Abstract
Purpose
Mesial temporal lobe epilepsy (MTLE) is the most prevalent type of partial epileptic disorders. In this study, we have analyzed the impact of levetiracetam (LEV) in the pilocarpine model of MTLE.
Methods
Sprague-Dawley rats (n = 19) were injected with pilocarpine (380 mg/kg, i.p.) to induce a status epilepticus. Twelve animals were used as controls and seven were treated with LEV. They were implanted with bipolar electrodes in the CA3 subfield of the hippocampus, entorhinal cortex (EC), dentate gyrus (DG) and subiculum and EEG-video monitored continuously from day 4 to day 14 after SE.
Results
Only 29% of LEV-treated animals had seizures compared to all controls following a latent period that was similar in duration. Seizure rates were lower in LEV-treated animals. In LEV-treated animals without seizures, lower interictal spike rates were found in all regions compared to controls. Analysis of interictal high-frequency oscillations (HFOs) revealed that LEV-treated animals without seizures had lower rates of interictal spikes with ripples (80–200 Hz) in CA3, EC and subiculum (p < 0.01), whereas rates of interictal spikes with fast ripples (250–500 Hz) were significantly lower in CA3 and subiculum, compared to controls.
Conclusion
Our findings indicate that the anti-ictogenic properties of LEV are mirrored by decreases of interictal spike rate in temporal lobe regions, and are accompanied by subregion-specific decreases of HFO occurrence in CA3 and subiculum. Overall, this evidence suggest that LEV may inhibit neural network activity in regions that are known to play important roles in MTLE.
Keywords: Levetiracetam, Pilocarpine, Seizures, Interictal spikes, High-frequency oscillations
1. Introduction
Levetiracetam (LEV; [(S)-α-ethyl-2-oxo-1-pyrrolidine acetamide]) is a second generation anti-epileptic drug (AED) that is widely used in patients with either generalized or partial epileptic disorders.1 LEV also has anti-ictogenic effects in animal models of epilepsy such as in the amygdala kindling model,2 audiogenic kindling,3 spontaneously epileptic rats4 and the kainic acid model.5 Unlike several other AEDs,6 LEV does not block voltage-dependent Na+ but may decrease glutamatergic excitatory transmission,7 thus preventing excessive neuronal synchronization.8 Although the exact mechanisms through which LEV controls seizures remain unclear, its anticonvulsive properties are thought to rely on its ability to modulate neurotransmission by binding to SV2A, a synaptic vesicle glycoprotein that interacts with synaptotagmin, a pre-synaptic Ca2+ sensor involved in regulating synaptic vesicle exocytosis.9
MTLE is the most prevalent type of partial epileptic disorders, with symptoms consisting of partial seizures that originate from the hippocampus, entorhinal cortex or amygdala many years after an initial brain insult such as status epilepticus (SE), encephalitis or febrile convulsions.10 MTLE is also one of the most refractory forms of epilepsy with approximately one third of patients being unresponsive to medication.11 In these patients, the surgical removal of the seizure onset zone often represents the only therapeutic alternative, which may sometimes necessitate the use of invasive intracranial EEG recordings to properly delineate the seizure onset zone.12
Recently, high-frequency oscillations (HFOs, ripples: 80–200 Hz, fast ripples: 250–500 Hz) have been recorded in the EEG of epileptic patients and in animal models of epilepsy.13 They were shown to occur in association with spikes or independently, and they are thought to reflect the activity of dysfunctional neural networks, since the surgical removal of regions with HFOs is associated to good post-surgical outcomes.14–18 Animal studies have also shown that HFOs may be linked to epileptogenesis and ictogenesis.8,19,20 The relation between seizures, interictal spikes, HFOs and the effect of anti-epileptic drugs is however still unclear. A study performed in epileptic patients has shown that HFOs may increase after medication reduction.21 However, these studies were carried out in patients with “well-established” epileptic conditions, and were limited by the variability of AEDs. We analyzed here the effects of LEV on seizures, interictal spikes and HFOs in the pilocarpine model of MTLE, and focused on the 4–14 day period that follows the initial pilocarpine-induced SE.
2. Methods
2.1. Ethical approval
All procedures were approved by the Canadian Council of Animal Care and all efforts were made to minimize the number of animals and their suffering.
2.2. Animal preparation
Male Sprague-Dawley rats (250–300 g) were obtained from Charles River (St-Constant, Qc, Canada) and let habituate for 72 h after delivery before pilocarpine treatment. They were housed in controlled conditions, at 22 (±2) °C and under a 12 h light/12 h dark cycle (lights on from 7:00 AM to 7:00 PM) with food and water ad libitum.
2.3. Pilocarpine treatment
Animals were injected with scopolamine methylnitrate (1 mg/kg i.p.; Sigma–Aldrich, Canada) and 30 min later with a single dose of pilocarpine hydrochloride (380 mg/kg, i.p.; Sigma–Aldrich, Canada).8 Their behaviour was scored according to the Racine scale22 and SE was defined as continuous stage 5 seizures. Status epilepticus (SE) was terminated after 1 h by an injection of diazepam (5 mg/kg, s.c.; CDMV, Canada) and ketamine (50 mg/kg, s.c.; CDMV, Canada).8
2.4. Treatment with levetiracetam
Approximately 4 h after SE termination, seven animals were anesthetized with isoflurane (2%) in 100% O2 and an osmotic pump (2 ml, flow rate: 5 μl/h) was installed subcutaneously (2ML2 ALZET osmotic mini-pumps, DURECT Corporation, Cupertino, CA, USA) in order to deliver LEV dissolved in saline at 300 mg/kg/day.23 These pumps deliver a continuous dosing over 2 weeks, circumventing the need for repetitive invasive blood sampling. Osmotic pumps filled with saline were installed subcutaneously in controls.
2.5. Surgery for the implantation of electrodes
All animals underwent surgery for the implantation of electrodes three days after pilocarpine treatment. Before surgery, animals received topical Lidocain (5%; Odan, Canada). An incision was then made in the skin to expose the skull plate, from bregma to lambda. Four stainless steel screws (2.4 mm length) were fixed to the skull and four small holes were drilled to allow the implantation of bipolar electrodes (20–30 kΩ; 5–10 mm length; distance between exposed tips: 500 μm) (MS303/2-B/spc, Plastics One, VA, USA). Electrodes were implanted in the CA3 subfield of the ventral hippocampus (AP: −4.4, ML: 4, DV: 7.8), medial entorhinal cortex (AP: −8.6, ML: 5.2, DV: 6.8), ventral subiculum (AP: −6.8, ML: 4, DV: 6) and dentate gyrus (AP: −4.4, ML: 2.4, DV: 3.4). Recordings were performed in the right CA3 and EC and in the left DG and subiculum. Screws and electrode pins were connected with a pin connector and fastened to the skull with dental cement. A fifth bipolar electrode was placed under the frontal bone, after the removal of insulating material, and used as reference. During surgery, animals received a preventive antibiotherapy (Enrofloxacine, 10 mg/kg, s.c.). After surgery, animals were injected with Ketoprofen (5 mg/kg, s.c. Merail, Canada), Buprenorphine (0.01–0.05 mg/kg, s.c., CDMV, Canada) and 2 ml of 0.9% sterile saline (s.c.).
2.6. EEG recordings
The pin connector was connected to a multichannel cable and electrical swivel (Slip ring T13EEG, Air Precision, France; or Commutator SL 18C, HRS Scientific, Canada) and EEG-video monitoring (24 h/day) was performed. EEGs were amplified via an interface kit (Mobile 36ch LTM ProAmp, Stellate, Montreal, QC, Canada), low-pass filtered at 500 Hz and sampled at 2 kHz per channel. Infrared cameras were used to record day/night video files that were time-stamped for integration with the electrophysiological data using monitoring software (Harmonie, Stellate, Montreal, QC, Canada). Continuous 24/7 EEG-video recordings were performed from day 4 to day 14 after SE.
2.7. Spontaneous seizures and onset patterns
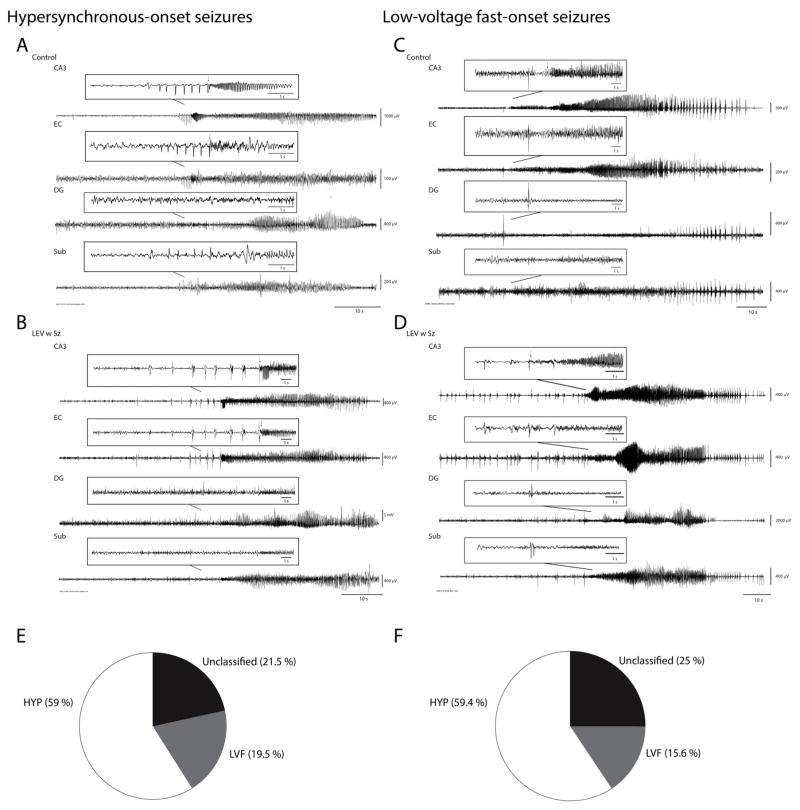
All EEG and video recordings were reviewed manually in order to detect seizures. The time corresponding to the onset and end of each seizure was recorded and the behaviour of the animal was graded on the Racine scale.22 Seizures were also classified in two groups according to their onset pattern. Low-voltage fast-onset seizures (LVF) were characterized by the occurrence of a positive-or negative-going spike that was followed by the appearance of low amplitude high frequency activity. Hypersynchronous-onset seizures (HYP) were characterized at onset by a pattern of focal periodic spiking at a frequency of approximately 2 Hz. Seizures for which a seizure onset pattern could not be identified were named “unclassified”. The duration of seizures was based on the first occurrence of activity in the 5–20 Hz range until the return to baseline activity and normal behaviour. The duration of seizures was calculated from the onset of activity in the 5–20 Hz range (see arrows in Figs. 2 and 3) until the return to baseline EEG activity.
Fig. 2.
(A and B) Representative HYP-onset seizures in a control (A) and in a LEV w Sz animal (B). Note the occurrence of multiple spikes preceding the onset of fast activity (arrow) in CA3, EC and subiculum in the control animal and in CA3 and EC in the LEV w Sz animal. Seizure duration was calculated from the onset of fast activity (15–20 Hz) (arrow) to the end of the seizure. (C and D) Representative LVF-onset seizures in a control (C) and in a LEV w Sz animal (D). Note the occurrence of a single pre-ictal spike preceding the onset of fast activity (arrow). (E and F) Pie chart showing the distribution of seizure-onset patterns in controls (E) and LEV w Sz animals (F). Both groups showed a similar distribution of seizure-onset patterns. CA3, CA3 region of the hippocampus; EC, entorhinal cortex; DG, dentate gyrus; Sub, subiculum.
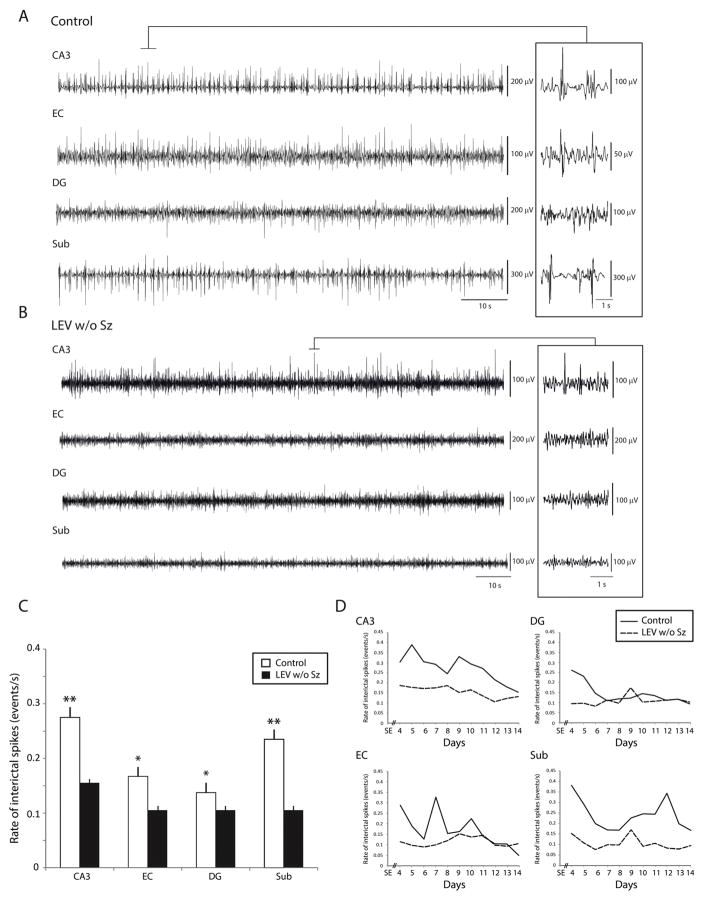
Fig. 3.
(A) Representative recordings showing interictal spikes in a control (A) and in a LEV w/o Sz animal (B). Interictal spikes could occur in any region of the temporal lobe, but they occurred at significantly lower rates in LEV w/o Sz animals. Insets show interictal spikes on an expanded time scale. (C) Bar graph showing the average rates (number of events/s) of interictal spikes in the control group and in the LEV w/o Sz group. Note that in all regions interictal spike rates were lower in LEV w/o Sz animals compared to controls. (D) Average interictal spike rates in all regions over time. Note the difference in interictal spike rate between controls and LEV w/o Sz animals over time (*p < 0.05, **p < 0.01). IIS, interictal spikes.
The occurrence of a spontaneous seizure (non-convulsive or convulsive) after SE marked the end of the latent period. Based on the presence or absence of spontaneous seizures between the 4th and the 14th day after SE, LEV-treated animals were divided in two groups, those with seizures (LEV w Sz) and those without seizures (LEV w/o Sz).
2.8. Analysis of interictal spikes and high-frequency oscillations
To reduce the potential variability in interictal spike occurrence due to seizures in the LEV-treated group, we excluded LEV w Sz animals from the analysis of interictal spikes and HFOs. For each control and LEV w/o Sz animal, two epochs of 10 min were selected for each day in order to analyze interictal spikes and HFOs. In order to reduce a possible bias on seizures and interictal activities caused by the light/dark cycle, we selected from each recording day one epoch during the light period (from 7 AM to 7 PM) and a second epoch during the dark period (from 7 PM to 7 AM). Only epochs of non-REM sleep were used for analysis, because of the low rate of movement artefacts and because HFOs are more prominent during this sleep stage.24 Non-REM sleep epochs were selected based on the occurrence of EEG activity in the 1–6 Hz range. In order to minimize the effects of seizure occurrence during the chronic phase, epochs were selected at least 1 h before or after seizures.
Interictal spikes were detected based on threshold crossings (mean and standard deviation (SD)), calculated over the entire period for the 10-min epoch. Events above 4 SD were considered as interictal spikes and movement artefacts were removed from the analysis. The rate of interictal spikes (number of events/s) was then calculated for each region (CA3, EC, DG and subiculum) and results obtained from the two epochs (dark and light period) were averaged in order to obtain a single value per day per animal.
The same epochs used to detect interictal spikes were used for the analysis of HFOs. The algorithm used to detect these events was previously published25 and will be briefly described here. The algorithm identified oscillations in each frequency range (ripples: 80–200 Hz, fast ripples: 250–500 Hz), using routines based on standardized functions (Matlab Signal Processing Toolbox, Matlab R2013a (Mathworks)). Raw EEG recordings were first bandpass filtered in the 80–200 Hz and in the 250–500 Hz frequency range. Filtered EEGs from each region were then normalized using their own average root mean square value calculated over the 10-min epoch. To be considered as an HFO candidate, oscillatory events in each frequency band had to show at least four consecutive cycles having amplitude of 3 SD above the mean. HFOs were considered as co-occurring with a spike if they occurred within a time window of ±300 ms from the peak of an interictal spike. Ripples were kept for analysis only if they were detected in the 80–200 Hz range, whereas fast ripples were kept if they were detected only in the 250–500 Hz range. Rates of HFOs were calculated for each day by averaging values obtained from the light and dark time-period.
2.9. Statistical analysis
Results are expressed as mean (±standard error of mean (SEM)). Statistical analyses were performed in Matlab R2013a. Non-parametric Kruskal–Wallis and Wilcoxon signed-rank tests were used to compare values obtained in each group, since values were not normally distributed according to the Kolmogorov–Smirnov test. Fisher’s exact tests were also used for the analysis of percentages in contingency tables. The level of significance was set at p < 0.05.
3. Results
3.1. Seizure occurrence and latent period
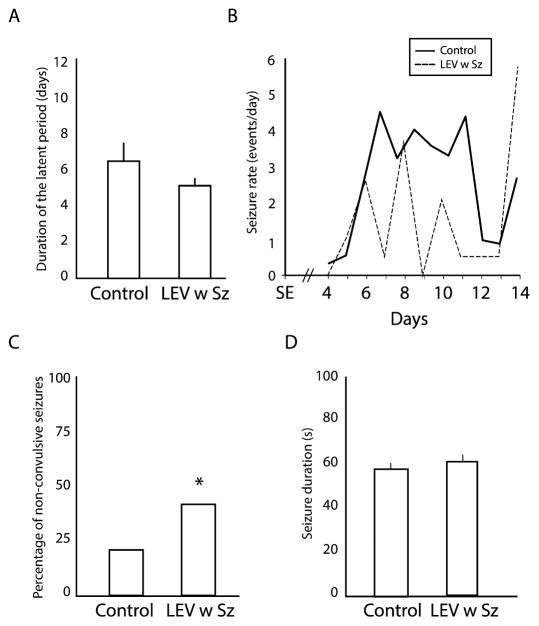
Seizures were recorded in both controls and LEV-treated animals. However, all controls (n = 12) showed seizures (n = 339 seizures) compared to only 29% (2/7) of LEV-treated animals (n = 32 seizures), after a latent period of similar duration (Fig. 1A). In both groups, seizures tended to occur in clusters, with a first cluster between day 6 and day 11 after SE (Fig. 1B). However, when analysing seizure rates from day 4 to day 14 after SE, they were significantly lower in the LEV w Sz group compared to the control group (control = 2.8 (±0.4) seizures per day, LEV w Sz = 1.5 (±0.5), p < 0.05). When comparing the proportions of convulsive and non-convulsive seizures in each group, LEV w Sz animals were more likely to show non-convulsive seizures compared to controls (p < 0.05, Fisher’s exact test) (Fig. 1C). Finally, the duration of seizures was not significantly different between the two groups (Fig. 1D).
Fig. 1.
(A) Bar graph showing the average duration of the latent period in each group. No significant differences were observed. (B) Average seizure rate per day for each group. Note that in each group seizures tend to occur in a cluster, between the 6th and the 11th days after SE. (C) Bar graph showing the percentage, in each group, of non-convulsive seizures (*p < 0.05). LEV w Sz animals showed significantly more non-convulsive seizures compared to controls. (D) Bar graph showing the average duration of seizures in each group. No significant differences were observed.
3.2. Seizure onset patterns
HYP and LVF seizures were observed in both groups (Fig. 2A–D). In control animals, HYP seizures occurred more frequently compared to LVF seizures and unclassified seizures (Fig. 2E). A similar distribution of seizure onset patterns was observed in the LEV w Sz group (Fig. 2F).
3.3. Interictal spikes
We compared LEV w/o Sz animals (n = 4) to control animals (n = 4) in order to know if the anti-ictogenic effect of LEV was mirrored by differences in interictal spike and HFO occurrence. A total of 15 h of interictal activity was analyzed for each group (two 10-min epochs in each animal from every day of recording between day 4 and day 14 after SE). This analysis was performed over 30,500 interictal spikes in the control group and 19,606 interictal spikes in the LEV-treated group.
Interictal spikes were recorded in all controls and LEV w/o Sz animals and could occur in any region of the temporal lobe (Fig. 3A and B). Analysis of interictal spike rates revealed that in all regions, LEV w/o Sz animals had significantly lower values than in controls (p < 0.05 and p < 0.01) (Fig. 3C). Fig. 3D shows the evolution of interictal spike rates overtime. InCA3 and subiculum, the low rates of interictal spikes in LEV w/o Sz animals were maintained over time.
3.4. Ripples (80–200 Hz)
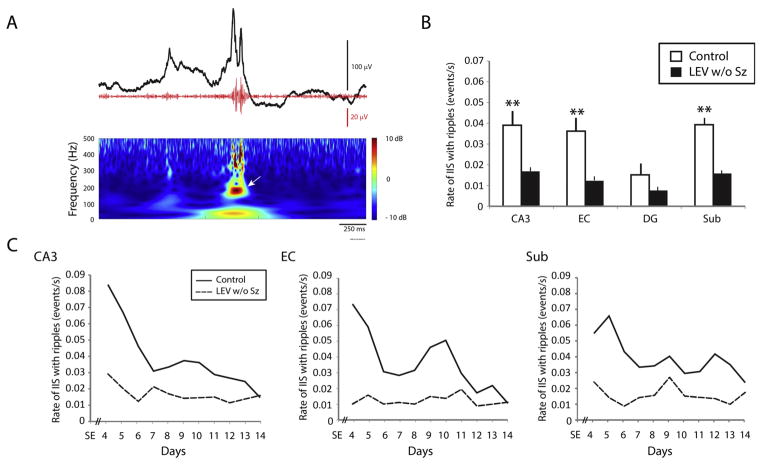
As previously reported in epileptic patients and animal models,19,26,27 interictal spikes could co-occur with HFOs. All controls and all LEV w/o Sz animals showed interictal spikes with ripples (Fig. 4A), on every day of recording after SE. However, in LEV w/o Sz animals, interictal spikes with ripples occurred at significantly lower rates compared to controls. This difference reached significance in CA3, EC and subiculum (p < 0.01) (Fig. 4B). Fig. 4C shows rates of interictal spikes with ripples over time. From day 4 to day 14 after SE, LEV w/o Sz animals showed lower rates of interictal spikes with ripples compared to controls in CA3, EC and subiculum.
Fig. 4.
(A) Representative example of an interictal spike (black line) co-occurring with a ripple (red line). The time frequency representation of the activity in the ripple frequency range (80–200 Hz) is also shown. The arrow points to the detected ripple. (B) Bar graph showing the average rate of interictal spikes with ripples in each region for the control and the LEV w/o Sz group. In CA3, EC and subiculum, significantly lower rates of interictal spike rates with ripples were recorded (**p < 0.01). (C) Average interictal spike rates over time. Note the difference between controls and LEV w/o Sz animals over time in CA3, EC and subiculum. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Fast ripples (250–500 Hz)
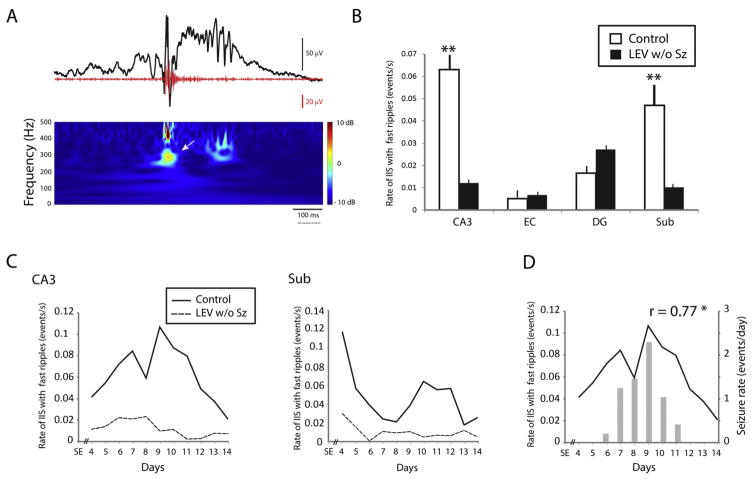
Interictal spikes with fast ripples were recorded from controls and LEV w/o Sz animals as well (Fig. 5A). As for ripples, interictal spikes with fast ripples occurred at significantly lower rates in LEV w/o Sz animals compared to controls, and this difference reached significance in CA3 and subiculum (p < 0.01) (Fig. 5B). Fig. 5C shows the average occurrence over time of interictal spikes with fast ripples in both groups. As it was observed for ripples, the low rates of interictal spikes with fast ripples in LEV w/o Sz animals were maintained over time.
Fig. 5.
(A) Representative example of an interictal spike (black line) co-occurring with a fast ripple (red line). The fast ripple is indicated by the arrow in the time frequency representation. (B) Bar graph showing the average rate of interictal spikes with fast ripples in each region for the control and the LEV w/o Sz group. In CA3 and subiculum, significantly lower rates of interictal spike rates with fast ripples were recorded (**p < 0.01). (C) Average rates of interictal spikes with fast ripples over time in CA3 and subiculum. Note the difference between controls and LEV w/o Sz animals over time. (D) Average rates of seizures (bar graph) and of interictal spikes with fast ripples (solid line) in CA3 in controls. Rates of interictal spikes with fast ripples in CA3 were significantly correlated with seizure rates (*p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Next, we wanted to confirm the hypothesis that interictal spikes with fast ripples in CA3 were the best predictor of seizure occurrence, as previously reported by Lévesque et al.19 We thus performed a linear regression analysis between rates of seizures and rates of interictal spikes with fast ripples in all regions. As previously shown,19 interictal spikes with fast ripples in CA3 were the best predictors of seizure occurrence in controls (p < 0.05) (Fig. 5D). This relation was not observed in LEV w Sz animals.
4. Discussion
The main findings of our study can be summarized as follows. First, from the 4th to the 14th day after a pilocarpine-induced SE, only 29% of LEV-treated animals showed seizures compared to all controls, after latent periods of similar durations. Second, LEV w Sz animals were more likely to have non-convulsive seizures compared to controls. Third, interictal spikes occurred in all regions, but at lower rates in LEV w/o Sz animals compared to controls. Fourth, LEV w/o Sz animals showed significantly lower rates of occurrence of interictal spikes with ripples in CA3, EC and subiculum, whereas rates of interictal spikes with fast ripples were significantly lower in CA3 and subiculum, compared to controls. Finally, in controls but not in LEV w Sz animals, rates of interictal spikes with fast ripples in CA3 predicted seizure occurrence.
4.1. Effects of levetiracetam on seizures
We have found that LEV has effective anti-ictogenic properties in the pilocarpine model of MTLE. This is in line with previous in vivo studies using either pilocarpine28 or kainic acid5 and it is consistent with the seizure-suppressing effect of LEV observed in epileptic patients.29 We cannot however exclude the fact that LEV w/o Sz animals may also develop seizures after 14 days post-SE, indicating that LEV may only delay the occurrence of the first spontaneous seizure. Future studies should be performed with long-term recordings under LEV since our study mainly focused on the early chronic period.
We have also found that the severity of seizures in LEV w Sz animals was significantly lower compared to controls. This is in line with what was reported by Van Vliet et al,30 who discovered that following an electrically induced SE, the severity of spontaneous seizures was reduced by LEV treatment. In addition, Zheng et al31 have found an attenuation of behavioural symptoms when LEV was administered intravenously 30 min after the onset of pilocarpine-induced acute seizures. The mechanisms through which LEV decreases the behavioural counterpart of seizures remain undefined but it has been proposed that it depends on the ability of LEV to reduce neuronal synchronization between the hippocampus and cortex.32
We have observed however that seizures had similar durations in the control and in the LEV w Sz group. Few studies have investigated the effect of LEV on the duration of spontaneous seizures and only contradictory results were obtained so far. For instance, no effect of LEV was observed on seizure duration in the pilocarpine model28 or following an electrically induced SE.30 However, a decrease in duration was shown in the kainic acid model5 and in the tetanus toxin model.33 These discrepancies could be due to multiple factors associated to the methods used to induce SE (e.g., electrical stimulation or chemoconvulsant treatments). Also, in these studies, different doses of LEV as well as different modalities of administration (i.e., systemic or intraventricular) were used. These methodological discrepancies could explain, at least in part, the different effects induced by LEV on the duration of seizures reported to date.
4.2. Effects of levetiracetam on interictal spikes and high-frequency oscillations
We have found that compared to the control group, LEV w/o Sz animals had significantly lower rates of interictal spikes in all recorded regions. This is in line with clinical studies, since a similar effect of LEV on interictal spikes was observed in patients with partial seizures.34 Furthermore, we found that LEV induced lower rates of occurrence of interictal spikes with ripples or fast ripples compared to controls. To our knowledge, this is the first study showing the effect of an AED on HFOs in an animal model of MTLE, and it is in line with studies performed in epileptic patients showing that ripples and fast ripples are sensitive to AEDs.21 Specifically, our results suggest that network activity in specific limbic regions such as CA3, EC and subiculum is affected by the administration of this AED.
It has been proposed that ripples represent population IPSPs generated by principal neurons entrained by synchronously active interneuron networks whereas fast ripples are thought to reflect the hypersynchronous firing of principal (glutamatergic) neurons.13 Fast ripples are considered as better predictors of seizure occurrence and of seizure onset zones compared to ripples.13 However, the fact that LEV modulates both fast ripples and ripples suggest that a particular subgroup of ripples could be pathological and highly sensitive to AEDs. In epileptic patients implanted with depth electrodes, ripples can occur in the hippocampus and entorhinal cortex, on either side of the brain, independently of the side of seizure onset.35 These results thus suggest that ripples occurring on interictal spikes could reflect the activity of neuronal networks that are not necessarily located in the seizure onset zone, but that are nonetheless dysfunctional in the epileptic brain.
In our study, the decrease of fast ripple activity occurred in temporal lobe regions that are known to play important roles in MTLE, namely CA3 and subiculum. The CA3 region of the hippocampus is highly sensitive to neuronal damage induced by the initial SE,36 is one of the first region to show epileptiform activity during the latent period37 and is often the onset zone of spontaneous seizures.8,19,38 Furthermore, we previously proposed that the occurrence of interictal spikes with fast ripples in CA3 may mirror the evolutionary state of the epileptogenic tissue and reveal pathologic activity from regions that will bring neural networks close to seizure onset.19 The results reported here support this hypothesis since we found a significant correlation in CA3 between rates of interictal spikes with fast ripples and seizure rates in controls. The anti-ictogenic properties of LEV could thus be due to its ability to inhibit excessive synchronization in CA332 that would be reflected by a decrease in fast ripple occurrence.
Finally, a decrease of fast ripple occurrence in the subiculum of LEV w/o Sz animals may mirror an inhibiting effect of this AED on pathological neuronal excitability associated to MTLE, as suggested by experiments performed in the resected human tissue.39,40 Spontaneous rhythmic spikes similar to interictal spikes recorded in vivo can propagate from the subiculum to the hippocampus in the tissue of patients with MTLE39 and glutamatergic pre-ictal discharges in the subiculum induced by low levels Mg2+ can trigger seizures.40 In vitro, reduced GABAergic inhibition and a corresponding increase in subicular excitability were found in pilocarpine-treated animals.41 This increased excitability may lead to spontaneous rhythmic activity, polysynaptic response and all-or-none bursts of action potentials in these animals.42 Thus, since LEV w/o Sz animals showed low rates of interictal spikes with HFOs in the subiculum, pathological network activity in this region may play an important role in the transition from interictal activity to ictal activity in MTLE and represent an important therapeutic target.
Further studies are however needed in order to investigate if this decrease in HFO occurrence reflects a disease-modifying property of LEV. It should be emphasized that previous studies have proposed that LEV may have antiepileptogenic properties since its anti-ictogenic effect can persist for many days after treatment cessation in spontaneously epileptic rats,4 in kainic-acid treated5 and in amygdala-kindled rats.2
5. Conclusion
LEV has potent anti-ictogenic properties in the pilocarpine model of MTLE that is mirrored by a decrease in the occurrence of interictal spikes and HFOs, considered to be biomarkers of abnormal network activity. Our results thus further illustrate the potential of this AED in the treatment of MTLE and possibly its ability to induce pharmacological changes that will inhibit neural networks reorganization leading to the occurrence of spontaneous seizures.
Acknowledgments
This study was supported by an investigator initiated grant from UCB Pharma as well as by operating grants from the Canadian Institutes of Health Research (8109 and 74609) and the Savoy Foundation. C.B. is a recipient of a Student Scholarship from the Savoy Foundation.
Footnotes
Conflict of interest statement
M.A. received an investigator initiated grant from UCB Pharma. The remaining authors have no conflict of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Lyseng-Williamson KA. Levetiracetam: a review of its use in epilepsy. Drugs. 2011;71(March 4):489–514. doi: 10.2165/11204490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Löscher W, Hönack D, Rundfeldt C. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J Pharmacol Exp Ther. 1998;284(February 2):474–9. [PubMed] [Google Scholar]
- 3.Vinogradova LV, van Rijn CM. Anticonvulsive and antiepileptogenic effects of levetiracetam in the audiogenic kindling model. Epilepsia. 2008;49(July 7):1160–8. doi: 10.1111/j.1528-1167.2008.01594.x. [DOI] [PubMed] [Google Scholar]
- 4.Yan H-D, Ji-qun C, Ishihara K, Nagayama T, Serikawa T, Sasa M. Separation of antiepileptogenic and antiseizure effects of levetiracetam in the spontaneously epileptic rat (SER) Epilepsia. 2005;46(August 8):1170–7. doi: 10.1111/j.1528-1167.2005.35204.x. [DOI] [PubMed] [Google Scholar]
- 5.Sugaya Y, Maru E, Kudo K, Shibasaki T, Kato N. Levetiracetam suppresses development of spontaneous EEG seizures and aberrant neurogenesis following kainate-induced status epilepticus. Brain Res. 2010;1352:187–99. doi: 10.1016/j.brainres.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 6.Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010;9(April 4):413–24. doi: 10.1016/S1474-4422(10)70059-4. [DOI] [PubMed] [Google Scholar]
- 7.Deshpande LS, DeLorenzo RJ. Mechanisms of levetiracetam in the control of status epilepticus and epilepsy. Front Neurol. 2014;5 doi: 10.3389/fneur.2014.00011. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3907711/ [cited 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lévesque M, Salami P, Gotman J, Avoli M. Two seizure-onset types reveal specific patterns of high-frequency oscillations in a model of temporal lobe epilepsy. J Neurosci. 2012;32(September 38):13264–72. doi: 10.1523/JNEUROSCI.5086-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Baijalieh SM, Matagne A, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101(June 26):9861–6. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel J., Jr Introduction to temporal lobe epilepsy. Epilepsy Res. 1996;26(December 1):141–50. doi: 10.1016/s0920-1211(96)00043-5. [DOI] [PubMed] [Google Scholar]
- 11.Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early Randomized Surgical Epilepsy Trial (ERSET) Study Group. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307(March 9):922–30. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiebe S, Blume WT, Girvin JP, Eliasziw M Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(August 5):311–8. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 13.Jefferys JG, Menendez de la Prida L, Wendling F, Bragin A, Avoli M, Timofeev I, et al. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol. 2012;98(September 3):250–64. doi: 10.1016/j.pneurobio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49(November 11):1893–907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88(October 4):1743–52. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- 16.Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130(September 9):2354–66. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- 17.Haegelen C, Perucca P, Châtillon CE, Andrade-Valença L, Zelmann R, Jacobs J, et al. High-frequency oscillations, extent of surgical resection, and surgical outcome in drug-resistant focal epilepsy. Epilepsia. 2013;54(May 5):848–57. doi: 10.1111/epi.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67(February 2):209–20. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lévesque M, Bortel A, Gotman J, Avoli M. High-frequency (80–500 Hz) oscillations and epileptogenesis in temporal lobe epilepsy. Neurobiol Dis. 2011;42(June 3):231–41. doi: 10.1016/j.nbd.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bragin A, Wilson CL, Almajano J, Mody I, Engel J. High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45(9):1017–23. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- 21.Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72(March 11):979–86. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(March 3):281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 23.Margineanu D-G, Matagne A, Kaminski RM, Klitgaard H. Effects of chronic treatment with levetiracetam on hippocampal field responses after pilocarpine-induced status epilepticus in rats. Brain Res Bull. 2008;77(November 5):282–5. doi: 10.1016/j.brainresbull.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J. Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia. 2009;50(April 4):617–28. doi: 10.1111/j.1528-1167.2008.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salami P, Lévesque M, Benini R, Behr C, Gotman J, Avoli M. Dynamics of interictal spikes and high-frequency oscillations during epileptogenesis in temporal lobe epilepsy. Neurobiol Dis. 2014;67C:97–106. doi: 10.1016/j.nbd.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bragin A, Wilson CL, Engel J. Voltage depth profiles of high-frequency oscillations after kainic acid-induced status epilepticus. Epilepsia. 2007;48(September 5):35–40. doi: 10.1111/j.1528-1167.2007.01287.x. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs J, Levan P, Châtillon C-E, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132(April Pt 4):1022–37. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glien M, Brandt C, Potschka H, Löscher W. Effects of the novel antiepileptic drug levetiracetam on spontaneous recurrent seizures in the rat pilocarpine model of temporal lobe epilepsy. Epilepsia. 2002;43(April 4):350–7. doi: 10.1046/j.1528-1157.2002.18101.x. [DOI] [PubMed] [Google Scholar]
- 29.Abou-Khalil B. Levetiracetam in the treatment of epilepsy. Neuropsychiatr Dis Treat. 2008;4(June 3):507–23. doi: 10.2147/ndt.s2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Vliet EA, Van Schaik R, Edelbroek PM, Lopes da Silva FH, Wadman WJ, Gorter JA. Development of tolerance to levetiracetam in rats with chronic epilepsy. Epilepsia. 2008;49(July 7):1151–9. doi: 10.1111/j.1528-1167.2007.01516.x. [DOI] [PubMed] [Google Scholar]
- 31.Zheng Y, Moussally J, Cash SS, Karnam HB, Cole AJ. Intravenous levetiracetam in the rat pilocarpine-induced status epilepticus model: behavioral, physiological and histological studies. Neuropharmacology. 2010;58(April 4–5):793–8. doi: 10.1016/j.neuropharm.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klitgaard H, Matagne A, Grimee R, Vanneste-Goemaere J, Margineanu D-G. Electrophysiological, neurochemical and regional effects of levetiracetam in the rat pilocarpine model of temporal lobe epilepsy. Seizure. 2003;12(March 2):92–100. doi: 10.1016/s1059131102001930. [DOI] [PubMed] [Google Scholar]
- 33.Doheny HC, Whittington MA, Jefferys JGR, Patsalos PN. A comparison of the efficacy of carbamazepine and the novel anti-epileptic drug levetiracetam in the tetanus toxin model of focal complex partial epilepsy. Br J Pharmacol. 2002;135(March 6):1425–34. doi: 10.1038/sj.bjp.0704606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stodieck S, Steinhoff BJ, Kolmsee S, van Rijckevorsel K. Effect of levetiracetam in patients with epilepsy and interictal epileptiform discharges. Seizure J Br Epilepsy Assoc. 2001;10(December 8):583–7. doi: 10.1053/seiz.2001.0582. [DOI] [PubMed] [Google Scholar]
- 35.Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid – treated rats with chronic seizures. Epilepsia. 1999;40(February 2):127–37. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 36.Ben-Ari Y, Tremblay E, Ottersen OP, Meldrum BS. The role of epileptic activity in hippocampal and “remote” cerebral lesions induced by kainic acid. Brain Res. 1980;191(June 1):79–97. doi: 10.1016/0006-8993(80)90316-9. [DOI] [PubMed] [Google Scholar]
- 37.Lothman EW, Collins RC, Ferrendelli JA. Kainic acid-induced limbic seizures: electrophysiologic studies. Neurology. 1981;31(July 7):806–12. doi: 10.1212/wnl.31.7.806. [DOI] [PubMed] [Google Scholar]
- 38.Li T, Ren G, Lusardi T, et al. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest. 2008;118(February 2):571–82. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298(November 5597):1418–21. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 40.Huberfeld G, Menendez de la Prida L, Pallud J, Cohen I, Le Van Quyen M, Adam C, et al. Glutamatergic pre-ictal discharges emerge at the transition to seizure in human epilepsy. Nat Neurosci. 2011;14(May 5):627–34. doi: 10.1038/nn.2790. [DOI] [PubMed] [Google Scholar]
- 41.de Guzman P, Inaba Y, Biagini G, Baldelli E, Mollinari C, Merlo D, et al. Subiculum network excitability is increased in a rodent model of temporal lobe epilepsy. Hippocampus. 2006;16(10):843–60. doi: 10.1002/hipo.20215. [DOI] [PubMed] [Google Scholar]
- 42.Knopp A, Kivi A, Wozny C, Heinemann U, Behr J. Cellular and network properties of the subiculum in the pilocarpine model of temporal lobe epilepsy. J Comp Neurol. 2005;483(March 4):476–88. doi: 10.1002/cne.20460. [DOI] [PubMed] [Google Scholar]