Abstract
GABAA receptor-mediated inhibition—which is due to Cl− and HCO3− currents controlled by KCC2 and carbonic anhydrase activity, respectively—contributes to short-and long-lasting interictal events recorded from the CA3 region of hippocampus during application of 4-aminopyridine (4AP, 50 μM). Here, we employed field potential recordings in an in vitro brain slice preparation to establish the effects induced by the KCC2 blockers VU0240551 (10 μM) or bumetanide (50 μM) and by the carbonic anhydrase inhibitor acetazolamide (10 μM) on the two types of interictal events. We found that blocking KCC2 activity decreased the amplitude of the short-lasting events. In addition, this pharmacological procedure increased the interval of occurrence of the long-lasting events and reduced their amplitude. Blocking carbonic anhydrase activity with acetazolamide reduced the interval of occurrence and the duration of the short-lasting events while increasing their amplitude; acetazolamide also reduced the duration and amplitude of the long-lasting events. Finally, blocking either KCC2 or carbonic anhydrase activity increased the interval of occurrence of pharmacologically isolated synchronous GABAergic events and decreased their duration and amplitude. These data substantiate further the role of GABAA receptor-mediated signaling in driving neuronal populations toward hypersynchronous states presumably by increasing extracellular [K+].
Keywords: 4-Aminopyridine, Carbonic anhydrase, CA3, GABA, Interictal discharge, KCC2
Introduction
Interictal discharges (or spikes) are short, electrographic events used for localizing the epileptogenic zone in patients presenting with focal epileptic disorders such as temporal lobe epilepsy [4, 9, 10]. Interictal discharges often consist of a large-amplitude, rapid component followed by a slow wave; however, they may present, even in the same patient, with a large amount of variability in both shape and duration [10].
Matsumoto and Ajmone-Marsan [24] were among the first to report that cortical neurons located in experimental epileptic foci induced by drugs interfering with GABAA receptor signaling generate interictal spikes mirrored by intracellular paroxysmal depolarizing shifts associated to sustained and synchronous action potential firing. Successive in vitro studies have shown that these interictal spikes result from enhanced synaptic excitation (due to pharmacologically induced weakening of inhibition) leading to regenerative Ca2+ currents [13, 36, 39]. Thus, under these experimental conditions, interictal activity depends on the activation of glutamate receptors of the AMPA and NMDA subtypes, and it is sustained by non-synaptic mechanisms such as voltage-gated currents, intercellular gap junctions, and ephaptic interactions [20].
However, interictal spikes can also be induced by drugs that boost both glutamatergic and GABAergic synaptic transmission such as the K+ channel blocker 4-aminopyridine (4AP) [1]. Within the hippocampal formation, 4AP discloses two types of interictal spikes [7, 30]. The first type is characterized by frequently occurring, short-lasting events that are driven by the CA3 network, correspond intracellularly to paroxysmal depolarizing shifts, and are abolished by AMPA receptor antagonists. The second type consists of “slow,” long-lasting spikes that continue to occur during application of ionotropic glutamatergic receptor antagonists but are abolished by drugs interfering with GABAA receptor-mediated transmission. These non-glutamatergic spikes, which presumably reflect the activation of postsynaptic GABAA receptors on principal cells consequent to synchronous firing of local interneurons, have been identified in several brain structures (e.g., neocortex, amygdala, entorhinal, perirhinal, insular, and piriform cortices) that are maintained in vitro both in brain slices and in the isolated guinea pig brain preparation [1].
The diversity in interictal spiking identified experimentally as well as in epileptic patients [11] suggests the involvement of distinct underlying neurobiological mechanisms. Indeed, the evidence obtained in the 4AP model underscores a different degree of contribution of GABAA receptor-mediated inhibition to the two types of interictal discharge recorded in the CA3 area. GABAA receptor activation opens channels that are permeable to Cl− and to a lesser extent to HCO3−. In adulthood, the GABAA receptor-mediated current is hyperpolarizing, thanks to a neuron-specific cotransporter (termed K+-Cl− cotransporter, KCC2) that extrudes Cl− [6, 16]; moreover, another prerequisite of hyperpolarization is that at resting membrane potential, the GABAA receptor-mediated HCO3− current is not larger than the Cl− current [21, 32]. To better define the contribution of GABAA receptor-mediated currents to interictal discharges generated by CA3 network during 4AP treatment, we tested here the effects induced by pharmacologically inhibiting either KCC2 or carbonic anhydrase activity on the two patterns of epileptiform synchronization.
Methods
Brain slice preparation and maintenance
Male, adult Sprague-Dawley rats (250–275 g) were decapitated under isoflurane anesthesia according to the procedures established by the Canadian Council of Animal Care. The brain was quickly removed and placed in cold (1–3 °C), oxygenated artificial cerebrospinal fluid (ACSF) with the following composition (mM): 124 NaCl, 2 KCl, 2 CaCl2, 2 MgSO4, 1.25 KH2PO4, 26 NaHCO3, and 10 D-glucose. Horizontal brain slices (450 μm) containing CA3 were cut from this brain block using a vibratome. Slices were then transferred to an interface tissue chamber where they were superfused with ACSF and humidified gas (95% O2, 5% CO2) at a temperature of 31–32 °C and a pH of 7.4. 4AP (50 μM), VU0240551 (10 μM), bumetanide (10 or 50 μM), acetazolamide (10 μM), CLP275 (100 μM), picrotoxin (50 μM), 6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX, 10 μM), and 3, 3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonate (CPP, 10 μM) were bath applied. Chemicals were acquired from Sigma-Aldrich Canada (Oakville, Ontario, Canada) except for CLP275 that was generously provided by Dr. Yves De Koninck from Laval University (Quebec City, Quebec, Canada). VU0240551 and CLP275 were dissolved in DMSO, and control experiments were performed to rule out any possible interference of this solvent on the epileptiform field activity induced by 4AP.
Electrophysiological recordings
Field potential recordings were made with ACSF-filled, glass pipettes (resistance, 5–10 MΩ) that were positioned in the CA3 close to stratum pyramidale. Intracellular recordings from presumptive CA3 pyramidal cells (n=12) were performed with glass pipettes that were filled with 4 M K-acetate (resistance, 50–80 MΩ). These neurons were impaled for periods lasting from 10 to 40 min and had resting membrane potential positive to −62 mV (−60.4±5.4 mV, mean± SEM; n=12), input resistance >22 MΩ (30±4.8 MΩ=10), and action potential amplitude >80 mV (96.3±9.5 mV, n=12).
Field and intracellular signals were fed to high-impedance amplifiers with a bridge circuit that allowed current to pass through the intracellular recording electrode. During intracellular recordings, the bridge was monitored carefully throughout the experiments and adjusted as necessary. Both types of signals were fed to a computer interface (Digidata 1322A, Molecular Devices, and Palo Alto, CA, USA), acquired, and stored using the pCLAMP 9 software (Molecular Devices). Subsequent data analyses were performed with Clampfit 9 (Molecular Devices). In some experiments, a bipolar stainless steel electrode was positioned in the CA3 close to stratum pyramidale to deliver electrical stimuli (0.02–0.4 mA, 50–90 μs).
Statistical analysis
We used Clampfit 9 (Molecular Devices) for offline analysis of the interval of occurrence, duration, and amplitude of interictal discharges. Frequency of interictal discharges was measured as the number of consecutive discharges divided by duration of the recording period(s). The intervals of occurrence of interictal discharges were defined as the time between the onsets of two consecutive discharges. The duration of interictal discharges were defined as the time between the first deflection of the discharge from baseline to its return to baseline. Amplitude of interictal discharges was measured from peak to peak. Since the kurtosis and skewness measures showed that values were not normally distributed, we transformed the raw data to Z-scores and performed one-way ANOVAs followed by Tukey’s post-hoc tests to identify differences between experimental conditions (i.e., 4AP alone, 4AP+VU0240551, 4AP+bumetanide, 4AP+CLP275, and 4AP+acetazolamide) in the CA3 area regarding the rate, duration, and amplitude of interictal discharges. Statistical tests were performed in Matlab R2012b (Mathworks, Natick, MA), and the level of significance was set at p<0.05. Results are expressed as mean±SEM, and n indicates the number of slices used for analysis.
Results
Epileptiform activity induced by 4AP in the CA3 subfield
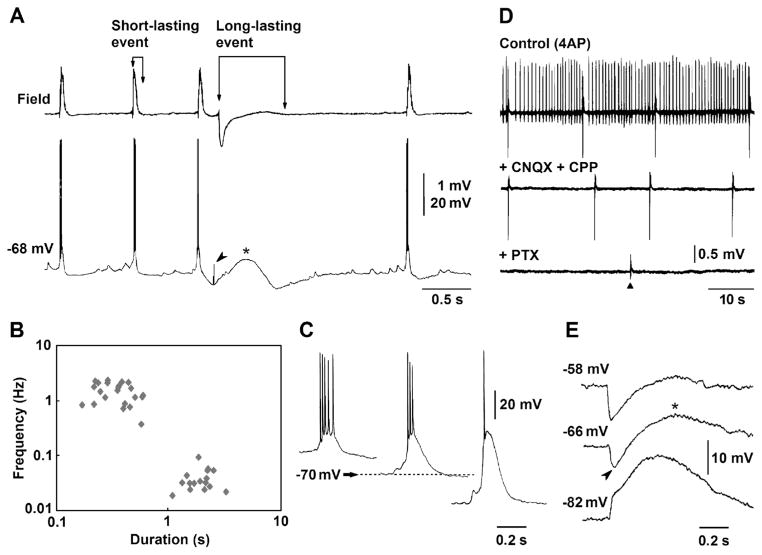
As previously reported in isolated hippocampal slices [23, 24, 42], 4AP application induced two types of spontaneous interictal discharge in the CA3 subfield of brain slices cut horizontally. Simultaneous field and intracellular recordings obtained from stratum pyramidale revealed that the first type consisted of “short-lasting events” (duration 200–320 ms), associated to negative-going population spikes, and recurring every 590–850 ms; these discharges were mirrored by intracellular depolarizations with overriding action potentials (Fig. 1a). The second type of interictal discharge identified as “long-lasting,” occurred every 23–41 s, lasted up to 1.6 s, and was usually characterized by a positive-negative field potential; these long-lasting discharges were intracellularly mirrored by sequences of hyper- and depolarizing events predominated by a long-lasting depolarization (asterisk in Fig. 1a). Presumptive ectopic action potentials of variable amplitude [2] could be recorded in coincidence with the peak of the early hyperpolarizing potential (arrowhead in Fig. 1a). The existence of two types of interictal activity generated by CA3 networks during 4AP application was confirmed by plotting their duration versus their frequency of occurrence (Fig. 1b).
Fig. 1.
Two types of interictal discharge are recorded from the CA3 subfield in horizontal brain slices during 4AP application. a Simultaneous field potential and intracellular recordings obtained from the stratum pyramidale demonstrate “short-lasting events” (associated to intracellular depolarizations/fast action potential firing) and a “long-lasting event,” (characterized by an intracellular sequence of hyperpolarizing-depolarizing events and predominated by a long-lasting depolarization, asterisk); note also that presumptive, ectopic action potentials coincide with the peak of the early hyperpolarizing potential (arrowhead). b Plot of the duration versus frequency of occurrence of the interictal discharge demonstrates the existence of the two types of interictal activity. c Changes induced by intracellular injection of negative or positive DC current on the depolarizations associated to the short-lasting interictal events; horizontal arrow points at resting membrane potential of this CA3 pyramidal cell (−70 mV). d Application of the ionotropic glutamatergic receptor antagonists CPP and CNQX abolishes the occurrence of the short-lasting interictal events while the long-lasting events continue to occur at a similar rate; these non-glutamatergic interictal discharges are later abolished by further application of picrotoxin. Triangle points at the time when a single-shock electrical stimulus was delivered; note that this stimulus fails in inducing a negative-going field response similar to those spontaneously occurring during application of CPP+CNQX. e Intracellular recording obtained from a CA3 pyramidal cell during application of 4AP+CPP+CNQX. The three samples were obtained at different membrane potentials during intracellular injection of DC current (resting membrane potential=−66 mV). Note that as seen under control conditions in panel A, this intracellular event is characterized at resting membrane potential by an early hyperpolarization (arrowhead) followed by a long-lasting depolarization (asterisk); note also that the early hyperpolarization inverts in polarity at −82 mV
Intracellular injection of negative or positive DC current revealed that the depolarizations associated to the short-lasting discharges decreased or increased in amplitude, respectively (Fig. 1c); hence, these events were presumably triggered by giant EPSPs [33]. In line with this view and in agreement with previous studies [1], application of the glutamatergic receptor antagonists CPP and CNQX abolished the occurrence of short-lasting interictal discharges while long-lasting events continued to occur at similar rates (n=4 slices) (Fig. 1d). These non-glutamatergic discharges were however abolished by picrotoxin application (n=3 slices) suggesting that they reflected the synchronous postsynaptic activation of GABAA receptors on pyramidal cells [1]. Changing the membrane potential in either hyperpolarizing or depolarizing directions with intracellular injection of DC current during blockade of glutamatergic transmission (n=4 cells) increased or decreased the amplitude of the long-lasting depolarization (Fig. 1e) [30].
Effects induced by KCC2 blockers
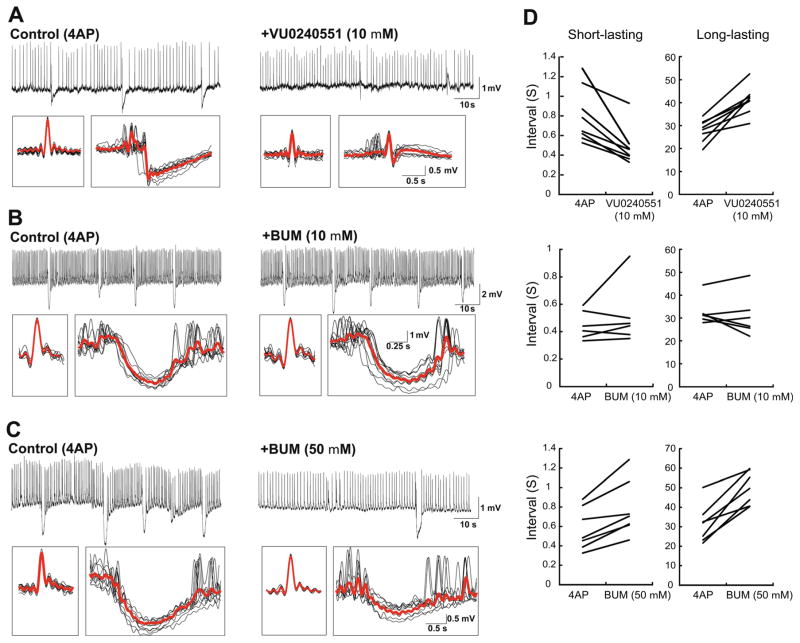
To investigate the role of GABAA receptor-mediated signaling on the short- and long-lasting interictal events recorded from CA3, we used the KCC2 blocker, VU0240551 as well as bumetanide, which has high affinity for NKCC1 and a low affinity for KCC2. Accordingly, it has been reported that at low doses (2–10 μM), bumetanide only blocks NKCC1, whereas at higher concentrations (50 μM), it inhibits both NKCC1 and KCC2 [24]. As illustrated in Fig. 2a, we found that application of VU0240551 changed both types of interictal events (n=8 slices). In addition, as described in detail below, application of low doses of bumetanide (10 μM) did not affect either short- or long-lasting interictal events (Fig. 2b, n=6 slices), whereas at high concentration (50 μM), it increased the interval of occurrence of both types of interictal discharge while decreasing their amplitude (Fig. 2c, n=7 slices). These effects are further shown in the averages in Fig. 2a–c. Also, application of VU0240551 decreased the interval of occurrence of short-lasting events while it increased the interval of occurrence of long-lasting events. High doses of bumetanide decreased the interval of occurrence of both short- and long-lasting events while low doses of bumetanide did not have any effect on their interval of occurrence (Fig. 2d).
Fig. 2.
Changes induced by interfering with KCC2 and/or NKCC1 activities on the two types of interictal discharge induced by 4AP in the CA3 subfield. The effects induced by VU0240551 (10 μM) (a) as well as by 10 μM (b) and 50 μM (c) bumetanide are shown on continuous recordings obtained under control conditions and after application of each of these drugs. In each panel, the inserts below represent averages of short- and long-lasting interictal events. d Graphs showing change in interval of occurrence of both short- and long-lasting events after application of each drug. Note that blocking KCC2 activity with either VU0240551 or 50 μM bumetanide influences both short- and long-lasting events, whereas blocking NKCC1 with low doses of bumetanide (10 μM) does not cause any appreciable change
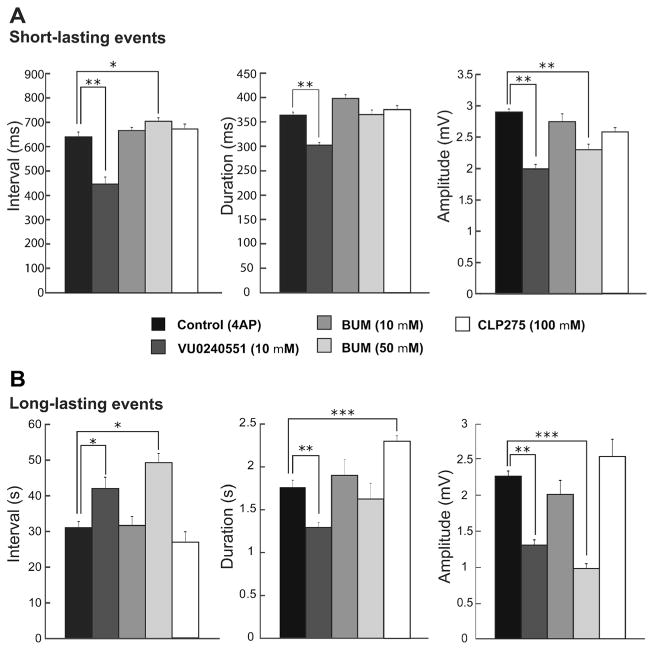
As illustrated in Fig. 3a, blocking KCC2 with VU0240551 (10 μM; n=8 slices) significantly reduced the interval of occurrence of short-lasting events from 632.8±20.2 to 442.2± 32.9 ms (p<0.0001) while high doses of bumetanide significantly increased the intervals of these events to 695.7± 14.9 ms (p<0.001; n=7 slices). However, blocking NKCC1 with low doses of bumetanide did not modulate the intervals of occurrence of these events (n=6 slices). Duration of short-lasting events was also reduced after application of VU0240551 from 364±5.9 to 302.4±5.5 ms (10 μM; p<0.0001) while bumetanide did not change the duration of these events. Also blocking KCC2 with VU0240551 (10 μM) or high doses of bumetanide (50 μM) significantly reduced the amplitude of short-lasting events from 2.88±0.04 to 1.99± 0.06 mV and 2.3±0.08 mV, respectively (Fig. 3a; p<0.0001) while low doses of bumetanide (10 μM) did not change the amplitude of these events.
Fig. 3.
Summary of the effects induced by interfering with KCC2 and/or NKCC1 activities on the two types of interictal discharge induced by 4AP in the CA3 subfield. Bar graphs showing the means (±SEM) of the interval of occurrence, duration, and amplitude of short-lasting (a) and long-lasting (b) events recorded from CA3 under control conditions (i.e., 4AP) and during application of 10 μM VU0240551, 10 μM bumetanide, 50 μM bumetanide, and 10 μM CLP275. Note in a that blocking KCC2 with VU0240551 decreases the interval of occurrence, the duration, and the amplitude of the short-lasting events while a similar, significant effect is induced by 50 μM bumetanide only on the amplitude. Note also in b that blocking KCC2 with VU0240551 increases the interval of occurrence of the long-lasting events while it decreases their duration and amplitude significantly; moreover, similar changes can be induced by high doses of bumetanide but not on the duration. Note in both a and b that blocking NKCC1 with bumetanide (10 μM) does not have a significant effect on the characteristics of the short- and long-lasting interictal events. Finally, note that CLP275 only increases the duration of long-lasting events. *p<0.001, **p<0.0001, and ***p<0.00001
Blocking KCC2 activity also modulated the interval of occurrence, duration and amplitude of long-lasting events. Application of VU0240551 or a high concentration of bumetanide (50 μM) significantly increased the intervals of long-lasting events from 31.2±1.9 to 42±3.1 s and 49.2±2.6 s, respectively, (Fig. 3b; p<0.001), whereas low doses of bumetanide did not change the intervals of these events (10 μM). Duration of long-lasting events was also reduced after application of VU0240551 from 1.76±0.08 to 1.29±0.05 s (10 μM; p<0.0001) while bumetanide (10 and 50 μM) did not change the duration of these events. Also, blocking KCC2 with VU0240551 (10 μM) or high doses of bumetanide (50 μM) significantly reduced the amplitude of long-lasting events from 2.26±0.11 to 1.3±0.07 mV (p<0.0001) and 0.97±0.06 mV (p<0.00001), respectively, whereas low doses of bumetanide (10 μM) did not change this parameter.
We also enhanced KCC2 activity by application of CLP275 (100 μM; n=5 slices) [17]. This procedure did not affect the interval of occurrence, the duration and the amplitude of short-lasting events (Fig. 3a). Moreover, CLP275 (100 μM) did not influence the interval of occurrence and amplitude of long-lasting events but significantly increased their duration to 2.29±0.06 s (Fig. 3b; p<0.0001).
Effects induced by blocking carbonic anhydrase activity
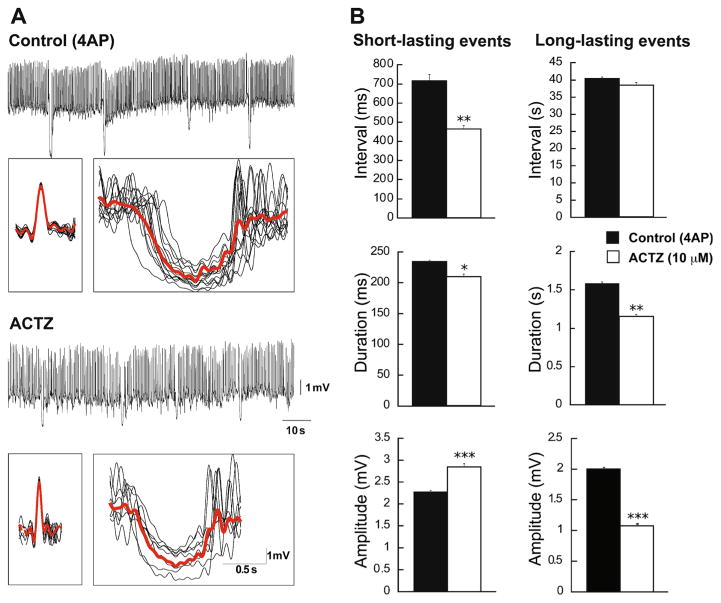
Next, we studied the effects of the carbonic anhydrase inhibitor acetazolamide (10 μM) on the short- and long-lasting events generated in CA3 region of the hippocampus during 4AP application (n=7 slices). Figure 4a shows example of traces in CA3 with averaged short- and long-lasting events under control condition and during the application of acetazolamide. We found that following application of acetazolamide, interval of occurrence and duration of short-lasting interictal events were significantly decreased from 715.6±33.3 to 463.8±17.2 ms (p<0.001) and from 234.5± 4.8 to 209.5±4.5 ms (p <0.05), respectively, while their amplitude was significantly increased from 2.26±0.02 to 2.84±0.07 mV (p<0.0001) (Fig. 4b). In addition, blocking carbonic anhydrase activity induced a significant decrease in the duration and the amplitude of the long-lasting interictal events from 1.58±0.01 to 1.15±0.01 s (p<0.001) and from 2±0.01 to 1.07±0.01 mV (p<0.0001), respectively, but it did not change their interval of occurrence.
Fig. 4.
Changes induced by inhibiting carbonic anhydrase activity on the two types of interictal discharge induced by 4AP in the CA3 subfield. a Continuous recordings obtained under control conditions and during the application of acetazolamide (10 μM). In each panel, the inserts below represent averages of the short- and long-lasting interictal events. b Bar graphs showing the means (±SEM) of the interval of occurrence, duration, and amplitude of short- and long-lasting interictal events recorded from CA3 under control conditions (i.e., 4AP) and during application of 10 μM acetazolamide. Note that blocking carbonic anhydrase activity decreases both the interval of occurrence and the duration of short-lasting interictal events while increasing the amplitudes of these events. Note also that this pharmacological procedure decreases the duration and the amplitude of the long-lasting interictal events without causing any effect on their interval of occurrence. *p<0.05, **p<0.001 and ***p<0.0001
Effects induced by KCC2 and carbonic anhydrase blockers on pharmacologically isolated synchronous events
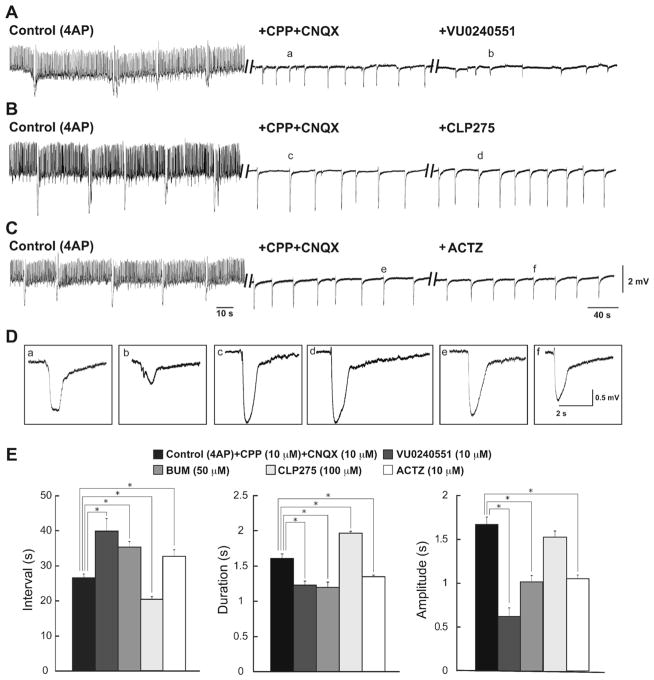
Finally, we pharmacologically blocked glutamatergic transmission with CPP and CNQX (10 μM each) to analyze the effects of VU0240551 (10 μM), bumetanide (50 μM), CLP275 (100 μM), and acetazolamide (10 μM) on the 4AP-induced synchronous GABAergic events that are recorded under these conditions [1]. As illustrated in Fig. 5a, b, short-lasting interictal events were abolished by concomitant application of CPP and CNQX while long-lasting events, which in control condition (4AP, 50 μM), occurred at interval of occurrence of 28.17±1.17 s, duration of 1.53±0.03 s, and amplitude of 1.87±0.07 mV continued to be recorded; under these pharmacological conditions, they had interval of occurrence of 26.42±0.8 s, duration of 1.58±0.06 s, and amplitude of 1.67±0.09 mV. Blocking KCC2 with either VU0240551 (10 μM; n=5 slices) or bumetanide (50 μM; n=4 slices) increased significantly (p<0.001) the interval of occurrence of the pharmacologically isolated long-lasting events to 39.88± 3.6 s and 35.35±1.58 s, respectively (Fig. 5c). These procedures also decreased significantly (p<0.001) the duration (to 1.23±0.05 s and 1.19±0.07 s, respectively) and the amplitude (to 0.61±0.09 mV and 1.01±0.07 mV, respectively) of these events. In addition, similar changes could be observed during application of acetazolamide in slices (n=5 slices) that were superfused with medium containing 4AP along with CPP and CNQX. Acetazolamide significantly increased the interval of occurrence of pharmacologically isolated long-lasting events to 32.72±1.86 s while it decreased their duration and amplitude to 1.34± 0.02 s and 1.05±0.04 mV, respectively (p<0.001). Finally, enhancing KCC2 activity with CLP275 (100 μM, n=4 slices) reduced the interval of occurrence of the isolated GABAergic events to 20.29±0.7 s (p <0.001). CLP275 also resulted in an increase in the duration of these events to 1.98±0.02 s (p<0.001) while it did not affect the amplitude of occurrence of these events.
Fig. 5.
Effects induced by KCC2 and carbonic anhydrase inhibitors on the pharmacologically isolated GABAergic events. Effects induced by VU0240551 (10 μM) (a), CLP275 (100 μM) (b), or acetazolamide (10 μM) (c) on the slow synchronous field events occurring in the CA3 subfield during concomitant application of 4AP+CPP+CNQX (10 μM). In both panels, the initial control activity under 4AP treatment is also shown. d Enlarged portion of slow synchronous field events shown in panels a–c. e Bar graphs summarizing the change in interval of occurrence, duration, and amplitude of the slow synchronous events under control conditions (i.e., 4AP+CPP+ CNQX) and during further application of VU0240551 (10 μM), bumetanide (50 μM), CLP275 (100 μM), or acetazolamide (10 μM). Note that inhibiting either KCC2 or carbonic anhydrase activity ecreases the rate of occurrence, duration, and amplitude of the slow events while enhancing KCC2 activity increases the rate of occurrence and duration of these events. *p<0.001
Discussion
The main findings of our study can be summarized as follows. First, VU0240551 led to a reduction in the interval of occurrence, duration, and amplitude of short-lasting events. It also increased the interval of occurrence of long-lasting events while reducing their duration and amplitude. Second, high doses of bumetanide led to an increase in the interval of occurrence of both short- and long-lasting events while it decreased their amplitude. Enhancing KCC2 activity with CLP275 did not have any effects on the short-lasting events and it only increased the duration of the long-lasting events without affecting their interval of occurrence and amplitude. Third, blocking carbonic anhydrase activity using acetazolamide induced a reduction in the interval of occurrence and duration of short-lasting events while it increased their amplitude. In addition, acetazolamide also reduced both duration and amplitude of the long-lasting events. Finally, blocking KCC2 or carbonic anhydrase activity induced an increase in the interval of occurrence of pharmacologically isolated synchronous GABAergic events and led to a decrease in their duration, while opposite effects were observed by enhancing KCC2 activity with CLP275.
Short- and long-lasting events induced by 4AP in the CA3
Consistent with previous studies from our laboratory performed in isolated hippocampal slices [2, 29, 30], we have found that 4AP induces two types of epileptiform (short-and long-lasting) events that could be discriminated by their interval of occurrence and duration. Short-lasting events occurred more frequently with short duration while long-lasting events had higher interval of occurrence and duration. We noted that in our experiments, the frequency of occurrence of short-lasting events was higher than what was reported originally by Perreault and Avoli [30]. Retrospective analysis of different papers [3, 5] suggests that short-lasting events are more frequent in horizontal brain slices that comprise the hippocampus and other limbic structures such as the entorhinal cortex. The mechanisms underlying this difference remain unclear. However, as reported in the isolated hippocampal slice preparation [30], we found here that application of ionotropic glutamatergic receptor antagonists abolished the short-lasting interictal events while long-lasting spikes continued to occur [1]. GABAA receptor signaling plays a significant role in the generation of these long-lasting events since application of GABAA receptor antagonists can suppress these events [1].
Modulation of short- and long-lasting events by blocking KCC2 activity
GABAA receptor-mediated hyperpolarization depends on the low intracellular [Cl−] that is maintained by the activity of KCC2. However, repetitive activation of GABAA receptor with high-frequency stimulation [22] or application of 4AP [25] can cause a depolarizing response in principal cells. We have found that blocking KCC2 activity with VU0240551 increases the rate of occurrence of the short-lasting events and induces a reduction in their duration and amplitude. This is presumably due to the fact that blocking KCC2 may alter Cl− homeostasis causing depolarizing GABAA receptor-mediated signaling and thus increasing the excitability of principal cells in the CA3 network [19]. Interestingly, low doses of bumetanide (10 μM), which only block NKCC1 activity, did not influence the patterns of the short-lasting interictal events while blocking both NKCC1 and KCC2 with high doses of bumetanide (50 μM) increased their interval of occurrence and decreased their amplitude. One study by Wahab et al. [43] has shown that the effects induced by low doses of bumetanide are age dependent since 10 μM bumetanide can block epileptiform activity induced by 4AP in the immature but not in the adult CA3 region. This may explain why blocking NKCC1 activity with low doses of bumetanide does not influence the short-lasting interictal events.
The opposite results induced by VU0240551 and high doses of bumetanide on the short-lasting but not on the long-lasting events may reflect the fact that high doses of bumetanide block both NKCC1 and KCC2, whereas VU0240551 only blocks KCC2. In fact, it has been shown that during reduced KCC2 expression and activity, application of low doses of bumetanide can decrease or suppress epileptiform activity [19]. The different effects induced by VU0240551 on the frequency of short- and long-lasting events may reflect primary involvement of the glutamatergic receptor-mediated signaling on the generation of the formers as long-lasting interictal discharges should mainly reflect GABAA receptor-mediated signaling [1]. Indeed, after blockade of glutamatergic receptor-mediated signaling with CPP and CNQX, both VU0240551 and bumetanide (50 μM) had similar effects on the pharmacologically isolated, slow GABAergic events. Moreover, we found that enhancing KCC2 activity did not change the interval of occurrence, duration, or amplitude of the short-lasting interictal events further supporting the view that they are most likely driven by the activity of principal neurons and glutamatergic receptor-mediated signaling [1].
We have also found that blocking KCC2 activity with either VU0240551 or high doses of bumetanide increased the interval of occurrence of the long-lasting interictal events. In addition, VU0240551 induced a decrease in their duration while application of either VU0240551 or high doses of bumetanide decreased their amplitude. Long-lasting interictal spikes recorded under control conditions (i.e., 4AP) in the CA3 area are accompanied by transient increases in extracellular [K+] that are larger than those associated to the frequent, short-lasting discharges and continue to occur during blockade of ionotropic glutamatergic transmission [27]. It has been shown that excessive activation of GABAA receptors leads to accumulation of Cl− inside the postsynaptic cells and to a subsequent increase in KCC2 activity that causes K+ and Cl− efflux [40]. Hence, we are inclined to interpret the ability of both VU0240551 or high doses of bumetanide to decrease the frequency and amplitude of long-lasting events as the result of the inability of GABAA receptor activation to cause transient, KCC2 dependent increases in extracellular [K+]. It should be however noted that VU0240551, in addition to its inhibitory action on KCC2 [11], might also interfere with some G protein-coupled receptors and ion channels [12]. We believe, however, that the effects of this compound identified here result from a decrease in KCC2 activity since almost similar findings could be obtained with high concentrations of bumetanide (50 μM) that are known to block both KCC2 and NKCC1 [23].
Our results are different from those published by Zhu et al. [44]; these authors have shown that in KCC2+/− mice, application of 4AP induced seizure-like events in the hippocampal CA1 region while it only induced interictal discharges in wild-type mice. This difference can be explained by experimental differences since Zhu et al. [44] used slices that presumably expressed half of the KCC2 protein content as compared to wild-type animals [44], whereas in our study, we employed slices from normal rats with normal expression of KCC2. Under-expression of KCC2 can in fact result in Cl− homeostasis changes leading to a switch in GABAA receptor signaling from hyperpolarizing to depolarizing [19, 26]. Experimental data have also shown that epileptiform activity induced by 4AP leads to downregulation of KCC2 expression [8, 31]. Therefore, we used CLP275 to enhance KCC2 activity [17] in order to determine if our results were biased by down-regulation of KCC2. We found that application of CLP275 did not have any effect on the interval of occurrence and amplitude of long-lasting events while it increased their duration. These results suggest that although there might be a downregulation in the expression of KCC2, the efficacy of this transporter to extrude Cl− is intact. In line with this view, Huberfeld et al. [19] have shown that in brain slices obtained from resected human epileptic tissue, the expression of KCC2 in dentate gyrus and CA2 region of hippocampus is not changed during epileptiform activity and in subiculum, only 20% of cells lacked expression of KCC2. In line with this evidence, we have found that blocking KCC2 or carbonic anhydrase activity reduced the rate of occurrence of glutamatergic independent synchronous events and shorten their duration, whereas application of CLP275 increased the rate of occurrence of independent synchronous events and their duration.
Role of carbonic anhydrase in the modulation of short- and long-lasting events
GABAA receptor-mediated signaling is also associated to an inward HCO3− current [18], and it has been demonstrated that carbonic anhydrase activity replenishes HCO3− inside the cell [22, 41]. We have found that blockade of carbonic anhydrase activity by application of acetazolamide reduced the interval of occurrence and duration of the short-lasting interictal events and increased their amplitude. Furthermore, this pharmacological procedure led to a decrease in the duration and amplitude of the long-lasting interictal events.
GABAA receptor-mediated elevations in extracellular [K+] depend on the presence of CO2/HCO3− and functional carbonic anhydrase [35, 40]. Cl− load during prolonged activation of GABAA receptor is driven by the depolarizing action of HCO3− efflux (i.e., an inward current) through Cl− channels [22, 41]. This depolarizing HCO3− current does not fade since the intracellular HCO3− is consistently replenished by the carbonic anhydrase activity [22, 41]. Blocking carbonic anhydrase activity abolishes the depolarizing HCO3− current and therefore prevents Cl− from loading into the cell and the subsequent increase in KCC2 activity and increase in extracellular [K+] [35]. In addition, accumulation of Cl− inside the postsynaptic cell can overwhelm KCC2 activity and eventually, results in depolarizing GABAA receptor-mediated signaling through degradation of the Cl− current and predominance of the HCO3− current [37]. In line with these mechanisms, Perez Velazquez [28] has reported that in both interneurons and principal cells, HCO3− efflux through GABAA receptors contribute to depolarizing GABAA-mediated potentials which can be suppressed by either carbonic anhydrase inhibitor or GABAA receptor antagonist. Ruusuvuori et al. [35] have also shown that carbonic anhydrase inhibitor can reduce GABAA receptor-mediated increase in extracellular [K+]. In addition, carbonic anhydrase activity has a key role in pH regulation in neurons, glia, and interstitial fluid in the brain [34]. It is well established that neuronal excitability can be enhanced or suppressed by alkalosis or acidosis, respectively [15, 38]. Blocking carbonic anhydrase activity can thus change intracellular and extracellular pH and modulate neuronal signaling, including voltage-gated ion channels, GABAA receptors, NMDA receptors, and gap junctions [34]. This evidence may explain the slightly different effect of VU0240551 and acetazolamide on the amplitude of short-lasting events as well as on the frequency of long-lasting events.
Interestingly and in support of the contribution of HCO3− currents to epileptiform synchronization, the antiepileptic drug topiramate exerts its effect by inhibiting carbonic anhydrase activity [14]. Moreover, bumetanide and acetazolamide have been used to treat seizures in epileptic patients [23]. Results obtained here may also explain the inefficacy of some GABA enhancer drugs in the treatment of epileptic patients.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (CIHR grants 8109 and 74609). We thank Dr. M. Levesque, Ms. R. Herrington and Ms. P. Salami for helping with the recording procedures and data analysis. We also thank Dr. Yves De Koninck for generously providing us with CLP275.
Footnotes
Conflicts of interest None of the authors has any conflict of interest to disclose.
References
- 1.Avoli M, de Curtis M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol. 2011;95:104–132. doi: 10.1016/j.pneurobio.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avoli M, Methot M, Kawasaki H. GABA-dependent generation of ectopic action potentials in the rat hippocampus. Eur J Neurosci. 1998;10:2714–2722. doi: 10.1046/j.1460-9568.1998.00275.x. [DOI] [PubMed] [Google Scholar]
- 3.Barbarosie M, Avoli M. CA3-driven hippocampal-entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci. 1997;17:9308–9314. doi: 10.1523/JNEUROSCI.17-23-09308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behr C, D’Antuono M, Hamidi S, Herrington R, Lévesque M, Salami P, Shiri Z, Kohling R, Avoli M. Limbic networks and epileptiform synchronization: the view from the experimental side. Int Rev Neurobiol. 2014;114:63–87. doi: 10.1016/B978-0-12-418693-4.00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benini R, Avoli M. Rat subicular networks gate hippocampal output activity in an in vitro model of limbic seizures. J Physiol. 2005;566:885–900. doi: 10.1113/jphysiol.2005.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Brückner C, Heinemann U. Effects of standard anticonvulsant drugs on different patterns of epileptiform discharges induced by 4-aminopyridine in combined entorhinal cortex-hippocampal slices. Brain Res. 2000;859:15–20. doi: 10.1016/s0006-8993(99)02348-3. [DOI] [PubMed] [Google Scholar]
- 8.Chamma I, Heubl M, Chevy Q, Renner M, Moutkine I, Eugène E, Poncer JC, Lévi S. Activity-dependent regulation of the K/Cl transporter KCC2 membrane diffusion, clustering, and function in hippocampal neurons. J Neurosci. 2013;33:15488–15503. doi: 10.1523/JNEUROSCI.5889-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatrian GE, Bergamini L, Dondey M, Klass DW, Lennox-Buchthal M, Petersen I. A glossary of terms most commonly used by clinical electroencephalographers. Electroencephalogr Clin Neurophysiol. 1974;37:538–548. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- 10.de Curtis M, Jefferys JGR, Avoli M. Interictal epileptiform discharges in partial epilepsy: complex neurobiological mechanisms based on experimental and clinical evidence. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s basic mechanisms of the epilepsies [internet] 4. National Center for Biotechnology Information (US); Bethesda: 2012. [PubMed] [Google Scholar]
- 11.Delpire E, Days E, Lewis LM, Mi D, Kim K, Lindsley CW, Weaver CD. Small-molecule screen identifies inhibitors of the neuronal K-Cl cotransporter KCC2. Proc Natl Acad Sci U S A. 2009;106:5383–5388. doi: 10.1073/pnas.0812756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delpire E, Baranczak A, Waterson AG, Kim K, Kett N, Morrison RD, Daniels JS, Weaver CD, Lindsley CW. Further optimization of the K-Cl cotransporter KCC2 antagonist ML077: development of a highly selective and more potent in vitro probe. Bioorg Med Chem Lett. 2012;22:4532–4535. doi: 10.1016/j.bmcl.2012.05.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dingledine R, Gjerstad L. Reduced inhibition during epileptiform activity in the in vitro hippocampal slice. J Physiol. 1980;305:297–313. doi: 10.1113/jphysiol.1980.sp013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodgson SJ, Shank RP, Maryanoff BE. Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia. 2000;41:S35–S39. doi: 10.1111/j.1528-1157.2000.tb06047.x. [DOI] [PubMed] [Google Scholar]
- 15.Dulla CG, Frenguelli BG, Staley KJ, Masino SA. Intracellular acidification causes adenosine release during states of hyperexcitability in the hippocampus. J Neurophysiol. 2009;102:1984–1993. doi: 10.1152/jn.90695.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signaling. Prog Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- 17.Gagnon M, Bergeron MJ, Lavertu G, Castonguay A, Tripathy S, Bonin RP, Perez-Sanchez J, Boudreau D, Wang B, Dumas L, Valade I, Bachand K, Jacob-Wagner M, Tardif C, Kianicka I, Isenring P, Attardo G, Coull JA, De Koninck Y. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med. 2013;11:1524–1528. doi: 10.1038/nm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grover LM, Lambert NA, Schwartzkroin PA, Teyler TJ. Role of HCO3− ions in depolarizing GABAA receptor-mediated responses in pyramidal cells of rat hippocampus. J Neurophysiol. 1993;69:1541–1555. doi: 10.1152/jn.1993.69.5.1541. [DOI] [PubMed] [Google Scholar]
- 19.Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jefferys JGR, Jiruska P, de Curtis M, Avoli M. Limbic network synchronization and temporal lobe epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s basic mechanisms of the epilepsies [internet] 4. National Center for Biotechnology Information (US); Bethesda: 2012. [PubMed] [Google Scholar]
- 21.Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 22.Kaila K, Lamsa K, Smirnov S, Taira T, Voipio J. Long-lasting GABA-mediated depolarization evoked by high-frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network-driven, bicarbonate-dependent K+ transient. J Neurosci Off J Soc Neurosci. 1997;17:7662–7672. doi: 10.1523/JNEUROSCI.17-20-07662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Löscher W, Puskarjov M, Kaila K. Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology. 2013;69:62–74. doi: 10.1016/j.neuropharm.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto H, Ajmone-Marsan C. Cortical cellular phenomena in experimental epilepsy: interictal manifestations. Exp Neurol. 1964;80:286–304. doi: 10.1016/0014-4886(64)90025-1. [DOI] [PubMed] [Google Scholar]
- 25.Michelson HB, Wong RK. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science. 1981;253:1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- 26.Miles R, Blaesse P, Huberfeld G, Wittner L, Kaila K. Chloride homeostasis and GABA signaling in temporal lobe epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s basic mechanisms of the epilepsies [internet] 4. National Center for Biotechnology Information (US); Bethesda: 2012. [PubMed] [Google Scholar]
- 27.Morris ME, Obrocea GV, Avoli M. Extracellular K+ accumulations and synchronous GABA-mediated potentials evoked by 4-aminopyridine in the adult rat hippocampus. Exp Brain Res. 1996;109:71–82. doi: 10.1007/BF00228628. [DOI] [PubMed] [Google Scholar]
- 28.Perez Velazquez JL. Bicarbonate-dependent depolarizing potentials in pyramidal cells and interneurons during epileptiform activity. Eur J Neurosci. 2003;18:1337–1342. doi: 10.1046/j.1460-9568.2003.02843.x. [DOI] [PubMed] [Google Scholar]
- 29.Perreault P, Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol. 1991;65:771–785. doi: 10.1152/jn.1991.65.4.771. [DOI] [PubMed] [Google Scholar]
- 30.Perreault P, Avoli M. 4-Aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. J Neurosci. 1992;12:104–115. doi: 10.1523/JNEUROSCI.12-01-00104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipilä S, Payne JA, Minichiello L, Saarma M, Kaila K. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J Neurosci. 2004;24:4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling: the K+-Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J Physiol. 2005;562:27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutecki PA, Lebeda FJ, Johnston D. 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol. 1987;57:1911–1924. doi: 10.1152/jn.1987.57.6.1911. [DOI] [PubMed] [Google Scholar]
- 34.Ruusuvuori E, Kaila K. Carbonic anhydrases and brain pH in the control of neuronal excitability. Subcell Biochem. 2014;75:271–290. doi: 10.1007/978-94-007-7359-2_14. [DOI] [PubMed] [Google Scholar]
- 35.Ruusuvuori E, Li H, Huttu K, Palva JM, Smirnov S, Rivera C, Kaila K, Voipio J. Carbonic anhydrase isoform VII acts as a molecular switch in the development of synchronous gamma-frequency firing of hippocampal CA1 pyramidal cells. J Neurosci Off J Soc Neurosci. 2004;24:2699–2707. doi: 10.1523/JNEUROSCI.5176-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartzkroin PA, Prince DA. Changes in excitatory and inhibitory synaptic potentials leading to epileptogenic activity. Brain Res. 1980;183:61–76. doi: 10.1016/0006-8993(80)90119-5. [DOI] [PubMed] [Google Scholar]
- 37.Staley KJ. Role of the depolarizing GABA response in epilepsy. In: Binder DK, Scharfman HE, editors. Recent advances in epilepsy research. Kluwer Academic/Plenum Publishers; USA: 2004. [DOI] [PubMed] [Google Scholar]
- 38.Tolner EA, Hochman DW, Hassinen P, Otahal J, Gaily E, Haglund MM, Kubova H, Schuchmann S, Vanhatalo S, Kaila K. Five percent CO2 is a potent, fast-acting inhalation anticonvulsant. Epilepsia. 2011;52:104–114. doi: 10.1111/j.1528-1167.2010.02731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Traub RD, Wong RK. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982;216:745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- 40.Viitanen T, Ruusuvuori E, Kaila K, Voipio J. The K+-Cl− cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J Physiol. 2010;588:1527–1540. doi: 10.1113/jphysiol.2009.181826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voipio J, Kaila K. GABAergic excitation and K(+)-mediated volume transmission in the hippocampus. Prog Brain Res. 2000;125:329–338. doi: 10.1016/S0079-6123(00)25022-X. [DOI] [PubMed] [Google Scholar]
- 42.Voskyul RA, Albus H. Spontaneous epileptiform discharges in hippocampal slices induced by 4-aminopyridine. Brain Res. 1985;342:54–66. doi: 10.1016/0006-8993(85)91352-6. [DOI] [PubMed] [Google Scholar]
- 43.Wahab A, Albus K, Heinemann U. Age- and region-specific effects of anticonvulsants and bumetanide on 4-aminopyridine-induced seizure-like events in immature rat hippocampal-entorhinal cortex slices. Epilepsia. 2011;52:94–103. doi: 10.1111/j.1528-1167.2010.02722.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhu L, Polley N, Mathews GC, Delpire E. NKCC1 and KCC2 prevent hyperexcitability in the mouse hippocampus. Epilepsy Res. 2008;79:201–212. doi: 10.1016/j.eplepsyres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]