Abstract
GABAA receptor-mediated inhibition is active and may contribute to epileptiform synchronization. The efficacy of inhibition relies on low levels of intracellular Cl−, which are controlled by KCC2 activity. This evidence has led us to analyze with field potential recordings the effects induced by the KCC2 blockers VU0240551 (10 μM) or bumetanide (50 μM) and by the KCC2 enhancer CLP257 (100 μM) on the epileptiform discharges generated by piriform and entorhinal cortices (PC and EC, respectively) in an in vitro brain slice preparation. Ictal- and interictal-like discharges along with high-frequency oscillations (HFOs, ripples: 80–200 Hz, fast ripples: 250–500 Hz) were recorded from these two regions during application of 4-aminopyridine (4AP, 50 μM). Blocking KCC2 activity with either VU024055 or high doses of bumetanide abolished ictal discharge in both PC and EC; in addition, these experimental procedures decreased the interval of occurrence and duration of interictal discharges. In contrast, enhancing KCC2 activity with CLP257 increased ictal discharge duration in both regions. Finally, blocking KCC2 activity decreased the duration and amplitude of pharmacologically isolated synchronous GABAergic events whereas enhancing KCC2 activity led to an increase in their duration. Our data demonstrate that in vitro ictogenesis is abolished or facilitated by inhibiting or enhancing KCC2 activity, respectively. We propose that these effects may result from the reduction of GABAA receptor-dependent increases in extracellular K+ that are known to rest on KCC2 function.
Keywords: 4-Aminopyridine, Entorhinal cortex, Epilepsy, GABA, High-frequency oscillations, KCC2, NKCC1, Piriform cortex
Introduction
In the adult brain, γ-Aminobutyric acid (GABA) acts as the main inhibitory neurotransmitter and its failure has been often cited as a contributing factor to the generation of epileptic seizures. Indeed, early work in the in vitro hippocampal slice preparation demonstrated that interictal-like (hereafter referred to as interictal) epileptiform activity results from weakened inhibition (Johnston and Brown, 1981; Schwartzkroin and Prince, 1978, 1980) but later, by employing experimental procedures that enhance GABAergic signaling such as the K+ channel blocker 4-aminopyridine (4AP) or Mg2+ free medium, we and other investigators have reported that ictal-like (hereafter referred to as ictal) discharges in adult rodents are paradoxically contributed by GABAA receptor-mediated signaling (see for review Avoli and de Curtis, 2011). In addition, under specific pharmacological manipulations, GABAA receptor-mediated currents are the sole synaptic mechanism contributing to ictogenesis in the hippocampus (Uusisaari et al., 2002).
GABAA receptor activation opens channels that are permeable to Cl− and to a lesser extent to . In adulthood, GABAA receptor-mediated current is hyperpolarizing due to the low intracellular [Cl−] that is maintained by the activity of cation chloride cotransporters (CCCs). CCCs are secondarily active transporters that use cation gradient for transporting Cl− (Rinehart et al. 2011) and two important CCCs in controlling neuronal [Cl−] are KCC2 and NKCC1. KCC2 is exclusively expressed in the central nervous system in mature brain and is the main extruder of Cl− from neurons (Viitanen et al., 2010). On the other hand, NKCC1 is responsible for the uptake of Cl− and renders GABA depolarizing in the immature brain (see for review Ben-Ari et al., 2012).
Some studies have linked failure in GABAergic inhibition to the Cl− accumulation in the postsynaptic neurons thus leading to GABAA receptor-mediated depolarization and even excitation (Ben-Ari and Holmes, 2005; Lillis et al., 2012; Staley et al., 1995). Also, downregulation of KCC2 is often observed in several pathological conditions associated with enhanced excitation such as epilepsy (Huberfeld et al., 2007; Rivera et al., 2002, 2004). Therefore, it has been proposed that the initiation and maintenance of ictal activity rest on the inability of KCC2 to control intracellular Cl− thus disclosing depolarizing (and potentially excitatory) GABAA receptor-mediated currents along with -mediated currents (Avoli and de Curtis, 2011; Jefferys et al., 2012). However, other studies have suggested that excessive activity of KCC2 during prolonged activation of GABAA receptor and accumulation of Cl− may result in an increase in extracellular [K+] (Viitanen et al., 2010), which in turn can lead to depolarization of neighboring neurons and excitation (see for review Avoli and de Curtis, 2011).
To date the role played by the GABAA receptor-mediated signaling in epileptiform synchronization and in particular its participation to ictogenesis remains unclear. Therefore, in this study we investigated the effects induced by the KCC2 inhibitor VU0240551 and the KCC2 enhancer, CLP257 on the epileptiform discharges and associated HFO that are generated by rat olfactory (PC) and limbic (EC) cortical networks maintained in vitro during 4AP treatment.
Methods
Brain slice preparation and maintenance—Male, adult Sprague-Dawley rats (250–275 g) were decapitated under isoflurane anesthesia according to the procedures established by the Canadian Council of Animal Care. The brain was quickly removed and placed in cold (1–3 °C), oxygenated artificial cerebrospinal fluid (ACSF) with the following composition (mM): 124 NaCl, 2 KCl, 2 CaCl2, 2 MgSO4, 1.25 KH2PO4, 26 NaHCO3, 10 D-glucose. Horizontal brain slices (450 μm) containing PC and EC were cut from this brain block using a vibratome. Slices were then transferred to an interface tissue chamber where they were superfused with ACSF and humidified gas (95% O2, 5% CO2) at a temperature of 31–32 °C and a pH of 7.4. 4AP (50 μM), VU0240551 (10 μM), bumetanide (10 μM or 50 μM), CLP257 (100 μM), 3, 3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonate (CPP, 10 μM), and 6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX, 10 μM) were bath applied. Chemicals were acquired from Sigma-Aldrich Canada (Oakville, Ontario, Canada) except for CLP257 that was generously provided by Dr. Yves De Koninck from Laval University (Quebec City, Que., Canada). VU0240551 and CLP257 were dissolved in DMSO and control experiments were performed to rule out any possible interference of this solvent on the epileptiform field activity induced by 4AP.
Electrophysiological recordings–Field potential recordings were obtained with ACSF-filled, glass pipettes (1B150F-4; World Precision Instruments, Sarasota, Florida, USA; tip diameter <10 μm, resistance 5–10 MΩ) that were connected to high-impedance amplifiers. The recording electrodes were positioned in the deep layers of the posterior PC and of the lateral EC (Fig. 1A). Field potential signals were fed to a computer interface (Digidata 1322A, Molecular Devices, and Palo Alto, CA, USA), acquired and stored using the PCLAMP 9.2 software (Molecular Devices). Subsequent data analyses were performed with CLAMPFIT 9.2 (Molecular Devices).
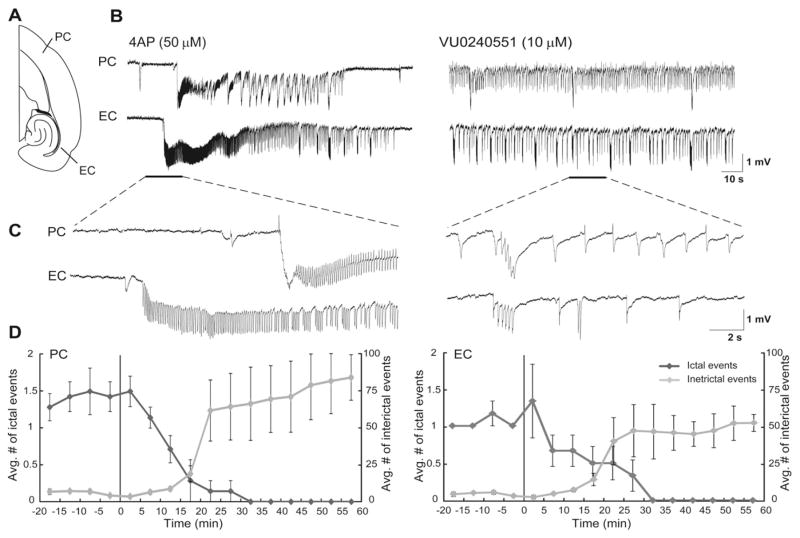
Fig. 1.
A: Schematic drawing of a horizontal slice containing PC and EC. B: Field potential recordings obtained from PC and EC areas during application of 4AP and VU0240551 (10 μM). Note that blocking KCC2 with VU0240551 virtually abolishes ictal discharges in both brain regions whereas interictal discharges continue to occur. Enlarged portion of the recordings is shown in insets with traces that are further expanded at the start of an ictal discharge during 4AP and during interictal activity after application of VU0240551. C: Plots showing the average number of ictal and interictal discharges in PC and EC. Vertical lines in both graphs indicate the application of VU0240551. Note that 30 min application of VU0240551 blocked the occurrence of ictal discharges in both PC and EC while it increased the average number of interictal events in both regions.
Detection of high-frequency oscillatory events—Time-periods containing interictal discharges recorded from the PC and EC were extracted. To identify oscillations in each frequency range (80–200 Hz and 250–500 Hz), a multi-parametric algorithm was employed using routines based on standardized functions (Matlab Signal Processing Tool-box) as described in detail in Hamidi et al. (2014) and Salami et al. (2012).
To be considered as HFOs, oscillatory events in each frequency range had to show at least four consecutive cycles, having amplitude of 3 SD above the mean of the reference period. The time lag between two consecutive cycles had to be between 5 and 12.5 ms for ripples (80–200 Hz) and between 2 and 4 ms for fast ripples (250–500 Hz). Oscillatory events containing overlapping ripples and fast ripples were excluded from the analysis (Bénar et al., 2010).
Statistical analysis—We used CLAMPFIT 9.2 (Molecular Devices) for offline analysis of the duration and interval of occurrence of ictal and interictal discharges. To segregate ictal from interictal discharges, we applied the k-means clustering algorithm with squared euclidean distances on the duration of all recorded events and a cutoff at 3 s was chosen to segregate ictal and interictal events. These values are similar to those “arbitrarily” chosen by Traub et al. (1996). The duration of both ictal and interictal discharges were defined as the time between the first deflections of the discharge from baseline to its return to baseline. The interval of occurrence of both ictal and interictal discharges were defined as the time between the onsets of two consecutive discharges. Amplitude of epileptiform events was measured from peak to peak. Since the kurtosis and skewness measures showed that values were not normally distributed, we transformed the raw data to Z-scores and performed one-way ANOVAs followed by Tukey post-hoc tests to identify differences between experimental conditions (i.e., 4AP alone, 4AP+ VU0240551, 4AP+ bumetanide and 4AP+ CLP257) in each region (PC and EC) regarding the rate and duration of ictal events and the rate, duration and amplitude of interictal discharges and isolated GABAergic events. Statistical tests were performed in Matlab R2012b (Matworks, Natick, MA) and the level of significance was set at p < 0.05. Results are expressed as mean ± SEM and n indicates the number of slices used for analysis.
Results
Effects of KCC2 blockers on 4AP-induced epileptiform activity
Field potential recordings—which were obtained simultaneously from PC and EC during 4AP application—revealed ictal and interictal discharges that occurred in both structures (Hamidi et al., 2014) (Fig. 1B). Ictal discharges recorded from PC lasted 96.43 ± 5.56 s, and recurred every 155.03 ± 9.71 s (90 events, n = 20 slices) whereas those occurring in EC lasted 127.62 ± 6.57 s, and recurred every 180.16 ± 8.65 s (78 events, n = 20 slices). Interictal discharges recorded from PC (375 events, n = 20 slices) and EC (392 events, n = 20 slices) in these experiments lasted 1.33 ± 0.05 s and 1.69 ± 0.04 s, occurred every 34.4 ± 3.1 s and 35.9 ± 4.4 s, and had amplitudes of 1.12 ± 0.03 mV and 1.21 ± 0.06 mV, respectively.
We studied the effect of VU0240551 (10 μM) on the epileptiform discharges recorded from PC and EC. As illustrated in Fig. 1B, we found that 30 min application of VU0240551 (10 μM) completely abolished the occurrence of ictal event in both PC and EC (n = 8 slices). In Fig. 1C we plotted the average number of ictal and interictal events over time during 4AP (50 μM) and application of VU0240551 (10 μM). We found that application of VU0240551 (10 μM) resulted in reduction in the average number of ictal events while it increased simultaneously the average number of interictal events in both PC and EC.
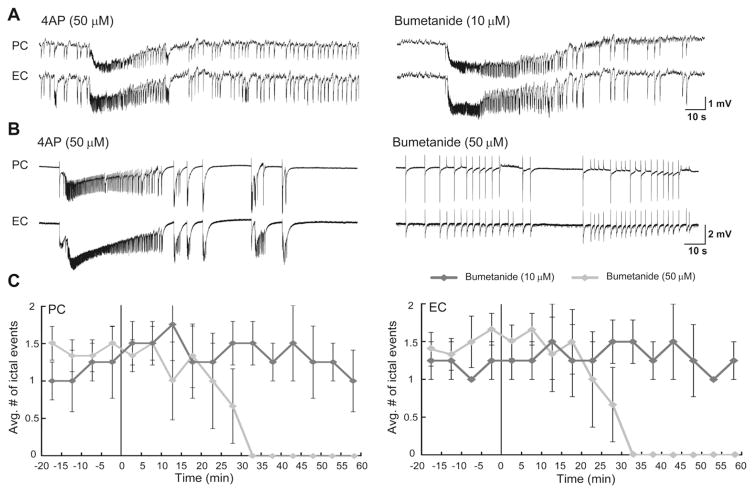
We also used bumetanide that has high affinity for NKCC1 and a low affinity for KCC2; accordingly, it has been reported that at low doses (2–10 μM), bumetanide blocks NKCC1 whereas at higher concentrations (50 μM) it inhibits both NKCC1 and KCC2 (Löscher et al., 2013). In both PC and EC, low doses of bumetanide (10 μM) did not significantly modulate the rate of occurrence of ictal discharges or their duration (Fig. 2A, n = 6 slices). In contrast, 30 min application of higher doses of bumetanide (50 μM) completely abolished ictal discharges in both PC and EC (Figs. 2B, n = 6 slices). The change induced by bumetanide in the average number of ictal events over time is plotted in Fig. 2C.
Fig. 2.
A and B: Effects induced by 10 μM (A) and 50 μM (B) bumetanide on the epileptiform discharges recorded from the PC and EC during bath application of 4AP. Note that low dosage of bumetanide does not have any effect on the epileptiform discharges while blocking KCC2 with 50 μM bumetanide virtually abolishes ictal discharges and discloses interictal discharges in both regions. C: Plots showing the average number of ictal discharges in PC and EC before and after the application of low and high dosage of bumetanide. Vertical lines in both graphs indicate the application of the drugs. Note that 30 min application of 50 μM bumetanide blocked the occurrence of ictal discharges in both regions.
In both PC and EC, blocking KCC2 with VU0240551 (10 μM; n = 8 slices) or high concentrations of bumetanide (50 μM; n = 6 slices) significantly (p < 0.0001) reduced the interval of occurrence of interictal events to 4.2 ± 0.9 s and 9.8 ± 0.7 s, respectively, in PC and to 3.8 ± 0.07 s and 9.1 ± 0.06 s, respectively, in EC (Fig. 3A). Application of VU0240551 (10 μM) or high concentrations of bumetanide (50 μM) also induced a significant reduction in the duration of the interictal discharges to 1.06 ± 0.04 s and 0.76 ± 0.03 s, respectively, in PC and to 1.15 ± 0.04 s and 1.03 ± 0.02 s, respectively, in EC (Fig. 3B, p < 0.05). Low concentrations of bumetanide (10 μM; n = 6 slices) did not influence the interval of occurrence and duration of interictal discharges in PC and EC.
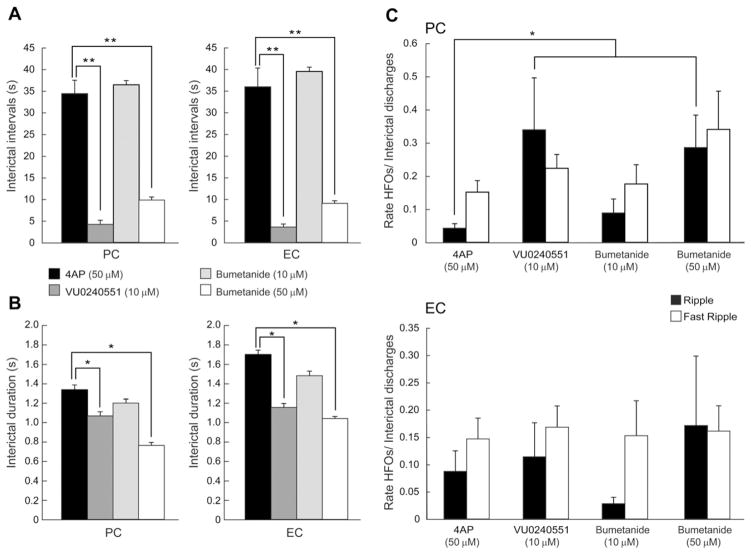
Fig. 3.
A and B: Bar graphs showing the average interval of occurrence (A) and duration (B) of interictal discharges recorded from PC and EC under control conditions (i.e., 4AP) and during application of 10 μM VU0240551, 10 μM bumetanide and 50 μM bumetanide. Note that blocking KCC2 decreases the interval of occurrence and the duration of interictal discharges in both regions whereas blocking NKCC1 with bumetanide (10 μM) does not have a significant effect on interval of occurrence and duration of interictal discharges. C: Bar graphs showing the proportion of interictal events, in PC and EC, co-occurring with ripples and fast ripples under control conditions (i.e., 4AP), and during application of 10 μM VU0240551, 10 μM bumetanide and 50 μM bumetanide. Note that application of VU0240551 or bumetanide (50 μM) induces a significant increase in the proportion of interictal spikes co-occurring with ripples in the PC. *p < 0.05 and **p < 0.0001.
We also analyzed the occurrence of HFOs in both the ripple (80–200 Hz) and fast ripple (250–500 Hz) frequency range that were associated to interictal discharges recorded from the PC and EC during 4AP (50 μM), VU0240551(10 μM) and bumetanide (10 μM or 50 μM). Application of VU0240051 (10 μM) induced a significant increase in the occurrence of ripples associated with interictal discharges in the PC (p < 0.05) whereas fast ripple occurrence did not change significantly (Fig. 3C, PC). In contrast, VU0240551 (10 μM) did not change the occurrence of ripples and fast ripples in the EC (Fig. 3C, EC). Following the application of a high concentration of bumetanide (50 μM), ripple occurrence significantly increased in PC (p < 0.05) whereas fast ripple occurrence did not change (Fig. 3C, PC). Also, application of bumetanide (50 μM) did not change the occurrence of ripples and fast ripples in EC (Fig. 3C, EC). Application of low doses of bumetanide did not influence the rate of occurrence of ripples and fast ripples in both PC and EC (Fig. 3C). These findings indicate that blocking KCC2 activity induces a significant increase in the proportion of ripples associated to interictal discharges in the PC but not in the EC.
Effects of KCC2 enhancer on 4AP-induced epileptiform activity
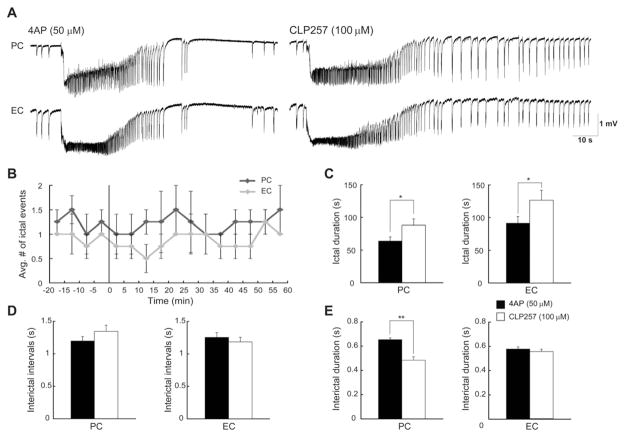
Next, we studied the effect of a KCC2 enhancer, CLP257 (100 μM; n = 5 slices) on the 4AP-induced epileptiform discharges recorded from PC and EC (Fig. 4A). As illustrated in Fig. 4B, we found that CLP257 (100 μM) did not affect the rate of occurrence of ictal events since the average number of ictal discharges over the time did not change after application of CLP257 (100 μM) in both PC and EC. Interestingly, CLP257 (100 μM) resulted in an increase in the duration of ictal events from 64.19 ± 6.10 s to 88.07 ± 9.46 s in PC, and from 91.51 ± 9.95 s to 126.54 ± 15.24 s in EC (Fig. 4C, p < 0.05). CLP257 (100 μM; n = 5 slices) did not influence the interval of occurrence of interictal discharges (Fig. 4D) in PC and EC but shortened the duration of interictal discharges significantly (p < 0.01) only in PC from 0.65 ± 0.01 s to 0.48 ± 0.02 s (Fig. 4E). This procedure did not induce any change in the rate of occurrence of ripples and fast ripples associated with ictal and interictal events.
Fig. 4.
A: Effects induced by 100 μM CLP257 on the epileptiform discharges recorded from the PC and EC during bath application of 4AP. B: Plot showing the average number of ictal discharges in PC and EC before and after the application of CLP257. Vertical line indicates the application of the drug. C: Bar graphs showing the duration of ictal discharges recorded from PC and EC under control conditions (i.e., 4AP) and during application of 100 μM CLP257. Note that enhancing KCC2 activity increases the duration of ictal discharges in both region without affecting their rate of occurrence. D and E: Bar graphs showing the average interval of occurrence (D) and duration (E) of interictal discharges recorded from PC and EC under control conditions and during application of CLP257. Note that CLP257 only decreases the duration of interictal discharges only in PC. *p < 0.05 and **p < 0.01.
KCC2 blockers and pharmacologically isolated synchronous events
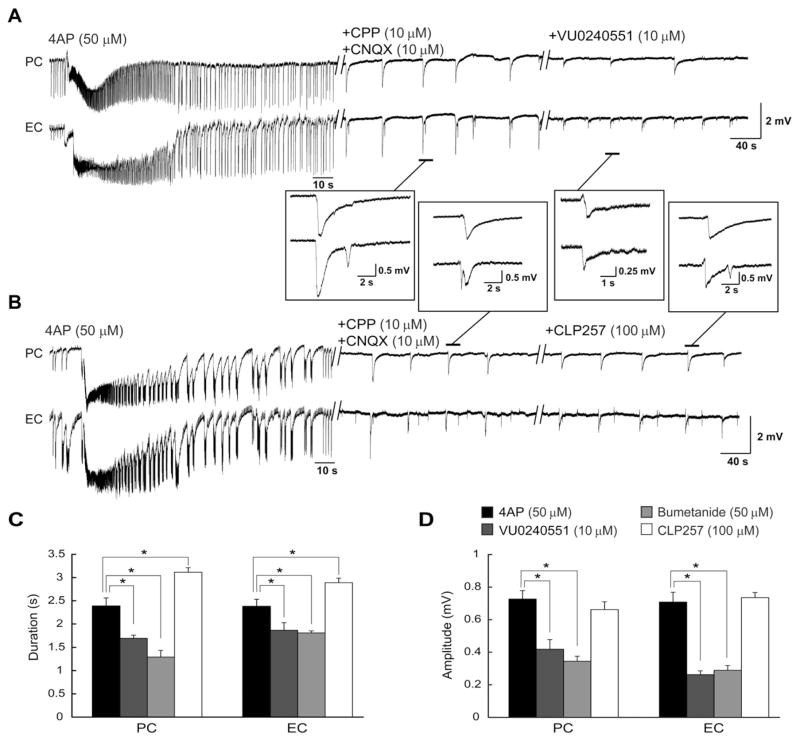
Finally, we pharmacologically blocked glutamatergic transmission with CPP (10 μM) and CNQX (10 μM) to analyze the effects of VU0240551, bumetanide (50 μM) and CLP257 on the 4AP-induced isolated GABAergic events that are recorded under these conditions (Hamidi et al., 2014). As shown in Figs. 5A and B, CPP (10 μM) and CNQX (10 μM) abolished ictal discharges revealing the recurrence of slow events that occurred in the both PC and EC at intervals of 41.2 ± 1.9 s and 38.7 ± 1.3 s with the duration of 2.38 ± 0.17 s and 2.37 ± 0.15 s and amplitude of 0.72 ± 0.05 mV and 0.70 ± 0.05 mV, respectively. HFOs were virtually abolished by CPP and CNQX application. Adding VU0240551 (10 μM, n = 5 slices) or bumetanide (50 μM, n = 5 slices) to medium containing 4AP, CPP, and CNQX decreased the duration of the isolated slow events significantly (p < 0.05) in both PC (to 1.69 ± 0.06 s and 1.28 ± 0.14 s, respectively) and EC (to 1.86 ± 0.16 s and 1.80 ± 0.04 s, respectively) (Fig. 5C). Interestingly, application of CLP257 (100 μM, n = 5 slices) induced an increase in the duration of these events in both PC and EC to 3.11 ± 0.09 s and 2.89 ± 0.09 s, respectively. Also, blocking KCC2 with VU0240551 (10 μM) or bumetanide (50 μM) decreased the amplitude of the isolated slow events significantly (p < 0.05) in both PC (to 0.41 ± 0.05 mV and 0.34 ± 0.03 mV, respectively) and EC (to 0.26 ± 0.02 mV and 0.28 ± 0.02 mV, respectively) while CLP257 did not have any significant effect on the amplitude of these events (Fig. 5D). Blocking or enhancing KCC2 failed to induce a change in the interval of occurrence of these slow field events.
Fig. 5.
A and B: Effects induced by VU0240551 (A) or CLP257 (B) on the slow field events recorded during concomitant application of 4AP, CPP (10 μM) and CNQX (10 μM). Insets show examples of the slow events at an expanded time scale. C and D: Bar graphs summarizing the change in duration (C) and amplitude (D) of the slow events under control conditions (i.e., 4AP + CPP + CNQX) and during further application of VU0240551 (10 μM), bumetanide (50 μM) and CLP257 (100 μM). Note that application of KCC2 blockers decreases the duration and amplitude of the slow events whereas KCC2 enhancer increases the duration of isolated GABAergic events. *p < 0.05.
Discussion
The main findings of our study can be summarized as follows. First, blocking KCC2 activity with either VU0240551 or bumetanide (50 μM) abolished the occurrence of ictal discharges in both PC and EC. Second, application of KCC2 blocker increased the rate of occurrence of interictal discharges and decreased their duration in both PC and EC, and increased the occurrence of ripples associated to interictal discharges in the PC. Third, enhancing KCC2 activity with CLP257 increased the duration of ictal discharges in both PC and EC and decreased the duration of interictal discharges only in PC. Finally, blocking KCC2 activity induced a decrease in the duration of pharmacologically isolated synchronous GABAergic events whereas enhancing KCC2 activity had a completely opposite effect on the duration of these events.
Modulation of epileptiform activity by blocking KCC2 activity
GABAA receptor activation sustains neuronal depolarizations that occur during epileptiform discharges in the 4AP model (Avoli and de Curtis, 2011). It has also been shown in the human subiculum that GABAA receptor-mediated depolarizations are associated to decreased KCC2 activity (Huberfeld et al., 2007). Therefore, we expected that blocking KCC2 activity would shift the reversal potential of GABAA receptor-mediated currents to less negative values thus increasing both ictal and interictal epileptiform activity. Contrary to our expectations, we found that ictal activity was abolished in both PC and EC during bath application of VU0240551 or bumetanide (50 μM). It has been shown that excessive activation of GABAA receptors leads to accumulation of Cl− inside the postsynaptic cells and to a subsequent increase in KCC2 activity that causes K+ and Cl− efflux (Viitanen et al., 2010). In line with this evidence we have previously shown that epileptiform and in particular ictal discharges induced by 4AP are accompanied by elevations in extracellular K+ (reviewed in Avoli and de Curtis, 2011). Hence, we are inclined to interpret the ability of both VU0240551 or high doses of bumetanide to abolish ictal activity as the result of the inability of GABAA receptor activation to cause transient, KCC2 dependent increases in extracellular K+.
We also found that blocking KCC2 activity with either VU0240551 or bumetanide (50 μM) induced an increase in the rate of occurrence of interictal discharges while decreasing their duration. Blocking KCC2 activity may change Cl− homeostasis that may contribute to generation of interictal events by affecting GABAA receptor-mediated signaling. In fact, Huberfeld et al. (2007) have shown that expression of KCC2 and Cl− homeostasis is perturbed in 20% of subicular pyramidal cell tissue from epileptic patients. In these 20% subicular pyramidal neurons, GABAA-mediated IPSPS reversed at depolarizing potentials and contributed to the generation of interictal events (Huberfeld et al., 2007).
We have discovered that blocking KCC2 activity with either VU0240551 or high doses of bumetanide induces an increase in the occurrence of ripples during interictal discharges. This is presumably due to the fact that blocking KCC2 may weaken inhibition by changing [Cl−]i thus increasing the excitability of interneurons (Viitanen et al., 2010). Interneuron activity has been indeed proposed to contribute to the generation of ripples (Buzsáki et al., 2007; Ylinen et al., 1995).
A recent, short study has identified that VU0240551, in addition to its inhibitory action on KCC2 (Delpire et al., 2009), might also interfere with G protein-coupled receptors (GPCRs) and ion channels (Delpire et al., 2012). We are however inclined to interpret the effects of this compound identified in our present study as resulting from a decrease in KCC2 activity since similar findings could be obtained with high concentrations of bumetanide (50 μM) that are known to block both KCC2 and NKCC1 (Löscher et al., 2013). Interestingly, low doses of bumetanide (10 μM), which only block NKCC1 activity, did not influence the patterns of epileptiform discharges recorded from the PC and EC; these negative findings are indeed in line with the view that NKCC1 is down regulated in the adult brain (Kahle and Staley, 2008; Wahab et al., 2011). Also one study by Wahab et al. (2011) has shown that the effects induced by low doses of bumetanide are age dependent since 10 μM bumetanide can block epileptiform activity induced by 4AP in the immature but not in the adult EC region.
Enhancing KCC2 activity modulates epileptiform activity
Epilepsy has often been linked to inefficient GABAergic signaling derived from downregulation of KCC2 and elevated internal [Cl−] (Huberfeld et al., 2007; Payne et al., 2003; Rivera et al., 2004). Expression and function of KCC2 are tightly regulated by neuronal activity. Suppression of KCC2 during enhanced excitation is thought to alter the balance of excitation and inhibition that in turn may lead to anomalous activity such as seizures (Huberfeld et al., 2007; Rivera et al., 2002, 2004). Surprisingly, we have found that enhancing KCC2 activity with CLP257 (Gagnon et al., 2013) increased the duration of ictal events without affecting their interval of occurrence. These results suggest that downregulation of KCC2 might not be the cause of seizures in epilepsy but rather an adaptive mechanism to prevent the increase in extracellular [K+] due to the excessive activation of GABAA receptors and KCC2 activity.
Modulation of isolated GABAergic events by KCC2 blockers and enhancer
We have confirmed here that ionotropic glutamatergic receptor-mediated signaling is required for the generation of ictal discharges in both PC and EC (Avoli et al., 2013; Hamidi et al, 2014; Herrington et al., 2014). In keeping with evidence obtained in these previous studies, we have found that blockade of glutamatergic signaling using NMDA and non-NMDA receptor antagonists led to the occurrence of isolated, slow GABA receptor-dependent potentials during application of 4AP. These glutamatergic independent field potentials result from the synchronous firing of interneurons that leads to GABA release and consequent postsynaptic activation of principal cells (Avoli and de Curtis, 2011).
We have found here that blocking KCC2 activity shortened the duration of these synchronous events, and reduced their amplitude in both PC and EC; in addition, enhancing KCC2 activity led to an increase in the duration of these events in both regions. These findings, therefore, demonstrate that GABAA receptor-dependent hyperpolarization, which is reduced and perhaps blocked by VU0240551 or high doses of bumetanide, contributes the generation of the synchronous GABAergic events induced by 4AP. Indeed, these field events are blocked by GABAA receptor antagonists and correspond to transient depolarizations of principal cells in several limbic structures (see for review Avoli and de Curtis, 2011). Blocking KCC2 activity should increase intra-cellular [Cl−] but Viitanen et al. (2010) have reported that this change does not influence GABAA receptor-mediated hyperpolarizations and slightly shifts their reversal potential which remains more negative than the resting membrane potential. The lack of meaningful effects on GABAA receptor-mediated hyperpolarizations could be due to the contribution of the Na+ dependent anion exchanger (NDAE), which extrudes Cl− and H+ and loads and Na+ (Payne et al., 2003; Hübner and Holthoff, 2013). Activation of NDAE can promote the inhibitory action of GABA by maintaining Cl− reversal potential negative to the resting membrane potential (Hübner and Holthoff, 2013). Although GABAA receptor-mediated signaling remains hyperpolarizing, KCC2 blockers may influence the duration of the pharmacologically isolated GABAergic events by slightly shifting the EGABA and altering the ability of postsynaptic neurons to accumulate Cl−. In addition, expression and function of KCC2 are tightly regulated by neuronal activity in a way that enhanced excitation results in suppression of KCC2 activity (Huberfeld et al., 2007; Rivera et al., 2002, 2004). Indeed, restoring KCC2 activity by application of CLP257 increased the duration of isolated GABAergic events (Gagnon et al., 2013).
Conclusions
In conclusion, our results show that reducing or blocking KCC2 activity abolishes the occurrence of 4AP-induced ictal discharges that are known to be contributed by the activation of GABAA receptor and to be associated to transient elevations in extracellular K+. These findings were unexpected since increasing the intracellular concentration of Cl− should cause a shift of the reversal potential of inhibitory currents in the positive direction and thus an enhancement of GABAA receptor dependent depolarizations which should reinforce ictal synchronization. However, our findings can be explained by taking into consideration the fact that GABAA receptor activation leads to increases in extracellular K+ that depend on KCC2 activity.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (CIHR grants 8109 and 74609). We thank Dr. M. Levesque, Ms. R. Herrington and Ms. P. Salami for helping with the recording procedures and data analysis. We also thank Dr. Yves De Koninck for generously providing us with CLP257.
Footnotes
Available online on ScienceDirect (www.sciencedirect.com).
Conflicts of interest
None of the authors has any conflict of interest to disclose.
References
- Avoli M, de Curtis M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol. 2011;95:104–132. doi: 10.1016/j.pneurobio.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, Panuccio G, Herrington R, D’Antuono M, de Guzman P, Lévesque M. Two different interictal spike patterns anticipate ictal activity in vitro. Neurobiol Dis. 2013;52:168–176. doi: 10.1016/j.nbd.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénar CG, Chauvière L, Bartolomei F, Wendling F. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on “false” ripples. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2010;121:301–310. doi: 10.1016/j.clinph.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Holmes GL. The multiple facets of gamma-aminobutyric acid dysfunction in epilepsy. Curr Opin Neurol. 2005;8:141–145. doi: 10.1097/01.wco.0000162855.75391.6a. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012;18:467–486. doi: 10.1177/1073858412438697. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Kaila K, Raichle M. Inhibition and brain work. Neuron. 2007;56:771–783. doi: 10.1016/j.neuron.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpire E, Days E, Lewis LM, Mi D, Kim K, Lindsley CW, Weaver CD. Small-molecule screen identifies inhibitors of the neuronal K–Cl cotransporter KCC2. Proc Natl Acad Sci U S A. 2009;106:5383–5388. doi: 10.1073/pnas.0812756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpire E, Baranczak A, Waterson AG, Kim K, Kett N, Morrison RD, Daniels JS, Weaver CD, Lindsley CW. Further optimization of the K–Cl cotransporter KCC2 antagonist ML077: development of a highly selective and more potent in vitro probe. Bioorg Med Chem Lett. 2012;22:4532–4535. doi: 10.1016/j.bmcl.2012.05.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon M, Bergeron MJ, Lavertu G, Castonguay A, Tripathy S, Bonin RP, Perez-Sanchez J, Boudreau D, Wang B, Dumas L, Valade I, Bachand K, Jacob-Wagner M, Tardif C, Kianicka I, Isenring P, Attardo G, Coull JA, De Koninck Y. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med. 2013;11:1524–1528. doi: 10.1038/nm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi S, Lévesque M, Avoli M. Epileptiform synchronization and high-frequency oscillations in brain slices comprising piriform and entorhinal cortices. Neuroscience. 2014;281:258–268. doi: 10.1016/j.neuroscience.2014.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington R, Lévesque M, Avoli M. Neurosteroids modulate epileptiform activity and associated high-frequency oscillations in the piriform cortex. Neuroscience. 2014;256:467–477. doi: 10.1016/j.neuroscience.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner CA, Holthoff K. Anion transport and GABA signaling. Front Cell Neurosci. 2013;7:177. doi: 10.3389/fncel.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JGR, Jiruska P, de Curtis M, Avoli M. Limbic network synchronization and temporal lobe epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet] 4. National Center for Biotechnology Information (US); Bethesda (MD): 2012. [PubMed] [Google Scholar]
- Johnston D, Brown TH. Giant synaptic potential hypothesis for epileptiform activity. Science. 1981;16:294–297. doi: 10.1126/science.7444469. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Staley KJ. The bumetanide-sensitive Na-K-2Cl cotransporter NKCC1 as a potential target of a novel mechanism-based treatment strategy for neonatal seizures. Neurosurg Focus. 2008;25:E22. doi: 10.3171/FOC/2008/25/9/E22. [DOI] [PubMed] [Google Scholar]
- Lillis KP, Kramer MA, Mertz J, Staley KJ, White JA. Pyramidal cells accumulate chloride at seizure onset. Neurobiol Dis. 2012;47:358–366. doi: 10.1016/j.nbd.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W, Puskarjov M, Kaila K. Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology. 2013;69:62–74. doi: 10.1016/j.neuropharm.2012.05.045. [DOI] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Rinehart J, Vázquez N, Kahle KT, Hodson CA, Ring AM, Gulcicek EE, Louvi A, Bobadilla NA, Gamba G, Lifton RP. WNK2 kinase is a novel regulator of essential neuronal cation-chloride cotransporters. J Biol Chem. 2011;286:30171–30180. doi: 10.1074/jbc.M111.222893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, Saarma M. BDNF-induced TrkB activation down-regulates the K+–Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J Cell Biol. 2002;159:747–752. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipila S, Payne JA, Minichiello L, Saarma M, Kaila K. Mechanism of activity-dependent downregulation of the neuron-specific K–Cl cotransporter KCC2. J Neurosci. 2004;24:4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami P, Lévesque M, Gotman J, Avoli M. A comparison between automated detection methods of high-frequency oscillations (80–500 Hz) during seizures. J Neurosci Methods. 2012;211:265–271. doi: 10.1016/j.jneumeth.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin PA, Prince DA. Cellular and field potential properties of epileptogenic hippocampal slices. Brain Res. 1978;147:117–130. doi: 10.1016/0006-8993(78)90776-x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Prince DA. Changes in excitatory and inhibitory synaptic potentials leading to epileptogenic activity. Brain Res. 1980;183:61–73. doi: 10.1016/0006-8993(80)90119-5. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- Traub RD, Borck C, Colling SB, Jefferys JG. On the structure of ictal events in vitro. Epilepsia. 1996;37:879–891. doi: 10.1111/j.1528-1157.1996.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Uusisaari M, Smirnov S, Voipio J, Kaila K. Spontaneous epileptiform activity mediated by GABA(A) receptors and gap junctions in the rat hippocampal slice following long-term exposure to GABA(B) antagonists. Neuropharmacology. 2002;43:563–572. doi: 10.1016/s0028-3908(02)00156-9. [DOI] [PubMed] [Google Scholar]
- Viitanen T, Ruusuvuori E, Kaila K, Voipio J. The K+–Cl cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J Physiol. 2010;588:1527–1540. doi: 10.1113/jphysiol.2009.181826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab A, Albus K, Heinemann U. Age- and region-specific effects of anticonvulsants and bumetanide on 4-aminopyridine-induced seizure-like events in immature rat hippocampal–entorhinal cortex slices. Epilepsia. 2011;52:94–103. doi: 10.1111/j.1528-1167.2010.02722.x. [DOI] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nádasdy Z, Jandó G, Szabó I, Sik A, Buzsáki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]