Abstract
Lymphedema of the penis and scrotum is a rare entity characterized by enlargement of the skin and subcutaneous tissue of the genital region due to lymphatic drainage impairment. This clinical condition is more frequent in tropical countries due to a higher incidence of filariasis, which, in turn, is the main etiology. We describe the case of a 33-year-old man with large lymphedema of the scrotum and penis due to an acute and chronic inflammatory process, foreign body granuloma, and marked hyalinization. Four consecutive surgical interventions were necessary to remove the great part of the affected tissue, which enabled satisfactory results and improved the patient's quality of life.
Keywords : Filariasis, Lymphedema, Penile Diseases, Scrotum
INTRODUCTION
Lymphedema is defined as an abnormal accumulation of a protein-rich fluid in soft tissues due to a derangement of the lymphatic drainage. There is an imbalance between the production and the absorption of the lymph.1 In chronic conditions, this is shown with fat storage and thickening of fibrous tissue. The obstruction to the lymphatic flow causes ductal dilation, which is accompanied by hypertrophy and hyperplasia of the connective tissue, interstitial edema, and chronic inflammation.2
Lymphedema is classified as primary (idiopathic) or secondary forms according to its etiology. Primary lymphedema is caused by an intrinsic defect of the lymphatic vessels, while secondary lymphedema may occur after surgery, radiation, tumors, and infections. In tropical countries, infection by Wuchereria bancrofti, a human parasitic roundworm, represents the most frequent etiology, followed by postsurgical lymphedema.
Lymphedema of the penis and scrotum produces mobility and voiding limitations, fatigue, pain, and recurrent subcutaneous infections due to the difficulty of self-hygiene. It also causes sexual limitations, social isolation, and impaired quality of life.2
The purpose of this paper is to report the case of a patient with a giant scrotal lymphedema, the surgical treatment undertaken and the early results.. Through searching the Cochrane Library, Medline, and Embase with the keywords Heartworm, Lymphedema, Penile Diseases, and Scrotum we retrieved 16 indexed online articles and one chapter in a book, which were used in this article.
CASE REPORT
A 33-year-old male patient sought the urology department with a history of the enlargement of the scrotum and prepuce during the last 6 months, which hampered sexual intercourse, the use of underwear, and walking without assistance.
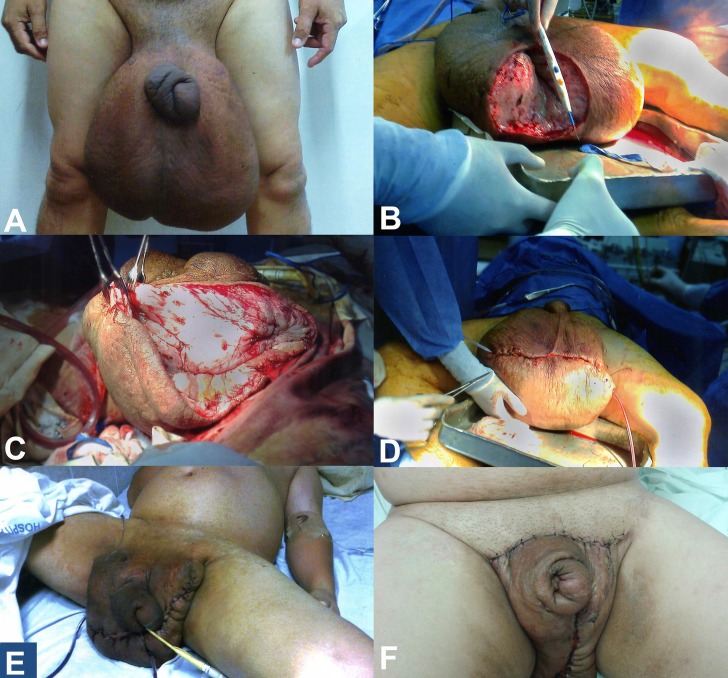
This patient lives in a rural area, which is polluted and the sanitation is poor. The physical examination disclosed an enlarged scrotum, increased testicular size, and a redundant and thickened foreskin. The scrotum measured 144 cm in circumference and 46 cm in length (Figure 1A).
Figure 1. A - Giant edema of the scrotum and thickened redundant foreskin; B - First surgery, dissection of the scrotum; C and D - A transverse incision, dividing the anterior two-thirds and the posterior one-third of the scrotum, accompanied by the drainage of the perirectal lymphatics; E - Postoperative result of the first surgery; note the skin flap and negative pressure drainage of the scrotum; F - Final result after the fourth surgical intervention.
Considering the clinical features and the epidemiological data, the filarial infection was highly considered. Therefore, the initial treatment consisted of albendazole 400 mg single dose, with doxycycline 100 mg every 12 hours, and loratadine as a daily tablet for 1 month, followed by another 30-day course 1 month after the first prescription.
Surgical treatment was scheduled to be performed in four stages. The initial approach involved two surgical sessions because of the anticipated heavy bleeding, which would hamper the total tissue removal in a single intervention. In the first approach, 2.5 kg of infiltrated tissue was removed. Then skin flaps were performed for covering the 20 cm denuded area (Figure 1B). The postoperative period was uneventful. The second surgical procedure was performed 5 days after the first one, when 2.7 kg of scrotal tissue were resected followed by genital reconstruction. A pediculated flap measuring 20 cm in length was created (Figure 1C, D).
One month later, the third surgical procedure was performed, and this time, besides the genital area reconstruction through a skin flap, 600 g of tissue was removed. The suction drains were placed in the perirectal space and remained until the drainage became scarce. This took approximately 1 week (Figure 1E).
One year later, the fourth surgical intervention was performed, which consisted in the resection of the myocutaneous flaps measuring up to 20 cm for the reconstruction of the penis and the scrotum (Figure 1F).
The patient's quality of life improved and sexual activity resumed. Unfortunately, the patient did not attend the scheduled monthly follow-up visits due to financial constraints.
The histological examination of the skin and soft tissue of the external genitalia showed marked acute and chronic inflammatory infiltration with lymphocytes, plasma cells, and polymorphonuclear leukocytes, foreign body granuloma, and marked hyalinization of the skin and soft tissues of the scrotum. The analysis of the glans skin depicted hyperkeratosis, papilloma, and koilocytosis, under which a moderate chronic inflammatory infiltration was present. Malignancy was absent.
DISCUSSION
Genital lymphedema is an accumulation of lymph in the superficial lymph vessels between the skin and fascia (Colles fascia in the scrotum and Buck's fascia of the penis).3 Lymphedema is classified into primary and secondary according to its etiology. Primary lymphedema occurs due to hypoplasia or aplasia of the lymphatic vessels. Approximately 14% of cases of primary lymphedema are hereditary.4,5 Primary lymphedema is classified by age as congenital lymphedema (10-20%), which is the less frequent presentation; the praecox, which involves patients in puberty, accounts for 80% of the cases. Late lymphedema usually occurs after 35 years old.
Secondary lymphedema occurs when the lymphatic flow is interrupted or hindered due to damage or resection of the lymph nodes during surgical procedures, granulomatous disease, Paget's disease of the scrotum, Down syndrome, infection, and radiation, among others.6,7 Infection is the most frequent etiology, usually as a result of lymphogranuloma venereum or an infestation by Wuchereria bancrofti filaria, which can account for 20% of the cases of the male population in tropical countries.8 Other infectious causes may also be associated with chronic lymphedema, such as recurrent cellulitis mainly caused by Streptococcus sp and urethritis caused by Chlamydia trachomatis.9,10 The superficial lymphatic vessels, at the inguinal region, drain the lymph from the skin of the penis and scrotum; therefore, their obstruction by filarial infection will result in scrotal edema.11 Clinically it is characterized by edema and painless enlargement of the penis and scrotum. Gradually, the skin becomes coarse and tough as fibrosis ensues, which causes genital disfigurement, urinary dribbling, impotence, and recurrent cellulitis,5,12 setting up a disease that affects the patient psychologically and deteriorates their quality of life.5
The diagnosis of scrotal lymphedema is clinical and can be confirmed by a lymphoscintigraphy with radiocolloid, which shows the anatomy and the lymphatic function. The magnetic resonance may be useful in detecting other causes of genital lymphedema as a malignant pelvic etiology.11
The diagnosis of filariasis in hospitals is based on clinical suspicion and eosinophilia. The confirmatory diagnosis is based on microscopic observation of smears or a thick film of blood samples stained by Giemsa. The detection of antibodies against filarial infection in serum has low sensitivity (56-98%) and high specificity (78-98%). As the diagnosis of the lymphedema occurs many years after the infection, serologic tests are most likely to be of little help.
Therapeutic management of mild cases comprises local hygiene, compression, elevation of the affected limbs, diuretics, and benzopyrones. Such measures decrease the amount of protein in the interstitial tissue, activate macrophages, enhance the capillary barrier to prevent exudation of proteins, lower the hydrostatic pressure, and lower the risk of infection.
The treatment of choice for a case of filariasis is diethylcarbamazine 6 mg/kg/day for 12 days. Patients with a high parasite load are recommended to start treatment with a low dose, which should be gradually increased; partnering this with a single dose of albendazole (400 mg) will enhance the macrofilaricidal activity. Adding doxycycline 100 mg or 200 mg/day for 6-8 weeks in the treatment of lymphatic filariasis causes a reduction in both the adult and the microfilariae. The general treatment of filariasis may require the use of antihistamines and corticosteroids to reduce allergic reactions elicited by components of dead microfilariae.13 In the case of our patient, the therapeutic regimen was established based on the chronic clinical presentation.
Surgical treatment is indicated in cases of moderate to severe scrotal lymphedema.14,15 Surgical techniques for the management of this condition are reconstructive. Partial or total resection of the skin and the subcutaneous tissue associated with lymphatic drainage and skin or muscle flaps, micro-vascular reconstructions, and skin grafts may be required.12,16 In our case, four surgical interventions involving drainage and resection of thickened lymphatic tissue of the scrotum and the penis (3 kg in total were removed) were necessary, due to the large size of lymphedema. The reconstruction with skin flaps and the negative pressure drainage were the very important steps in this case.
The technique used in the case of our patient was described by Dr. Steven W. Siegel.17 It consists of a transverse incision, dividing the scrotal anterior two-thirds and the posterior one-third. After the identification and isolation of the testes and the spermatic cord, the redundant scrotal tissue and skin mass are removed. The lymphedema and the subcutaneous tissue must be excised extensively for scrotal reconstruction. The remaining posterior one-third of the scrotum remains spared to provide an alternate drainage through the perirectal lymphatics.
Lymphangioplasty is undertaken to restore the lymphatic drainage with vascular anastomoses. It is limited to cases with minor stasis, well-isolated lymphatic channels, and no fibrosis.2
Due to the magnitude of the resected tissue, the surgical approach was scheduled in four stages to prevent excessive bleeding and consequent hemodynamic imbalance.
Our patient underwent an uneventful postoperative course and presented a satisfactory surgical result. He regained unaided walking, normal sexual activity, and assumed the usual daily pursuits. The patient belonged to an indigenous community, which was quite distant from the hospital. This meant that he was unable to attend the scheduled long-term follow-up due to his low financial resources and the costs of travel.
CONCLUSIONS
Lymphedema of the penis and scrotum is a rare entity, mostly caused by filarial infection, which substantially impairs the patient's ability to function fully as a normal human being. In severe cases, surgical management is the treatment of choice to improve the patient’s overall quality of life.
Footnotes
Vives F, García-Perdomo HA, Ocampo-Flórez GM. Giant lymphedema of the penis and scrotum: a case report. Autopsy Case Rep [Internet]. 2016;6(1):57-61. http://dx.doi.org/10.4322/acr.2016.026
REFERENCES
- 1.Godoy JMP, Facio FN Jr, Carvalho ECM, Godoy MFG. New compression mechanism in penile-scrotal lymphedema and sexual rehabilitation. Urol Ann. 2014;6(1):88-90. http://dx.doi.org/10.4103/0974-7796.127025. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh V, Sinha RJ, Sankhwar SN, Kumar V. Reconstructive surgery for penoscrotal filarial lymphedema: a decade of experience and follow-up. Urology. 2011;77(5):1228-31. http://dx.doi.org/10.1016/j.urology.2010.10.026. PMid: [DOI] [PubMed] [Google Scholar]
- 3.Al-Shaham AA, Sood S. Recurrent furunculosis as a cause of isolated penile lymphedema: a case report. J Med Case Reports. 2010;4(1):196. http://dx.doi.org/10.1186/1752-1947-4-196. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naimer SA, Trattner A. Transient idiopathic primary penoscrotal edema. Indian J Dermatol. 2013;58(5):408. http://dx.doi.org/10.4103/0019-5154.117340. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thejeswi P, Prabhu S, Augustine AJ, Ram S. Giant scrotal lymphoedema: a case report. Int J Surg Case Rep. 2012;3(7):269-71. http://dx.doi.org/10.1016/j.ijscr.2012.03.005. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji R-C. Lymphatic endothelial cells, lymphedematous lymphangiogenesis, and molecular control of edema formation. Lymphat Res Biol. 2008;6(3-4):123-37. http://dx.doi.org/10.1089/lrb.2008.1005. PMid: [DOI] [PubMed] [Google Scholar]
- 7.Reitsma W, Wiegman MJ, Damstra RJ. Penile and scrotal lymphedema as an unusual presentation of Crohn’s disease: case report and review of the literature. Lymphology. 2012;45(1):37-41. PMid: [PubMed] [Google Scholar]
- 8.Carrasco López C, Torremadé Barreda J, Palacín Porté JA, Franco Miranda E, Viñals Viñals JM. Linfedema escrotal gigante. Cirugía Plástica Ibero-Latinoamericana. Reparadora y Estética. 2013;39(2):187-91. [Google Scholar]
- 9.Fernández RCG, Rodríguez RF, Iglesias IS, Carballo CF. Elefantiasis genital. Galicia Clin. 2011;72(3):129-30. [Google Scholar]
- 10.Pastor C, Granick MS. Scrotal lymphedema. Eplasty. 2011;11:ic15. PMid: [PMC free article] [PubMed] [Google Scholar]
- 11.Recalde-Losada C, Rubio-Verdú R, Solesio-Pilarte F, Lorda-Barraguer E, Lobato JJ. Abordaje quirúrgico de la elefantiasis escrotal a propósito de dos casos graves. Cir. Plást. Iberolatinoam. 2014;40(2):205-12. http://dx.doi.org/10.4321/S0376-78922014000200010. [Google Scholar]
- 12.Champaneria MC, Workman A, Kao H, Ray AO, Hill M. Reconstruction of massive localised lymphoedema of the scrotum with a novel fasciocutaneous flap: A rare case presentation and a review of the literature. J Plast Reconstr Aesthet Surg. 2013;66(2):281-6. http://dx.doi.org/10.1016/j.bjps.2012.06.024. PMid: [DOI] [PubMed] [Google Scholar]
- 13.Díaz-Menéndez M, Norman F, Monge-Maillo B, Pérez-Molina JA, López-Vélez R. Las filariasis en la práctica clínica. Enferm Infecc Microbiol Clin. 2011;29(Suppl 5):27-37. http://dx.doi.org/10.1016/S0213-005X(11)70041-6. PMid: [DOI] [PubMed] [Google Scholar]
- 14.Parmar HD. The surgical approach in huge scrotal lymphedema. Int J Med Sci Public Health. 2013;2(1):153-5. http://dx.doi.org/10.5455/ijmsph.2013.2.153-155. [Google Scholar]
- 15.Sapountzis S, Ciudad P, Lim SY, et al. Modified Charles procedure and lymph node flap transfer for advanced lower extremity lymphedema. Microsurgery. 2014;34(6):439-47. http://dx.doi.org/10.1002/micr.22235. PMid: [DOI] [PubMed] [Google Scholar]
- 16.Machol JA 4th, Langenstroer P, Sanger JR. Surgical reduction of scrotal massive localized lymphedema (MLL) in obesity. J Plast Reconstr Aesthet Surg. 2014;67(12):1719-25. http://dx.doi.org/10.1016/j.bjps.2014.07.031. PMid: [DOI] [PubMed] [Google Scholar]
- 17.Streem SB, Novick AC, Pontes JE. Stewart’s operative urology. 2nd ed. Baltimore: Williams & Wilkins; 1989. chapt. 72. vol. 2. [Google Scholar]