Abstract
Although the regenerative potential of adult skeletal muscle is maintained by satellite cells, other stem/progenitor cell populations also reside in skeletal muscle. These heterogeneous cellular pools with mesenchymal lineage potentially play important roles in tissue homeostasis, with reciprocal collaborations between these cells and satellite cells appearing critical for effective regeneration. However, in disease settings, these mesenchymal stem/progenitors adopt a more sinister role – likely providing a major source of fibrosis, fatty tissue and extracellular matrix protein deposition in dystrophic tissue. Development of therapies for muscle degeneration therefore requires complete understanding of the multiple cell types involved and their complex interactions.
Keywords: fibro/adipogentic progenitors, mesenchymal stem cells, muscular dystrophy, myogenesis, regenerative medicine, satellite cells, skeletal muscle, stem cells, tissue regeneration
Introduction
Despite its post-mitotic nature, skeletal muscle maintains remarkable plasticity. Muscle fibres (myofibres) are capable of large alterations in size as well as an enormous ability to regenerate following injury. Like most postnatal tissues, the regenerative potential of skeletal muscle is maintained by a pool of resident adult stem cells. The principal stem cell responsible for this potential in adult skeletal muscle is the satellite cell – a quiescent bi-potent [1] tissue-specific cell population located between the basal lamina and sarcolemma of myofibres and most reliably identified by expression of paired box transcription factor Pax7 [2,3]. Serial transplantation and lineage tracing studies have conclusively demonstrated that, as well as acting as a progenitor, primed to enter myogenic differentiation to fuse into and repair damaged myofibres following injury, satellite cells (or at least a subset of satellite cells [4,5]) are also capable of replenishing the existing stem cell pool via self-renewal [5–9]. Genetic ablation of Pax7+ satellite cells in adult mice has been shown to entirely block regenerative myogenesis, demonstrating an absolute requirement for these cells in myogenic repair and providing evidence that satellite cells are an exclusive source of stem cells in skeletal muscle regeneration [10–12].
However, in addition to satellite cells, a variety of other stem/progenitor and immune cell populations are also found in skeletal muscle (Fig. 1) and play important and increasingly appreciated roles in maintaining tissue homeostasis. Indeed, evidence suggests that collaborative interactions between multiple heterogeneous cell types are required for effective repair processes. This collective ‘society’ of cells in skeletal muscle contributes to the regenerative potential by locally providing the correct environmental settings for stem cell function. This ensures that the biological processes taking place in the multiple types of progenitors are temporally coordinated during regeneration. As discussed in this review, ‘social unrest’ in the skeletal muscle niche may have deleterious consequences.
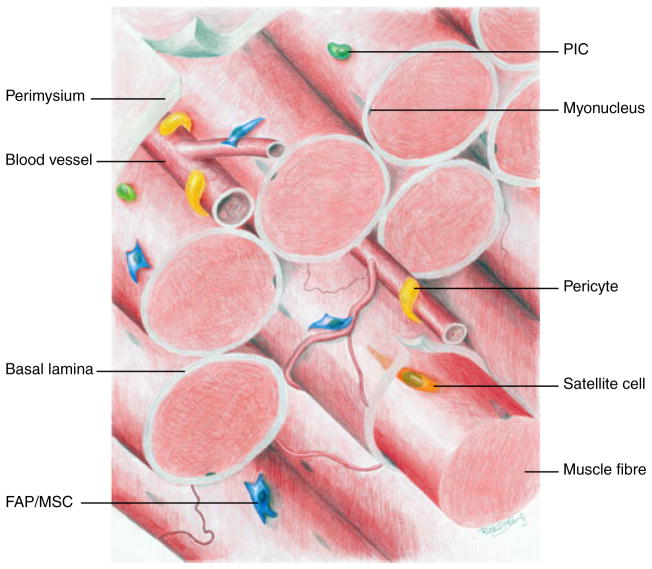
Fig. 1.
Illustration of the various populations of tissue-resident progenitors in skeletal muscle: PICs (PW1+/Pax7− interstitial cells, green), FAPs/MSCs (fibroadipogenic progenitors/mesenchymal stem/progenitor cells, blue), vessel-associated pericytes (yellow) and satellite cells (orange) beneath the basal lamina.
This review discusses the current literature on identification and characterization of skeletal muscle-resident stem/progenitor cell populations involved in regeneration that are distinct from satellite cells. These heterogeneous cell populations possess the capacity to differentiate in multiple lineages (Table 1) and may hold great therapeutic promise for the treatment of debilitating degenerative diseases such as muscular dystrophies, which are characterized by both impaired myogenic stem cell function and excessive deposition of extracellular matrix proteins (fibrosis).
Table 1.
Summary of differing populations of skeletal muscle-resident progenitors. FAPs, fibro-adipogenic progenitors; MSCs, mesenchymal stem/progenitor cells; PICs, PW1+/Pax7− interstitial cells; AP, alkaline phosphatise; NG2, neuro-glial 2 proteoglycan; PDGFRα/β, platelet-derived growth factor receptor α/β; Tcf4, transcription factor 7-like 2 (Tcf7L2). For most of these cell types, potentials other than myogenic have not been investigated thoroughly in vivo.
| Described developmental potential in regenerating skeletal muscle
| ||||
|---|---|---|---|---|
| Population | Cellular markers | In vitro | In vivo | References |
| Satellite cells | Pax7, CD34, Myf5, caveolin-1 and Vcam-1 | Myogenic and fibrogenic (?) | Myogenic | [5–9,40,48,49] |
| FAPs/MSCs | Sca-1, CD34, PDGFRα, Adam12 and Tcf4 (?) | Adipogenic, fibrogenic, osteogenic and chondrogenic | Adipogenic, fibrogenic, osteogenic and chondrogenic | [28–33] |
| PICs | PW1, Sca-1 and CD34 | Myogenic (smooth muscle) | Myogenic | [21] |
| Pericytes | AP, PDGFRβ and NG2 | Myogenic, adipogenic (smooth muscle) and osteogenic | Myogenic | [26,27] |
| Side population cells | Hoechst-negative | Myogenic, adipogenic, fibrogenic, osteogenic and haematopoietic | Myogenic | [13,15,20] |
Non-satellite skeletal muscle-resident stem/progenitors with myogenic potential
Although the satellite cell is the critical stem cell in regenerative myogenesis, there are additional muscle-resident cell populations that are also believed to hold myogenic potential. Gussoni et al. [13] first demonstrated that ‘side population’ cells found in skeletal muscle, identifiable by the preferential exclusion of fluorescent dye Hoechst 33342, give rise to dystrophin-positive myofibres when intravenously injected into mdx mice. However, defining progenitor pools based on Hoechst 33342 dye exclusion is not a particularly stringent parameter in terms of population homogeneity compared to presentation of defined cell-surface antigens [14]. Indeed, there still appears to be a great deal of inconsistency in the gating strategies used to define populations that are Hoechst-positive or -negative. Nevertheless, muscle-resident ‘side population’ cells have been described and characterized by several groups. These studies have found that ‘side population’ cells reside in the muscle interstitium, share close proximity to the endothelium, and contain subsets positive for Sca-1 and CD34 and also for satellite cell markers such as Pax7 [14–20]. Not surprisingly given its heterogeneity, this cell population holds some myogenic potential when exposed to appropriate environmental cues, such as stimulation in vitro [16,18], co-culturing with myoblasts [15], injection into regenerating muscle [13,17,18,20] or over-expression of MyoD [15]. However, whether, to what extent and which ‘side population’ cells participate in regenerative myogenesis following injury in vivo, or even whether muscle ‘side population’ cells are functionally different from their antigenically similar counterparts found in the so-called ‘main population’ (a term that comprises all the remaining cells) with regard to anything other than their ability to efflux dye remains under investigation.
Recently, Mitchell et al. [21] made progress in identifying and characterizing a non-satellite cell myogenic progenitor in the neonate. Here, a cell population in the muscle interstitium identifiable by the expression of stress mediator PW1, but negative for Pax7 (PW1+/Pax7− interstitial cells, PICs), is described. PICs were isolated based on lineage-negative CD45−/Ter119− and Sca1+/CD34+ cell-surface antigen presentation using fluorescence-activated cell sorting. These cells showed some capacity to spontaneously enter myogenic differentiation in vitro in a Pax7-dependent manner, and this was greatly enhanced when PICs were co-cultured with myoblasts [21]. Most interestingly, when transplanted into a regenerating muscle environment in vivo, PICs were able to contribute to new myofibre formation with great efficiency, as well as generating satellite cells and PICs [21]. However, as the majority of data collected in this study were from the neonate, it remains unclear whether a phenotypically comparable cell population is present in adult skeletal muscle, and whether this progenitor functions in normal regenerative myogenesis. In addition, as endothelial markers were not used when prospectively isolating PICs, it remains to be seen whether these cells phenotypically resemble myoendothelial cells [22] or mesoangioblasts [23–25] (endothelial-like mesodermal progenitors with pericytic features and myogenic potential found in several tissues). Having said this, PW1+ cells were negative for CD31 when analysed immunohistochemically [21].
Muscle-resident pericytes have also been shown to possess myogenic potential, and efforts have been made to understand the phenotypic links between them and mesoangioblasts. By dissecting, plating and culturing human muscle interstitium tissue under specific culture conditions, Dellavalle et al. [26] isolated a cell population consistent with pericytes. In vivo, this cell population was negative for myogenic markers (Myf5, MyoD and MyoG) and showed heterogeneous expressed of the pericyte markers neuro-glial 2 proteoglycan, platelet-derived growth factor receptor β (PDGFRβ) and α smooth muscle actin (αSMA) but was most reliably identified by expression of alkaline phosphatase (AP). A proportion of these cells were capable of myogenic differentiation when stimulated in vitro. Most interestingly, these cells were able to produce enough dystrophin-positive muscle fibres to yield a measurable improvement in muscle function when intravenously injected into SCID-mdx mice after expansion in culture [26]. Based on these findings, the same group recently developed a mouse strain in which tamoxifen-inducible Cre recombinase (Cre-ERT2) is expressed under the control of the AP locus, allowing, when crossed with a reporter line, inducible lineage tracing of AP+ pericytes in skeletal muscle development and regeneration [27]. Interestingly, they demonstrate that AP+ pericytes enter myogenesis and contribute to maturing myofibres during development as well as in response to injury during the early postnatal period. They also provide evidence that a small percentage of adult satellite cells are descendants of AP+ progeny; however, AP+ cells appear to have a minimal role in adult regenerative myogenesis [27]. Although such elegant lineage tracing strategies have become an important tool in stem cell biology, their reliability remains dependent on the efficiency of Cre-mediated recombination, on the assumption that Cre expression mimics that of the endogenous locus despite the fact that transgenes often do not include sequences involved in post-translational regulation, and last but not least, on the assumption that a given promoter is only expressed in a specific cell type. The exact expression pattern of AP may be dynamically and heterogeneously regulated during muscle development, and it remains unclear whether myofibres themselves are capable of expressing AP during these events. Nevertheless, these studies provided strong evidence for the involvement of pericytic cells in myofibre formation during specific developmental stages.
Skeletal muscle-resident mesenchymal progenitors
In 2010, two groups characterized a population of skeletal muscle-resident progenitors with bipotent fibro/adipogenic potential [28,29]. Using fluorescence-activated cell sorting on digested mouse muscle preparations, Joe et al. [28] isolated fibro/adipogenic progenitors (FAPs) based on CD45−/CD31− (lineage-negative), α7 integrin−, Sca1+ and CD34+ cell-surface antigen presentation. Similarly, Uezumi et al. [29] isolated a functionally and phenotypically equivalent population of mesenchymal progenitors based on CD45−/CD31−, SM/C2.6− and PDGFRα+ expression. FAPs [28] and mesenchymal progenitors [29] readily entered adipocyte and fibroblast differentiation spontaneously in vitro in bulk cultures as well as in clonal assays, producing both αSMA-expressing fibroblasts and perilipin/peroxisome proliferator-activated receptor γ-positive adipocytes. Both groups demonstrated that this cell population was capable of in vivo adipogenic differentiation when transplanted into glycerol-injected skeletal muscle (a fatty degeneration model) [28,29]. The fibrogenic potential of this PDGFRα+ population has also been verified in vivo following transplantation of genetically labelled cells (PDGFRα–GFP) into γ-irradiated skeletal muscle after cardiotoxin injury [30]. Here, GFP-labelled cells accumulated in areas of fibrosis within the muscle interstitium, presumably consistent with differentiation into collagen type I-producing cells. Recent work by Wosczyna et al. [31] revealed further developmental potency of this progenitor population in vivo. Lineage tracing of muscle-resident cells based on a Tie2-driven Cre-dependent GFP reporter revealed a significant contribution of this cell type to cartilage and bone formation in a model of heterotopic ossification. Analysis of cell-surface antigen expression in lineage-negative, Tie2–GFP+ cells revealed that ~ 90% were PDGFRα+ Sca1+, while the PDGFRα− Sca1− fraction did not contribute to bone or cartilage formation [31]. These results provide evidence that an equivalent or closely related skeletal muscle progenitor as described by Joe et al. [28] and Uezumi et al. [29] is also capable of osteogenic and chrondogenic differentiation in vivo.
Immunohistochemistry demonstrates that these progenitors are localized to the muscle interstitium and adjacent to myofibre-associated blood vessels [28–31], although they do not express markers such as neuroglial 2 proteoglycan [31], defining a cell population distinct from pericytes. Although provisionally labelled as FAPs, evidence highlighting the perivascular localization and capacity to differentiate down multiple skeletal lineages in vivo (as determined by the microenvironment they are transplanted into) suggests these cells may be best recognized as skeletal muscle-resident mesenchymal progenitors/stromal cells (MSCs).
In response to muscle injury, skeletal muscle mesenchymal progenitors become activated and expand rapidly [28,32]. However, unlike satellite cells, which enter myogenic differentiation and repair damaged myofibres, mesenchymal progenitors do not contribute directly to regenerative myogenesis. Transplantation of genetically labelled FAPs (lineage-negative, α7 intrigin−, Sca1+, CD34+) or mesenchymal progenitors (lineage-negative, SM/C2.6−, PDGFRα+) into regenerating muscle made little or no contribution to the regenerating myofibres [28]. Similarly, no myosin heavy chain-positive (MyHC+) myotubes were observed in clonal assays or following stimulation by low serum conditions in vitro, indicating minimal myogenic capacity of these cells [28,29]. Instead, Joe et al. [28] demonstrated that mesenchymal progenitors play an important non-cell autonomous role in facilitating myogenesis. Using co-culture assays in vitro, mesenchymal progenitors were shown to promote myotube formation and differentiation of muscle progenitors [28]. Although the precise ‘pro-myogenic’ signals/factors released from mesenchymal cells during muscle injury remain under investigation, interleukin-6 was significantly up regulated and remains an obvious candidate. The important role of non-myogenic mesenchymal cells was further highlighted by Murphy et al. [11] and Mathew et al. [33]. Here, creation of Tcf4-Cre/CreERT2 alleles used in combination with the Cre-responsive ablator allele R26-DTA allowed the authors to investigate the consequences of ablation of Tcf4+ (transcription factor 7-like 2, Tcf7L2) connective tissue fibroblasts on developmental and regenerative myogenesis in vivo. During development, muscle-resident fibroblasts were shown to be important for slow MyHC expression and the maturation of fetal myofibres [33]. In the adult, despite effective deletion of only 40% of Tcf4+ fibroblasts, skeletal muscle regeneration was significantly impaired following injury [11]. Loss of skeletal muscle fibroblasts altered the proliferative kinetics of satellite cells and induced premature differentiation as determined by the early appearance of embryonic MyHC+ myofibres only 3 days after injury. Although regeneration still proceeded with a depleted fibroblast population in Tcf4-CreERT2/R26-DTA mice, regenerated myofibres were smaller in diameter and cross-sectional area. Using the same genetic strategy, Murphy et al. [11] also ablated satellite cells under the Pax7 locus (Pax7CreERT), and found that Tcf4+ fibroblasts failed to expand effectively in response to injury and did not decrease to pre-injury levels 28 days after injury. These results demonstrate nicely the important reciprocal interactions that occur between satellite cells and other progenitor populations during skeletal muscle regeneration. Interestingly, premature myogenic differentiation in the absence of fibrogenic cells differs from the results obtained by co-culture experiments by Joe et al. [28], who showed that mesenchymal cells provide a micro-environment that supports myotube formation in vitro. Such differences in findings probably reflect the differing models, cellular systems and precise cellular populations examined. Indeed, precise phenotypic and functional distinctions, if any, between ‘fibroblasts’ and MSCs in skeletal muscle and other tissues remain largely uncharacterized. In a classical model, a fibroblast perhaps represents a more lineage-committed cell type capable of producing extracellular matrix proteins such as collagen and other connective tissue components. However, an MSC may be distinguished by its broadened potency, being capable of differentiating into bone or fat as well as connective tissues, and perhaps, although yet to be shown, capable of self-renewal. Such controversies and conceptual ideas are the subject of an excellent recent review [34].
The above evidence highlights that skeletal muscle comprises, in addition to satellite cells, populations of tissue-resident mesenchymal progenitors with multipotent lineage potential in vivo. Although not directly contributing to regenerative myogenesis in normal settings, such progenitors represent a critical component of the cellular niche required for effective satellite cell-mediated regeneration. Indeed, the paracrine effect of MSCs in assisting tissue regeneration has provided the underlining rationale for a plethora of clinical trials investigating stem cell transplantation therapies in various tissues, such as the heart [35,36]. However, it seems unlikely that such progenitor populations exist solely to provide the correct environmental cues to facilitate myogenesis. Little is know about the contribution of mesenchymal progenitors to normal muscle repair processes. Do these progenitors differentiate into collagen-producing cells to restore damaged extracellular matrix and repair muscle architecture needed for myofibre/myofibril support? Are these cells capable of self-renewal? What are the molecular networks involved in their regulation? Answering of such questions will provide a more complete picture of the mechanisms and processes involved in muscle regeneration in the future.
Tissue-resident mesenchymal stem/progenitor cells in skeletal muscle degeneration
Although tissue-resident mesenchymal progenitors appear to be important contributors to the cellular environment required for muscle regeneration in healthy settings, evidence suggests these cells play a more sinister role in conditions of disease. Consistent with degenerative disease states in many tissues, failed skeletal muscle regeneration is associated with fibrosis, accumulation of extracellular matrix proteins and fat deposition. Such histopathological features are commonly observed in dystrophic and ageing muscle, and contribute to the impaired contractile and metabolic functions of this tissue [37]. Indeed, elevated levels of transforming growth factor β (TGFβ) consistent with a ‘pro-fibrotic’ environment, have been well described in dystrophic muscle [38,39].
Efforts have been made to identify the cellular source of fibro-adipogenic tissue formation in degenerative skeletal muscle. Initial studies provided evidence that satellite cells, when exposed to certain environmental cues, may trans-differentiate into a fibroblastic lineage. Brack et al. [40] showed that exposure of primary myoblasts to serum from aged mice induced a proportion of cells to adopt a ‘non-myogenic’ cell fate in vitro – a phenotype that is apparently dependent on activation of canonical Wnt signalling. Accordingly, pharmacological inhibition of Wnt signalling in vivo resulted in a small reduction in skeletal muscle fibrosis following acute injury in aged mice [40]. Other studies reported that isolated myoblasts from normal and dystrophic muscle are capable of conversion into fibroblastic collagen-producing cells [41–43], suggesting that fibrogenesis from satellite cells may contribute to degenerative processes.
However, more recent work has suggested that populations of tissue-resident mesenchymal progenitors are likely to be the major source of pro-fibrotic cells in skeletal muscle. Comparisons of the mRNA expression profiles of muscle-resident cell populations in dystrophic muscle have found that expression of the fibrosis-related genes encoding collagen type 1a (Col1a), Collagen type 3a1 (Col3a1), connective tissue growth factor and αSMA occurs almost exclusively in PDGFRα+ cells [30]. Similarly, PDGFRα+ cells treated with TGFβ show conversion into Col1a- and αSMA-producing cells with far greater sensitivity compared to muscle progenitors and muscle-resident endothelial/hematopoietic cell populations [30]. These findings have been extended by data obtained by Dulauroy et al. [32]. Using an elegant lineage tracing strategy, Dulauroy et al. showed that a population of gp23+, PDGFa+, Sca-1+ progenitors transiently expressing Adam12 are a major source of fibrotic tissue accumulation following muscle damage. The population of Adam12+ cells rapidly expanded upon muscle injury in vivo, peaking 4 days after injury and then decreasing over time, resembling the proliferation kinetics of mesenchymal progenitors observed by Joe et al. [28]. In vitro, Adam12+ cells readily differentiated into αSMA-expressing myofibroblasts upon TGFβ stimulation, but showed poor differentiation into adipocytes. These data suggest that Adam12 may represent a marker for a more committed ‘fibrogenic’ sub-population of mesenchymal progenitors in skeletal muscle. Interestingly, Adam12+ cells displayed phenotypic features of pericytes, sharing close proximity to blood vessel walls and expressing molecular markers such as neuro-glial 2 proteoglycan. Reasoning that Adam12+ cells are a major source of pro-fibrotic cells, Dulauroy et al. [32] next investigated whether selective ablation of Adam12+ cells dampened the fibrotic response following acute muscle injury. Indeed, inducible expression of diphtheria toxin under the control of the Adam12 promoter led to depletion of Adam12+ cells and a significant reduction in interstitial collagen formation 3 weeks following muscle injury. These findings have provided the most convincing demonstration to date of the contribution of mesenchymal progenitors to tissue fibrosis during skeletal muscle regeneration. In the future, it will be important to examine whether ablation or inhibition of similar progenitor populations affects the progression of fibrosis in longer-term degenerative models of muscle disease such as mdx mice.
When the above studies are considered in combination, it is clear that skeletal muscle-resident MSCs adopt some menacing characteristics in reparative disorders. Instead of cooperating to exert a positive influence on muscle repair, failed regeneration triggers the accumulation of MSCs and deposition of fibrogenic, adipogenic and extracellular matrix proteins.
Targeting skeletal muscle-resident mesenchymal progenitors for the treatment of muscle disease
As skeletal muscle mesenchymal progenitors appear to possess dual roles, both influencing myogenesis and contributing to fibrosis, these cells represent an attractive target for treatment of chronic degenerative diseases such as muscular dystrophies. Indeed, muscular dystrophies are classically characterized by fatty and connective tissue accumulation, and therefore manipulation of the differentiation, survival, proliferation or lineage choices of MSCs represents a logical therapeutic strategy to impair fibrotic accumulation and enable other strategies to boost regeneration. Indeed, the results obtained by Dulauroy et al. [32] provide a conceptual framework for this idea by demonstrating that ablation of a sub-population of mesenchymal progenitors in skeletal muscle significantly reduced fibrosis in response to muscle damage.
From a pharmacological point of view, several groups have shown that the tyrosine receptor kinase inhibitor imatinib appears to be effective at inhibiting connective tissue accumulation in several models of fibrosis, including mdx mice [44–46]. Recent findings revealed that the anti-tumoral kinase inhibitor imatinib appears to have a specific influence on PDGFRα-expressing mesenchymal progenitors in skeletal muscle. Ito et al. [46] demonstrated that, unlike satellite cells, isolated PDGFRα+ cells from damaged muscle displayed decreased proliferation and reduced expression of ‘pro-fibrotic’ genes (αSMA, tissue inhibitor of metalloproteinases 1 and Col3a1) when treated with imatinib in vitro. These findings suggest that imatinib may represent a pharmacological means to target muscle-resident mesenchymal progenitors and prevent fibrosis; however, further research is required to understand which other cell types that imatinib affects and the precise molecular pathways upon which this tyrosine receptor kinase inhibitor acts. Other groups have examined the affects of blocking TGFβ – a secreted ligand known to stimulate fibrosis development. Promising initial results have revealed that systemic administration of a TGFB neutralising antibody reduces connective tissue accumulation in mdx mice; however, off-target effects were observed [47]. In summary, we consider that identifying drugs to target mesenchymal progenitors or the ‘pro-fibrotic’ molecules that trigger fibrosis is an important research goal in the treatment of muscular dystrophies.
In addition, further work is required to shed light onto the molecular regulation of MSCs in muscle. Understanding the signalling networks and mechanisms that regulate MSC differentiation, proliferation, gene expression and lineage choice is likely to open up further therapeutic avenues to target these cells in settings of acute/chronic fibrosis.
Conclusion
In summary, accumulating evidence indicates that tissue-resident mesenchymal progenitors play important roles in skeletal muscle repair. In healthy tissue, reciprocal interactions between MSCs and satellite cells exist to facilitate effective stem cell function and repair. However, in disease settings, MSCs appear to adopt a more sinister role – providing a major source of fibrosis, fatty tissue accumulation and deposition of extracellular matrix proteins. It is therefore clear that, in order to unlock the regenerative potential of skeletal muscle in therapeutic settings, a complete understanding of the multiple cell types involved and their complex interactions is required.
Acknowledgments
Keith Gourlay is greatly acknowledged for proofreading the manuscript and providing insightful comments and suggestions on article layout and content.
Abbreviations
- AP
alkaline phosphatase
- Col1a
collagen type 1a
- Col3a1
collagen type 3a1
- CreERT2
tamoxifen-inducible Cre recombinase
- FAPs
fibroadipogenic progenitors
- MSCs
mesenchymal stem/progenitor cells
- MyHC
myosin heavy chain
- PDGFRα/β
platelet-derived growth factor receptor α/β
- PICs
PW1+/Pax7− interstitial cells
- Tcf4
transcription factor 7-like 2 Tcf7L2
- TGFβ
transforming growth factor β
- αSMA
α smooth muscle actin
References
- 1.Yin H, Pasut A, Soleimani VD, Bentzinger CF, Antoun G, Thorn S, Seale P, Fernando P, Van Ijcken W, Grosveld F, et al. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab. 2013;17:210–224. doi: 10.1016/j.cmet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 4.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012;148:112–125. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 6.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 9.Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 13.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 14.Kallestad KM, McLoon LK. Defining the heterogeneity of skeletal muscle-derived side and main population cells isolated immediately ex vivo. J Cell Physiol. 2010;222:676–684. doi: 10.1002/jcp.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamaki T, Akatsuka A, Okada Y, Matsuzaki Y, Okano H, Kimura M. Growth and differentiation potential of main- and side-population cells derived from murine skeletal muscle. Exp Cell Res. 2003;291:83–90. doi: 10.1016/s0014-4827(03)00376-8. [DOI] [PubMed] [Google Scholar]
- 17.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, Roy RR, Edgerton VR. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol. 2002;157:571–577. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamaki T, Akatsuka A, Yoshimura S, Roy RR, Edgerton VR. New fiber formation in the interstitial spaces of rat skeletal muscle during postnatal growth. J Histochem Cytochem. 2002;50:1097–1111. doi: 10.1177/002215540205000812. [DOI] [PubMed] [Google Scholar]
- 20.Uezumi A, Ojima K, Fukada S, Ikemoto M, Masuda S, Miyagoe-Suzuki Y, Takeda S. Functional heterogeneity of side population cells in skeletal muscle. Biochem Biophys Res Commun. 2006;341:864–873. doi: 10.1016/j.bbrc.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell KJ, Pannérec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- 22.Zheng B, Cao B, Crisan M, Sun B, Li G, Logar A, Yap S, Pollett JB, Drowley L, Cassino T, et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25:1025–1034. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 23.Cossu G, Bianco P. Mesoangioblasts – vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev. 2003;13:537–542. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthélémy I, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 25.Tonlorenzi R, Dellavalle A, Schnapp E, Cossu G, Sampaolesi M. Isolation and characterization of mesoangioblasts from mouse, dog, and human tissues. Curr Protoc Stem Cell Biol. 2007;chapter 2(unit 2B.1) doi: 10.1002/9780470151808.sc02b01s3. [DOI] [PubMed] [Google Scholar]
- 26.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 27.Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- 28.Joe AWB, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 30.Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 31.Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012;27:1004–1017. doi: 10.1002/jbmr.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012;18:1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 33.Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, Hansen MS, Angus-Hill M, Kardon G. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2011;138:371–384. doi: 10.1242/dev.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012;2:CD006536. doi: 10.1002/14651858.CD006536.pub3. [DOI] [PubMed] [Google Scholar]
- 36.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 37.Goldspink G, Fernandes K, Williams PE, Wells DJ. Age-related changes in collagen gene expression in the muscles of mdx dystrophic and normal mice. Neuromuscul Disord. 1994;4:183–191. doi: 10.1016/0960-8966(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 38.Yamazaki M, Minota S, Sakurai H, Miyazono K, Yamada A, Kanazawa I, Kawai M. Expression of transforming growth factor-β1 and its relation to endomysial fibrosis in progressive muscular dystrophy. Am J Pathol. 1994;144:221–226. [PMC free article] [PubMed] [Google Scholar]
- 39.Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R. Expression of transforming growth factor-β1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J Clin Invest. 1995;96:1137–1144. doi: 10.1172/JCI118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 41.Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol. 2007;293:C661–C669. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- 42.Ono Y, Sensui H, Okutsu S, Nagatomi R. Notch2 negatively regulates myofibroblastic differentiation of myoblasts. J Cell Physiol. 2007;210:358–369. doi: 10.1002/jcp.20838. [DOI] [PubMed] [Google Scholar]
- 43.Zhou L, Wang L, Lu L, Jiang P, Sun H, Wang H. Inhibition of miR-29 by TGF-β–Smad3 signaling through dual mechanisms promotes transdifferentiation of mouse myoblasts into myofibroblasts. PLoS ONE. 2012;7:e33766. doi: 10.1371/journal.pone.0033766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bizario JC, Cerri DG, Rodrigues LC, Oliveira GL, Nomizo A, De Araujo DD, Fukuhara PS, Ribeiro JC, De Castro FA, Costa MC. Imatinib mesylate ameliorates the dystrophic phenotype in exercised mdx mice. J Neuroimmunol. 2009;212:93–101. doi: 10.1016/j.jneuroim.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Huang P, Zhao XS, Fields M, Ransohoff RM, Zhou L. Imatinib attenuates skeletal muscle dystrophy in mdx mice. FASEB J. 2009;23:2539–2548. doi: 10.1096/fj.09-129833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito T, Ogawa R, Uezumi A, Ohtani T, Watanabe Y, Tsujikawa K, Miyagoe-Suzuki Y, Takeda S, Yamamoto H, Fukada S. Imatinib attenuates dystrophic condition in severe mouse dystrophy and inhibits both proliferation and fibrosis-marker expression in muscle mesenchymal progenitors. Neuromuscul Disord. 2013;23:349–356. doi: 10.1016/j.nmd.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 47.Andreetta F, Bernasconi P, Baggi F, Ferro P, Oliva L, Arnoldi E, Cornelio F, Mantegazza R, Confalonieri P. Immunomodulation of TGF-β1 in mdx mouse inhibits connective tissue proliferation in diaphragm but increases inflammatory response: implications for antifibrotic therapy. J Neuroimmunol. 2006;175:77–86. doi: 10.1016/j.jneuroim.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Calhabeu F, Hayashi S, Morgan JE, Relaix F, Zammit PS. Alveolar rhabdomyosarcoma-associated proteins PAX3/FOXO1A and PAX7/FOXO1A suppress the transcriptional activity of MyoD-target genes in muscle stem cells. Oncogene. 2013;32:651–662. doi: 10.1038/onc.2012.73. [DOI] [PubMed] [Google Scholar]
- 49.Gnocchi V, White R, Ono Y, Ellis J, Zammit P. Further characterisation of the molecular signature of quiescent and activated mouse muscle satellite cells. PLoS ONE. 2009;4:e5205. doi: 10.1371/journal.pone.0005205. [DOI] [PMC free article] [PubMed] [Google Scholar]