Summary
Primary immune thrombocytopenia (ITP) is caused by platelet autoantibodies and T-cell dysregulation. Both platelets and their precursor megakaryocytes may be targeted leading to platelet destruction and underproduction. Current treatments for ITP are inadequate since they do not reverse the disease process and generally do not result in durable remissions. In addition, many treatments are limited by side effects including infection and potentially thrombosis. Novel agents that are currently in development target certain key steps in the disease process, including: (1) the interaction between T-cell and antigen presenting cells (CD40–CD154 interaction); (2) the binding of the Fc portion of platelet autoantibodies to Fc-receptors on macrophages (soluble Fc-RIIb); and (3) the signaling pathways leading to platelet phagocytosis by macrophages (Syk inhibition). Other strategies have been to augment platelet production by simulating thrombopoiesis or by neutralizing physiological inhibitors of megakaryopoiesis. Targeted therapies in ITP have the potential to improve disease morbidity and mortality while limiting systemic side effects. Before these agents can be used in practice, additional clinical studies are needed with rational study outcomes including platelet count, bleeding and quality of life. An individualized treatment strategy is needed for patients since ITP is a distinctly heterogeneous disease.
Immune thrombocytopenia (ITP) is an autoimmune disease characterized by reduced numbers of platelets which can cause an increased risk of bleeding. In most adults, ITP is typically a chronic condition and often requires treatment. Major bleeding including intracerebral hemorrhage (ICH) is rare and occurs predominantly in patients with platelet counts below 10 × 109/L [1]; however, the bleeding risk rises with increasing age and other comorbidities [2]. Patients with ITP have a 4–5-fold increased risk of death from bleeding or infection [3,4], and quality of life is often reduced [5,6]. Given the morbidity and mortality associated with ITP, better treatments are needed to achieve and maintain disease control.
In this article, we discuss the limitations of current therapies and highlight new treatments for ITP that are currently in development. We begin with a summary of the immune pathways that are disrupted in ITP and how these pathways may be targets for novel therapies (figure 1).
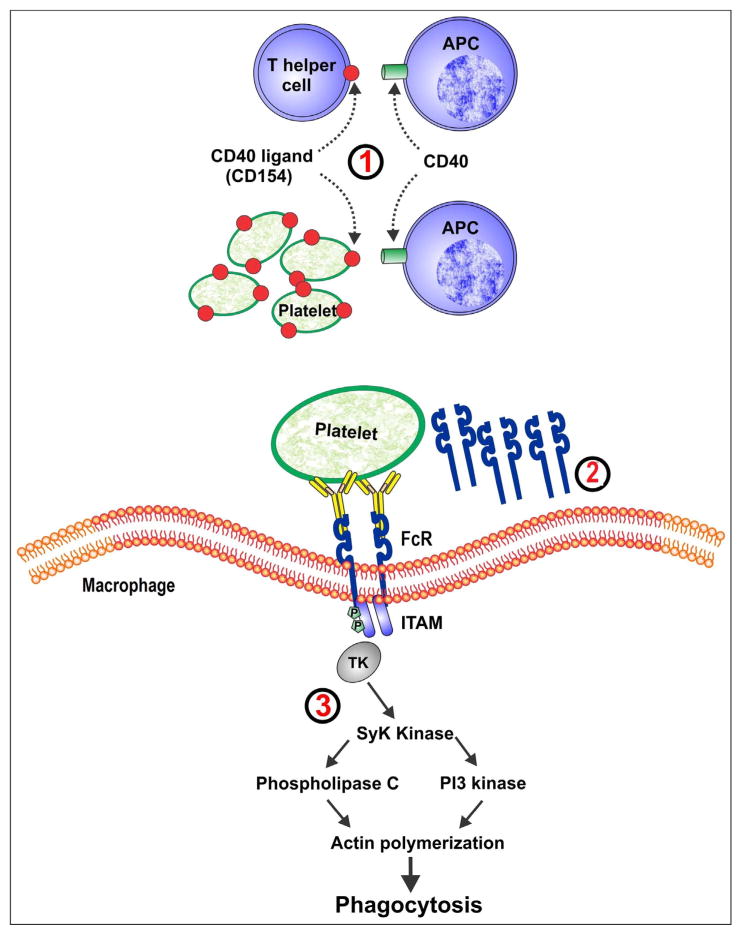
Figure 1. Targets for new treatments for immune thrombocytopenia.
1) interruption of CD40–CD154 interaction.
2) soluble FcγRIIb.
3) inhibition of Syk signaling.
APC: antigen presenting cell; ITAM: immunoreceptor tyrosine-based activation motifs.
Overview of pathophysiology of ITP
Primary ITP represents a spectrum of pathophysiological events, which together result in a reduction in the number of circulating platelets. Additionally, increasing clinical and experimental evidence suggests that ITP results from the loss of self-tolerance for platelet proteins. As a result, platelets and possibly their precursor megakaryocytes are rapidly destroyed by autoantibodies and by cell-mediated toxicity. Experiments from the 1950s implicated a circulating plasma factor as the cause of platelet destruction in ITP and further studies demonstrated a platelet-specific immunoglobulin G (IgG) in many patients [7,8]. More recently, the cellular mechanisms underlying the immune dysregulation in ITP patients have been better defined, including defects in regulatory [9,10] and cytotoxic T-cells [11,12].
Loss of tolerance to platelet autoantigens
The normal response to antigens is mediated through helper (CD4+) and cytotoxic (CD8+) T-cells. During maturation in the thymus, T-cells that react strongly with self-antigens are deleted [13] to ensure that immune cells can differentiate between self and non-self. In addition, safeguards are in place in the peripheral circulation to avoid T-cell autoreactivity. For example, for T-cells to become activated, they must be properly stimulated by binding to major histocompatibility complex (MHC) molecules and CD40 on antigen presenting cells [14] via the T-cell receptor (TcR) and CD154 (CD40 ligand) on T-cells [15]. This activation process initiates the humoral immune response. If platelet-autoreactive T-cells are stimulated by this process [16], auto-antibodies are produced that react with platelets and/or megakaryocytes. Thus, interruption of the CD40–CD154 interaction is a potential target for therapy in ITP.
Autoantibodies against platelets and megakaryocytes
IgG autoantibodies have been identified in many ITP patients, with the most frequent target being platelet glycoproteins (GP) IIbIIIa and IbIX [17]. Autoantibody-coated platelets bind to Fc-receptors (FcR) on macrophages in reticuloendothelial tissues which leads to phagocytosis and platelet destruction [18,19]. Blocking FcR binding or interrupting the signaling pathways that ultimately lead to phagocytosis are also being explored as potential targets for ITP treatment [20]. Bone marrow megakaryocytes also express platelet proteins; thus, these cells may also be affected by platelet autoantibodies. In vitro experiments using plasma or isolated IgG from ITP patients have demonstrated that some IgG antibodies can bind megakaryocytes and impair their growth and proliferation [17]. Consistent with these observations, in vivo platelet survival studies using autologous radiolabelled platelets have shown that platelet production is often impaired [21]. In recent years, the use of thrombopoietin (TPO) receptor agonists to augment platelet production has been shown to be effective in up to 75% of ITP patients [22] by correcting the relative impairment in platelet production.
Cytotoxic T-cells against platelets and megakaryocytes
Although platelet autoantibodies are thought to play an important role in ITP, only about 50–65% of patients have detectable anti-GPIIbIIIa or anti-GPIbIX autoantibodies on their platelets, and far fewer have autoantibodies detectable in the circulation [23,24]. Other potential mechanisms of thrombocytopenia in ITP include a direct lytic effect of cytotoxic T-lymphocytes on platelets [11] and/or megakaryocytes [25]; and an abnormal effector T-cell response [25,26]. In addition, T-regulatory cells are dysfunctional or reduced in some ITP patients [27], a defect that may be corrected after successful treatment with TPO receptor agonists, rituximab or dexamethasone [9,10,28].
Current treatment of primary ITP
ITP represents a heterogeneous spectrum of disease in terms of its presentation and pathophysiology; therefore, patient management must be individualized. A small proportion of patients with stable thrombocytopenia and platelet counts above 50 × 109/L may undergo spontaneous remissions [29]; but for those who require treatment, corticosteroids, intravenous immune globulin (IVIG) and Rh-immune globulin (RhIg) are accepted first-line treatments [30]. Corticosteroids often produce responses that last weeks or months but typically require ongoing drug exposure. Clinical data suggest that 3 to 6 cycles of high-dose dexamethasone administered at 2- to 4-week intervals may be associated with durable responses in some patients [31] and may be more effective than daily prednisone. RhIg is currently used less often due to a black box warning from national health authorities about the possibility of fatal reactions related to intravascular hemolysis.
Second-line treatments include rituximab, splenectomy, TPO receptor agonists [30] and immunosuppressant medications [32]. Of all available medical treatments, only rituximab can achieve durable responses in a significant proportion of patients [33]. In an observational study (n = 77 adults and n = 66 children), 21% of adults and 26% of children with chronic ITP treated with rituximab maintained a response for at least 5 years [34]. In contrast, splenectomy is associated with a higher rate of durable responses (60–70%) that are generally longer-lasting (continuing after up to 12-year follow-up) [35]. TPO receptor agonists are a novel class of medications that increase platelet production through activation of the TPO receptor on megakaryocytes and hematopoietic progenitor cells. TPO is endogenously produced in the liver, but plasma levels are normal or decreased in patients with ITP [36]. The two TPO receptor agonists that are licensed for ITP are romiplostim and eltrombopag. Both agents have been shown to be effective in phase III trials [37,38] and in long-term follow-up studies [39,40]. The risk of thromboembolism with these agents remains uncertain [41].
The limitations of current medical treatments for ITP are their inability to induce sustained platelet count responses, and their associated risks of infection and thrombosis. Novel treatments that are safe and that target specific pathways in the disease process may provide better alternatives.
Novel agents for the treatment of ITP
Therapies targeting the CD40–CD154 interaction
The initiation of the T-cell dependent B-cell response depends on the interaction between CD40 (on antigen presenting cells) and CD154 (on T-cells). Activated platelets also express CD154 which can directly stimulate B-cells [42] but the function of CD154 on platelet remains uncertain. Blocking the CD40-CD154 interaction on T-cells can interrupt the stimulation of auto-reactive T-cells in patients with ITP [43] (figure 1).
Blocking antibodies against CD154 have been developed. In a phase I, multicenter, dose-escalating study, 20 patients with ITP with a platelet count less than 50 × 109/L (mean 29 × 109/L) were treated with toralizumab (IDEC-131), a humanized monoclonal anti-CD154 antibody. Of 5 patients who received the highest dose (10 mg/kg), 3 patients achieved a platelet count above 60 × 109/L that was sustained for 6 weeks. Side effects included hot flashes, fatigue, dizziness, and gastrointestinal symptoms. There was a significant decrease in anti-GPIIbIIIa antibodies and antibody-producing B-cells and a trend towards decreased GPIIbIIIa-induced T-cell proliferation after treatment [44]. Subsequent open-label studies with toralizumab (n = 31) and ruplizumab (hu5c8; n = 15), another humanized anti-CD154 monoclonal were done over a decade ago. All patients had ITP for at least 2 months with platelet counts at or below 30 × 109/L and had failed multiple therapies. Of the 31 patients initially treated with toralizumab, one achieved a platelet count above 150 × 109/L and 4 achieved a platelet count above 50 × 109/L. Of 15 patients treated with ruplizumab, 4 achieved a platelet count above 150 × 109/L lasting at least one week and 2 patients achieved a platelet count above 50 × 109/L. Three patients who relapsed were salvaged successfully with toralizumab. The overall response with anti-CD154 mAb was 24% [45].
The occurrence of thromboembolic events in both human and animals halted further trials with the monoclonal anti-CD154 [44,46]. As CD154 is expressed on the surface of activated platelets, thrombotic events were thought to be mediated by FcR binding on platelets and subsequent platelet activation. Newer monoclonal anti-CD154 antibodies with a modified Fc portion are currently being tested in clinical trials. These molecules may potentially be less thrombogenic because the Fc portion cannot bind or activate platelet FcR [47].
Therapies targeting FcR binding and signaling
In patients with ITP, IgG-coated platelets are rapidly cleared via FcR on macrophages [20]. Occupancy of FcR is believed to be one of the main mechanisms of action of RhIg and IVIG, since Fc fragments isolated from IVIG have a similar effect on increasing platelet counts [48,49] and blocking FcγRIII can reverse ITP in a mouse model [50]. Therefore, interference with FcR binding or its downstream effects are promising targets for ITP therapies. Rozrolimupab is a mixture of 25 recombinant human anti-RhD monoclonal antibodies. The combination of multiple recombinant antibodies may produce a more potent biological effect than human-derived RhIg while avoiding donor exposures and minimizing the risk of infection [51]. Rozrolimupab was evaluated in a multicenter, phase I/II dose-finding trial of 61 patients with ITP. At the highest dose, 62% of patients achieved a platelet count response and only one patient required rescue treatment. However, hemolytic anemia was a limiting side effect. A fall in hemoglobin of 25 g/L by 7 days occurred in 25% of patients, 4 patients required red blood cell transfusion, and 2 patients had a decrease in hemoglobin level of 30 g/L or more [52].
Crosslinking Fc-receptors on the surface of macrophages induces intracellular signaling via phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs). Downstream recruitment and activation of the protein tyrosine kinase Syk results in cytoskeletal changes and ultimately phagocytosis [53]. Inhibition of Syk decreases the efficiency of phagocytosis [54] and in clinical studies has been shown to improve outcomes in patients with rheumatoid arthritis [53] and ITP. In a pilot open-label, single-arm cohort dose-escalation study, 16 adults with chronic ITP with platelet counts below 30 × 109/L were treated with the oral Syk inhibitor R788 [20]. Of 16 patients, 8 had improved platelet counts over 50 × 109/L and 4 had transient responses. Toxicities occurred in some patients including diarrhea, vomiting, and increased liver enzymes.
Inhibitory Fc gamma receptors, such as FcγRIIb, prevent the consumption of platelets by macrophages. These receptors mediate the protective effect of IVIG [49]. FcγRIIb receptors are also expressed on B-cells and control B-cell activation [55]. A soluble FcγRIIb molecule (SM101) has been developed to compete for pathogenic immune complexes and prevent stimulation of memory B-cells that can lead to pathogenic auto-antibody production. This compound was recently evaluated in a randomized, double-blinded, placebo-controlled, dose-escalation phase I study of 36 ITP patients. Three patients who received the highest dose achieved sustained platelet count responses over 50 × 109/L [56].
Therapies targeting platelet production
New TPO receptor agonists are currently under investigation. Avatrombopag, which may be more potent than eltrombopag, has additive effects when combined with recombinant human TPO [57] and clinical data are promising [58]. Other TPO receptor agonists that have undergone clinical development include LGA-4665, NIP-004, NIP-002, and butyzamide [59].
A different approach to augmenting platelet production is to neutralize physiological inhibitors of megakaryopoiesis. Megakaryocytes and platelets express VPAC1, a stimulatory G-protein coupled receptor. In vivo, pituitary adenylyl cyclase-activating peptide (PACAP) and vasoactive intestinal peptide (VIP) bind to this receptor to inhibit megakaryocyte growth and differentiation. Blocking this interaction may improve thromopoiesis. The anti-VPAC1 monoclonal IgG1 antibody has been shown to stimulate thrombopoiesis in murine models of both immune and non-immune thrombocytopenia [60].
Amifostine is another molecule that has been found to improve thrombopoiesis. It is a cytoprotective agent that also has the effect of increasing hematopoiesis by mechanisms that remain unclear [61]. In one ITP study, all 24 amifostine-treated patients responded and 22 of 24 achieved a platelet count above 100 × 109/L [62]. In another study, amifostine was associated with a normalization of the platelet count in 17 ITP patients. Responses persisted for 2 months after the medication was discontinued [63].
Novel treatments are shown in table I.
Table I.
Novel treatments for immune thrombocytopenia (ITP)
| Therapy | Mechanism of action | Efficacy | Adverse effects |
|---|---|---|---|
| Therapies targeting T-cell co-stimulation | |||
| Toralizumab (IDEC-151) [44] | Humanized anti-CD154 Antibody | Toralizumab (phase I): 20 patients with ITP, 3 of 5 with highest dose had sustained response | Thromboembolic events [49,51] |
| Ruplizumab (hu5c8) [45] | Combined analysis of phase I/II studies of toralizumab and ruplizumab showed 24% overall response | ||
|
| |||
| Therapies targeting FcR binding and signaling | |||
|
| |||
| GMA161 [50] | Humanized anti-human FcγRIII antibody | Efficacy in transgenic murine model of ITP | Hypersensitivity reactions |
|
| |||
| Rozrolimupab [52] | 25 recombinant human anti-RhD monoclonal antibodies | Multicenter phase I/II dose-escalation study showed 62% overall response in the group who received the highest dose | Hemolysis |
|
| |||
| SM101 [56] | Soluble FcγRIIB | Dose-escalation part of a phase Ib, randomized clinical trial in ITP: 0–25% required rescue mediation in the highest dose group compared with 42% on placebo. Sustained responses (> 50 × 109/L) were observed after one cycle | No dose limiting toxicity or serious adverse events observed |
|
| |||
| R788 [20] | Syk inhibitor | Open-label, single-arm cohort dose-escalation study: 8/16 patients, had a significant response and 4 had transient responses | Mild adverse effects–gastrointestinal symptoms and increased liver enzymes |
|
| |||
| Therapies targeting platelet production | |||
|
| |||
| Avatrombopag (E5501) [58] | Small molecule TPO receptor agonist | Multicenter, randomized, double-blind, placebo-controlled phase II study: 52.8% response rate | Mostly mild adverse effects - fatigue, headache, epistaxis |
|
| |||
| 23A11 [60] | Murine anti-VPAC1 monoclonal IgG1 antibody, improves thrombopoiesis | Efficacy in murine models of ITP | |
|
| |||
| Amifostine | Improve thrombopoiesis | In one study, all 24 patients showed a response and 22/24 achieved platelet counts > 100 × 109/L [62]. In another study, all 17 patients had achieved a platelet count response that persisted for 2 months after discontinuation [63] | Dizziness, nausea, vomiting, fatigue, and mild hypocalcemia |
Future directions
Targeting the neonatal FcR
Reducing the half-life of pathologic IgG is another potential method for treating autoimmune disease. The function of the neonatal FcR (FcRn) is to recycle IgG, thus effectively increasing its half-life [64]. Blocking FcRn may increase clearance of the pathogenic (and normal) IgG. In a murine model of myasthenia gravis, anti-FcRn mAb reduced the serum IgG concentration by approximately 40% and improved disease manifestations [65].
Interference with TNF signaling
TNF neutralizing therapies such as etanercept and infliximab have improved the treatment of certain autoimmune disorders [66,67]. Etanercept has been effective in a small number of ITP patients [68]. Recent discoveries of other ligands in the TNF family such as BAFF (B-cell activating factor belonging to the TNF family) and APRIL (a proliferation-inducing ligand) may also be promising for novel therapeutic targets. BAFF and APRIL are secreted by monocytes, dendritic cells, macrophages, and T-cells in response to inflammatory mediators [69]. They are required for B-cell survival during maturation in the spleen, and excess BAFF levels may lead to survival of autoreactive B-cells, autoantibody production, and development of autoimmune disease [70–72]. Studies of a humanized mAb that blocks BAFF have been effective in rheumatoid arthritis and systemic lupus erythematosus (SLE) [73,74]. Belimumab, a humanized mAb that blocks soluble BAFF, is now licensed for the treatment of SLE in several countries [75]; however, only a modest response has been reported for hematological manifestations of SLE [76]. Atacicept, a humanized fusion protein that binds both BAFF and APRIL, has had less success [74,77]. The utility of these agents in ITP is not yet known.
Induction of immune tolerance
The mechanisms of antibody response to autoantigens could reveal new targets for therapy. IgG responses that are driven primarily by CD4+ helper T-cells are associated with an anamnestic response to platelet autoantigens and loss of peripheral T-cell tolerance [78,79]. T-cells recognize short peptide antigens displayed on MHC class II molecules by antigen presenting cells [14] and subsequently stimulate B-cells to produce IgG [80,81]. Re-induction of tolerance can be achieved by exposing T-cells to soluble synthetic peptides containing MHC class II restricted epitopes, which can leads to the expansion of regulatory T-cells and deletion of antigen-specific memory cells [82].
Sukati et al. successfully mapped epitopes of GPIIIa that could stimulate T-cells from 31 ITP patients by synthesizing a panel of 86 overlapping peptides of GPIIIa [83]. While there was variability in the pattern of stimulation by peptides within and between patients, dominant sequences that induced proliferation were identified. Many of these sequences were predicted to have low affinity for restricting MHC molecules, suggesting that an inefficient presentation of self-peptides rather than antigen specificity may cause loss of tolerance [84,85]. These peptides may eventually form the basis of vaccine therapy in ITP [83].
Treatment with TPO receptor agonists may re-induce tolerance by increasing exposure to platelet antigens. In a retrospective study of 31 chronic ITP patients treated with either romiplostim or eltrombopag, Ghadaki et al. observed 9 patients who achieved a durable platelet count response after discontinuing the medication, including 3 patients who met all criteria for a TPO receptor agonist-induced remission. A progressive decline in platelet autoantibody titre was documented in one patient with ongoing exposure to the drug [86]. Another case series showed similar findings [87]. Durable remission after exposure to TPO receptor agonists may also be explained by improved regulatory T-cell function [28].
Conclusion
ITP is a heterogeneous disorder that is often associated with relapses and refractoriness to multiple therapies. Because of this, an individualized rather than an empiric approach to management may be beneficial. Given the significant morbidity associated with over-treatment of the disease, advancements in the management of ITP are necessary to improve the health and quality of life of patients. Novel agents that target specific steps in the mechanistic pathways may lead to more definitive treatments.
Acknowledgments
Funding support: this study was funded in part by the Canadian Institutes for Health Research in partnership with Amgen Canada (Grant #102446).
Footnotes
Disclosure of interest: D. Arnold: Amgen (consulting, research grants, speaker fees, advisory board); GlaxoSmithKline (research grant, speakers fees); Bristol Myers Squibb (advisory board); J. Kelton: GlaxoSmithKline (advisory board). The other authors declare that they have no conflicts of interest concerning this article.
References
- 1.Lee MS, Kim WC. Intracranial hemorrhage associated with idiopathic thrombocytopenic purpura: report of seven patients and a meta-analysis. Neurology. 1998;50:1160–3. doi: 10.1212/wnl.50.4.1160. [DOI] [PubMed] [Google Scholar]
- 2.Nrgaard M, Jensen AÕ, Engebjerg MC, Farkas DK, Thomsen RW, Cha S, et al. Long-term clinical outcomes of patients with primary chronic immune thrombocytopenia: a Danish population-based cohort study. Blood. 2011;117:3514–20. doi: 10.1182/blood-2010-10-312819. [DOI] [PubMed] [Google Scholar]
- 3.Kühne T, Berchtold W, Michaels LA, Wu R, Donato H, Espina B, et al. Newly diagnosed immune thrombocytopenia in children and adults: a comparative prospective observational registry of the Intercontinental Cooperative Immune Thrombocytopenia Study Group. Haematologica. 2011;96:1831–7. doi: 10.3324/haematol.2011.050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Portielje JE, Westendorp RG, Kluin-Nelemans HC, Brand A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood. 2001;97:2549–54. doi: 10.1182/blood.v97.9.2549. [DOI] [PubMed] [Google Scholar]
- 5.McMillan R, Bussel JB, George JN, Lalla D, Nichol JL. Self-reported health-related quality of life in adults with chronic immune thrombocytopenic purpura. Am J Hematol. 2008;83:150–4. doi: 10.1002/ajh.20992. [DOI] [PubMed] [Google Scholar]
- 6.Kuter DJ, Mathias SD, Rummel M, Mandanas R, Giagounidis AA, Wang X, et al. Health-related quality of life in nonsplenectomized immune thrombocytopenia patients receiving romiplostim or medical standard of care. Am J Hematol. 2012;87:558–61. doi: 10.1002/ajh.23163. [DOI] [PubMed] [Google Scholar]
- 7.Harrington WJ, Sprague CC, Minnich V, Moore CV, Aulvin RC, Dubach R. Immunologic mechanisms in idiopathic and neonatal thrombocytopenic purpura. Ann Intern med. 1953;38:433–69. doi: 10.7326/0003-4819-38-3-433. [DOI] [PubMed] [Google Scholar]
- 8.van Leeuwen EF, van der Ven JT, Engelfriet CP, von dem Borne AE. Specificity of autoantibodies in autoimmune thrombocytopenia. Blood. 1982;59:23–6. [PubMed] [Google Scholar]
- 9.Stasi R, Cooper N, Del Poeta G, Stipa E, Laura Evangelista M, Abruzzese E, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112:1147–50. doi: 10.1182/blood-2007-12-129262. [DOI] [PubMed] [Google Scholar]
- 10.Ling Y, Cao X, Yu Z, Ruan C. Circulating dendritic cells subsets and CD4+ Foxp3+ regulatory T cells in adult patients with chronic ITP before and after treatment with high-dose dexamethasome. Eur J Haematol. 2007;79:310–6. doi: 10.1111/j.1600-0609.2007.00917.x. [DOI] [PubMed] [Google Scholar]
- 11.Olsson B, Andersson PO, Jernas M, Jacobsson S, Carlsson B, Carlsson LM, et al. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9:1123–4. doi: 10.1038/nm921. [DOI] [PubMed] [Google Scholar]
- 12.Zhang F, Chu X, Wang L, Zhu Y, Li L, Ma D, et al. Cell-mediated lysis of autologous platelets in chronic idiopathic thrombocytopenic purpura. Eur J Haematol. 2006;76:427–31. doi: 10.1111/j.1600-0609.2005.00622.x. [DOI] [PubMed] [Google Scholar]
- 13.Kappler JW, Staerz U, White J, Marrack PC. Self-tolerance eliminates T cells specific for Mls-modified products of the major histo-compatibility complex. Nature. 1988;332:35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- 14.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–50. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 15.Chambers CA. The expanding world of co-stimulation: the two-signal model revisited. Trends Immunol. 2001;22:217–23. doi: 10.1016/s1471-4906(01)01868-3. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H, Chess L. An integrated view of suppressor T cell subsets in immunoregulation. J Clin Invest. 2004;114:1198–208. doi: 10.1172/JCI23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang M, Nakagawa PA, Williams SA, Schwartz MR, Imfeld KL, Buzby JS, et al. Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal auto-antibodies inhibit megakaryocytopoiesis in vitro. Blood. 2003;102:887–95. doi: 10.1182/blood-2002-05-1475. [DOI] [PubMed] [Google Scholar]
- 18.McMillan R, Longmire RL, Yelenosky R, Donnell RL, Armstrong S. Quantitation of platelet-binding IgG produced in vitro by spleens from patients with idiopathic thrombocytopenic purpura. N Engl J Med. 1974;291:812–7. doi: 10.1056/NEJM197410172911602. [DOI] [PubMed] [Google Scholar]
- 19.Clarkson SB, Bussel JB, Kimberly RP, Valinsky JE, Nachman RL, Unkeless JC. Treatment of refractory immune thrombocytopenic purpura with an anti-Fc gamma-receptor antibody. N Engl J Med. 1986;314:1236–9. doi: 10.1056/NEJM198605083141907. [DOI] [PubMed] [Google Scholar]
- 20.Podolanczuk A, Lazarus AH, Crow AR, Grossbard E, Bussel JB. Of mice and men: an open-label pilot study for treatment of immune thrombocytopenic purpura by an inhibitor of Syk. Blood. 2009;113:3154–60. doi: 10.1182/blood-2008-07-166439. [DOI] [PubMed] [Google Scholar]
- 21.Ballem PJ, Segal GM, Stratton JR, Gernsheimer T, Adamson JW, Slichter SJ. Mechanisms of thrombocytopenia in chronic autoimmune thrombocytopenic purpura. Evidence of both impaired platelet production and increased platelet clearance. J Clin Invest. 1987;80:33–40. doi: 10.1172/JCI113060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377:393–402. doi: 10.1016/S0140-6736(10)60959-2. [DOI] [PubMed] [Google Scholar]
- 23.Warner MN, Moore JC, Warkentin TE, Santos AV, Kelton JG. A prospective study of protein-specific assays used to investigate idiopathic thrombocytopenic purpura. Br J Haematol. 1999;104:442–7. doi: 10.1046/j.1365-2141.1999.01218.x. [DOI] [PubMed] [Google Scholar]
- 24.McMillan R, Wang L, Tani P. Prospective evaluation of the immunobead assay for the diagnosis of adult chronic immune thrombocytopenic purpura (ITP) J Thromb Haemost. 2003;1:485–91. doi: 10.1046/j.1538-7836.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- 25.Olsson B, Ridell B, Carlsson L, Jacobsson S, Wadenvik H. Recruitment of T cells into bone marrow of ITP patients possibly due to elevated expression of VLA-4 and CX3CR1. Blood. 2008;112:1078–84. doi: 10.1182/blood-2008-02-139402. [DOI] [PubMed] [Google Scholar]
- 26.Chow L, Aslam R, Speck ER, Kim M, Cridland N, Webster ML, et al. A murine model of severe immune thrombocytopenia is induced by antibody- and CD8+ T cell-mediated responses that are differentially sensitive to therapy. Blood. 2010;115:1247–53. doi: 10.1182/blood-2009-09-244772. [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Zhao H, Poon MC, Han Z, Gu D, Xu M, et al. Abnormality of CD4(+)CD25(+) regulatory T cells in idiopathic thrombocytopenic purpura. Eur J Haematol. 2007;78:139–43. doi: 10.1111/j.1600-0609.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 28.Bao W, Bussel JB, Heck S, He W, Karpoff M, Boulad N, et al. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116:4639–45. doi: 10.1182/blood-2010-04-281717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stasi R, Stipa E, Masi M, Cecconi M, Scimò MT, Oliva F, et al. Long-term observation of 208 adults with chronic idiopathic thrombocytopenic purpura. Am J Med. 1995;98:436–42. doi: 10.1016/s0002-9343(99)80342-8. [DOI] [PubMed] [Google Scholar]
- 30.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 31.Mazzucconi MG, Fazi P, Bernasconi S, De Rossi G, Leone G, Gugliotta L, et al. Therapy with high-dose dexamethasone (HD-DXM) in previously untreated patients affected by idiopathic thrombocytopenic purpura: a GIMEMA experience. Blood. 2007;109:1401–7. doi: 10.1182/blood-2005-12-015222. [DOI] [PubMed] [Google Scholar]
- 32.Arnold DM, Nazi I, Santos A, Chan H, Heddle NM, Warkentin TE, et al. Combination immunosuppressant therapy for patients with chronic refractory immune thrombocytopenic purpura. Blood. 2010;115:29–31. doi: 10.1182/blood-2009-06-222448. [DOI] [PubMed] [Google Scholar]
- 33.Arnold DM, Dentali F, Crowther MA, Meyer RM, Cook RJ, Sigouin C, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146:25–33. doi: 10.7326/0003-4819-146-1-200701020-00006. [DOI] [PubMed] [Google Scholar]
- 34.Patel VL, Mahevas M, Lee SY, Stasi R, Cunningham-Rundles S, Godeau B, et al. Outcomes 5 years after response to rituximab therapy in children and adults with im mu ne th rom bocytopenia. Blood. 2012;119:5989–95. doi: 10.1182/blood-2011-11-393975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kojouri K, Vesely SK, Terrell DR, George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood. 2004;104:2623–34. doi: 10.1182/blood-2004-03-1168. [DOI] [PubMed] [Google Scholar]
- 36.Stasi R, Bosworth J, Rhodes E, Shannon MS, Willis F, Gordon-Smith EC. Thrombopoietic agents. Blood Rev. 2010;24:179–90. doi: 10.1016/j.blre.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371:395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]
- 38.Bussel JB, Provan D, Shamsi T, Cheng G, Psaila B, Kovaleva L, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:641–8. doi: 10.1016/S0140-6736(09)60402-5. [DOI] [PubMed] [Google Scholar]
- 39.Kuter DJ, Bussel JB, Newland A, Baker RI, Lyons RM, Wasser J, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol. 2013;161:411–23. doi: 10.1111/bjh.12260. [DOI] [PubMed] [Google Scholar]
- 40.Saleh MN, Bussel JB, Cheng G, Meyer O, Bailey CK, Arning M, et al. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood. 2013;121:537–45. doi: 10.1182/blood-2012-04-425512. [DOI] [PubMed] [Google Scholar]
- 41.Siegal D, Crowther M, Cuker A. Thrombopoietin receptor agonists in primary immune thrombocytopenia. Semin Hematol. 2013;50(Suppl 1):S18–21. doi: 10.1053/j.seminhematol.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sprague DL, Elzey BD, Crist SA, Waldschmidt TJ, Jensen RJ, Ratliff TL. Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood. 2008;111:5028–36. doi: 10.1182/blood-2007-06-097410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Datta SK, Kalled SL. CD40-CD40 ligand interaction in autoimmune disease. Arthritis Rheum. 1997;40:1735–45. doi: 10.1002/art.1780401002. [DOI] [PubMed] [Google Scholar]
- 44.Kuwana M, Nomura S, Fujimura K, Nagasawa T, Muto Y, Kurata Y, et al. Effect of a single injection of humanized anti-CD154 monoclonal antibody on the platelet-specific autoimmune response in patients with immune thrombocytopenic purpura. Blood. 2004;103:1229–36. doi: 10.1182/blood-2003-06-2167. [DOI] [PubMed] [Google Scholar]
- 45.Patel VL, Schwartz J, Bussel JB. The effect of anti-CD40 ligand in immune thrombocytopenic purpura. Br J Haematol. 2008;141:545–8. doi: 10.1111/j.1365-2141.2008.07039.x. [DOI] [PubMed] [Google Scholar]
- 46.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 47.Mirabet M, Barrabes JA, Quiroga A, Garcia-Dorado D. Platelet pro-aggregatory effects of CD40L monoclonal antibody. Mol Immunol. 2008;45:937–44. doi: 10.1016/j.molimm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Debre M, Bonnet MC, Fridman WH, Carosella E, Philippe N, Reinert P, et al. Infusion of Fc gamma fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet. 1993;342:945–9. doi: 10.1016/0140-6736(93)92000-j. [DOI] [PubMed] [Google Scholar]
- 49.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–6. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 50.Flaherty MM, MacLachlan TK, Troutt M, Magee T, Tuaillon N, Johnson S, et al. Nonclinical evaluation of GMA161–an anti-human CD16 (FcgammaRIII) monoclonal antibody for treatment of autoimmune disorders in CD16 transgenic mice. Toxicol Sci. 2012;125:299–309. doi: 10.1093/toxsci/kfr278. [DOI] [PubMed] [Google Scholar]
- 51.Frandsen TP, Naested H, Rasmussen SK, Hauptig P, Wiberg FC, Rasmussen LK, et al. Consistent manufacturing and quality control of a highly complex recombinant polyclonal antibody product for human therapeutic use. Biotechnol Bioeng. 2011;108:2171–81. doi: 10.1002/bit.23166. [DOI] [PubMed] [Google Scholar]
- 52.Robak T, Windyga J, Trelinski J, von Depka Prondzinski M, Giagounidis A, Doyen C, et al. Rozrolimupab, a mixture of 25 recombinant human monoclonal RhD antibodies, in the treatment of primary immune thrombocytopenia. Blood. 2012;120:3670–6. doi: 10.1182/blood-2012-06-438804. [DOI] [PubMed] [Google Scholar]
- 53.Kyttaris VC, Tsokos GC. Syk kinase as a treatment target for therapy in autoimmune diseases. Clin Immunol. 2007;124:235–7. doi: 10.1016/j.clim.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greuber EK, Pendergast AM. Abl family kinases regulate FcgammaR-mediated phagocytosis in murine macrophages. J Immunol. 2012;189:5382–92. doi: 10.4049/jimmunol.1200974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13-aminoacid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature. 1994;368:70–3. doi: 10.1038/368070a0. [DOI] [PubMed] [Google Scholar]
- 56.Konstaninova TS, Leonidovna IV, Hellmann A, et al. Interim results from a phase Ib/IIa clinical trial with the soluble Fc-Gamma IIb receptor SM101 for the treatment of primary immune thrombocytopenia. Blood. 2012;120 Abstract 3388. [Google Scholar]
- 57.Desjardins RE, Tempel DL, Lucek R, Kuter DJ. Single and multiple oral doses of AKR-501 (YM477) increase the platelet count in healthy volunteers. Blood. 2006;108 Abstract 477. [Google Scholar]
- 58.Bussel J, Zhang Y, Tang S, McIntosh J, Kuter D. A phase II 6-month extension study of the efficacy, safety, and tolerability of E5501 (AKR501) in subjects with chronic immune thrombocytopenia (CITP) Haematologica. 2011;96 Abstract 0221. [Google Scholar]
- 59.Kuter DJ. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol. 2013;98:10–23. doi: 10.1007/s12185-013-1382-0. [DOI] [PubMed] [Google Scholar]
- 60.Peeters K, Loyen S, Van Kerckhoven S, Stoffels K, Hoylaerts MF, Van Geet C, et al. Thrombopoietic effect of VPAC1 inhibition during megakaryopoiesis. Br J Haematol. 2010;151:54–61. doi: 10.1111/j.1365-2141.2010.08327.x. [DOI] [PubMed] [Google Scholar]
- 61.Santini V, Giles FJ. The potential of amifostine: from cytoprotectant to therapeutic agent. Haematologica. 1999;84:1035–42. [PubMed] [Google Scholar]
- 62.Fan H, Zhu HL, Li SX, Lu XC, Zhai B, Guo B, et al. Efficacy of amifostine in treating patients with idiopathic thrombocytopenia purpura. Cell Biochem Biophys. 2011;59:7–12. doi: 10.1007/s12013-010-9100-5. [DOI] [PubMed] [Google Scholar]
- 63.Fan H, Zhu HL, Li SX, Lu XC, Yang Y, Yao SQ. Therapy of 17 cases of idiopathic thrombocytopenia purpura by amifostine. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008;16:192–6. [PubMed] [Google Scholar]
- 64.Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG - Fc-coupled drugs. J Immunol. 2003;170:3528–33. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- 65.Liu L, Garcia AM, Santoro H, Zhang Y, McDonnell K, Dumont J, et al. Amelioration of experimental autoimmune myasthenia gravis in rats by neonatal FcR blockade. J Immunol. 2007;178:5390–8. doi: 10.4049/jimmunol.178.8.5390. [DOI] [PubMed] [Google Scholar]
- 66.Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–9. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 67.Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–93. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- 68.McMinn JR, Jr, Cohen S, Moore J, Lilly S, Parkhurst J, Tarantino MD, et al. Complete recovery from refractory immune thrombocytopenic purpura in three patients treated with etanercept. Am J Hematol. 2003;73:135–40. doi: 10.1002/ajh.10331. [DOI] [PubMed] [Google Scholar]
- 69.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–9. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 72.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–98. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 73.Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–31. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 74.Davidson A. Targeting BAFF in autoimmunity. Curr Opin Immunol. 2010;22:732–9. doi: 10.1016/j.coi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harvey PR, Gordon C. B-cell targeted therapies in systemic lupus erythematosus: successes and challenges. BioDrugs. 2013;27:85–95. doi: 10.1007/s40259-013-0015-8. [DOI] [PubMed] [Google Scholar]
- 76.Manzi S, Sanchez-Guerrero J, Merrill JT, Furie R, Gladman D, Navarra SV, et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two-phase III trials. Ann Rheum Dis. 2012;71:1833–8. doi: 10.1136/annrheumdis-2011-200831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ginzler EM, Wax S, Rajeswaran A, Copt S, Hillson J, Ramos E, et al. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res Ther. 2012;14:R33. doi: 10.1186/ar3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Semple JW, Freedman J. Increased anti-platelet T helper lymphocyte reactivity in patients with autoimmune thrombocytopenia. Blood. 1991;78:2619–25. [PubMed] [Google Scholar]
- 79.Filion MC, Bradley AJ, Devine DV, Decary F, Chartrand P. Autoreactive T cells in healthy individuals show tolerance in vitro with characteristics similar to but distinct from clonalanergy. Eur J Immunol. 1995;25:3123–7. doi: 10.1002/eji.1830251120. [DOI] [PubMed] [Google Scholar]
- 80.Kuwana M, Kaburaki J, Ikeda Y. Autoreactive T cells to platelet GPIIb-IIIa in immune thrombocytopenic purpura. Role in production of anti-platelet autoantibody. J Clin Invest. 1998;102:1393–402. doi: 10.1172/JCI4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuwana M, Okazaki Y, Kaburaki J, Kawakami Y, Ikeda Y. Spleen is a primary site for activation of platelet-reactive T and B cells in patients with immune thrombocytopenic purpura. J Immunol. 2002;168:3675–82. doi: 10.4049/jimmunol.168.7.3675. [DOI] [PubMed] [Google Scholar]
- 82.Larche M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med. 2005;11:S69–76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- 83.Sukati H, Watson HG, Urbaniak SJ, Barker RN. Mapping helper T-cell epitopes on platelet membrane glycoprotein IIIa in chronic autoimmune thrombocytopenic purpura. Blood. 2007;109:4528–38. doi: 10.1182/blood-2006-09-044388. [DOI] [PubMed] [Google Scholar]
- 84.Liu GY, Fairchild PJ, Smith RM, Prowle JR, Kioussis D, Wraith DC. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 1995;3:407–15. doi: 10.1016/1074-7613(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 85.Fairchild PJ, Wraith DC. Lowering the tone: mechanisms of immunodominance among epitopes with low affinity for MHC. Immunol Today. 1996;17:80–5. doi: 10.1016/0167-5699(96)80584-6. [DOI] [PubMed] [Google Scholar]
- 86.Ghadaki B, Nazi I, Kelton JG, Arnold DM. Sustained remissions of immune thrombocytopenia associated with the use of thrombopoietin receptor agonists. Transfusion. 2013 doi: 10.1111/trf.12139. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thachil J, Salter I, George JN. Complete remission of refractory immune thrombocytopenia (ITP) with a short course of Romiplostim. Eur J Haematol. 2013;91:376–7. doi: 10.1111/ejh.12165. [DOI] [PubMed] [Google Scholar]