Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma comprising a heterogeneous group of disorders with variable histological and clinical behavior. Although other lymphomas may present in the leukemic phase more frequently, this appearance is unusually observed among DLBCL cases. Diagnosing lymphoma is not always easy, and the patient's clinical status quite often may hamper invasive procedures for diagnosis pushing the clinician to look for alternatives to reach the nearest possible accurate diagnosis. The authors report the case of a middle-aged man who presented the history of malaise, weight loss, and low-grade fever. The peripheral blood count showed leukocytosis with the presence of blasts and thrombocytopenia. The cytological morphology and immunophenotyping of the peripheral blood and bone marrow aspirate, as well as the bone marrow biopsy accompanied by a thorough immunohistochemical analysis, rendered the diagnosis of DLBCL in the leukemic phase. The patient was prescribed R-CHOP with a favorable outcome. Intra-abdominal lymph node biopsy was avoided because of the patient's critical medical condition. The authors highlight this rare form of presentation of DLBCL as well as the combination of peripheral blood, bone marrow aspirate, and bone marrow biopsy for reaching the diagnosis in cases were a lymph node sample is unavailable for the diagnostic work-up.
Keywords : Lymphoma, Large B-cell, Diffuse, Leukemia, Flow citometry, Diagnosis
CASE REPORT
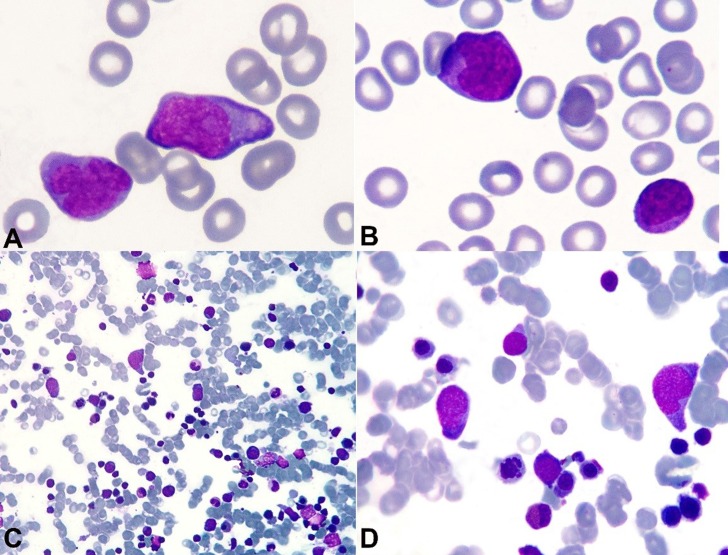
A 60-year-old man was admitted to the emergency facility complaining of syncope following two episodes of melena that occurred the night before. He referred weight loss of 7 kg during the last 3 months (10% of his usual weight), and over the last week asthenia and low-grade fever. His past medical history included a sigmoidectomy for colonic adenocarcinoma 4 years ago, without evidence of relapse or metastatic disease. The physical examination disclosed a hydrated, pale, hypotensive, and tachycardic patient. The lungs and heart examinations were normal, and the abdomen showed an enlarged spleen. Mildly enlarged lymph nodes were palpable in the groin and bilaterally in the axillary region. During the examination, the patient presented episodes of vomiting and hematemesis. After hemodynamic stabilization with crystalloids and blood transfusion, an upper digestive endoscopy was undertaken, which showed a bleeding arteriolar stub in a normal gastric mucosa, classified as Forrest Ib (Dielafoy lesion). Bleeding was controlled with the aid of metallic hemoclips. The peripheral blood count, before the blood transfusion, showed hemoglobin of 3.9 g/dL (reference value [RV]: 13.5-17.5 g/dL), hematocrit of 12.5% (RV: 41-53%), and leukocytes count of 31,470/mm3 (RV: 4,500-11,000/ mm3) with 19% of medium-sized cells characterized by basophilic cytoplasm, medium nucleus/cytoplasm relationship, loose chromatin, evident nucleolus (Figure 1A, B), and platelets of 52,000/mm3 (RV: 150,000-400,000/mm3). Lactate dehydrogenase determination was 2612 U/L (RV: 120-246 U/L). Bone marrow aspiration showed erythroid hyperplasia and 23.6% of lymphocytes with varied morphology–2.8% of which were represented by large-sized cells with medium nucleus/cytoplasm relationship, basophilic cytoplasm, and loose chromatin (Figure 1C, D). Renal function, electrolytes, hepatic enzymes, and coagulogram were normal.
Figure 1. Photomicrography of the peripheral blood smear A, B - and bone marrow aspiration smear; C, D - (Leishman stain), showing large cells with abundant basophilic cytoplasm, and nuclei with loose chromatin and evident nucleolus.
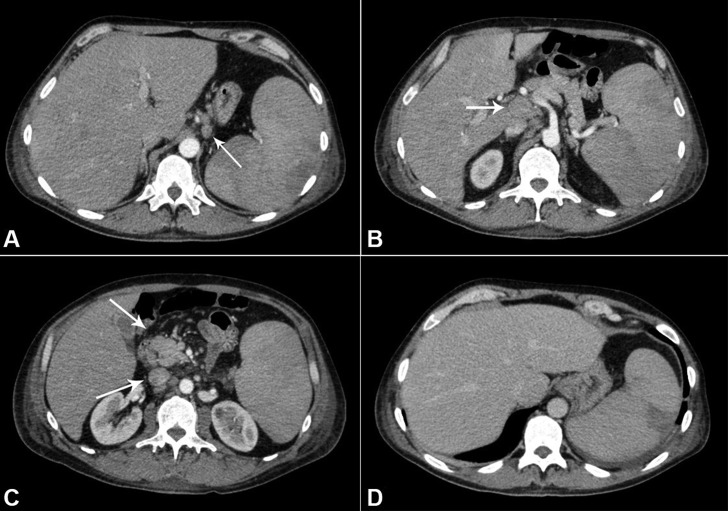
The computed tomography (CT) of the abdomen and thorax showed heterogeneous, enlarged, and confluent lymph nodes measuring 4.1 × 1.6 cm in the retroperitoneum (periaortic, interaortocaval, and portocaval), and in the perigastric, peri-esophageal, and pericardic regions. The spleen was enlarged and exhibited heterogeneous attenuation coefficient (Figure 2). The axillary lymph nodes measured 1.5 × 1.3 cm
Figure 2. Abdominal CT showing lymphadenomegaly. A - Perigastric; B - Periportal space; C - Mesenteric region; D - Hepatosplenomegaly with perfusional heterogeneity in the spleen consistent with infarction.
Immunophenotyping and cell morphology of bone marrow and peripheral blood samples resulted in mature monoclonal cells of B lineage consistent with leukemic large B-cell lymphoma (heterogeneous CD20+, CD79b++, CD79a+, mIgM-, cyIgM+, cykappa+, cylambda−, besides faint coexpression of CD200 and negativity for CD5.
During hospitalization, the patient presented daily high-grade fever (mostly in the evening) accompanied by diaphoresis.
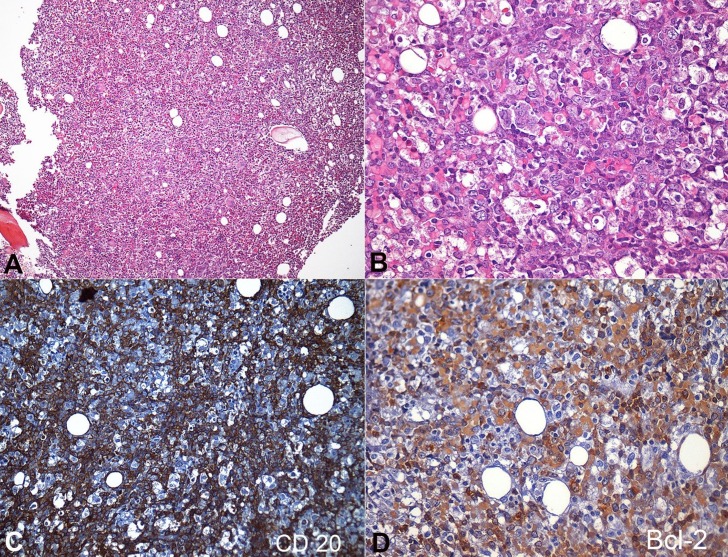
An inguinal lymph node was biopsied and resulted in reactive lymphadenitis on histology. An intra-abdominal lymph node biopsy was not performed due to the patient's poor clinical condition. A bone marrow biopsy showed massive lymphomatous cells infiltration (60-65%), composed by large cells–some of them with homogeneous eosinophilic cytoplasm (Figure 3). The immunohistochemical analysis was positive for CD20 and BCL-2, and negative for CD10 (Figure 3), CD5, CD10, Cycline-D1, and TdT. The Ki67 was 70%.
Figure 3. Photomicrography of the bone marrow. A - Massive marrow infiltration by lymphoma cells (H&E, 100X); B - Lymphoma cells, composed of large atypical cells with blue cytoplasm; round, irregular and vesicular nuclei with prominent nucleoli; and abundant cytoplasm. Some cells present homogeneous eosinophilic cytoplasm (H&E, 400X); C - Positivity for CD 20 in the lymphomatous cells (200X); D - Positivity for BCL-2 in the lymphomatous cells (400X).
Therefore, the analysis of the bone marrow biopsy added to the peripheral and bone marrow cytology (morphology and immunophenotype) permitted the diagnosis of diffuse large B-cell lymphoma (DLBCL) in the leukemic phase. The Ann Arbor staging was IV (bone marrow and blood), and the revised International Prognostic Index (R-IPI) was 4 (LDH, ECOG 3, Stage IV, EN>1) regardless of the negative nodal confirmation. Other diagnoses, such as the indolent lymphomas (CD10 negative, Cyclin-D1 negative, CD5 negative) or even the precursor cell lymphomas/leukemias (TdT negative) could be ruled out.
The patient signed the informed consent form, authorizing the publication of this report.
DISCUSSION
Lymphomas differ from chronic lymphoid leukemia by the major or primary site of the disease, which is usually peripheral lymphoid tissues rather than bone marrow. However, bone marrow infiltration by neoplastic circulating cells may occur in lymphomas, such as in leukemia, giving rise to a leukemic phase.1 This leukemic phase may take place during the lymphoma progression or rarely (in DLBCL) can be present at diagnosis, as occurred in the present case.
Although the diagnosis of lymphoma is based predominantly on histological examination of tissues, the presence of circulating lymphoma cells during the leukemic phase can give rise to the diagnosis, based on the cell's immunophenotype analysis by flow cytometry. Interestingly, the lymph node biopsy in this case was negative for malignancy, showing that the site for biopsy was not suitable for the histological examination.
DLBCL is a heterogeneous group of disorders with variable histological and clinical behavior. It is characterized morphologically by the large atypical lymphoid cell appearance with pale blue cytoplasm; round, irregular, and vesicular nuclei with prominent nucleoli, and relatively abundant cytoplasm.2
Immunophenotypically, DLBCL is by definition, positive for B-lineage markers (mostly CD20) and variable immunoglobulin (surface or cytoplasmic). The follicle center cell markers CD10 and BCL6 are expressed in 40% and 60% of cases, respectively. Some cases may express post-germinal center cell or plasma cell-associated markers such as CD38, VS38, and MUM1. Up to 50% of the cases express the BCL-2 protein increasing the possibility of double hit lymphoma (when the positivity is confirmed by FISH on an MYC-related locus), which is related to a worse prognosis. A minority of DLBCs express CD30, usually in a heterogeneous pattern. CD5 is expressed in 10% of cases, and the Ki-67 is usually high.2
DLBCL accounts for 30-40% of the newly diagnosed adult non-Hodgkin lymphomas, but rarely presents a leukemic phase, which is different from other lymphomas where this feature is well-recognized, such as mantle cell lymphoma, follicular lymphoma, anaplastic large cell lymphoma, and in terminal phases of all refractory lymphomas.3,4 We searched on PubMed for DLBCL presenting in the leukemic phase, but could only retrieve publications of case reports or short series.
The pathologic mechanisms involving the migration of lymphoma cells into the bloodstream and, the reasons for different leukemic presentations among the different lymphomas are unclear. A differential expression of adhesion molecules was proposed as a possible mechanism for the migration of lymphocytes from the lymph nodes, but the current studies are not conclusive. The available data on DLBC do not support the hypothesis that a different profile of adhesion molecules plays a role in the pathogenesis of bone marrow involvement.5,6
Patients with DLBC presenting in the leukemic phase are prone to have extranodal involvement besides the bone marrow, bearing a high tumor burden. Data from one cohort of 29 patients showed the involvement of the spleen in 62%, pleura/lung in 41%, liver in 21%, bone in 17%, bowels in 7%, and cerebrospinal fluid in 14% of cases. Of these patients, 90% received anthracycline-based regimens with rituximab, which was associated with early mortality (14% vs. 6% in patients with DLBCL treated with R-CHOP), but yields approximately 50% at the 4-year survival analysis, which is somehow comparable to the overall survival in patients with DLBCL and a high International Prognostic Index Score, and no leukemic presentation.5 There is no available data concerning randomized or prospective studies to guide the better treatment for these cases Currently, the therapeutic choice remains as rituximab and anthracycline-based protocols.
CONCLUSION
We described a rare clinical presentation of DLBCL with early leukemic phase, and highlighted the use of flow cytometry analysis of peripheral blood and bone marrow samples to perform the diagnosis.
Footnotes
Pires PP, Kanegae MY, Rays J, et al. Diffuse large B-cell lymphoma presenting in the leukemic phase. Autopsy Case Rep [Internet]. 2016;6(1):41-45. http://dx.doi.org/10.4322/acr.2016.027
REFERENCES
- 1.Bain BJ, Catovsky D. The leukaemic phase of non-Hodgkin’s lymphoma. J Clin Pathol. 1995;48(3):189-93. http://dx.doi.org/10.1136/jcp.48.3.189. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan AC, Chan JKC. Diffuse large B-cell lymphoma In: Jaffe ES, Harris NL, Vardiman J, Campo E, Arber DA, editors. Hematopathology. Philadelphia: Saunders; 2010. [Google Scholar]
- 3.Nogai H, Dorken B, Lenz G. Pathogenesis of Non-Hodgkin’s lymphoma. J Clin Oncol. 2011;29(14):1803-11. http://dx.doi.org/10.1200/JCO.2010.33.3252. PMid: [DOI] [PubMed] [Google Scholar]
- 4.De Paepe P, De Wolf-Peeters C. Diffuse large B-cell lymphoma: a heterogeneous group of non-Hodgkin lymphomas comprising several distinct clinicopathological entities. Leukemia. 2007;21(1):37-43. http://dx.doi.org/10.1038/sj.leu.2404449. PMid: [DOI] [PubMed] [Google Scholar]
- 5.Muringampurath-John D, Jaye DL, Flowers CR, et al. Characteristics and outcomes of diffuse large B-cell lymphoma presenting in leukaemic phase. Brit J Haem. 2012;158(5):608-14. http://dx.doi.org/10.1111/j.1365-2141.2012.09209.x. PMid: [DOI] [PubMed] [Google Scholar]
- 6.Matos DM, Rizzatti EG, Garcia AB, Gallo DA, Falcão RP. Adhesion molecule profile of B-cell non-Hodgkin’s lymphoma in the leukemic phase. Braz J Med Biol Res. 2006;39(10):1349-55. http://dx.doi.org/10.1590/S0100-879X2006001000011. PMid: [DOI] [PubMed] [Google Scholar]