Abstract
Introduction
People with vestibular disorders often experience space and motion discomfort when exposed to moving or highly textured visual scenes. The purpose of this study was to measure the type and severity of symptoms in people with vestibular dysfunction during coordinated head and eye movements in optic flow environments.
Methods
Seven subjects with vestibular disorders and 25 controls viewed four different full-field optic flow environments on six different visits. The optic flow environments consisted of textures with various contrasts and spatial frequencies. Subjects performed 8 gaze movement tasks, including eye saccades, gaze saccades, and gaze stabilization tasks. Subjects reported symptoms using Subjective Units of Discomfort (SUD) and the Simulator Sickness Questionnaire (SSQ). Self-reported dizziness handicap and space and motion discomfort were also measured.
Results/ Conclusion
Subjects with vestibular disorders had greater discomfort and experienced greater oculomotor and disorientation symptoms. The magnitude of the symptoms increased during each visit, but did not depend on the optic flow condition. Subjects who reported greater dizziness handicap and space motion discomfort had greater severity of symptoms during the experiment. Symptoms of fatigue, difficulty focusing, and dizziness during the experiment were evident. Compared with controls, subjects with vestibular disorders had less head movement during the gaze saccade tasks. Overall, performance of gaze pursuit and gaze stabilization tasks in moving visual environments elicited greater symptoms in subjects with vestibular disorders compared with healthy subjects.
Keywords: VOR, virtual reality, simulator sickness
INTRODUCTION
People with vestibular disorders often experience symptoms of disorientation in full-field visual environments or in situations with moving visual patterns.[3, 4, 9] These symptoms have been defined as “visual vertigo”,[4, 27] and “space and motion discomfort”.[6, 10-12] Supermarkets, crowds, or even fence posts viewed within a person's peripheral vision can cause significant distress (increased dizziness, nausea, or even disorientation) in persons with complaints of visual vertigo.[4, 5, 19] Symptoms of visual vertigo have also been reported in persons without vestibular dysfunction.[9, 16]
One proposed explanation of visual vertigo is an over-reliance on vision for postural orientation in people with vestibular dysfunction.[3, 4, 9, 19] Others have suggested that visual vertigo occurs in patients with vestibular disorders who are visually dependent and who have difficulty with vestibular-proprioceptive and visual cues that are conflicting.[9] Bronstein [5] has suggested that when pursuit-optokinetic eye movements and the vestibulo-ocular reflex (VOR) are in conflict, instead of working synergistically, visual input may suppress the VOR. An example where visual input may suppress the VOR is sitting on a moving bus while reading.[5] The sensation of vection (i.e. perception that person is moving rather than the visual surroundings) suggests that it is difficult sometimes for the CNS to suppress inappropriate sensory cues.[15] It is possible that a mismatch between perceived movement in the visual field and vestibular sensation of head movement is partly responsible for the symptoms of disorientation that persons with vestibular disorders experience.
A hallmark of vestibular rehabilitation is to gradually habituate people with vestibular disorders to more challenging motor and sensory exercises over time. [7, 8, 17, 18, 21-23] Habituation exercises may be instrumental for reducing symptoms in visual vertigo that arise from sensory conflict. Bronstein[4] and Pavlou et al.[19] have suggested that optic flow and visual/vestibular conflict exercises may enhance the rehabilitation of persons experiencing visual vertigo. Optokinetic stimulation has been shown to decrease symptoms in persons with vestibular dysfunction.[26, 27]
Despite the identification of visual vertigo and space and motion discomfort over ten years ago, many aspects of these conditions are not well understood. For instance, what are the contributions of the visual environment (contrast and optic flow speed), and duration of exposure (both within and between sessions) to symptom generation. Understanding these factors will help us to design clinical trials that use optic flow or virtual reality for habituation. The primary purpose of this study was to examine which environmental factors were related to symptoms elicited in people with vestibular disorders as they performed gaze movements in optic flow environments. A secondary objective was to see if gaze behavior differed between subjects with vestibular disorders and controls. Finally, we examined how the reported symptoms related to validated self-report and performance measures.
METHODS
Subjects
Seven subjects with vestibular disorders and 25 control subjects agreed to participate in a study that had been approved by the University of Pittsburgh Institutional Review Board. The age range was 27-77 years (mean = 53, sd 18) for the patients and 22-83 years (mean = 52, sd 18) for the control subjects. The vestibular disorder group consisted of 4 women and 3 men and the control subjects consisted of 12 women and 13 men. One subject had undergone a resection of a left acoustic neuroma and the remaining subjects had various non-surgical vestibular pathologies. Demographic characteristics and vestibular function test results of the subjects are displayed in Table 1. Diagnoses were made by an otoneurologist. Time from symptom onset ranged from 4-14 months, except for the person with left acoustic neuroma, who was 6 years post resection. All patients and control subjects underwent oculomotor, positional, binaural bithermal closed-loop caloric irrigation, and sinusoidal earth-vertical axis rotational testing at frequencies between 0.02 and 0.16 HZ. The control subjects were free of spontaneous and gaze-evoked nystagmus, had normal saccadic eye movements, optokinetic nystagmus, and smooth pursuits. Control subjects were within age-adjusted normal for all vestibular testing results and audiometry.
Table 1.
Demographic characteristics and vestibular function test results for subjects with vestibular disorders.
| Sub | Age | Gender | Symptom duration (months) | Caloric | Rotational Gain | Diagnosis |
|---|---|---|---|---|---|---|
| P1 | 27 | F | 8 | Left 54% RVR | 0.43 | Left peripheral vestibular dysfunction of uncertain etiology |
| P2 | 35 | F | 5 | Right 54% RVR | 0.14 | Right vestibular neuritis |
| P3 | 45 | F | 6 | Right 73% RVR | 0.25 | Right vestibular neuritis |
| P4 | 59 | M | 6 | Left 42% RVR | 0.68 | Left labyrinthine concussion |
| P5 | 62 | M | 4 | Right 13% RVR | 0.56 | Uncompensated non-localized peripheral vestibular dysfunction* |
| P6 | 69 | M | 85 | Left 87% RVR | 0.25 | Left acoustic neuroma resection |
| P7 | 77 | F | 8 | Left 57% RVR | 0.21 | Left labyrinthine infarction |
based on direction-fixed persistent positional nystagmus
Procedures
Clinical Tests
Prior to the first test day, subjects with vestibular disorders completed two self-report measures of how dizziness symptoms affect daily activities and two balance performance measures. The self-report measures were the Dizziness Handicap Inventory (DHI) and Situational Characteristics Questionnaire (SCQ). The DHI measures self-perception of handicap due to dizziness, across physical, functional and emotional domains.[13] The SCQ has 3 subscales that provide information about space and motion discomfort (SMD-1 and SMD-2) and also agoraphobic discomfort (SMD-AG) that is not related to space and motion symptoms.[10, 11] Higher scores represent worse function for the DHI and SCQ. The balance performance measures included the Dynamic Gait Index (DGI) and Sensory Organization Test (SOT, Neurocom, Inc.). The DGI assesses the amount of impairment in subjects walking while moving their head or avoiding obstacles. [24] The SOT is a type of computerized dynamic posturography that examines the ability of subjects to maintain upright stance during different sensory conditions. Higher scores indicate better function for the DGI and SOT.
Experimental Tests
Subjects performed visually-cued gaze movements while standing in a Balance NAVE Automatic Virtual Environment (BNAVE), a 180° wide field of view projection-based display system that was developed to investigate multi-sensory interactions in postural control (Figure 1).[25, 28] Subjects performed the same tasks on each of six visits, with at least 2 days between each visit. A different visual environment was used on each visit.[25] During the first visit, the visual environment was a solid gray texture. During the second visit, a stationary pattern of high contrast black and white stripes was displayed. During visits 3 to 6, the environment consisted of stripes moving toward the subjects at a velocity of 0.5 m/s. Therefore, moving stripes created an optic flow field that simulated the perception of moving through a highly-patterned corridor. The 4 combinations of contrast (2 levels) and spatial frequency (2 levels) of the environment varied on each of the last 4 visits. High and low levels of contrast were based on the relative shading of the stripes. The high contrast level had alternating black and white stripes with luminances of 1 and 170 cd/m2, respectively; the low contrast level had alternating intermediate shades of gray, with luminances of 15 and 34 cd/m2. The low contrast condition was based on average measurements of luminance obtained from products sampled at a local grocery store. High (4.2 cycles/meter) and low (1.4 cycles/meter) levels of spatial frequency were based on the width of the stripes; the high spatial frequency had three times as many stripes as the low spatial frequency.
Figure 1.
The subject is standing in a full field of view BNAVE while viewing Optic Flow conditions of high contrast with high (left) and low (right) spatial frequency.
Subjects performed a total of 8 different head and eye coordination tasks during each visit; each task was performed as one trial of data collection for approximately 90 s. After each task, the subject sat and rested for 3 minutes. The tasks were as follows:
Calibration task (always performed as first trial of each day)
A calibration of the head tracker was performed by having the subjects move their head in the yaw plane from 60 deg to the left of midline to 60 deg to the right of midline in 10 deg increments. A target consisting of a yellow star superimposed on a black square that subtended 1° of visual arc was placed at eye level at the requested yaw position, and provided the head movement cue.
Control task (always performed as second trial of each day)
Head remained stationary while subject viewed optic flow.
The remaining 6 tasks were randomized during trials 3 to 8.
Eye Saccade task - midline
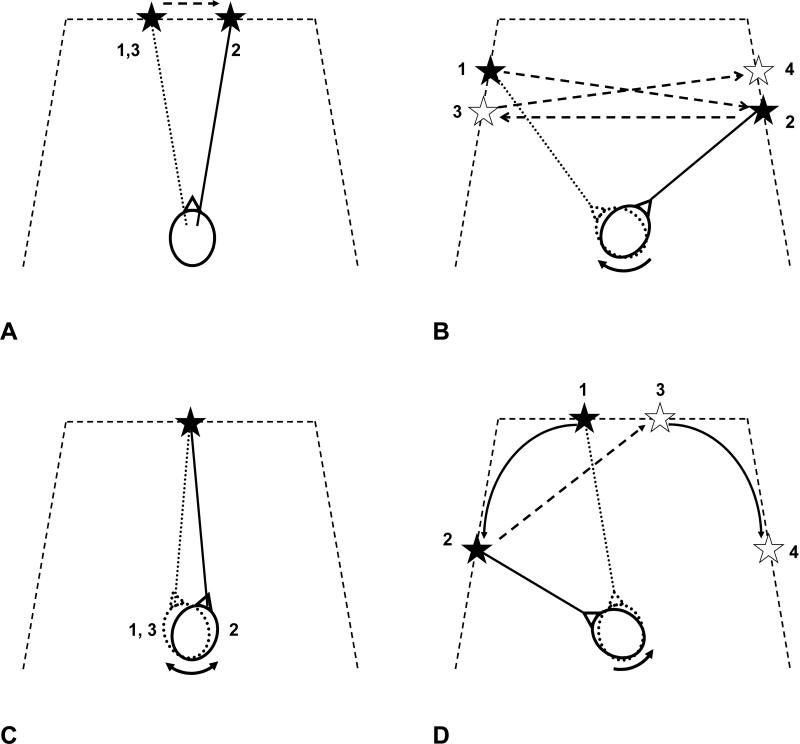
Subjects maintained head position constant at 0° (i.e. straight forward) while fixating the eyes on a target that moved ±10° horizontally from the midline at a random interval of 3-6 seconds (Figure 2A). During the 90-second trial, about nine saccades were performed to both the right and to the left.
Figure 2.
Schematic of different head and eye movement tasks. A) Eye saccade task: subjects shifted gaze using eye movements only when target moved ±10° from midline at random 3 to 6 s intervals. B) Gaze saccade task: subjects shifted gaze using head and eye movements when target moved ±40° and ±50° from midline at random 3 to 6 s intervals. C) Gaze stabilization task: subjects fixed gaze on stationary target while moving head at 0.25 Hz. D) Gaze pursuit task: subjects viewed target as it moved from central viewing area of display to 60° left and right of midline.
Eye Saccade task - left
Same as Eye Saccade Task-midline with head yaw position at 50 deg left of midline.
Eye Saccade task - right
Same as Eye Saccade Task-midline with head yaw position at 50 deg right of midline.
Gaze Saccade task
Subjects fixated on a target that moved ± 40° and 50° horizontally from midline at a random interval of 3-6 seconds (Figure 2B). Subjects were told to perform the task primarily with head movement, i.e. to point their nose at the target throughout the 90 second trial. The gaze saccades consisted of the following movements from midline (looking straight forward): −40° to +50°, +50° to −50°, −50° to +40°, +40° to −40°.
Gaze Stabilization task
Subjects continuously fixated on a stationary target placed at 0° while moving the head approximately ±20° in the yaw plane at a frequency of about 0.25 Hz (Figure 2C). Prior to testing, all participants practiced this task while being paced with a metronome until the subject had maximized their ability to replicate the timing of the metronome.
Gaze pursuit task
Subjects continuously pursued a target that first moved from 10° to 60° left of midline, and then from 10° to 60° right of midline (Figure 2D). The target moved at a velocity that increased from 0-20°/s, as the target moved from the center to the periphery. Each trial for this task had 4 leftward and 3 rightward movements from 10° to 60°. Subjects were told to perform the task primarily with head movement.
Symptom Measures
Prior to the first trial and after every trial during the rest break, the Subjective Units of Discomfort measure (SUD, 0-100 range) was rated according to how much “anxiety” the subject perceived during the trial. Likewise, the Simulator Sickness Questionnaire (SSQ) was completed.[14] The SSQ contains 16 items on which subjects rate the degree of particular symptoms on a severity scale from 0 to 3 (0=none, 1=slight, 2=moderate, 3=severe). The SSQ has three subscales that were comprised of the following symptom types: nausea (general discomfort, increased salivation, sweating, nausea, difficulty concentrating, stomach awareness, burping), oculomotor stress (general discomfort, fatigue, headache, eyestrain, difficulty focusing, difficulty concentrating, blurred vision), and disorientation (difficulty focusing, nausea, head fullness, blurred vision, dizzy with eyes open, dizzy with eyes closed, vertigo). For each subscale, the sum of the ratings for each of the items in the subscale was computed.
Data Analysis
Head and eye data collection
Initially, video-oculography (VOG) was used to record eye movements during the tasks. However, this method was discontinued after the first 12 control subjects were tested because of several complaints of headache that occurred while wearing the VOG equipment for an extended period of time. We subsequently switched to the use of electro-oculography (EOG) for the remaining 13 controls and the 7 subjects with vestibular dysfunction. Horizontal eye-in-head position was measured using standard EOG techniques. After cleansing the skin with alcohol, Ag-AgCl surface electrodes were placed lateral to the lateral angle of each eye, with the reference electrode placed in the midline superior to the nasion. The EOG signal was amplified (gain = 5000) and low pass filtered using a cutoff frequency of 40 Hz. The EOG data were filtered (Butterworth low pass filter, 4th order, phaseless, cutoff frequency = 25 Hz) and differentiated to compute the eye velocity. The cutoff frequency was determined by a residual analysis. The EOG signal was recorded by a digital computer at a sampling rate of 120 Hz.
Head yaw position was measured using a 6-DOF electromagnetic tracker (Polhemus Fastrak), sampled at 120 Hz. The head position data were low-pass filtered using a 4th order low-pass phaseless Butterworth filter with a cutoff frequency of 5 Hz. Head yaw velocity was computed using single difference numerical differentiation. The magnitude of yaw position excursions and peak velocity of head movements were computed for all conditions.
Peak eye velocities were computed for each of the saccades performed in the left and right directions. Mean values for each direction were then tabulated for each testing visit/visual environment.
Statistical analysis
The primary hypothesis related to the effect of experimental factors on the symptom ratings. Because of the small number of subjects and the non-normal distribution of the ratings, nonparametric statistics were used to examine the effect of the independent variables on the symptom measures; consequently, only main effects were able to be examined. The frequency of non-zero responses and average SUD and SSQ ratings were computed for each subject across each level of the independent variable. The Mann-Whitney U test for independent samples was used to examine the effect of subject group on the symptom ratings. The Friedman test for related samples was used to determine the effect of Visit (6 levels), Optic Flow condition (6 levels), Trial number (9 levels), and Gaze task (8 levels) on the symptom ratings. The false discovery rate method was used to adjust the p-value for multiple tests, using a familywise error rate of α = 0.05.[2]
Similar non-parametric analyses (i.e. the Mann-Whitney U test for between group main effects and the Friedman test for within-group main effects) were conducted for the head and eye movements during selected tasks. In particular, peak eye velocity was investigated during the eye saccade task performed with head at midline. The head excursion and peak velocity in the yaw plane were examined during the gaze saccade task. Finally, the peak head velocity in the yaw plane was computed during the gaze stabilization task.
To gain a greater understanding of the relationship between the symptom ratings that were elicited in the environment and the functional difficulties experienced by the subjects with vestibular dysfunction, a post-hoc exploratory analysis was conducted. Spearman correlation coefficients were computed between the average symptom rating (SUD, SSQ) and the DHI, SCQ, DGI, and SOT.
RESULTS
Symptom ratings
The average percentage of trials in which each subject had a symptom rating greater than zero is shown in Table 2. Subjects with vestibular disorders had a significantly higher proportion of non-zero responses for the SUD and the oculomotor and disorientation subscales of the SSQ. Accordingly, average SUD ratings were greater in subjects with vestibular disorders compared with control subjects (Table 3). Likewise, subjects with vestibular disorders had significantly greater Oculomotor and Disorientation subscales of the SSQ. Specific items that were most frequently cited were dizziness with eyes open (51% of patient trials) and closed (29%), general discomfort (41%), fatigue (24%) and difficulty focusing (22%).
Table 2.
The mean (SD) percentage of non-zero responses for the Subjective Units of Discomfort (SUD) and the Simulator Sickness Questionnaire (SSQ) for controls (CON) and subjects with vestibular disorders (VEST), across all trials and visits.
| Symptom Scale | CON | VEST | p |
|---|---|---|---|
| SUD | 15 (19) | 60 (37) | 0.006* |
| SSQ | |||
| Nausea | 11 (17) | 43 (43) | 0.135 |
| Oculomotor | 20 (25) | 55 (35) | 0.023* |
| Disorientation | 5 (8) | 56 (42) | 0.015* |
The asterisks indicate significant findings, using the false discovery rate method to correct for the multiple comparisons, using a familywise error rate of α = 0.05 for the grouping of 4 symptom scales.[2]
Table 3.
The mean (SD) Subjective Units of Discomfort (SUD) and summed Simulator Sickness Questionnaire (SSQ) ratings given by controls (CON) and subjects with vestibular disorders (VEST), averaged across all trials and visits.
| Symptom Scale | CON | VEST | p |
|---|---|---|---|
| SUD | 0.25 (0.7) | 1.4 (1.8) | 0.020* |
| SSQ | |||
| Nausea | 0.2 (0.5) | 1.1 (2.0) | 0.112 |
| Oculomotor | 0.4 (1.2) | 1.5 (2.2) | 0.023* |
| Disorientation | 0.1 (0.5) | 1.7 (2.4) | 0.018 |
The asterisks indicate significant findings, using the false discovery rate method to correct for the multiple comparisons, using a familywise error rate of α = 0.05 for the grouping of 4 symptom scales.[2]
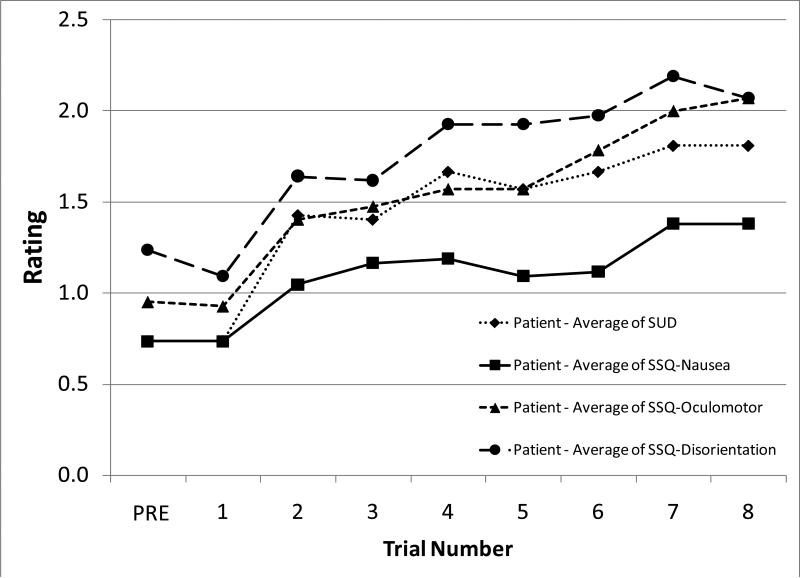
Given the small amount of symptoms experienced by the control subjects, examination of the influence of Visit, Optic Flow condition, Gaze task, and Trial number on the symptom measures was limited to the subjects with vestibular disorders. Neither Visit nor Optic Flow condition had a significant effect on the frequency or magnitude of symptoms. However, there were significant increases in the magnitude of ratings as a function of the Trial number, most notably after trial 1 (Figure 3, SUD p = 0.029, SSQ-Nausea p = 0.016, SSQ-Oculomotor p = 0.003, SSQ-Disorientation p = 0.013). The amount of change in ratings from before trial 1 to the final rating after trial 8 did not depend on how symptomatic the subjects were at the beginning of the visit. When the effect of the Gaze task was investigated, a general increase in ratings for all tasks compared with the first task (i.e. the calibration task) was observed. None of the individual Gaze tasks elicited substantially greater ratings than the others.
Figure 3.
Mean Subjective Units of Discomfort (SUD) and Simulator Sickness Questionnaire ratings for subjects with vestibular disorders, across trials.
Head and Eye Movements
There was no difference in head and eye movement parameters due to the effect of Visit or Optic Flow condition.
Eye Saccade Task
There was no difference in the peak eye velocity between the subjects with vestibular disorders (peak eye velocity: 388 ± 68 °/s to the right, 396 ± 78 °/s to the left) and controls (n=13; peak eye velocity: 379 ± 71 °/s to the right, 376 ± 65 °/s to the left) for either direction of eye movement.
Gaze Saccade Task
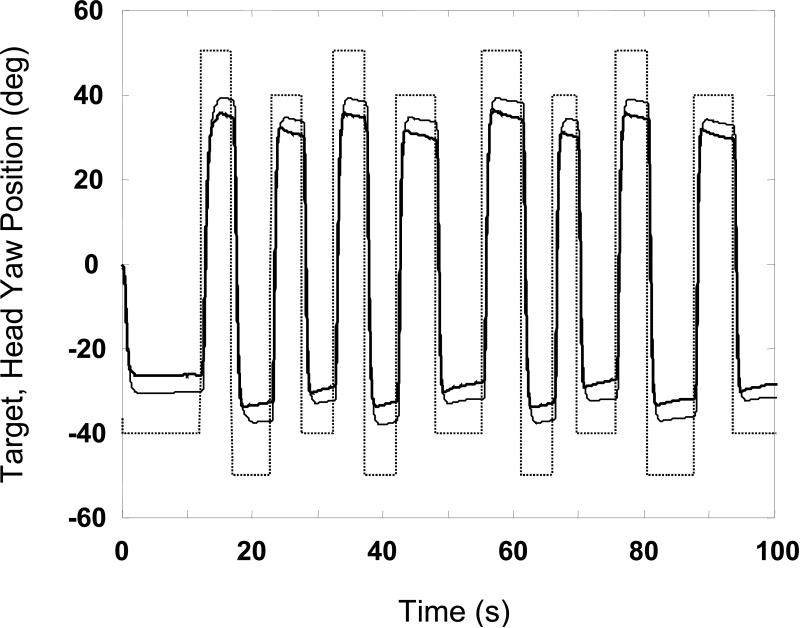
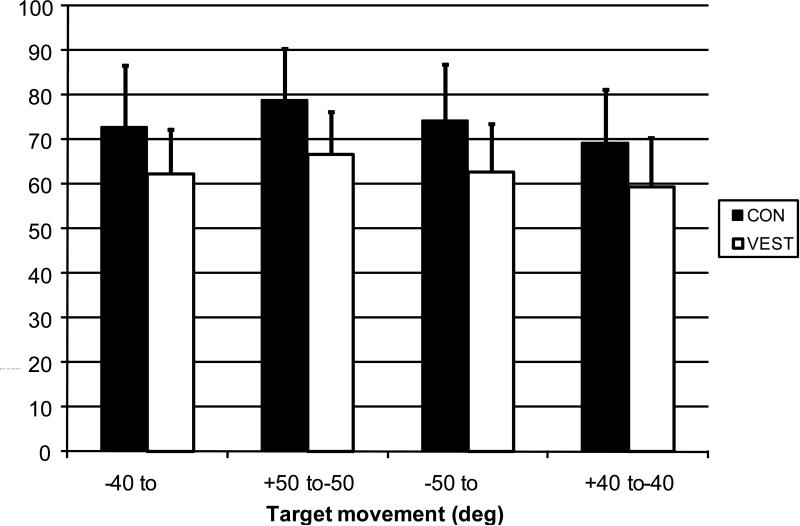
The ensemble average time series of the yaw head position during the gaze saccade task for the subjects with vestibular disorders and controls are shown in Figure 4. The plot reveals that the total excursion of the movements was greater in the control subjects compared with the vestibular subjects. The peak head yaw excursion was 10 to 12 degrees greater (p < 0.05) in the control subjects compared with the subjects with vestibular disorders across the four types of movement (Figure 5). However, there was no statistical difference in the timing or peak velocity between subject groups for any of the left or right head movements.
Figure 4.
Target (dashed line) and mean head yaw position of controls (solid, thin line) and subjects with vestibular disorders (solid, thick line) obtained during gaze saccade trials. Tracing for each group is ensemble average computed by pooling across subjects and visits.
Figure 5.
Mean peak head yaw excursion amplitude obtained during gaze saccades to the right (+ 40°, + 50°) and left (−40°, −50°) of midline.
CON: control subjects. VEST: subjects with vestibular disorders. Error bars: standard deviation of each subject group.
Gaze Stabilization Task
The peak head yaw position and velocity of head movements did not differ between subject groups (control subjects: mean peak head velocity to the right was 42°/s ± 10.3; to the left was 41°/s ± 10.7; Patients with vestibular disorders: mean peak head velocity to the right was 46°/s ± 10.2; to the left was 45°/s ± 8.3).
Correlation of symptoms with clinical measures
Subjects with vestibular disorders completed self-reports of dizziness handicap (DHI) and space and motion discomfort (SCQ). Scores displayed in Table 4 demonstrate large variation in scores, indicating that this sample of subjects with vestibular disorders had very different experiences with their dizziness. The 4 self-report measures (DHI and 3 subscales of the SCQ) were highly correlated with one another (Spearman ρ > 0.76, p < 0.05). Based on a sum of the rank order of these 4 scores, subjects were assigned a single rank order of self-reported dizziness and space and motion symptoms. Likewise, subjects were assigned a single rank order of balance function based on their performance of standing (SOT) and walking (DGI) balance function (Spearman ρ = 0. 6, p = 0.15). Finally, SUD and SSQ ratings obtained during the head and eye movements performed in the optic flow environment were compared with the summary symptom severity and balance function rank order measures (Table 5). Correlation analysis revealed that the SUD and SSQ ratings were significantly correlated with the self-report measures of dizziness and space and motion discomfort, indicating that subjects who had greater self-reported dysfunction also had greater symptom ratings in the optic flow environments. However, the symptom ratings were not correlated with balance function (Table 6). None of the symptom ratings were correlated with any of the gaze movement variables.
Table 4.
Clinical measures of self-reported dizziness handicap (DHI), space and motion discomfort (SMD-1, SMD-2, and SMD-AG), and balance performance assessed with Dynamic Gait Index (DGI) and Sensory Organization Test composite (SOT). The rank is a summary measure computed from the sum of the ranks of the 4 self-report and 2 balance performance measures. Higher numbers of rank indicate greater dysfunction.
| Sub | Self-Report | Balance | ||||||
|---|---|---|---|---|---|---|---|---|
| DHI | SMD-1 | SMD-2 | SMD-AG | Rank | DGI | SOT | Rank | |
| P7 | 0 | 0 | 0 | 0 | 1 | 15 | 38 | 7 |
| P6 | 8 | 0 | 0 | 1.1 | 2 | 22 | 68 | 6 |
| P4 | 12 | 0 | 1 | 5.6 | 3 | 23 | 76 | 2 |
| P1 | 8 | 4.3 | 1 | 2.2 | 4 | 24 | 90 | 1 |
| P2 | 32 | 1.4 | 4.5 | 7.8 | 5 | 23 | 64 | 3.5 |
| P3 | 60 | 7.1 | 5 | 14.4 | 6 | 16 | 73 | 5 |
| P5 | 72 | 8.6 | 7 | 23.3 | 7 | 22 | 72 | 3.5 |
Table 5.
Comparison of self-report and balance measures with average Subjective Units of Discomfort (SUD) and Simulator Symptom Questionnaire (SSQ) ratings.
| Sub | Self-Report Rank | Balance Rank | SUD | SSQ Nausea | SSQ Oculomotor | SSQ Disorientation |
|---|---|---|---|---|---|---|
| P7 | 1 | 7 | 0.7 | 0.3 | 0.6 | 0.6 |
| P6 | 2 | 6 | 0.0 | 0.0 | 0.0 | 0.0 |
| P4 | 3 | 2 | 0.2 | 0.0 | 0.3 | 0.0 |
| P1 | 4 | 1 | 0.7 | 0.1 | 0.9 | 0.8 |
| P2 | 5 | 3.5 | 1.8 | 0.7 | 1.8 | 1.5 |
| P3 | 6 | 5 | 2.9 | 2.1 | 4.1 | 4.6 |
| P5 | 7 | 3.5 | 3.8 | 4.5 | 3.1 | 4.7 |
Table 6.
Spearman Rank-order Correlations between self-report and balance measures and symptom ratings indicated by Subjective Units of Discomfort (SUD) and Simulator Symptom Questionnaire (SSQ).
| SUD | SSQ Nausea | SSQ Oculomotor | SSQ Disorientation | |
|---|---|---|---|---|
| Self-Report Rank | 0.85 p = 0.016* |
0.78 p = 0.041 |
0.86 p = 0.014* |
0.88 p = 0.008* |
| Balance Rank | −0.07 p = 0.88 |
0.11 p = 0.82 |
−0.13 p = 0.79 |
−0.109 p = 0.82 |
The asterisks indicate significant findings, using the false discovery rate method to correct for the multiple comparisons, using a familywise error rate of α = 0.05 for the grouping of 4 symptom scales (Benjamini and Hochberg, 1995).
DISCUSSION
One of the primary findings of this study is that subjects with vestibular disorders had greater symptoms compared with controls while performing coordinated head and eye movements within optic flow environments. Furthermore, the magnitude of symptoms appeared to be related to the baseline level of dizziness handicap and space and motion discomfort perceived by the subjects with vestibular disorders.
Another finding was that subjects with vestibular disorders had less head excursion during the gaze saccade tasks than the control subjects. One potential reason for the limited head excursion may be that subjects with vestibular disorders adopt a pattern of restricting their head movements to reduce provocation of motion-induced symptoms. This altered behavior strategy may be expected to result in self-limited head velocity, but to the contrary, in this study, the head velocity of the subjects of vestibular disorders was equivalent to the control subjects. Furthermore, as shown in Figure 4, both groups appeared to undershoot the target. Barnes[1] had reported that during large eye-head movements (targets > 60° from the central position) healthy subjects have a tendency to undershoot the target. The undershooting of the large gaze saccade was apparent in both control subjects and subjects with vestibular disease, but was of greater magnitude in patients in our study.
The coordinated gaze movement tasks were chosen because they either simulated real activities, e.g. in a grocery store, or because they were a commonly prescribed exercise (gaze stabilization) as part of a vestibular rehabilitation program. The high contrast, high spatial frequency condition was designed to simulate a busy grocery store environment, which is thought to induce visual vertigo or space and motion discomfort in people with vestibular disease.[4, 5, 9] Consequently, we expected that this visual environment would induce greater symptoms compared with the stationary environments tested on visits 1 and 2. However, we did not find the movements and symptoms of the subjects with vestibular disease to be affected by the visual environment. It may have been that the head movements were so innate and well-practiced that the visual environments did not influence the movements. It is also possible that the symptoms induced during the stationary visual environments were artificially inflated by the order of testing, which would have affected the comparisons that were later made to baseline. An increase in symptoms is common in studies using virtual reality.[25] We have previously reported that healthy adults were more symptomatic during their first exposure to VR than during subsequent trials,[25] yet this was not demonstrated in the present study with patients. Results may have been different if the patients had been experiencing more acute symptoms of dizziness.
The level of symptoms in the patients was affected by the length of time of the experiment. Table 4 demonstrated an increase in all of the symptoms as the trials progressed from 1 to 8. Gradual exposure to more complex visual scenes has been proposed as a rehabilitative strategy for persons with space and motion symptoms.[19, 20, 27] Given the increase in symptoms as a function of time, a gradual exposure appears prudent. Exposure time may be critical with patients, as too long an exposure might make the patient worse. Dosing of the exposure requires further study in order to determine the optimal dose for patients experiencing visual vertigo symptoms.
A limitation of the study is that it was assumed that when the subjects were asked to acquire the target, that they actually did locate the target. During practice they were instructed to line up their nose with the target so that their face was in line with the projected target. The authors were unable to determine if the subjects moved their eyes more than the controls, if they were miscalculating their head/eye position to acquire the target, or if they were more willing to view a target off the fovea.
CONCLUSION
Persons with vestibular disorders and control subjects are influenced by moving visual scenes. Generally, symptoms of patients and control subjects increased over the duration of the session, and patients had greater symptoms of anxiety, oculomotor stress and disorientation. Different scene contrasts and optic flow conditions did not differentially affect patients versus control subjects. During the largest gaze saccade task, patients with unilateral vestibular hypofunction moved their heads less than control subjects.
Acknowledgments
This research was supported in part by funding from the National Institutes of Health (R21 DC005372, K23 DC005384, P30 DC005205) and the Eye and Ear Foundation. The authors gratefully acknowledge the assistance of Prof. Larry Hodges, Sabarish Babu, and Jeffrey Jacobson in developing the hardware and software, and Leigh Mahoney and Theresa Yi for data collection and analysis.
Footnotes
This work was presented as a poster presentation at the Association for Research in Otolaryngology Feb 2006 (Baltimore, Maryland).
REFERENCES
- 1.Barnes GR. Vestibulo-Ocular Function During Co-Ordinated Head and Eye Movements to Acquire Targets. Journal of Physiology. 1979;287:127–147. doi: 10.1113/jphysiol.1979.sp012650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B. 1995;57:289–300. [Google Scholar]
- 3.Bronstein AM. Visual vertigo syndrome: clinical and posturography findings. Journal of Neurology, Neurosurgery, and Psychiatry. 1995;59:472–476. doi: 10.1136/jnnp.59.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronstein AM. Vision and vertigo: some visual aspects of vestibular disorders. J Neurol. 2004;251:381–387. doi: 10.1007/s00415-004-0410-7. [DOI] [PubMed] [Google Scholar]
- 5.Bronstein AM. Visual symptoms and vertigo, in: Vertigo and imbalance: Clinical neurophysiology of the vestibular system. In: Eggers SDZ, Zee DS, editors. Handbook of Clinical Neurophysiology. Elsevier B.V.; London: 2010. pp. 523–533. [Google Scholar]
- 6.Brown KE, Whitney SL, Wrisley DM, Furman JM. Physical therapy outcomes for persons with bilateral vestibular loss. Laryngoscope. 2001;111:1812–1817. doi: 10.1097/00005537-200110000-00027. [DOI] [PubMed] [Google Scholar]
- 7.Cohen HS, Kimball KT. Changes in a repetitive head movement task after vestibular rehabilitation. Clin Rehabil. 2004;18:125–131. doi: 10.1191/0269215504cr707oa. [DOI] [PubMed] [Google Scholar]
- 8.Cohen HS, Kimball KT, Jenkin HA. Factors affecting recovery after acoustic neuroma resection. 2002;122:841–850. [PubMed] [Google Scholar]
- 9.Guerraz M, Yardley L, Bertholon P, Pollak L, Rudge P, Gresty MA, Bronstein AM. Visual vertigo: symptom assessment, spatial orientation and postural control. Brain. 2001;124:1646–1656. doi: 10.1093/brain/124.8.1646. [DOI] [PubMed] [Google Scholar]
- 10.Jacob R, Furman J, Balaban C. Psychiatric Aspects of Vestibular Disorders. In: Baloh RW, Halmagyi GM, editors. Disorders of the Vestibular System. Oxford University Press; New York: 1996. pp. 509–528. [Google Scholar]
- 11.Jacob R, Woody SR, Clark DB, Lilienfeld SO, Hirsch BE, Kucera GD, Furman JF, Durrant JD. Discomfort with space and motion: A possible marker of vestibular dysfunction assessed by the Situational Characteristics Questionnaire. Journal of Psychopathology and Behavioral Assessment. 1993;15:299–324. [Google Scholar]
- 12.Jacob RG, Jacob RG. Panic disorder and the vestibular system. Psychiatry Clinics of North America. 1988;11:361–374. [PubMed] [Google Scholar]
- 13.Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116:424–427. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy RS, Lane NE. Simulator Sickness Questionnaire: An Enhanced Method for Quantifying Simulator Sickness. The International Journal of Aviation Psychology. 1993;3:203–220. [Google Scholar]
- 15.Keshner EA, Kenyon RV. Postural and spatial orientation driven by virtual reality. Stud Health Technol Inform. 2009;145:209–228. doi: 10.3233/978-1-60750-018-6-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keshner EA, Streepey J, Dhaher Y, Hain T. Pairing virtual reality with dynamic posturography serves to differentiate between patients experiencing visual vertigo. J Neuroeng Rehabil. 2007;4:24. doi: 10.1186/1743-0003-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norre ME, Beckers A. Vestibular habituation training for positional vertigo in elderly patients. Arch Gerontol Geriatr. 1989;8:117–122. doi: 10.1016/0167-4943(89)90055-1. [DOI] [PubMed] [Google Scholar]
- 18.Norre ME, De Weerdt W. Vestibular habituation training. Technique and first results. Preliminary report. Acta Otorhinolaryngolica Belgica. 1979;33:347–364. [PubMed] [Google Scholar]
- 19.Pavlou M, Lingeswaran A, Davies RA, Gresty MA, Bronstein AM. Simulator based rehabilitation in refractory dizziness. J Neurol. 2004;251:983–995. doi: 10.1007/s00415-004-0476-2. [DOI] [PubMed] [Google Scholar]
- 20.Pavlou M, Quinn C, Murray K, Spyridakou C, Faldon M, Bronstein AM. The effect of repeated visual motion stimuli on visual dependence and postural control in normal subjects. Gait and Posture. 2011;33:113–118. doi: 10.1016/j.gaitpost.2010.10.085. [DOI] [PubMed] [Google Scholar]
- 21.Rine RM, Schubert MC, Balkany TJ. Visual-vestibular habituation and balance training for motion sickness. Physical Therapy. 1999;79:949–957. [PubMed] [Google Scholar]
- 22.Shepard NT, Telian SA. Programmatic vestibular rehabilitation. Head and Neck Surgery. 1995:173–182. doi: 10.1016/S0194-59989570317-9. [DOI] [PubMed] [Google Scholar]
- 23.Shepard NT, Telian SA, Smith-Wheelock M. Habituation and balance retraining therapy. A retrospective review. Neurologic Clinics. 1990;8:459–475. [PubMed] [Google Scholar]
- 24.Shumway-Cook A, Woollacott M. Motor Control: Theory and Practical Applications. Williams and Wilkins; Baltimore: 1995. [Google Scholar]
- 25.Sparto PJ, Whitney SL, Hodges LF, Furman JM, Redfern MS. Simulator sickness when performing gaze shifts within a wide field of view optic flow environment: preliminary evidence for using virtual reality in vestibular rehabilitation. J Neuroengineering Rehabil. 2004;1:14. doi: 10.1186/1743-0003-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szturm T, Ireland DJ, Lessing-Turner M. Comparison of different exercise programs in the rehabilitation of patients with chronic peripheral vestibular dysfunction. Journal of Vestibular Research. 1994;4:461–479. [PubMed] [Google Scholar]
- 27.Vitte E, Semont A, Berthoz A. Repeated optokinetic stimulation in conditions of active standing facilitates recovery from vestibular deficits. Experimental Brain Research. 1994;102:141–148. doi: 10.1007/BF00232446. [DOI] [PubMed] [Google Scholar]
- 28.Whitney SL, Marchetti GF, Morris LO. Usefulness of the dizziness handicap inventory in the screening for benign paroxysmal positional vertigo. Otol Neurotol. 2005;26:1027–1033. doi: 10.1097/01.mao.0000185066.04834.4e. [DOI] [PubMed] [Google Scholar]