Abstract
Abdominal aortic aneurysm (AAA) is a dynamic vascular disease characterized by inflammatory cell invasion and extracellular matrix (ECM) degradation. Damage to elastin in the ECM results in release of elastin-derived peptides (EDPs), which are chemotactic for inflammatory cells such as monocytes. Their effect on macrophage polarization is less well known. Pro-inflammatory M1 macrophages initially are recruited to sites of injury but, if their effects are prolonged, they can lead to chronic inflammation that prevents normal tissue repair. Conversely, anti-inflammatory M2 macrophages reduce inflammation and aid in wound healing. Thus, a proper M1/M2 ratio is vital for tissue homeostasis. AAA tissue reveals a high M1/M2 ratio where pro-inflammatory cells and their associated markers dominate. In the present study, in vitro treatment of bone marrow-derived macrophages with EDPs induced M1 macrophage polarization. By using C57Bl/6 mice, antibody-mediated neutralization of EDPs reduced aortic dilation, matrix metalloproteinase activity, and pro-inflammatory cytokine expression at early and late time points after aneurysm induction. Furthermore, direct manipulation of the M1/M2 balance altered aortic dilation. Injection of M2 polarized macrophages reduced aortic dilation after aneurysm induction. EDPs promoted a pro-inflammatory environment in aortic tissue by inducing M1 polarization and neutralization of EDPs attenuated aortic dilation. The M1/M2 imbalance is vital to aneurysm formation.
Introduction
Abdominal aortic aneurysms (AAAs) are abnormal dilations of the aorta between the diaphragm and iliac bifurcation. If left untreated, they can lead to aortic rupture with rapid exsanguination into the retroperitoneum or abdominal cavity which is commonly fatal. Approximately 15,000 deaths occur each year in the United States due to rupture of aortic aneurysms.1 Currently, no pharmacological interventions exist to slow AAA growth or prevent rupture.2 The standard of care is mechanical intervention once the aneurysm reaches 5.0 cm in women or 5.5 cm in men. AAAs are characterized by inflammatory cell infiltration, extracellular matrix (ECM) degradation, and matrix metalloproteinase (MMP) upregulation.3 ECM destruction leads to proteolysis of elastin, the predominant ECM protein in the aortic wall.4 Elastin proteolysis releases elastin-derived peptides (EDPs), including peptides with the xGxxPG motif, a commonly repeated sequence in elastin.5 Of those EDPs released by elastin degradation, the VGVAPG repeat sequence in the human tropoelastin molecule has been shown to have the highest affinity for elastin-binding protein.5–8 EDPs are upregulated in the serum of patients with AAA, and an increase in their level is predictive of AAA expansion.9–11 Previous reports have demonstrated that EDPs recruit inflammatory cells to sites of ECM damage and neutralizing their effect with BA4, a monoclonal antibody that binds to VGVAPG and other xGxxPG motifs, prevents elastin damage.12–15 The precise mechanism through which EDPs affect macrophages and subsequently lead to enhanced tissue damage has not been fully elucidated.
Macrophages play critical roles in the innate immune system, responding to various stimuli in their microenvironment. They can exist in a pro-inflammatory M1 phenotype as well as an anti-inflammatory M2 phenotype.16 M1 macrophages are characteristically described by their release of pro-inflammatory cytokines, such as TNF-α and IL-1β. TNF-α, has been shown to be required for experimental aneurysm formation in mice.17 Previous studies have demonstrated an increase in M1 macrophages in human AAA tissue.9,18 In contrast, M2 macrophages are considered anti-inflammatory, aiding in the healing process by release of IL-10 and profibrotic factors such as TGF-β.19 Their role in AAA formation and progression is less well known. The chronic inflammatory process in AAA promotes aneurysm expansion by active ECM degradation and pro-inflammatory cell recruitment to areas of tissue damage, potentially caused by an abnormally high ratio of M1/M2 macrophages. Thus, altering the M1/M2 ratio may play an important role in slowing or preventing AAA expansion.
The purpose of this study was to evaluate the role of the M1/M2 ratio in AAA and the effect of EDPs on macrophage polarization. These studies were performed by three different methods. First, EDPs were used to examine whether they polarize macrophages to a pro-inflammatory M1 or anti-inflammatory M2 phenotype in vitro. Next, M1 or M2 macrophages were injected into mice in order to directly alter the M1/M2 ratio in the CaCl2 AAA model. Finally, the effect of direct inhibition of EDPs on AAA and macrophage polarization was examined by administration of BA4 after aneurysm induction.
Materials and Methods
Reagents
Monoclonal anti-elastin antibody BA4, IgG, soluble elastin, and LPS were purchased from Sigma-Aldrich (St. Louis, MO, USA). VGVAPG peptide was purchased from Elastin Products Company (Owensville, MO, USA). Mouse IFN-γ and IL-4 were purchased from R&D systems (Minneapolis, MN, USA).
Mouse aneurysm induction model
The Institutional Animal Care and Use Committee (IACUC) approved all animal procedures. Male C57BL/6 mice at 8 weeks of age were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Mice underwent surgery as described previously.20 To induce AAA, mice were anesthetized with tribromethanol (250 mg/kg; Sigma Aldrich) and underwent laparotomy. The abdominal aorta between the iliac artery bifurcation and renal arteries was isolated from the surrounding retroperitoneal structures. Next, the external diameter of the abdominal aorta was measured in triplicate midway between the renal arteries and iliac artery bifurcation. After baseline measurements, periaortic application of 0.25 M CaCl2 was administered for 15 minutes followed by two washes with 0.9% sterile saline. Sterile saline (0.9% NaCl) replaced 0.25 M CaCl2 in sham control mice. The laparotomy incision was closed followed immediately by subcutaneous injections of buprenorphine (0.15 mg/ml) for analgesic after surgery.
Anti-EDP antibody injections
After aortic exposure, application of CaCl2, and closure of the laparotomy incision, antibody treatment was administered by intraperitoneal injection of BA4 (10 mg/kg) or IgG (10 mg/kg) in CaCl2-treated mice. NaCl-treated mice received no treatment after laparotomy. Weekly injections of BA4 or IgG continued for six weeks after initial injection. One week or six weeks after surgery, mice underwent laparotomy and isolation of the infrarenal aorta. Previous work has demonstrated BA4 remains detectable in the serum one week after i.p. injection. 21 For this reason, aortic tissues from BA4-treated mice were harvested one week after aneurysm induction. Measurements were repeated in triplicate at the same locations as baseline measurements. Mice were sacrificed and the entire infrarenal aorta was removed for protein or mRNA isolation or histological studies. For histological studies, aortic tissue was perfusion-fixed with 10% neutral buffered formalin.
Bone marrow-derived macrophage isolation
Bone marrow-derived macrophages (BMDMs) were isolated from C57Bl/6 or C57Bl/6J-Tg(UBC-GFP)30Scha/J (GFP) mice as described previously.22 Briefly, mice were euthanized by cervical dislocation. The femur and tibia were isolated from the surrounding muscle tissue and sterilized in 70% ethanol for 15 seconds then washed in PBS (1x). The ends of each femur and tibia were removed and the bone marrow was flushed out with Dulbecco’s modified eagle medium (DMEM) supplemented with 1% fetal bovine serum (FBS) using a 25-gauge needle. Bone marrow cells were then passed through a cell strainer, washed with 1x PBS after centrifugation, then plated in a T75 dish at 1×107 cells per 10 ml of BMDM media. BMDM media is defined as DMEM supplemented with 10% FBS, 20% conditioned medium from L929 cells (a source of M-CSF), 1% Penicillin/Streptomycin, and 1% glutamine. BMDMs were grown at 37°C for seven days and media was removed and replaced with fresh BMDM media every two days. The purity of the macrophages at one week (98.6%) was confirmed by flow cytometry. After seven days of growth, cells were treated for 24 hours with one of the five following treatments: No Tx (no treatment), M1 [IFN-γ (20 ng/ml) and LPS (100 ng/ml)], M2 [IL-4 (20 ng/ml)], EDP (1 μg/ml), or VGVAPG (1 μg/ml). Cell media was collected and RNA was isolated from the cells using TriZol according to manufacturer’s instructions. RNA levels were examined using qPCR analysis with SYBR Green (BioRad). GAPDH was used as an internal control.
Bone marrow-derived macrophage polarization and injection
Bone marrow cells were isolated and differentiated into macrophages as described above. After differentiation, BMDMs were treated with IFN-γ and LPS (M1) or IL-4 (M2). After polarization, 5×106 M1 or M2 BMDMs were injected into the mouse circulation via the tail vein. Mice were subjected to CaCl2-induced aneurysm induction 24 hours after M1 or M2 injection. As a control, a group of mice received no M1 or M2 macrophages prior to CaCl2 treatment. Mice were monitored for three days or six weeks. The initial time point for observation (3 days), was chosen for several reasons. Supplementary Figure 1 demonstrates peak expression of M1- and M2-associated markers at this time. Additionally, we found divergence in aortic diameter between groups at this early time point. This suggested that key immune regulatory changes were occurring in the first several days after aneurysm induction. Mice were sacrificed and the infrarenal aortic tissue was collected for histological, flow cytometry, RNA, or protein analysis.
Histology and microscopy
After perfusion-fixation with 10% neutral buffered formalin, aortic tissue samples were embedded in paraffin and sectioned into 4 μm sections. For Verhoeff-Van Gieson (VVG) staining, sections were stained with Verhoeff’s solution, ferric chloride, sodium thiosulfate, and Van Gieson’s solution. After each staining cycle, a fixing and washing procedure followed. Slides were then examined and photographed using light microscopy (40×; Nikon). Four sections were viewed per mouse.
Flow Cytometry
Aortic tissue from mice was isolated and digested for one hour at 37°C with collagenase and elastase in RPMI with 1% FBS.23 In order to isolate cells, the suspension from the digested tissue was filtered through a 40 μm filter. Cells were then pelleted by centrifugation and resuspended in flow cytometry staining buffer (1×PBS with 1% FBS). Cells were blocked with anti-mouse CD16/32 (1 μg/ml; BD Biosciences, San Diego, CA, USA) before surface labeling with the following antibodies: AlexaFluor 700-labeled CD86 (BioLegend, San Diego, CA, USA) APC-labeled F4/80 (BioLegend) and RPE-labeled CD206 (Bio-Rad Laboratories, Hercules, CA, USA). Flow cytometry data were analyzed using BD FACSDIVA software.
Detection of TNF-α concentration
Bone marrow-derived macrophages were isolated as described above. Media was collected from cells after incubation with increasing doses of EDP (0.01, 0.1, 1.0, 10, 100 μg/ml), VGVAPG (1 μg/ml), IFN-γ (20 ng/ml) and LPS (100 ng/ml), or IL-4 (20 ng/ml) for 24 hours. Cell media was used in a TNF-α ELISA from R&D Systems (Minneapolis, MN, USA) according to manufacturer’s instructions.
Immunofluorescence
Mice that were injected with M1 or M2 macrophages were sacrificed at three days after aneurysm induction for macrophage staining performed on paraffin-embedded 4 μm aortic sections. After deparaffinizing the sections, antigen retrieval was performed using Proteinase K (Viagen Biotech, Los Angeles, CA, USA; 20 μg/ml in TE buffer pH 8.0). Sections were incubated in the Proteinase K working solution for three minutes at room temperature. Sections were then washed and blocked with 10% normal goat serum. Rat monoclonal anti-F4/80 (ab6640, Abcam, Cambridge, MA, USA) diluted 1:400 was applied to the sections and incubated at room temperature for one hour. Sections were then washed and incubated with AlexaFlour 488 goat anti-rat IgG (A-11006, Life Technologies, Grand Island, NY, USA) diluted 1:200 for one hour at room temperature. Slides were then mounted with ProLong gold antifade mountant with DAPI (P-36931, Life Technologies). Fluorescence images were captured with a Leica epifluorescence widefield microscope (DMRXA2) and CCD camera (Orca C4742; Hamamatsu Photonics, Bridgewater, NJ, USA), with Hamamatsu software (HCImage 4.0).
Immunohistochemistry
Mice underwent AAA induction according to the method described above. Three to four mice from the NaCl, CaCl2-IgG, CaCl2-BA4 were sacrificed six weeks after aneurysm induction. Paraffin-embedded aortic sections were stained for CD206 (M2) and CD86 (M1). Sections were incubated with monoclonal rabbit anti-mouse CD86 (Abcam) diluted 1/500 or monoclonal rabbit anti-mannose receptor (CD206; Abcam) diluted 1/100 for 30 minutes at 37°C. The sections were then washed in citrate solution followed by incubation with a biotin-conjugated rat anti-rabbit IgG. CD206 and CD86 stained sections were examined by light microscopy (40×). The number of CD206 or CD86 positive cells per high powered field were counted and displayed in a bar graph. Four separate sections from each aorta were stained and evaluated.
Gelatin zymography
Aortic tissue protein was extracted as previously described and protein concentration was measured using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA).24 Gelatin zymography was performed as previously described.20 Standardized protein samples (5 μg) were resolved by nondenaturing electrophoresis with 0.8% gelatin in a 10% SDS-polyacrylamide gel. Molecular sizes of gelatinolytic activity were determined using protein standards (Bio-Rad). Signal intensities were quantified using ImageJ version 1.38x software (NIH, Bethesda, MD, USA).
Statistical analysis
The change in external aortic diameter over the course of aneurysm development was compared to baseline for each animal, and results for each group were expressed as mean ± SEM. ANOVA and post-hoc t-test with Bonferroni’s correction were used to compare multiple groups. Statistical significance was accepted at a P-value < .05.
Results
Elastin-derived peptides promoted a pro-inflammatory M1 phenotype
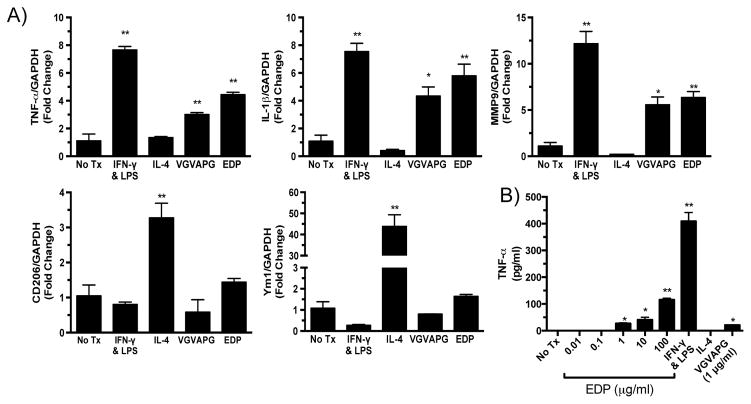
To determine the role that EDPs have on macrophage phenotype, bone marrow cells were isolated and differentiated into BMDMs. To establish control M1 or M2 cells, BMDMs were treated with IFN-γ and LPS (M1), IL-4 (M2), or left untreated (No Tx) for 24 hours (Fig. 1A). To examine the effect of elastin fragments on macrophage polarization, BMDMs were treated with 1 μg/ml of EDPs or VGVAPG for 24 hours. EDP and VGVAPG treatment induced mRNA expression of M1 associated markers, such as TNF-α and IL-1β (Fig. 1A). In contrast, mRNA expression of M2 markers, CD206 and Ym1, was not affected by EDP or VGVAPG treatment (Fig. 1A). MMP-9 mRNA expression was also increased by EDP or VGVAPG treatment (Fig. 1A). To determine if the EDP-mediated M1 polarization was concentration dependent, BMDMs were treated with increasing doses of EDPs. An ELISA demonstrated a stepwise increase in TNF-α levels in the media with increasing doses of EDPs (Fig. 1B).
Figure 1.
Elastin-derived peptides polarize macrophages to a pro-inflammatory M1 phenotype. A) qPCR analysis of M1 and M2 phenotype markers after treatment of macrophages with IFN-γ and LPS (M1), IL-4 (M2), VGVAPG (1 μg/ml), or EDP (1 μg/ml) for 24 hours. GAPDH was used as an internal control. B) ELISA measurement of TNF-α levels from EDP- or VGVAPG-treated macrophage media. Statistics performed using ANOVA and Student’s t-tests. Data expressed as mean ± SEM. *, P < .05; **, P < .01 versus no treatment (No Tx).
M2 macrophages reduced aortic dilation in an experimental AAA model
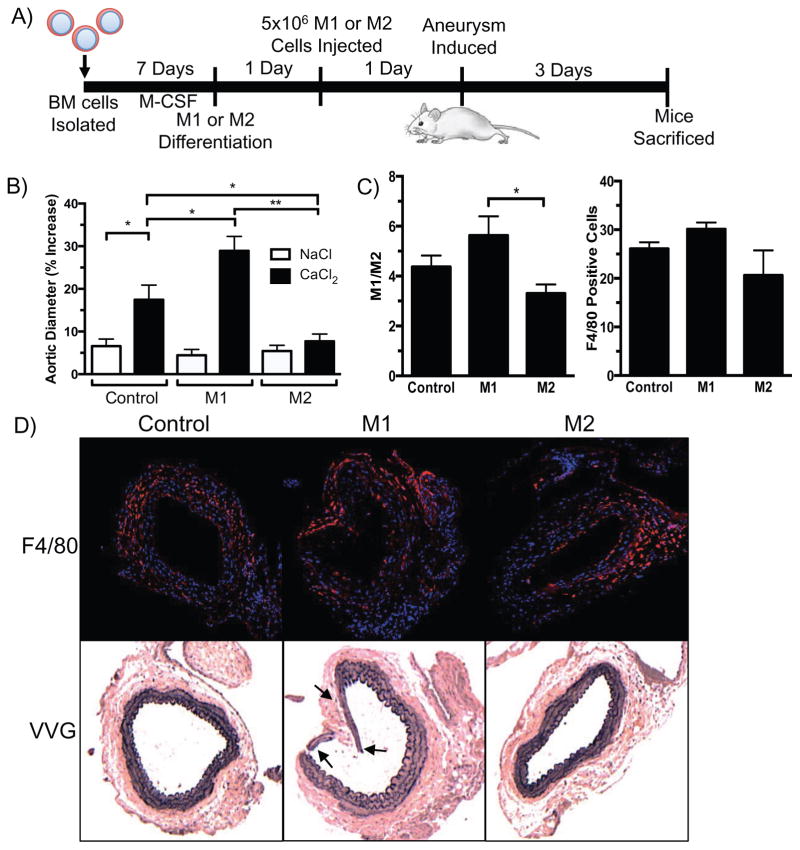
To determine the in vivo effects of influencing the M1/M2 ratio in an experimental AAA model, macrophages polarized to the M1 or M2 phenotype were injected intravenously into mice 24 hours prior to aneurysm induction. Cells were sampled from each treatment group prior to injection and M1 or M2 polarization was confirmed by protein expression of M1 (iNOS) and M2 (Ym1) markers (Fig. 2B). A control group of mice underwent aneurysm induction without injection of macrophages (Control). Aortic diameters were measured six weeks after aneurysm induction. Three of the eight mice that received M1 macrophages were sacrificed prior to the six-week time point due to severe systemic illness; necropsy revealed severe aortic damage in these mice. All of the mice that received M2 macrophages (9/9) survived to the six-week time point. Comparing survival (5/8 vs. 9/9) between the two groups by Fisher’s exact test demonstrated a P-value of 0.08. While M1 macrophage injection did not show a statistically significant increase in aortic diameter, the trend toward larger aneurysms may have been attenuated by the three mice that required sacrifice prior to six weeks. The aortic diameters from mice injected with M2 macrophages were significantly smaller than CaCl2-treated control mice or mice injected with M1 macrophages (Fig. 2C). Connective tissue staining indicated robust elastin degradation in aortas from the M1 injected mice compared to CaCl2-treated control mice. The most severe elastin degradation was seen in in the aortas of the three M1-injected mice who required sacrifice within the first week after aneurysm induction because of systemic illness. (Fig. 2D). Aortas from M2-injected mice displayed overall elastin preservation six weeks after aneurysm induction (Fig. 2D). The elastin preservation seen in these animals coincided with a decrease in levels of both MMP-9 and MMP-2 in M2-injected mice compared to M1-injected mice (Fig. 3). No differences in MMP levels were seen between Control and M1-injected mice.
Figure 2.
M1 and M2 macrophage influence on aortic size six weeks after aneurysm induction. A) Schematic representation of method of BMDM isolation, injection, and aortic aneurysm induction. B) Representative image of protein expression of M1 (iNOS) or M2 (Ym1) markers of macrophages treated with IFN-γ (20 ng/ml) and LPS (100 ng/ml) or IL-4 (20 ng/ml) prior to injection. C) Aortic diameter percent increase six weeks after aneurysm induction with M1 or M2 macrophage injection (n = 5–9 per group). Statistics performed using ANOVA and Student’s t-tests. Data expressed as mean ± SEM. *, P < .05; **, P < .01. D) Representative VVG images of aortas six weeks after aneurysm induction with injection of M1, M2, or no macrophages (Control) (n = 3–4 per group). VVG image of aorta from a mouse that required sacrifice within one week after aneurysm induction. Arrows indicate sites of elastin fragmentation.
Figure 3.

Active MMP-2 and MMP-9 levels are decreased in mice injected with M2 macrophages. Signal intensities of ProMMP-9, MMP-9, ProMMP-2, and MMP-2 were quantified using ImageJ software. Statistics performed using ANOVA and Student’s t-tests. Data are presented as mean ± SEM (n = 4–5 aortas per group). *, P < .05.
Infleunce of M1 and M2 macrophages at three days
To determine the effect of M1 or M2 macrophages on early aortic aneurysm formation, CaCl2- or NaCl-treated mice that had undergone M1 or M2 injection were sacrificed three days after aneurysm induction. The effect of the M2 macrophages observed at six weeks was also seen at the earlier three-day time point (Fig. 4B). In contrast to the six-week time point, M1 macrophage injection did show a significant increase in aortic diameter compared to CaCl2-treated control mice. M1 or M2 injection had no effect on aortic diameter in NaCl-treated mice (Fig. 4B). In order to assess that the injection of M1 or M2 cells were having a local effect on the macrophage population in the aortic tissue, cells were isolated from the aorta by enzymatic digestion three days after aneurysm induction. Cells were analyzed by flow cytometry to determine the ratio of M1 to M2 polarized cells (Fig 4C). These data demonstrated a higher M1/M2 ratio in the aortas of M1-injected mice and a decreased ratio in M2-injected mice. By immunofluorescence staining, there did not appear to be a difference in the total number macrophages in aortic tissue when comparing M1- to M2-injected mice (Fig. 4D). This was consistent with flow cytometry data showing no difference in the percentage of F4/80+ macrophages in the aortic tissue. Connective tissue staining revealed that M1 macrophage injection resulted in severe elastin fragmentation three days after aneurysm induction; the corresponding immunofluorescence studies indicated a high concentration of macrophages adjacent to the sites of marked elastin damage (Fig. 4D). Aortas from M2-injected mice showed preserved elastin architecture.
Figure 4.
M1 and M2 macrophage influence on aortic size three days after aneurysm induction. A) Schematic representation of methods three days after aneurysm induction and macrophage injection. B) Aortic diameter percent increase three days after aneurysm induction with injection of M1, M2, or no macrophages (Control) (n = 5–6 per group). NaCl was used as sham control. C) Bar graph on left represents M1/M2 ratio of M1 (CD86) to M2 (CD206) positive macrophages (F4/80) from flow cytometry (n = 5–6 per group). Right bar graph shows proportion of F4/80+ macrophages found in tissue. D) Representative immunofluorescence and VVG stained images of aortas three days after surgery and M1 or M2 macrophage injection (n = 3 per group). Top panel shows aortas stained with a rat anti-mouse F4/80 antibody (red), cell nuclei stained with DAPI (blue); bottom panel shows VVG stained aortas. Arrows indicate sites of elastin fragmentation or abnormal structure. Statistics performed using ANOVA and Student’s t-tests. Data expressed as mean ± SEM. *, P < .05; **, P < .01.
In order to determine whether intravenously injected macrophages migrated to the aorta, GFP+ BMDMs were incubated with IFN-γ and LPS (M1) and IL-4 (M2). After polarization, GFP+ M1 or GFP+ M2 cells were injected into the mouse tail vein 24 hours prior to aneurysm induction. Three days later, aortic tissue was harvested, digested, and a single cell suspension was analyzed by flow cytometry. GFP+ cells were found in aortas of 6/7 mice injected with M1 cells and 5/6 mice injected with M2 cells. Further analysis revealed that 1.18% ± 0.22 of the total M1 cells detected in the M1-injected mice were GFP+ and that 9.25% ± 2.74 of the total M2 cells detected in the M2-injected mice were GFP+.
BA4 reduced aortic diameter and elastin degradation
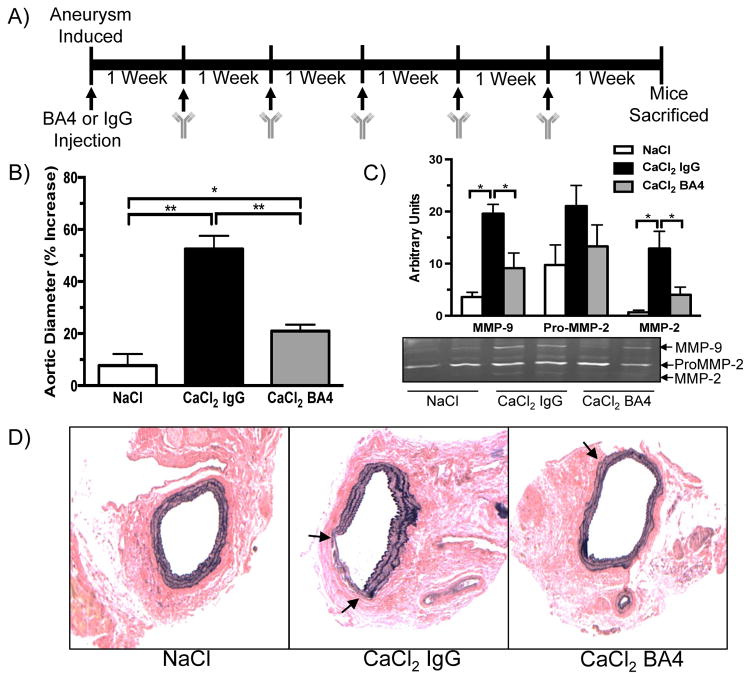
BA4 is a monoclonal antibody that specifically binds xGxxPG motifs, particularly VGVAPG, which is a common motif repeated in elastin. Previous studies have shown that BA4 reduces monocyte/macrophage chemotaxis in diseases associated with elastin fragmentation, such as Marfan syndrome and emphysema.12,21 To determine if BA4 can reduce aortic dilation in AAA, mice were treated with weekly i.p. injections of BA4 (10 mg/kg) after CaCl2 aneurysm induction. A control group of mice received weekly injections of non-specific IgG (10 mg/kg). Six weeks after aneurysm induction, mice were sacrificed and aortas harvested for analysis. BA4 reduced aortic dilation compared to IgG treatment (Fig. 5B). Active forms of both MMP-9 and MMP-2 in aortic tissue were also decreased in BA4-treated mice, whereas the inactive form of MMP-2 was not significantly reduced (Fig. 5C). Connective tissue staining revealed general elastin preservation in aortas from BA4-treated mice (Fig. 5D). Treatment with NaCl had no effect on elastin fragmentation. Immunohistochemical staining revealed an increase in CD206 positive cells (M2 macrophage marker) in aortic tissue from BA4-treated mice compared to NaCl and IgG-treated mice (Supplementary Fig. 2). The number of CD86 positive cells was higher in aortas from IgG-treated mice but not significantly different from NaCl or BA4-treated mice.
Figure 5.
BA4 attenuates aortic dilation and elastin degradation. A) Schematic representation of methods for weekly anti-EDP treatment. B) Percent increase in aortic diameter six weeks after aneurysm induction and treatment with IgG or BA4 (n = 7–8 mice per group). C) Representative gelatin zymogram of mouse aortic tissue six weeks after aneurysm induction. Signal intensities of MMP-2, ProMMP-2, and MMP-9 were quantified using ImageJ software. Statistics performed using ANOVA and Student’s t-tests. Data are presented as mean ± SEM (n = 4–5 aortas per group). *, P < .05; **, P < .01. C) Representative VVG staining of aortic tissue sections from NaCl, CaCl2 IgG, and CaCl2 BA4 treated mice (n = 3–4 aortas per group). Arrows indicate sites of elastin damage.
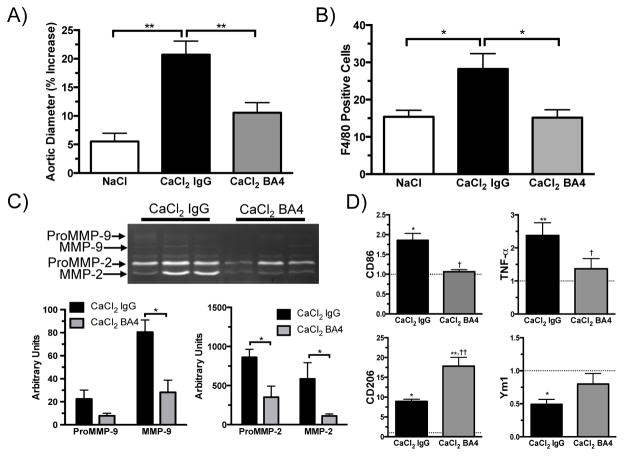
In order to better understand the early pathogenesis of aneurysm formation, the effects of EDP neutralization were evaluated one week after aneurysm induction. Aortic diameter was increased in both BA4-treated and IgG-treated mice; however, IgG-treated mice exhibited a markedly greater increase in diameter (Fig. 6A). A previous study showed that monocyte/macrophage chemotaxis in response to human AAA explants was reduced by preincubation of the tissue with BA4.13 In this present study, BA4’s ability to reduce macrophage migration in vivo was assessed by flow cytometry. As expected, migration of F4/80+ macrophages to aortic tissue was increased after CaCl2-IgG treatment compared to NaCl treatment (Fig. 6B). BA4 reduced the frequency of macrophage migration to the baseline levels seen in NaCl-treated aortas. Gelatin zymography demonstrated that BA4 treatment reduced the active forms of both MMP-2 and MMP-9 in aortic tissue at one week (Fig. 6C). M1 or M2 polarization was assessed in aortic tissue after CaCl2 treatment. Quantitative PCR revealed downregulation of M1 associated genes (TNF-α, CD86) and upregulation of M2 associated genes (CD206, Ym1) with BA4 treatment (Fig. 6D). Many typical pro-inflammatory markers identified in aneurysm tissue were decreased by BA4 treatment (Supplementary Fig. 3A) while anti-inflammatory markers were increased (Supplementary Fig. 3B). By Western blot analysis, the M2-associated marker Ym1 was increased in BA4-treated aortic tissue compared to IgG treatment (Supplementary Fig. 4).
Figure 6.
BA4 reduced macrophage migration, MMP production, and the ratio of M1/M2 markers in aortic tissue one week after aneurysm induction. A) Percent increase aortic diameter one week after aneurysm induction (n = 8–10 per group). B) Number of F4/80 positive cells in NaCl, CaCl2-IgG, and CaCl2-BA4 treated aortas (n = 7–8 per group). C) Representative gelatin zymogram of aortic tissue one week after aneurysm induction. Bar graphs represent signal intensities of ProMMP-9, MMP-9, ProMMP-2, and MMP-2 by quantification with ImageJ software (n = 3–4 per group). Data expressed as mean ± SEM. *, P < .05; **, P < .01. D) Fold change in mRNA expression of M1 and M2 associated markers from aortic tissue of mice treated with CaCl2-IgG or CaCl2-BA4 compared to NaCl-treated mice one week after aneurysm induction (n = 4 per group). GAPDH was used as internal control. Dashed line indicates NaCl control (fold change = 1). Statistics performed using ANOVA and Student’s t-tests. Data expressed as mean ± SEM. *, P < .05; **, P < .01 versus NaCl. †, P < .05; ††, P < .01 versus CaCl2-IgG treatment.
Discussion
Using a murine model of AAA, we found that M1 macrophages strongly promoted aneurysm formation. This effect was seen as early as three days after aneurysm induction; aortic diameters were larger in the M1-infused group. This increase was not significant compared to CaCl2-treated control at six weeks. This may be explained, however, by the fact that three of the eight mice treated with M1 macrophages required sacrifice within one week of aneurysm induction due to severe systemic illness. Post-mortem examination of the aortic tissue from these three mice demonstrated severe elastin damage not typically seen until six weeks. This early morbidity is atypical for this model and was not seen in any of the M2-treated mice, suggesting that an excess number of M1 macrophages may be toxic. Conversely, M2 macrophage infusion was highly protective against elastin degradation and aneurysm formation. Furthermore, EDPs, systemic levels of which are elevated in AAA patients,25 promoted polarization of macrophages to a pro-inflammatory M1 phenotype. EDPs also enhanced the transcription of MMP-9, which has previously been shown to be required for AAA development.20,26 In vitro studies showed that EDPs or the VGVAPG peptide did not upregulate expression of M2-associated genes. Administration of the antibody BA4, which binds the repeated elastin motif VGVAPG, dramatically reduced aortic dilation, elastin fragmentation, and MMP production. BA4 lowered M1 and raised M2 markers early after aneurysm induction, leading to a reduction in aneurysm formation at six weeks.
EDPs are created as a result of breakdown of elastin, a key extracellular matrix protein that is present in the skin, lungs, arteries, and other structures. Elastin makes up 50% of the aortic wall proteins by weight.27 Once elastin is incorporated into the tissue structure, there is little to no turnover except under pathological conditions.12,28–30 Previous studies have shown that EDPs are increased in the serum of patients with AAAs.10,11,25 EDPs have been shown to have inflammatory properties by examination of their effects on lymphocytes and MMP expression; they increase Th1 associated cytokines and MMP-9 synthesis.31 This polarization of lymphocytes appears to be mediated through a receptor for specific EDPs, since it can be blocked by neutralization of the elastin receptor or with lactose, which causes shedding of the receptor from the cell membrane.5 Similar treatment inhibited monocyte migration when monocytes were exposed to human AAA tissue extract.13 A previous study has suggested that EDPs may have an anti-inflammatory effect on macrophages under specific conditions.32 In contrast, the present study indicated that EDPs had a profound pro-inflammatory effect. Furthermore, we demonstrated that this effect was mediated by promoting M1 macrophage polarization.
Chronic inflammation occurs in the aortic tissue of patients with AAA; the predominate cell types found in this infiltrate are T lymphocytes and macrophages.33,34 Inflammation induced by ablumenal application of CaCl2 in a model of AAA recapitulates many features seen in human AAA tissue including macrophage and lymphocyte infiltration, MMP upregulation, elastin degradation, and aortic dilation.20,35–37 Pro-inflammatory M1-associated cytokines such as TNF-α, IL-6, and IFN-γ are increased in both human and experimental AAAs.9,18 Deletion or neutralization of these M1-associated cytokines resulted in reduced aortic dilation or complete aneurysm inhibition.17,24,38,39 M1 or M2 macrophage polarization can have a significant role in regulating chronic inflammatory processes; little is known about the role of M1 or M2 macrophage polarization in aortic aneurysms. We demonstrated that the pro-inflammatory state induced by CaCl2 was enhanced by supplementation with M1-polarized macrophages. M2 macrophage injection rescued the aorta from expansion and elastin disruption. The pathogenesis of aneurysm development was altered early in its course by M2 macrophages, where the impact on aortic dilation and elastin fragmentation could be seen as early as three days after aneurysm induction. Taken together, these data demonstrate that the M1/M2 ratio plays a fundamental role in aneurysm development, and could prove an important therapeutic target.
By using flow cytometry, we were able to determine that injected macrophages are able to migrate to the damaged aorta. This small proportion of injected cells found in the aorta three days after aneurysm induction may not completely account for how these cells induce the distinct aortic phenotypes observed. It is known that small populations of cells, such as T regulatory cells, can have a major effect on inflammatory processes in both human and murine AAAs. 40,41 These injected M1 or M2 cells may also be acting systemically by increasing circulating levels of cytokines levels vital to aneurysm formation. Injection of M1 macrophages led to early mortality in three mice within a week of aneurysm induction suggesting a profound systemic inflammatory response not seen in M2-injected mice. These injected M1 or M2 cells may also be influencing other cell types important in aneurysm formation such as T regulatory cells. 42 In the case of injected M2 cells, they may be creating a positive feedback loop by enhancing other anti-inflammatory cell populations. These populations may further enhance the M2 phenotype leading to decreased damage to the aortic wall and preservation of elastin. Previous studies have shown that macrophages are not terminally differentiated and have the ability to change phenotypes over time. 43–45 Future studies will focus on better understanding these mechanisms and, particularly, the potential benefit of injecting macrophages polarized to the M2 phenotype.
The elastin-binding protein is assumed to have a role in normal elastin assembly by secreting the elastin precursor, tropoelastin, into the ECM.46 This protein is found on many cell types, including cells that don’t normally produce elastin.47 This study suggests that the elastin-binding protein, through its interaction with EDPs, contributes to disease pathogenesis when present on inflammatory cells such as macrophages. Previous studies have shown that the peptide sequence predominantly recognized by the elastin-binding protein is VGVAPG. Other peptides of this motif, xGxxPG, have a lower affinity for the elastin-binding protein.48 These particular motifs are found primarily in elastin, but are also present to a lesser extent in other connective tissue proteins. Binding of the BA4 antibody to elastin fragments with the xGxxPG motif reduces or inhibits cellular activation in response to the presence of EDPs. 12,13,15,21, 49 In this study, we demonstrate this critical role of EDPs in potentiating aneurysm formation, in part, through the elastin-binding protein on macrophages. The in vitro studies indicated that the mechanism for this effect is mediated by altering macrophage polarization. As expected, BA4 reduced macrophage migration to the site of aortic injury. Importantly, the BA4 treatment also influenced production of M1 and M2 related proteins, reducing the pro-inflammatory microenvironment caused by EDPs. Since M2 macrophages are known to play a role in healing, the effects seen with BA4 may represent attempted tissue repair.
In conclusion, this study demonstrated that EDPs have an important role in promoting aneurysm expansion by altering the macrophage phenotype. This increase in M1/M2 ratio would be expected to create a positive feedback loop by further degradation of the elastin matrix and continued release of EDPs. The profound inhibitory effects of the M2 macrophages suggest a key role in preventing small aneurysm expansion. All current treatments for aortic aneurysms rely on mechanical intervention. Yet, when most AAAs are detected, they are below the threshold for repair leading to a significant observation period during which there is currently no medical therapy to prevent or slow aneurysm growth. The results presented herein suggest that decreasing the M1/M2 ratio, either by neutralization of pro-inflammatory EDPs or enhancing the anti-inflammatory environment created by M2 macrophages, could prove a useful therapeutic target for small aortic aneurysms.
Supplementary Material
Acknowledgments
We Pamela K. Carmines, PhD for assistance with manuscript preparation. We also thank the University of Nebraska Medical Center Flow Cytometry Research Facility for help with the flow cytometry experiments and the University of Nebraska Medical Center Tissue Sciences Facility in assistance with histological analysis.
Abbreviations used in this paper
- AAA
abdominal aortic aneurysm
- ECM
extracellular matrix
- EDP
elastin-derived peptide
- MMP
matrix metalloproteinase
- BMDM
bone marrow-derived macrophage
- VVG
Verhoeff-Van Geison
Footnotes
This study was supported by NIH Grant R01HL062400.
References
- 1.Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Baxter BT, Terrin MC, Dalman RL. Medical management of small abdominal aortic aneurysms. Circulation. 2008;117:1883–1889. doi: 10.1161/CIRCULATIONAHA.107.735274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 4.Botnar RM, Wiethoff AJ, Ebersberger U, Lacerda S, Blume U, Warley A, Jansen CH, Onthank DC, Cesati RR, Razavi R, Marber MS, Hamm B, Schaeffter T, Robinson SP, Makowski MR. In vivo assessment of aortic aneurysm wall integrity using elastin-specific molecular magnetic resonance imaging. Circ Cardiovasc Imaging. 2014;7:679–689. doi: 10.1161/CIRCIMAGING.113.001131. [DOI] [PubMed] [Google Scholar]
- 5.Blanchevoye C, Floquet N, Scandolera A, Baud S, Maurice P, Bocquet O, Blaise S, Ghoneim C, Cantarelli B, Delacoux F, Dauchez M, Efremov RG, Martiny L, Duca L, Debelle L. Interaction between the elastin peptide VGVAPG and human elastin binding protein. J Biol Chem. 2013;288:1317–1328. doi: 10.1074/jbc.M112.419929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duca L, Floquet N, Alix AJ, Haye B, Debelle L. Elastin as a matrikine. Crit Rev Oncol Hematol. 2004;49:235–244. doi: 10.1016/j.critrevonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Mochizuki S, Brassart B, Hinek A. Signaling pathways transduced through the elastin receptor facilitate proliferation of arterial smooth muscle cells. J Biol Chem. 2002;277:44854–44863. doi: 10.1074/jbc.M205630200. [DOI] [PubMed] [Google Scholar]
- 8.Senior RM, Griffin GL, Mecham RP, Wrenn DS, Prasad KU, Urry DW. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J Cell Biol. 1984;99:870–874. doi: 10.1083/jcb.99.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juvonen J, Surcel HM, Satta J, Teppo AM, Bloigu A, Syrjala H, Airaksinen J, Leinonen M, Saikku P, Juvonen T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1997;17:2843–2847. doi: 10.1161/01.atv.17.11.2843. [DOI] [PubMed] [Google Scholar]
- 10.Lindholt JS, Heickendorff L, Henneberg EW, Fasting H. Serum-elastin-peptides as a predictor of expansion of small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 1997;14:12–16. doi: 10.1016/s1078-5884(97)80219-5. [DOI] [PubMed] [Google Scholar]
- 11.Lindholt JS, Ashton HA, Heickendorff L, Scott RA. Serum elastin peptides in the preoperative evaluation of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2001;22:546–550. doi: 10.1053/ejvs.2001.1516. [DOI] [PubMed] [Google Scholar]
- 12.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116:753–759. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hance KA, Tataria M, Ziporin SJ, Lee JK, Thompson RW. Monocyte chemotactic activity in human abdominal aortic aneurysms: role of elastin degradation peptides and the 67-kD cell surface elastin receptor. J Vasc Surg. 2002;35:254–261. doi: 10.1067/mva.2002.120382. [DOI] [PubMed] [Google Scholar]
- 14.Hunninghake GW, Davidson JM, Rennard S, Szapiel S, Gadek JE, Crystal RG. Elastin fragments attract macrophage precursors to diseased sites in pulmonary emphysema. Science. 1981;212:925–927. doi: 10.1126/science.7233186. [DOI] [PubMed] [Google Scholar]
- 15.Guo G, Booms P, Halushka M, Dietz HC, Ney A, Stricker S, Hecht J, Mundlos S, Robinson PN. Induction of macrophage chemotaxis by aortic extracts of the mgR Marfan mouse model and a GxxPG-containing fibrillin-1 fragment. Circulation. 2006;114:1855–1862. doi: 10.1161/CIRCULATIONAHA.105.601674. [DOI] [PubMed] [Google Scholar]
- 16.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong W, MacTaggart J, Knispel R, Worth J, Persidsky Y, Baxter BT. Blocking TNF-alpha attenuates aneurysm formation in a murine model. J Immunol. 2009;183:2741–2746. doi: 10.4049/jimmunol.0803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlsson L, Bergqvist D, Lindback J, Parsson H. Expansion of small-diameter abdominal aortic aneurysms is not reflected by the release of inflammatory mediators IL-6, MMP-9 and CRP in plasma. Eur J Vasc Endovasc Surg. 2009;37:420–424. doi: 10.1016/j.ejvs.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo G, Munoz-Garcia B, Ott CE, Grunhagen J, Mousa SA, Pletschacher A, von Kodolitsch Y, Knaus P, Robinson PN. Antagonism of GxxPG fragments ameliorates manifestations of aortic disease in Marfan syndrome mice. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds439. [DOI] [PubMed] [Google Scholar]
- 22.Weischenfeldt J, Porse B. Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. CSH Protoc. 2008;2008 doi: 10.1101/pdb.prot5080. pdb.prot5080. [DOI] [PubMed] [Google Scholar]
- 23.Sharma AK, Lu G, Jester A, Johnston WF, Zhao Y, Hajzus VA, Saadatzadeh MR, Su G, Bhamidipati CM, Mehta GS, Kron IL, Laubach VE, Murphy MP, Ailawadi G, Upchurch GR., Jr Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation. 2012;126:S38–45. doi: 10.1161/CIRCULATIONAHA.111.083451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong W, Zhao Y, Prall A, Greiner TC, Baxter BT. Key roles of CD4+ T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model. J Immunol. 2004;172:2607–2612. doi: 10.4049/jimmunol.172.4.2607. [DOI] [PubMed] [Google Scholar]
- 25.Petersen E, Wagberg F, Angquist KA. Serum concentrations of elastin-derived peptides in patients with specific manifestations of atherosclerotic disease. Eur J Vasc Endovasc Surg. 2002;24:440–444. doi: 10.1053/ejvs.2002.1750. [DOI] [PubMed] [Google Scholar]
- 26.Thompson RW, Curci JA, Ennis TL, Mao D, Pagano MB, Pham CT. Pathophysiology of abdominal aortic aneurysms: insights from the elastase-induced model in mice with different genetic backgrounds. Ann N Y Acad Sci. 2006;1085:59–73. doi: 10.1196/annals.1383.029. [DOI] [PubMed] [Google Scholar]
- 27.D’Armiento J. Decreased elastin in vessel walls puts the pressure on. J Clin Invest. 2003;112:1308–1310. doi: 10.1172/JCI20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hornebeck W, Emonard H, Monboisse JC, Bellon G. Matrix-directed regulation of pericellular proteolysis and tumor progression. Semin Cancer Biol. 2002;12:231–241. doi: 10.1016/s1044-579x(02)00026-3. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinohara T, Suzuki K, Okada M, Shiigai M, Shimizu M, Maehara T, Ohsuzu F. Soluble elastin fragments in serum are elevated in acute aortic dissection. Arterioscler Thromb Vasc Biol. 2003;23:1839–1844. doi: 10.1161/01.ATV.0000085016.02363.80. [DOI] [PubMed] [Google Scholar]
- 31.Debret R, Antonicelli F, Theill A, Hornebeck W, Bernard P, Guenounou M, Le Naour R. Elastin-derived peptides induce a T-helper type 1 polarization of human blood lymphocytes. Arterioscler Thromb Vasc Biol. 2005;25:1353–1358. doi: 10.1161/01.ATV.0000168412.50855.9f. [DOI] [PubMed] [Google Scholar]
- 32.Baranek T, Debret R, Antonicelli F, Lamkhioued B, Belaaouaj A, Hornebeck W, Bernard P, Guenounou M, Le Naour R. Elastin receptor (spliced galactosidase) occupancy by elastin peptides counteracts proinflammatory cytokine expression in lipopolysaccharide-stimulated human monocytes through NF-kappaB down-regulation. J Immunol. 2007;179:6184–6192. doi: 10.4049/jimmunol.179.9.6184. [DOI] [PubMed] [Google Scholar]
- 33.Koch AE, Haines GK, Rizzo RJ, Radosevich JA, Pope RM, Robinson PG, Pearce WH. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am J Pathol. 1990;137:1199–1213. [PMC free article] [PubMed] [Google Scholar]
- 34.Pearce WH, Koch AE. Cellular components and features of immune response in abdominal aortic aneurysms. Ann N Y Acad Sci. 1996;800:175–185. doi: 10.1111/j.1749-6632.1996.tb33308.x. [DOI] [PubMed] [Google Scholar]
- 35.Longo GM, Buda SJ, Fiotta N, Xiong W, Griener T, Shapiro S, Baxter BT. MMP-12 has a role in abdominal aortic aneurysms in mice. Surgery. 2005;137:457–462. doi: 10.1016/j.surg.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Krishna S, Golledge J. The calcium chloride-induced rodent model of abdominal aortic aneurysm. Atherosclerosis. 2013;226:29–39. doi: 10.1016/j.atherosclerosis.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Tsuruda T, Kato J, Hatakeyama K, Kojima K, Yano M, Yano Y, Nakamura K, Nakamura-Uchiyama F, Matsushima Y, Imamura T, Onitsuka T, Asada Y, Nawa Y, Eto T, Kitamura K. Adventitial mast cells contribute to pathogenesis in the progression of abdominal aortic aneurysm. Circ Res. 2008;102:1368–1377. doi: 10.1161/CIRCRESAHA.108.173682. [DOI] [PubMed] [Google Scholar]
- 38.Johnston WF, Salmon M, Pope NH, Meher A, Su G, Stone ML, Lu G, Owens GK, Upchurch GR, Jr, Ailawadi G. Inhibition of interleukin-1beta decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation. 2014;130:S51–9. doi: 10.1161/CIRCULATIONAHA.113.006800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y, Wu W, Lindholt JS, Sukhova GK, Libby P, Yu X, Shi GP. Regulatory T cells in human and angiotensin II-induced mouse abdominal aortic aneurysms. Cardiovasc Res. 2015 doi: 10.1093/cvr/cvv119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng X, Yang J, Dong M, Zhang K, Tu E, Gao Q, Chen W, Zhang C, Zhang Y. Regulatory T cells in cardiovascular diseases. Nat Rev Cardiol. 2016;13:167–179. doi: 10.1038/nrcardio.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao Q, Wang Y, Zheng D, Sun Y, Wang Y, Lee VW, Zheng G, Tan TK, Ince J, Alexander SI, Harris DC. IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol. 2010;21:933–942. doi: 10.1681/ASN.2009060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu G, Ma H, Qiu L, Li L, Cao Y, Ma J, Zhao Y. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol. 2011;89:130–142. doi: 10.1038/icb.2010.70. [DOI] [PubMed] [Google Scholar]
- 44.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 46.Hinek A, Rabinovitch M. 67-kD elastin-binding protein is a protective “companion” of extracellular insoluble elastin and intracellular tropoelastin. J Cell Biol. 1994;126:563–574. doi: 10.1083/jcb.126.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Privitera S, Prody CA, Callahan JW, Hinek A. The 67-kDa enzymatically inactive alternatively spliced variant of beta-galactosidase is identical to the elastin/laminin-binding protein. J Biol Chem. 1998;273:6319–6326. doi: 10.1074/jbc.273.11.6319. [DOI] [PubMed] [Google Scholar]
- 48.Wrenn DS, Griffin GL, Senior RM, Mecham RP. Characterization of biologically active domains on elastin: identification of a monoclonal antibody to a cell recognition site. Biochemistry. 1986;25:5172–5176. doi: 10.1021/bi00366a028. [DOI] [PubMed] [Google Scholar]
- 49.Grosso L, Scott M. Peptide sequences selected by BA4, a tropoelastin-specific monoclonal antibody, are ligands for the 67-kilodalton bovine elastin receptor. 1993;32:13369–13374. doi: 10.1021/bi00211a052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.