Abstract
Gastric glomus tumors (GGTs) are unusual benign, subepithelial, mesenchymal neoplasms of modified smooth muscle cells representing a neoplastic counterpart of glomus bodies. A 38-year-old woman was admitted to our clinic presenting morbid obesity. Routine preoperative evaluations, such as laboratory analysis, abdominal ultrasonography, and upper gastrointestinal endoscopy, were performed. She underwent a classical laparoscopic sleeve gastrectomy (LSG). The postoperative course was uneventful and she was discharged for outpatient control. Her histopathology report revealed a GGT 0.8 cm in diameter. No further treatment was done and she had lost 28 kg at the postoperative sixth month. Here, we present the case of GGT, which was diagnosed incidentally after LSG.
Keywords : Glomus Tumor, Stomach, Gastrectomy, Bariatric Surgery, Incidental Findings
INTRODUCTION
A glomus tumor is a neoplastic lesion of subepithelial mesenchymal origin of the glomus body (neuromyoarterial canal; Sucquet-Hoyer).1-4 These tumors usually occur in peripheral soft tissue and in the distal part of extremities.4 In the gastrointestinal system, glomus tumors are mostly found in the stomach, and present as solitary, submucosal, nodular masses.3
A gastric glomus tumor (GGT) is rare and was first reported in 1951 by Kay et al.5 The occurrence of this tumor shows a female predominance and is mostly seen in the fifth or sixth decade.3,6 Usually, GGT are benign, but a malignant transformation–although extremely rare–may occur.7 Patients are usually asymptomatic and the diagnosis is frequently incidental. Symptomatic patients complain of epigastric pain/discomfort, upper gastrointestinal bleeding, and gastric ulcer.8-10
The gross appearance of the tumor is usually represented by polypoid lesions recovered by intact mucosa. Microscopic examination reveals a distinct morphology characterized by round, uniform glomus cells and a hypervascular structure. Tumor cells are positive for mesenchymal markers; for example, α-smooth muscle actin, laminin, collagen type IV, and vimentin.8-10 They can be diagnosed preoperatively by upper gastrointestinal endoscopy, endoscopic ultrasound, and computed tomography.
We present the case of a 38-year-old woman with GGT, which was incidentally diagnosed after a current procedure: laparoscopic sleeve gastrectomy (LSG) for morbid obesity.
CASE REPORT
A 38-year-old woman was admitted presenting obesity with a body mass index of 43. Comorbidities were restricted to diabetes mellitus. The laboratory work-up was normal except for the glycosylated hemoglobin, which was 9.4% (reference value < 5.6%). The abdominal ultrasound revealed hepatomegaly and grade 2 hepatosteatosis. Her upper gastrointestinal system endoscopy was normal. She underwent a classical LSG. The postoperative period was uneventful. On the first postoperative day she was mobilized, and on the third postoperative day the nasogastric drainage tube was removed. On the fourth postoperative day, she started feeding orally after a gastric contrasted x-ray ruled out any gastric leakage. The drainage tubes were removed and she was discharged asymptomatic and referred to an outpatient clinic for follow-up.
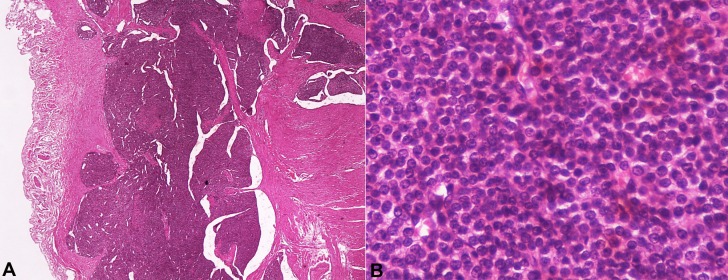
The histopathological examination of the resected gastric specimen showed a submucosal intramural GGT 0.8 cm in diameter 1 cm away from the surgical margin. No ulceration, nor vascular and/or perineural invasion (Figure 1A) was found. The immunohistochemical study showed positivity for S-100; strong positivity for actin and calponin; and negativity for chromogranine A, non-specific enolase (NSE), synaptophysin, CD56, thyroglobulin, TTF1, CD10, CD117, renal-cell carcinoma (RCC), and PanCK. The Ki-67 index was approximately 1%. The vascular structures showed positivity with CD34. Therefore, the histopathology confirmed the diagnosis of glomus tumor of the stomach (Figure 1B).
Figure 1. The histopathological photomicrography of the tumor. A - Solid lesion composed by sheets of uniform cells. Small blood vessels are uniformly distributed within the tumor but may not be apparent (H&E, 2X); B - Solid glomus tumor showing monomorphic round cells with eosinophilic cytoplasm and central punched-out nuclei. (H&E, 40X).
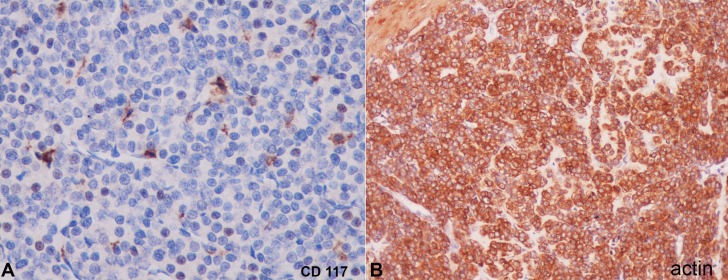
In the gastrointestinal tract, CD117 is a highly specific marker of gastrointestinal stromal tumors; it is not expressed by tumors in the differential diagnosis of a glomus tumor (Figure 2A). These glomus cells may show intense positivity for actin antibodies (Figure 2B).
Figure 2. Photomicrography of the tumor. A - Negative reaction for CD117 (40X); B - Positive reaction for actin (40X).
DISCUSSION
GGT is a rare benign mesenchymal neoplasm arising from the glomus body and was first reported by Kay et al.5 in 1951. Glomus body or apparatus is a component of the dermis involved in the regulation of body temperature.11 GGTs are commonly observed in the extremities but are rarely in the viscera. Nevertheless, the occurrence in bone joints, skeletal muscle, soft tissue, tympanum, mediastinum, trachea, kidney, uterus and vagina have also been described.12 The epidemiology is characterized by a slight predominance among women (female: male ratio = 1.6:1) and may appears between 28 and 79 years. GGT is mostly solitary, although multiple tumors have been reported;13-15 the gastric antrum is most often involved and the sizes range from 0.8 cm to 11 cm.2
GGT is usually benign but malignant transformation, although rare, can be observed. According to the WHO Classification of Tumors of Soft Tissue and Bone,16 “glomus tumor” should be defined as a malignant tumor when its size is larger than 2 cm and it is located under the fascia or viscera, with atypical mitotic figures or marked nuclear atypia, and any level of mitotic activity.
Several cases of malignant or metastatic GGTs have been reported.2 GGTs should be differentiated from other lesions, such as gastrointestinal stromal tumors, paragangliomas, and carcinoid tumors.
Histologically, GGTs are well circumscribed and located in gastric submucosa or muscularis mucosa, and comprise glomus cells surrounding capillaries. The glomus cells are small, uniform, and round without nuclear pleomorphism, mitotic figures, or necrosis. Immunohistochemical staining showed positivity for smooth muscle actinin, vimentin, actin, calponin, type IV collagen, and laminin.
The clinical features are nonspecific, but the patients may complain of epigastric pain and bloody stools. The diagnosis–by upper GIS endoscopy–is challenging, and endoscopic biopsy is usually not helpful because of the intramural nature of the tumor. In our case, no pathological sign was seen on the upper gastrointestinal endoscopy. Submucosal GGT smaller than 1 cm in diameter can be misdiagnosed. Endoscopic ultrasonography can suggest the layer of origin of the tumor, but it is insufficient for diagnosis. Fine-needle aspiration biopsy by endoscopic ultrasonography guidance can be useful for diagnosis.17
Surgical treatment of GGT consists of subtotal gastrectomy, wedge resection, and excision of the tumor depending on the location and size. Wedge resection of the tumor with negative clear margins was the treatment of choice.2
In the case reported herein, GGT was not diagnosed during the preoperative work-up due to the limitations of the diagnostic methods given the relatively small size of the lesion. This incidental finding exemplifies the importance of a thorough pathological examination of all the surgical specimens.
Footnotes
Oruç MT, Çakir T, Aslaner A, Çekiç S, Sakar A, Yardimci EC. Incidental gastric glomus tumor after laparoscopic sleeve gastrectomy. Autopsy Case Rep [Internet]. 2016;6(1):47-50. http://dx.doi.org/10.4322/acr.2016.028
REFERENCES
- 1.Orellana F, Onetto C, Balbontin P, et al. Gastric glomus tumor: report of one case and review. Endoscopy. 2011;43(Suppl 2):71-2. http://dx.doi.org/10.1055/s-0030-1256041. PMid: [DOI] [PubMed] [Google Scholar]
- 2.Fang HQ, Yang J, Zhang FF, Cui Y, Han AJ. Clinicopathological features of gastric glomus tumor. World J Gastroenterol. 2010;16(36):4616-20. http://dx.doi.org/10.3748/wjg.v16.i36.4616. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miettinen M, Paal E, Lasota J, Sobin LH. Gastrointestinal glomus tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol. 2002;26(3):301-11. http://dx.doi.org/10.1097/00000478-200203000-00003. PMid: [DOI] [PubMed] [Google Scholar]
- 4.Tsuneyoshi M, Enjoji M. Glomus tumor: a clinicopathologic and electron microscopic study. Cancer. 1982;50(8):1601-7. http://dx.doi.org/10.1002/1097-0142(19821015)50:8<1601::AID-CNCR2820500823>3.0.CO;2-5. PMid: [DOI] [PubMed] [Google Scholar]
- 5.Kay S, Callahan WP Jr, Murray MR, Randall HT, Stout AP. Glomus tumors of the stomach. Cancer. 1951;4(4):726-36. http://dx.doi.org/10.1002/1097-0142(195107)4:4<726::AID-CNCR2820040410>3.0.CO;2-Z. PMid: [DOI] [PubMed] [Google Scholar]
- 6.Wang LM, Chetty R. Selected unusual tumors of the stomach: a review. Int J Surg Pathol. 2012;20(1):5-14. http://dx.doi.org/10.1177/1066896911429300. PMid: [DOI] [PubMed] [Google Scholar]
- 7.Song SE, Lee CH, Kim KA, Lee HJ, Park CM. Malignant glomus tumor of the stomach with multiorgan metastases: report of a case. Surg Today. 2010;40(7):662-7. http://dx.doi.org/10.1007/s00595-008-4113-z. PMid: [DOI] [PubMed] [Google Scholar]
- 8.Bauerova L, Gabris V, Honsova E, Povysil C. Glomus tumor of the stomach: a case report and review of the literature. Cesk Patol. 2011;47(3):128-9. PMid: [PubMed] [Google Scholar]
- 9.Kang G, Park HJ, Kim JY, et al. Glomus tumor of the stomach: a clinicopathologic analysis of 10 cases and review of the literature. Gut Liver. 2012;6(1):52-7. http://dx.doi.org/10.5009/gnl.2012.6.1.52. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HW, Lee JJ, Yang DH, Lee BH. A clinicopathologic study of glomus tumor of the stomach. J Clin Gastroenterol. 2006;40(8):717-20. http://dx.doi.org/10.1097/00004836-200609000-00011. PMid: [DOI] [PubMed] [Google Scholar]
- 11.Vassiliou I, Tympa A, Theodosopoulos T, et al. Gastric glomus tumor: a case report. World J Surg Oncol. 2010;8(1):19-23. http://dx.doi.org/10.1186/1477-7819-8-19. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nascimento EF, Fonte FP, Mendonça RL, Nonose R, de Souza CA, Martinez CA. Glomus tumor of the stomach: a rare cause of upper gastrointestinal bleeding. Case Rep Surg. 2011;2011:371082. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alempijevic T, Knezevic S, Knezevic D, et al. Gastric multicentric glomangioma: a case report of this rare cause of abdominal pain. Med Sci Monit. 2008;14(1):CS5-8. PMid: [PubMed] [Google Scholar]
- 14.Haque S, Modlin IM, West AB, Path MR. Multiple glomus tumors of the stomach with intravascular spread. Am J Surg Pathol. 1992;16(3):291-9. http://dx.doi.org/10.1097/00000478-199203000-00010. PMid: [DOI] [PubMed] [Google Scholar]
- 15.Nadkarni KM, Deshpande RB, Patel VC, Mody BB, Kinare SG, Bhalerao RA. Multicentric glomus tumour of the stomach (a case report). J Postgrad Med. 1982;28(2):118-20B. PMid: [PubMed] [Google Scholar]
- 16.Folpe AL. Glomus tumours In: Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization classification of tumours: Pathology and Genetics of tumours of soft tissue and bone. Lyon: IARC Press; 2002. p. 136-7. [Google Scholar]
- 17.Chak A. EUS in submucosal tumors. Gastrointest Endosc. 2002;56(4, Suppl):43-8. http://dx.doi.org/10.1016/S0016-5107(02)70085-0. PMid: [DOI] [PubMed] [Google Scholar]