Multiple myeloma (MM) is a neoplastic proliferation of a clonal population of plasma cells, which produces monoclonal protein (immunoglobulin G [IgG], IgA, and light chain protein κ or λ are the most common).1 The plasma cells neoplasia affects the bones—including the skull—leading to extensive skeletal destruction due to osteolytic lesions and osteopenia, with consequent pathologic fractures.2 Other cardinal features of MM are bone pain, fatigue, weight loss, anemia, hypercalcemia, hypergammaglobulinemia with a monoclonal protein in serum or in the urine, renal insufficiency, and secondary infections.1
MM accounts for almost 1% and 10% of all neoplasia and hematologic malignancies, respectively, and has a worldwide distribution.1 In the United States, the incidence of MM is approximately 4–5 per 100,000 inhabitants.3 In Brazil, the incidence is not well known. In a survey involving 16 different Brazilian health centers, Hungria et al.4 found 1,112 cases of MM, from 1998 to 2004. Currently, The International Myeloma Foundation computes that there are approximately 30,000 MM patients under treatment in Brazil.5 MM is two to three times more common in Africans and African-Americans, with the lowest incidence among Japanese and Mexicans. The ratio of men: women is approximately 1.4:1. The neoplasia affects elderly people, with a median age of 66 years.6,7 Some of the risk factors for MM are older age, environmental exposure to radiation, organic solvents (such as benzene), herbicides and insecticides, and genetic factors (familial cases, ethnicity, variations at the 3p22.1 or 7p15.3 loci).6,8
The lytic lesions are the main finding of MM, which were described in the first reports of the disease by Solly9 and Macintyre10 as fractured bones were replaced by gelatiniform, a reddish and unctuous substance.9,10 Later, Wright11 associated MM with neoplastic proliferation of plasma cells. The myeloma lytic lesions are in agreement with the Paget’s12 concept of “seed and soil,” where the neoplastic cells (“seeds”: neoplastic plasma cells) have tropism for certain microenvironments (“soil”: bones).
The pathogenesis of the bone lytic lesions in the MM is complex and involves an imbalance between osteoblastic and osteoclastic functions. The osteoclastic activation is increased in MM, with bone reabsorption stimulated by the receptor activator of nuclear factor κ-β ligand (RANKL). The RANKL is overexpressed by osteoblasts and plasma cells, which are associated with low levels of osteoprotegerin, the RANKL decoy receptor. In addition, bone stromal cells produce factors that activate the osteoclastic function, such as interleukin-3 (IL-3), IL-6, macrophage inflammatory protein-1 alpha (MIP-1α) and stromal derived factor 1α (SDF-1α).13,14 The osteoblast differentiation is inhibited by an increased expression of IL-3, IL-7 and dickkopf 1 (DKK1).15
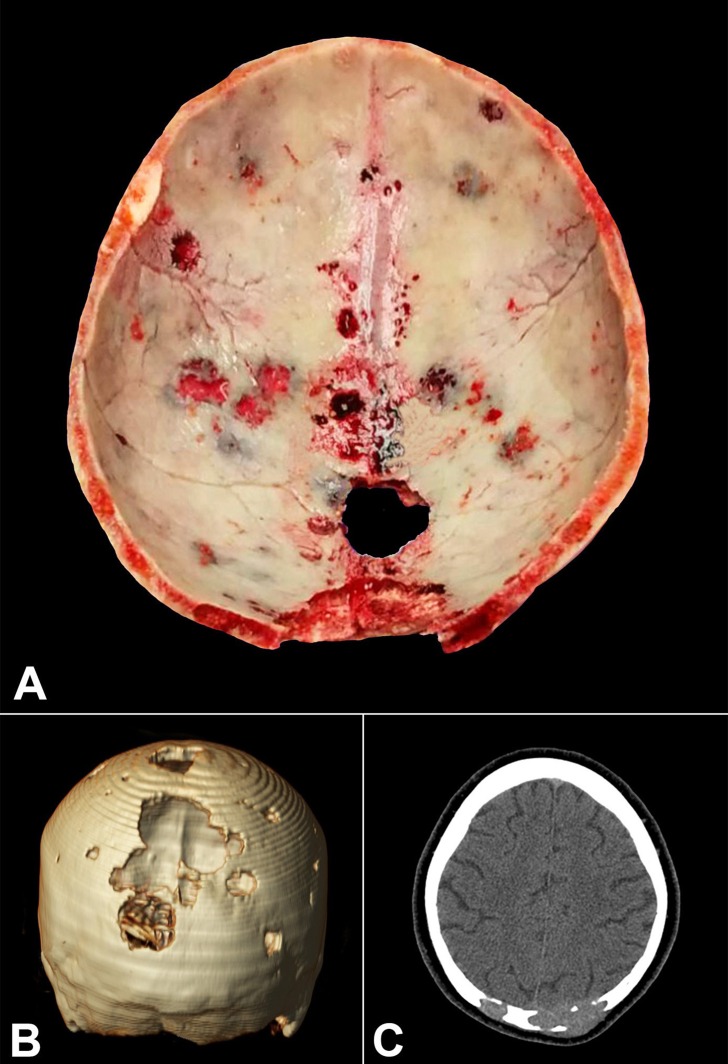
Figure 1 refers to the case of a 62-year-old woman who presented headache, fatigue, weight loss, episodes of blurred vision and diplopia, bone pain, and the appearance of numerous nodules in the scalp. The laboratory work-up revealed hypercalcemia, azotemia, multiple lytic bone lesions (skull, sternum, ribs, vertebrae, ilium), and urinary kappa Bence-Jones protein. The bone marrow biopsy showed infiltration of atypical plasma cells, with monoclonal expression of κ-light chain. The patient died due to a sudden cardiac arrest, which the autopsy revealed to be caused by a massive pulmonary embolism.
Figure 1. A – Gross appearance of the calvarium obtained at autopsy showing multiple bone lytic lesions (“punched out,” “pepper pot skull,” or “raindrop skull”) ranging from 0.5 cm to 2.5 cm in diameter, filled by fleshy, soft tissue. B – Computed tomography (CT) of the brain (3D reformation) showing multiple lytic lesions, which are more prominent in the occipital area. C – Axial brain CT showing bone lytic lesions invading soft tissues.
Footnotes
Bitelman VM, Lopes JADO, Nogueira AB, Frassetto FP, Duarte-Neto AN. “Punched out” multiple myeloma lytic lesions in the skull. Autopsy Case Rep [Internet]. 2016;6(1):7-9. http://dx.doi.org/10.4322/acr.2016.020
REFERENCES
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. Blood 2008;111(6):2962-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kesari S, Bundock EA. Punched-out skull. Arch Neurol. 2004;61(6):958-9. http://dx.doi.org/10.1001/archneur.61.6.958. PMid: [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. http://dx.doi.org/10.3322/caac.21332. PMid: [DOI] [PubMed] [Google Scholar]
- 4.Hungria VT, Maiolino A, Martinez G, et al. Confirmation of the utility of the International Staging System and identification of a unique pattern of disease in Brazilian patients with multiple myeloma. Haematologica. 2008;93(5):791-2. http://dx.doi.org/10.3324/haematol.11637. PMid: [DOI] [PubMed] [Google Scholar]
- 5.Minnicelli C, Maciel JF, Hassan R, Lemos TM. Clinical and epidemiological features of multiple myeloma patients from a low socio-economic region of Brazil. Rev Bras Hematol Hemoter. 2015;37(5):354-5. http://dx.doi.org/10.1016/j.bjhh.2015.05.007. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33. http://dx.doi.org/10.4065/78.1.21. PMid: [DOI] [PubMed] [Google Scholar]
- 7.Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population-based study. Blood. 2010;116(25):5501-6. http://dx.doi.org/10.1182/blood-2010-07-298760. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broderick P, Chubb D, Johnson DC, et al. Common variation at 3p22.1 and 7p15.3 influences multiple myeloma risk. Nat Genet. 2012;44(1):58-61. http://dx.doi.org/10.1038/ng.993. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solly S. Remarks on the pathology of mollities ossium; with cases. Med Chir Trans Lond. 1844;27(1):435-98. http://dx.doi.org/10.1177/095952874402700129. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macintyre W. Case of mollities and fragilitas ossium, accompanied with urine strongly charged with animal matter. Med Chir Trans. 1850;33(1):211-32. http://dx.doi.org/10.1177/095952875003300113. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright JH. A case of multiple myeloma. J Boston Soc Med Sci. 1900;15(8):137-47. PMid: [PMC free article] [PubMed] [Google Scholar]
- 12.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133(3421):571-3. http://dx.doi.org/10.1016/S0140-6736(00)49915-0. [PubMed] [Google Scholar]
- 13.Roodman GD. Role of the bone marrow microenvironment in multiple myeloma. J Bone Miner Res. 2002;17(11):1921-5. http://dx.doi.org/10.1359/jbmr.2002.17.11.1921. PMid: [DOI] [PubMed] [Google Scholar]
- 14.Sezer O. Myeloma bone disease: recent advances in biology, diagnosis, and treatment. Oncologist. 2009;14(3):276-83. http://dx.doi.org/10.1634/theoncologist.2009-0003. PMid: [DOI] [PubMed] [Google Scholar]
- 15.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483-94. http://dx.doi.org/10.1056/NEJMoa030847. PMid: [DOI] [PubMed] [Google Scholar]