microscope equipped with DAPI, Endow GFP, and Texas red zero pixel-shift filter sets (Chroma Technology) and a Coolsnap HQ camera (Roper Scientific) controlled by MetaMorph 6 (Universal Imaging). Confocal microscopy was performed with a Carl Zeiss LSM 510 laser-scanning confocal microscope equipped with a ×25 water-immersion objective. Beams of 488 nm and 534 nm from an Ag/Kr laser and 361 nm from a UV laser were used for excitation. Green and red fluorescence emissions were detected through LP505 and 585 filters, respectively. The two different fluorochromes were scanned sequentially by using the multitracking function to avoid any bleed through among these fluorescent dyes. For control experiments, the sections were incubated with primary antibody omitted or primary antibody adsorbed with excess of immunizing peptide.
Isolation of Human Small Intestinal and Colonic Plasma Membrane Vesicles
Small intestine and colon from healthy adult organ donors (primarily trauma victims) were obtained immediately after harvest of transplantation organs. The small intestine was divided into two equal parts after one-third of the middle portion was discarded. The first half was considered jejunum and the second half ileum. After the cecum was discarded, the remaining large intestine was divided equally into proximal and distal colon. The mucosa was scraped from the sero-muscular layer of these segments and was stored at −80°C.
Apical Membrane Vesicles
The apical membranes from these four regions of the intestine were prepared as described previously (9, 11). The purity of apical membrane vesicles (AMVs) and the degree of contamination with intra-cellular organelles were assessed by using appropriate marker enzymes. The small intestinal membranes exhibited 15- to 20-fold purity over the crude homogenate as assessed by the activities of alkaline phosphatase and sucrase-isomaltase. The colonic membranes showed 7- to 10-fold purity over the crude homogenate as assessed by the activity of cysteine-sensitive alkaline phosphatase.
Immunoblotting
For immunoblotting studies, briefly, 150 μg protein from each of the purified human small intestinal and colonic apical membranes was solubilized in Laemmli sample buffer (2% SDS, 100 mM dithiothreitol, 60 mM Tris, pH 6.8, 0.01% bromophenol blue) and were separated on 10% Tris/glycine SDS-polyacrylamide gel. The separated proteins were electroblotted onto nitrocellulose membranes and were stained with Ponceau S to ensure equal loading of the protein in each lane. The blot was then probed with rat anti-SERT antibody (Immunostar) (overnight, 4°C, diluted 1:500). The bands were visualized by enhanced chemiluminescence according to the manufacturer’s instructions (Amersham).
[3H]5-HT Uptake Studies
Uptake of [3H]5-HT into intestinal AMVs was measured by using a rapid filtration technique as previously described by us (9). The apical membrane vesicles (AMVs) were prepared in 300 mM mannitol, 10 mM Tris-HEPES, pH 7.4, and 0.1 mM MgSO4. BBMVs (20 μl) containing 60–80 μg protein were incubated in incubation media (80 μl) with a known buffer composition (100 mM NaCl or KCl, or K-gluconate; 100 mM mannitol; 10 mM Tris-HEPES, pH 7.4; and 0.1 mM MgSO4). The transport was studied at 30°C in a water bath with uniform temperature. The uptake was stopped at various time points by using 5 ml of ice-cold stop solution containing 280 mM mannitol and 20 mM Tris-HEPES, pH 7.5. The diluted sample was rapidly filtered by using a rapid filtration technique, employing 0.25-μm nitrocellulose filters. Filters were further washed twice with 5 ml ice-cold stop solution. The filters were then dissolved in Filter-Count, and the radioactivity was measured in a Packard TR1600 liquid scintillation counter (Packard Instruments, Downers Grove, IL). All values were corrected for nonspecific [3H]5-HT binding to filters and/or vesicles by subtracting radioactivity present in time 0 vesicle blank.
Statistical Analysis
Student’s t-test was used for statistical analysis. P < 0.05 was considered statistically significant.
RESULTS
SERT mRNA Levels
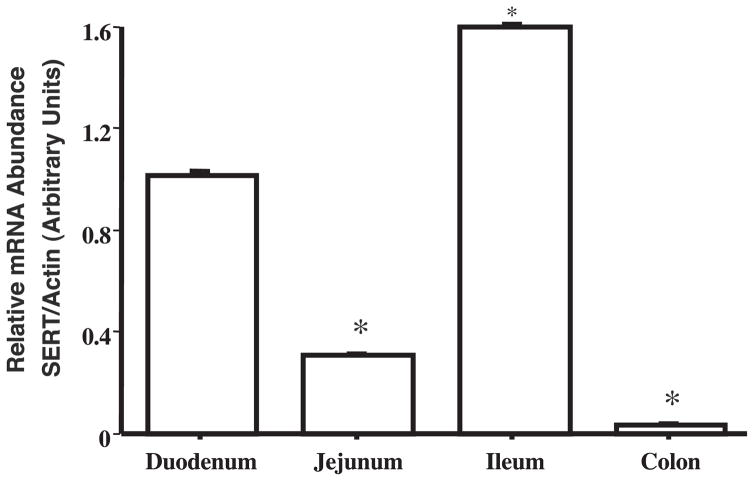
Initial studies examined the relative expression of SERT mRNA in human small intestine and colon by utilizing real-time QRT-PCR. The amplification plot for SERT mRNA showed comparatively lesser PCR cycle numbers to reach a detectable threshold (Ct; threshold cycle = 19) in the small intestine compared with the colon sample (Ct = 23), indicating higher SERT mRNA expression in the small intestine. Based on the differences in this Ct, the data were quantified by normalizing to actin used as an internal control. The relative abundance of SERT mRNA was ~16-fold higher in the small intestine compared with the colon [relative SERT abundance expressed as SERT mRNA/actin mRNA (arbitrary units): Colon 6.0 ± 0.5% vs. Small intestine, taken as 100%]. Therefore, it was of interest to examine SERT mRNA expression in different regions of the small intestine. For this, commercially available cDNAs from human duodenum, jejunum, ileum, and colon were amplified for SERT expression by using real-time PCR. As shown in Fig. 1, SERT expression was detected in the duodenum and ileum, with highest expression in the ileum. However, SERT expression was relatively very low in the jejunum and almost absent in colon.
Fig. 1.
Human serotonin (5-HT) transporter (SERT) mRNA expression in different regions of human intestine: Commercial cDNA from different regions of human intestine were subjected to real-time PCR using SYBR green fluorescent dye as described in MATERIALS AND METHODS. Data were quantified by normalizing human SERT mRNA levels to human β-actin mRNA and were expressed as arbitrary units. Data are means ± SE of values obtained from at least 3 different experiments performed on separate occasions. *P < 0.001 compared with duodenum (taken as control).
Northern Blot Analysis
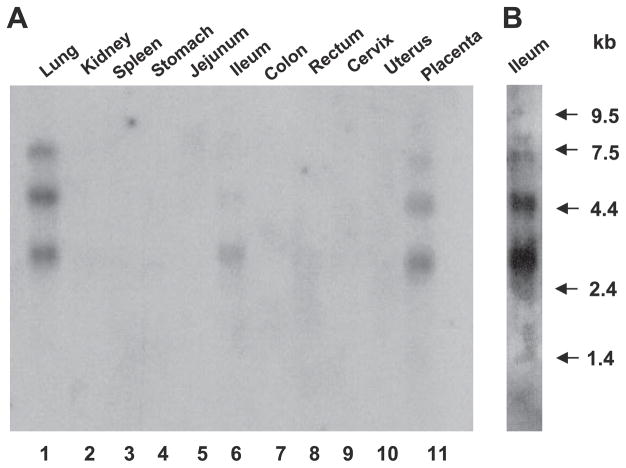
Previous studies have shown that cDNA for the human placental and lung 5-HT transporter hybridizes with three mRNA species corresponding to 6.8, 4.9, and 3.0 kb (18, 25). To ascertain the presence of mRNA species encoding SERT in various human intestinal tissues, commercially available human multiple-tissue Northern blots (BioChain) were probed with 32P-labeled 491-bp human SERT cDNA fragment. As previously reported, examination of mRNA distribution revealed multiple hybridizing mRNA species present in the lung and placenta. A single hybridizing mRNA species of ~3.0 kb was detected in the ileum (Fig. 2A); however, additional hybridizing mRNA species at 4.9 kb and 6.8 kb were also detected in the ileum at higher exposure (Fig. 2B). No message was detected in other intestinal regions such as the jejunum, colon, or rectum, even at higher exposures. Kidney, spleen, stomach, cervix, and uterus were also negative for SERT mRNA.
Fig. 2.
Northern blots of poly(A)+ RNA from different human tissues were probed with a 491-bp fragment of human SERT cDNA (A). Arrows indicate RNA size markers (in bp). Higher exposure of ileum lane is shown in B.
SERT Expression by Immuofluorescence Staining
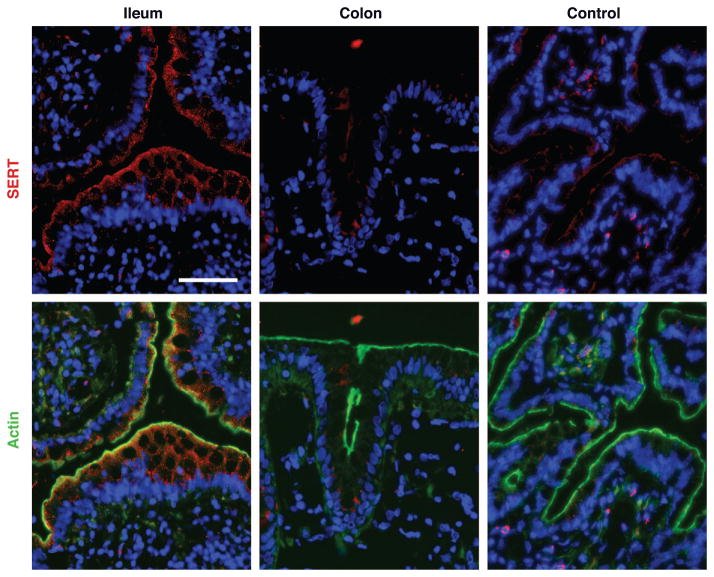
To determine the expression of SERT and its membrane localization across the length of the human intestine, immunofluorescence studies using SERT antibody were carried out in sections of human small intestine and colon. SERT (red) was detected in the ileum, where reactivity colocalized with the F-actin (green) at the apical brush-border membrane. SERT immunoreactivity was also present in the intracellular compartments of ileal epithelia, but SERT was almost not detected at basolateral membranes (Fig. 3). Preincubation of the SERT antibody with the immunizing peptide abolished the staining, thus confirming the specificity of immunodetection. Consistent with the absence of SERT mRNA, SERT staining was not detected in human colon (Fig. 3).
Fig. 3.
Immunofluorescent localization of SERT in human resected intestinal tissues. Immunofluorescent staining for SERT and actin was performed on sections of normal human ileum and colon. SERT staining is seen in red and actin in green, contrasting with blue counterstained nuclei. Ileum shows strong staining for SERT, which was absent in colon. SERT antibody preincubated with immunizing peptide was used as a control. Scale bar = 5 μm. A representative of 3 different experiments is shown.
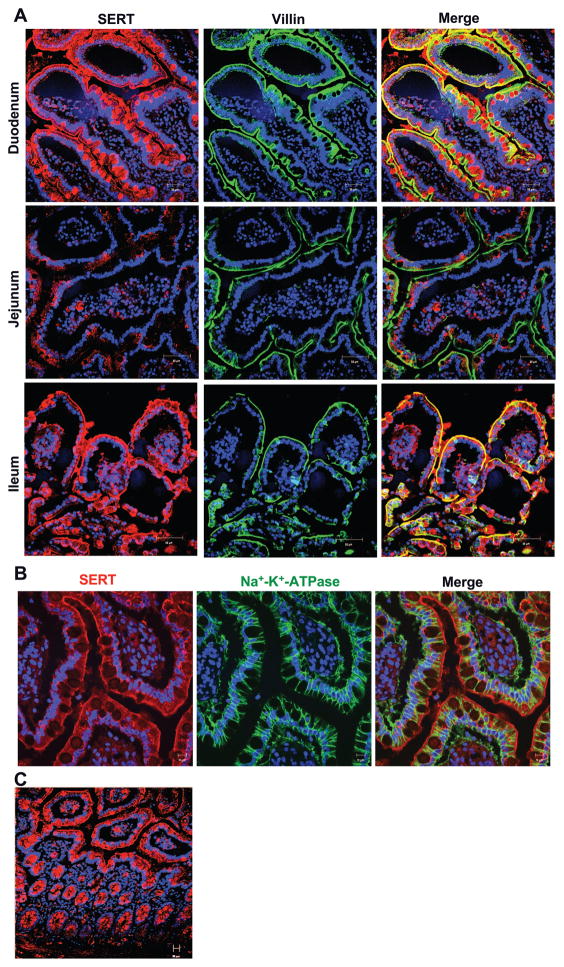
We next characterized the SERT expression and membrane localization in different regions of the human small intestine. For these studies, paraffin-embedded sections from resected human duodenum, jejunum, and ileum were stained with SERT antibody (red). Villin was used as a marker to localize the apical membranes (green). Figure 4A shows that epithelial cells of both duodenal and ileal regions were strongly positive for SERT expression. Interestingly, SERT expression colocalized with villin at the apical membrane (yellow) and also exhibited cytoplasmic staining. Incubation of the tissue sections with antibody preincubated with excess peptide did not show prominent staining, indicating the specificity of SERT antibody (data not shown). Jejunum showed the least reactivity for SERT staining, which was similar to its negative control when the antibody was preincubated with excess peptide. To further confirm the membrane localization of SERT, we investigated SERT colocalization with a basolaterally expressed marker, Na+-K+-ATPase. As expected, Na+-K+-ATPase staining was strictly restricted to the basolateral membranes of the ileal region; however, staining for SERT was distinctly present in the apical and subapical compartment with no colocalization with Na+-K+-ATPase (Fig. 4B). We further analyzed the distribution of SERT along the crypt-villus axis in the human ileum. As shown in Fig. 4C, SERT expression was distributed uniformly along the crypt-villus axis.
Fig. 4.
SERT expression and membrane localization in paraffin-embedded sections of human intestinal tissues. A: immunofluorescent staining for SERT and villin was performed on paraffin-embedded healthy human small intestinal resected sections obtained from duodenum, ileum, and jejunum. Duodenum and ileum showed SERT staining that colocalized on apical membrane with villin. SERT staining was also localized to intracellular compartments (small vesicular structures). Staining of goblet cell mucin vacuoles was not seen uniformly and is not present in stains of frozen tissue, suggesting that this is an artifact of staining paraffin-embedded tissue. Jejunum was essentially negative, similar to its negative control. Scale bar = 50 μm. A representative of 3 different experiments is shown. B: immunofluorescent staining of SERT (red) and Na+-K+-ATPase (green) in ileum. No colocalization of SERT was detected with Na+-K+-ATPase. C: SERT protein is present along crypt-villus axis in ileum.
SERT Protein Levels
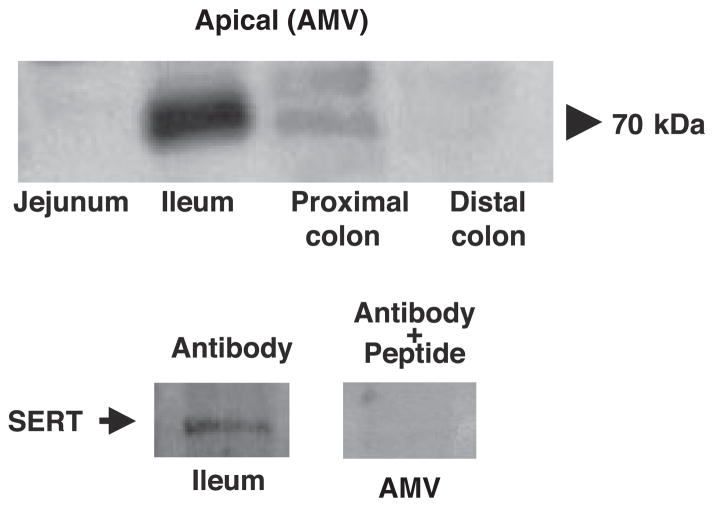
Immunoblotting studies in purified apical membranes isolated from different regions of the human intestine were performed to further determine the expression of SERT across the length of the human intestine. SERT protein expression (detected as a band of ~70 kDa) was predominantly found in the ileum region, whereas very faint bands were detected in the proximal colon (Fig. 5, top). The specificity of the protein bands was confirmed by peptide-competition experiments showing that the predominant band in the ileal brush-border membrane (but not proximal colon) was completely competed out when antibody was pretreated with excess peptide (Fig. 5, bottom). These results correspond to our immunofluorescence data showing the presence of SERT only in the ileal and not in the colonic region.
Fig. 5.
Expression of SERT in purified apical plasma membrane vesicles isolated from different regions of human intestine. Top: immunoblotting for human SERT was performed on luminal membrane vesicles isolated from different regions of human intestine. SERT expression was predominantly restricted to ileal membranes. Bottom: peptide competition experiments showing that specific SERT protein band was competed out when antibody is preincubated with 5-fold excess peptide. Data are representative of 4 different blots.
Functional Characterization of SERT
Because predominant SERT expression was found in the ileum, the functional activity of 5-HT transporter was examined in ileal AMVs.
Time course of [3H]5-HT uptake
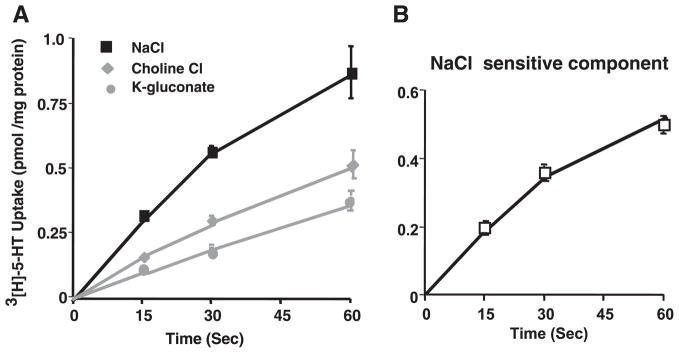
The initial rate of 5-HT uptake in ileal AMVs was first examined. AMVs were incubated with extravesicular medium containing 150 nM [3H]5-HT in the presence of NaCl. As shown in Fig. 6A, with increase in time, 5-HT uptake was significantly increased at all early time points. However, substitution of Na+ with choline or Cl− with gluconate significantly decreased the uptake. Figure 6B shows the NaCl-sensitive component, representing the actual SERT activity, which was linear for up to 1 min.
Fig. 6.
Time course of [3H]5-HT uptake. Ileal apical membrane vesicles preloaded with 300 mM mannitol, 10 mM Tris-HEPES, pH 7.4, and 0.1 mM MgSO4 were incubated in a reaction medium containing 100 mM NaCl, KCl, or K-gluconate; 100 mM mannitol; 10 mM Tris-HEPES, pH 7.4; 0.1 mM MgSO4; and 0.15 μM [3H]5-HT (final concentration). Uptake was determined at 30°C for indicated time periods. Values are means ± SE for 3 separate membrane preparations.
Effect of unlabeled 5-HT
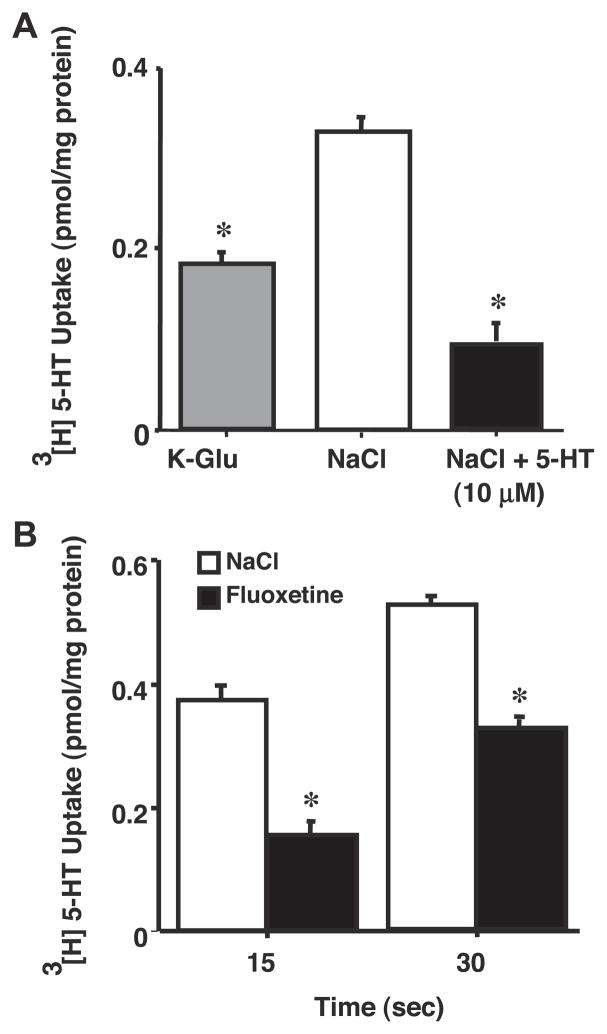
We next examined the 5-HT uptake in the presence of excess of cold 5-HT. The presence of 10 μM 5-HT in the extravesicular medium markedly reduced the uptake of 5-HT, which was essentially similar to that observed in the absence of NaCl (Fig. 7A). These data suggest that 5-HT transport occurs through a specific carrier-mediated process.
Fig. 7.
Effect of unlabeled 5-HT and fluoxetine on Na+/Cl− gradient-stimulated [3H]5-HT uptake. Ileal apical membrane vesicles were prepared in 300 mM mannitol, 10 mM Tris-HEPES, pH 7.4, and 0.1 mM MgSO4. Uptake was determined at 30°C for 10 s by incubating the vesicles in medium containing 100 mM NaCl, KCl, or K-gluconate; 100 mM mannitol; 10 mM Tris-HEPES, pH 7.4; 0.1 mM MgSO4; and 0.15 μM [3H]5-HT with 10 μM unlabeled 5-HT (A) or fluoxetine (B). Values are means ± SE of 3 separate membrane preparations. *P < 0.05 compared with 5-HT uptake in presence of NaCl.
Effects of fluoxetine
Fluoxetine is a well-known inhibitor of the neuronal SERT. Therefore, we also examined whether 5-HT uptake in ileal brush-border membrane was inhibited by fluoxetine. Fluoxetine treatment of apical membrane (Fig. 7B) significantly decreased the 5-HT uptake at both 15 and 30 s.
Kinetics of SERT
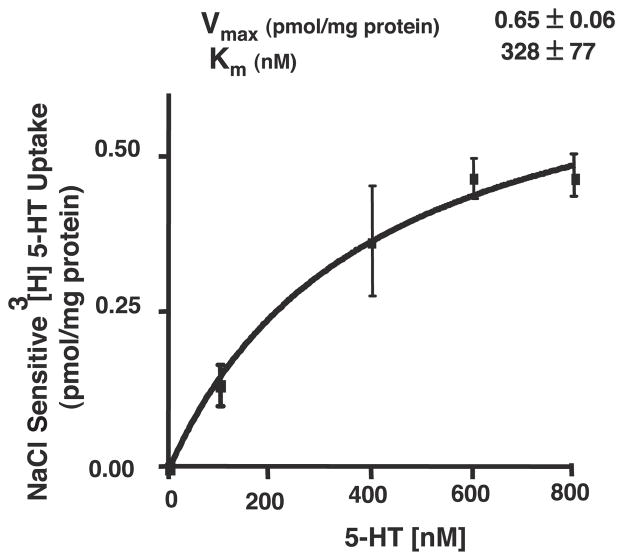
To further characterize the existence of a carrier-mediated process, kinetic studies were performed in which AMVs were incubated with increasing concentrations of the substrate. 5-HT transport in AMVs exhibited saturation kinetics, indicating the presence of a carrier-mediated process at the apical membranes (Fig. 8). Analysis of kinetic parameters revealed that 5-HT uptake in ileal brush-border membrane exhibited a Vmax of 0.65 ± 0.06 pmol · mg protein−1 · 10 s−1 and an apparent Km value of 328 nM.
Fig. 8.
Kinetic analysis of [3H]5-HT uptake. Initial rates of [3H]5-HT uptake in presence of increasing extravesicular concentrations of total 5-HT over range of 150–800 nM were determined. Brush-border membrane vesicles were preloaded with 300 mM mannitol, 10 mM Tris-HEPES, pH 7.4, and 0.1 mM MgSO4. Uptake was measured at 10 s by diluting vesicles in medium containing 100 mM NaCl or K-gluconate; 100 mM mannitol; 10 mM Tris-HEPES, pH 7.4; 0.1 mM MgSO4; [3H]5-HT; and varying concentrations of unlabeled 5-HT. Results are means ± SE of NaCl-sensitive component of total 5-HT uptake and are representative of 3 separate membrane preparations.
DISCUSSION
The mechanisms involved in reuptake of 5-HT in the native human intestine are not fully understood. To our knowledge, this is the first report showing comprehensive analysis of SERT expression and characterization in the native human intestine by utilizing several molecular and cellular approaches, including real-time PCR, Northern blot analysis, immunofluorescence, immunoblotting, and functional activity measurements.
Our initial studies using real-time QRT-PCR demonstrated relatively higher SERT mRNA expression in the human small intestine compared with the colon. This is consistent with studies of Meier et al. (22) showing predominant expression of SERT mRNA in biopsies obtained from human small intestine. Interestingly, our studies showed that SERT mRNA expression exhibits marked variability across different regions of the human small intestine, with highest expression in the ileum, followed by duodenum and jejunum. Furthermore, the presence of three transcripts hybridizing to human SERT cDNA probe in the ileum is consistent with multiple hybridizing species detected in human lung, brain, and placenta (25). These studies indicate the existence of more than one form of SERT mRNA in the ileum, which could arise as a result of alternative splicing. Whether these transcripts encode for distinct isoforms of SERT in the ileum needs to be established.
Corresponding to SERT mRNA levels, immunofluorescence studies showed that SERT expression was high in the duodenum and ileum but was very low in the colon. The differential expression of SERT across different regions of the human intestine is intriguing. The relatively low SERT expression in the colon might indicate the presence of alternate mechanisms of 5-HT transport through related monoamine transporters, including organic cation transporter (OCT), norepinephrine transporter (NET), and dopamine transporter (DAT). This is likely supported by upregulation of OCT-1 in the intestine of SERT knockout mice (4). Alternatively, SERT expression in the colonic epithelial cells may be induced depending on the 5-HT availability in the lumen of colonic mucosa.
SERT expression in the ileum and duodenum was detected in the apical and subapical compartments but not in the basolateral membranes. Immunoblotting studies also detected a single protein band of ~70 kDa (the predicted molecular size of SERT) predominantly in the apical membranes isolated from ileum. Because an antibody generated against neuronal SERT was used in the current studies, it is apparent that the apical 5-HT transporter in the small intestine epithelial cells is encoded by the neuronal SERT. In contrast to the apical localization of SERT observed in current studies, recent studies (17, 21) have shown the presence of a functional SERT capable of transporting 5-HT both at the apical and basolateral membranes of cultured human intestinal cells, Caco-2. These studies, however, did not show immunohistochemical evidence to confirm the identity of the 5-HT transporter. Because EC cells release 5-HT both into the lumen and interstitial space, we speculate that 5-HT uptake into the basolateral membrane of intestinal epithelial cells might be encoded by either the alternatively spliced variant of SERT or by two entirely different genes. Further studies to investigate the identity of the basolateral transporter would be of interest.
The distribution of SERT was found to be abundant along the crypt-villus axis. This is in contrast to the rat small intestine, where most of the immunoreactivity of the 5-HT transporter was observed in the crypt epithelial cells (31). These observations suggest variations in the expression of the 5-HT transporter among species. The presence of SERT along the crypt-villus axis in the human intestine suggests that SERT might play crucial role in epithelial functions of 5-HT. In fact, 5-HT has been shown to play important role in regulation of both ion secretion as well as absorption, the functions primarily attributed to crypts and villus cells, respectively.
Functional characterization of SERT was assessed in ileal apical plasma membranes because it showed predominant expression of SERT. Our laboratory has established the human organ donor intestinal mucosa as a viable source for purifying plasma membrane vesicles for transport studies (7–9, 11, 14, 15). About 70–80% of AMVs are in a right-side orientation. Our functional studies demonstrated that inwardly directed gradient of both Na+ and Cl− stimulated the uptake of 5-HT in ileal AMVs. Interestingly, the SSRI fluoxetine inhibited the SERT-specific uptake in ileal AMVs. Consistent with this, previous studies demonstrated a fluoxetine-sensitive, Na+- and Cl−-dependent transporter in Caco-2 cells encoded by the neuronal SERT (21). In the current studies, 5-HT uptake in ileal AMVs was inhibited in the presence of fluoxetine by ~50%, which was essentially similar to inhibition observed in the absence of NaCl gradient or in the presence of unlabeled 5-HT (60–70%; Fig. 7). These findings suggest that majority of the specific 5-HT uptake in ileal AMVs is NaCl dependent and fluoxetine sensitive. However, these studies do not completely exclude the possible involvement of other nonspecific components, which might be NaCl independent and fluoxetine insensitive or might involve binding components. The physiological significance of these is not clear at present. Nonetheless, our data convincingly suggested that 5-HT uptake by the human ileal BBMVs involves a carrier-mediated system. This conclusion was based on the observations that 1) 5-HT uptake was inhibited by unlabeled 5-HT, 2) saturation of the uptake process by increasing the substrate concentration in the incubation medium; and 3) the 5-HT uptake is sensitive to increasing medium osmolarity (data not shown). These findings suggest that the apical membranes of the human ileum are capable of transporting 5-HT and thus removing it from the luminal compartments. Kinetic parameters of the 5-HT uptake system in ileal AMVs showed Vmax of 0.65 ± 0.06 pmol · mg protein−1 · 10 s−1, with an apparent Km value of 328 nM. The above Km value of 5-HT transport in the human ileal AMVs was similar to neuronal SERT-mediated transport, previously reported to be in the range of 300–600 nM (1, 23).
In summary, our studies for the first time demonstrate the SERT message, protein expression, and membrane localization across the length of the human intestine. Our studies also show the presence of a functional Na+- and Cl−-dependent carrier-mediated process in the ileum AMVs, which might be encoded by the neuronal SERT. These studies may provide the basis for further detailed studies on the identification of functional SERT isoforms in the antipodal plasma membrane of human intestinal epithelial cells. An increased understanding of the detailed characterization of SERT-mediated transport of 5-HT might have major implications in understanding the pathophysiology of inflammatory bowel diseases associated with altered 5-HT availability.
Acknowledgments
GRANTS
These studies were supported by the Department of Veterans Affairs Merit Award and REAP grants (P. K. Dudeja) and the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-68324 and DK-54016 (P. K. Dudeja), DK-71596 (W. A. Alrefai), and K01-DK-74458 (R. K. Gill).
References
- 1.Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- 2.Brenner B, Harney JT, Ahmed BA, Jeffus BC, Unal R, Mehta JL, Kilic F. Plasma serotonin levels and the platelet serotonin transporter. J Neurochem. 2007;102:206–215. doi: 10.1111/j.1471-4159.2007.04542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang AS, Chang SM, Starnes DM, Schroeter S, Bauman AL, Blakely RD. Cloning and expression of the mouse serotonin transporter. Brain Res Mol Brain Res. 1996;43:185–192. doi: 10.1016/s0169-328x(96)00172-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JX, Pan H, Rothman TP, Wade PR, Gershon MD. Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. Am J Physiol Gastrointest Liver Physiol. 1998;275:G433–G448. doi: 10.1152/ajpgi.1998.275.3.G433. [DOI] [PubMed] [Google Scholar]
- 6.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Dudeja PK, Baldwin ML, Harig JM, Cragoe EJ, Jr, Ramaswamy K, Brasitus TA. Mechanisms of Na+ transport in human distal colonic apical membrane vesicles. Biochim Biophys Acta. 1994;1193:67–76. doi: 10.1016/0005-2736(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 8.Dudeja PK, Harig JM, Baldwin ML, Cragoe EJ, Jr, Ramaswamy K, Brasitus TA. Na+ transport in human proximal colonic apical membrane vesicles. Gastroenterology. 1994;106:125–133. doi: 10.1016/s0016-5085(94)94837-2. [DOI] [PubMed] [Google Scholar]
- 9.Dudeja PK, Tyagi S, Kavilaveettil RJ, Gill R, Said HM. Mechanism of thiamine uptake by human jejunal brush-border membrane vesicles. Am J Physiol Cell Physiol. 2001;281:C786–C792. doi: 10.1152/ajpcell.2001.281.3.C786. [DOI] [PubMed] [Google Scholar]
- 10.Gill R, Alrefai W, Ramaswamy K, Dudeja P. Mechanisms and regulation of NaCl absorption in the human intestine. Recent Res Devel Physiol. 2003;1:643–677. [Google Scholar]
- 11.Gill RK, Saksena S, Alrefai WA, Sarwar Z, Goldstein JL, Carroll RE, Ramaswamy K, Dudeja PK. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am J Physiol Cell Physiol. 2005;289:C846–C852. doi: 10.1152/ajpcell.00112.2005. [DOI] [PubMed] [Google Scholar]
- 12.Gill RK, Saksena S, Tyagi S, Alrefai WA, Malakooti J, Sarwar Z, Turner JR, Ramaswamy K, Dudeja PK. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKC alpha in human intestinal epithelial cells. Gastroenterology. 2005;128:962–974. doi: 10.1053/j.gastro.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Gronstad KO, Zinner MJ, Nilsson O, Dahlstrom A, Jaffe BM, Ahlman H. Vagal release of serotonin into gut lumen and portal circulation via separate control mechanisms. J Surg Res. 1988;44:146–151. doi: 10.1016/0022-4804(88)90042-x. [DOI] [PubMed] [Google Scholar]
- 14.Harig JM, Dudeja PK, Knaup SM, Shoshara J, Ramaswamy K, Brasitus TA. Apical plasma membrane vesicles formed from organ donor colon demonstrate Na+ and H+ conductances and Na+/H+ exchange. Biochem Biophys Res Commun. 1990;167:438–443. doi: 10.1016/0006-291x(90)92042-x. [DOI] [PubMed] [Google Scholar]
- 15.Harig JM, Ng EK, Dudeja PK, Brasitus TA, Ramaswamy K. Transport of n-butyrate into human colonic luminal membrane vesicles. Am J Physiol Gastrointest Liver Physiol. 1996;271:G415–G422. doi: 10.1152/ajpgi.1996.271.3.G415. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman BJ, Mezey E, Brownstein MJ. Cloning of a serotonin transporter affected by antidepressants. Science. 1991;254:579–580. doi: 10.1126/science.1948036. [DOI] [PubMed] [Google Scholar]
- 17.Iceta R, Mesonero JE, Aramayona JJ, Alcalde AI. Molecular characterization and intracellular regulation of the human serotonin transporter in Caco-2 cells. J Physiol Pharmacol. 2006;57:119–130. [PubMed] [Google Scholar]
- 18.Kekuda R, Torres-Zamorano V, Leibach FH, Ganapathy V. Human serotonin transporter: regulation by the neuroprotective agent aurintricarboxylic acid and by epidermal growth factor. J Neurochem. 1997;68:1443–1450. doi: 10.1046/j.1471-4159.1997.68041443.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee SL, Fanburg BL. Serotonin uptake by bovine pulmonary artery endothelial cells in culture. I. Characterization. Am J Physiol Cell Physiol. 1986;250:C761–C765. doi: 10.1152/ajpcell.1986.250.5.C761. [DOI] [PubMed] [Google Scholar]
- 20.Martel F. Recent advances on the importance of the serotonin transporter SERT in the rat intestine. Pharmacol Res. 2006;54:73–76. doi: 10.1016/j.phrs.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Martel F, Monteiro R, Lemos C. Uptake of serotonin at the apical and basolateral membranes of human intestinal epithelial (Caco-2) cells occurs through the neuronal serotonin transporter (SERT) J Pharmacol Exp Ther. 2003;306:355–362. doi: 10.1124/jpet.103.049668. [DOI] [PubMed] [Google Scholar]
- 22.Meier Y, Eloranta JJ, Darimont J, Ismair MG, Hiller C, Fried M, Kullak-Ublick GA, Vavricka SR. Regional distribution of solute carrier mRNA expression along the human intestinal tract. Drug Metab Dispos. 2007;35:590–594. doi: 10.1124/dmd.106.013342. [DOI] [PubMed] [Google Scholar]
- 23.Miller GM, Yatin SM, De La Garza R, 2nd, Goulet M, Madras BK. Cloning of dopamine, norepinephrine and serotonin transporters from monkey brain: relevance to cocaine sensitivity. Brain Res Mol Brain Res. 2001;87:124–143. doi: 10.1016/s0169-328x(00)00288-6. [DOI] [PubMed] [Google Scholar]
- 24.Mortensen OV, Kristensen AS, Rudnick G, Wiborg O. Molecular cloning, expression and characterization of a bovine serotonin transporter. Brain Res Mol Brain Res. 1999;71:120–126. doi: 10.1016/s0169-328x(99)00178-3. [DOI] [PubMed] [Google Scholar]
- 25.Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci USA. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramamoorthy S, Cool DR, Mahesh VB, Leibach FH, Melikian HE, Blakely RD, Ganapathy V. Regulation of the human serotonin transporter. Cholera toxin-induced stimulation of serotonin uptake in human placental choriocarcinoma cells is accompanied by increased serotonin transporter mRNA levels and serotonin transporter-specific ligand binding. J Biol Chem. 1993;268:21626–21631. [PubMed] [Google Scholar]
- 27.Saier MH., Jr A functional-phylogenetic system for the classification of transport proteins. J Cell Biochem Suppl. 1999;32–33:84–94. doi: 10.1002/(sici)1097-4644(1999)75:32+<84::aid-jcb11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.Saksena S, Gill RK, Tyagi S, Alrefai WA, Sarwar Z, Ramaswamy K, Dudeja PK. Involvement of c-Src and protein kinase C delta in the inhibition of Cl−/OH− exchange activity in Caco-2 cells by serotonin. J Biol Chem. 2005;280:11859–11868. doi: 10.1074/jbc.M411553200. [DOI] [PubMed] [Google Scholar]
- 29.Takayanagi S, Hanai H, Kumagai J, Kaneko E. Serotonin uptake and its modulation in rat jejunal enterocyte preparation. J Pharmacol Exp Ther. 1995;272:1151–1159. [PubMed] [Google Scholar]
- 30.Tobe T, Fujiwara M, Tanaka C. Distribution of serotonin (5-hydroxytryptamine) in the human gastroinetestinal tract. Am J Gastroenterol. 1966;46:34–37. [PubMed] [Google Scholar]
- 31.Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]