Abstract
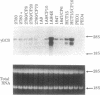
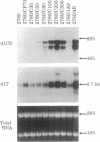
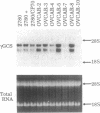
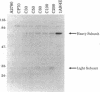
Exposure of human ovarian tumor cell lines to cisplatin led to development of cell lines that exhibited increasing degrees of drug resistance, which were closely correlated with increase of the levels of cellular glutathione. Cell lines were obtained that showed 30- to 1000-fold increases in resistance; these cells also had strikingly increased (13- to 50-fold) levels of glutathione as compared with the drug-sensitive cells of origin. These levels of resistance to cisplatin and the cellular glutathione levels are substantially greater than previously reported. Very high cisplatin resistance was associated with enhanced expression of mRNAs for gamma-glutamylcysteine synthetase and gamma-glutamyl transpeptidase; immunoblots showed increase of gamma-glutamylcysteine synthetase but not of glutathione synthetase. Glutathione S-transferase activity was unaffected, as determined with chlorodinitrobenzene as a substrate. These studies suggest the potential value of examining regulation of glutathione synthesis as an indicator of clinical prognosis. The highly resistant cell lines are proving useful for studying the multiple mechanisms by which tumor cells acquire drug- and radiation-resistance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S., Okine L., Wood R., Aljian J., Vistica D. T. gamma-Glutamyl transpeptidase (gamma-GT) and maintenance of thiol pools in tumor cells resistant to alkylating agents. J Cell Physiol. 1987 May;131(2):240–246. doi: 10.1002/jcp.1041310214. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Transport and direct utilization of gamma-glutamylcyst(e)ine for glutathione synthesis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):707–711. doi: 10.1073/pnas.80.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Cheng S., Nassar K., Levy D. gamma-Glutamyltranspeptidase activity in normal, regenerating and malignant hepatocytes. FEBS Lett. 1978 Jan 15;85(2):310–312. doi: 10.1016/0014-5793(78)80480-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Eva A., Robbins K. C., Andersen P. R., Srinivasan A., Tronick S. R., Reddy E. P., Ellmore N. W., Galen A. T., Lautenberger J. A., Papas T. S. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982 Jan 14;295(5845):116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- Fiala S., Fiala A. E., Dixon B. -Glutamyl transpeptidase in transplantable, chemically induced rat hepatomas and "spontaneous" mouse hepatomas. J Natl Cancer Inst. 1972 May;48(5):1393–1401. [PubMed] [Google Scholar]
- Fiala S., Fiala E. S. Activation by chemical carcinogens of gamma-glutamyl transpeptidase in rat and mouse liver. J Natl Cancer Inst. 1973 Jul;51(1):151–158. doi: 10.1093/jnci/51.1.151. [DOI] [PubMed] [Google Scholar]
- Fiala S., Fiala E. S. Activation of -glutamyl transpeptidase in rat liver by toxic doses of dimethylnitrosamine. Naturwissenschaften. 1971 Jun;58(6):330–330. doi: 10.1007/BF00624754. [DOI] [PubMed] [Google Scholar]
- Fiala S., Mohindru A., Kettering W. G., Fiala A. E., Morris H. P. Glutathione and gamma glutamyl transpeptidase in rat liver during chemical carcinogenesis. J Natl Cancer Inst. 1976 Sep;57(3):591–598. doi: 10.1093/jnci/57.3.591. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodspeed D. C., Dunn T. J., Miller C. D., Pitot H. C. Human gamma-glutamyl transpeptidase cDNA: comparison of hepatoma and kidney mRNA in the human and rat. Gene. 1989 Mar 15;76(1):1–9. doi: 10.1016/0378-1119(89)90002-4. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Anderson M. E., Meister A. Inhibition of glutathione biosynthesis by prothionine sulfoximine (S-n-propyl homocysteine sulfoximine), a selective inhibitor of gamma-glutamylcysteine synthetase. J Biol Chem. 1979 Feb 25;254(4):1205–1210. [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Lai G. M., Rothenberg M. L., Fojo A. T., Young R. C., Ozols R. F. Mechanisms of resistance to cisplatin and alkylating agents. Cancer Treat Res. 1989;48:151–169. doi: 10.1007/978-1-4613-1601-5_10. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Winker M. A., Louie K. G., Batist G., Behrens B. C., Tsuruo T., Grotzinger K. R., McKoy W. M., Young R. C., Ozols R. F. Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem Pharmacol. 1985 Jul 15;34(14):2583–2586. doi: 10.1016/0006-2952(85)90551-9. [DOI] [PubMed] [Google Scholar]
- Hansen M. B., Nielsen S. E., Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989 May 12;119(2):203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Huberman E., Montesano R., Drevon C., Kuroki T., St Vincent L., Pugh T. D., Goldfarb S. gamma-Glutamyl transpeptidase and malignant transformation of cultured liver cells. Cancer Res. 1979 Jan;39(1):269–272. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai G. M., Ozols R. F., Smyth J. F., Young R. C., Hamilton T. C. Enhanced DNA repair and resistance to cisplatin in human ovarian cancer. Biochem Pharmacol. 1988 Dec 15;37(24):4597–4600. doi: 10.1016/0006-2952(88)90325-5. [DOI] [PubMed] [Google Scholar]
- Li Y. C., Seyama T., Godwin A. K., Winokur T. S., Lebovitz R. M., Lieberman M. W. MTrasT24, a metallothionein-ras fusion gene, modulates expression in cultured rat liver cells of two genes associated with in vivo liver cancer. Proc Natl Acad Sci U S A. 1988 Jan;85(2):344–348. doi: 10.1073/pnas.85.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K. G., Behrens B. C., Kinsella T. J., Hamilton T. C., Grotzinger K. R., McKoy W. M., Winker M. A., Ozols R. F. Radiation survival parameters of antineoplastic drug-sensitive and -resistant human ovarian cancer cell lines and their modification by buthionine sulfoximine. Cancer Res. 1985 May;45(5):2110–2115. [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol Ther. 1991;51(2):155–194. doi: 10.1016/0163-7258(91)90076-x. [DOI] [PubMed] [Google Scholar]
- Meister A., Griffith O. W. Effects of methionine sulfoximine analogs on the synthesis of glutamine and glutathione: possible chemotherapeutic implications. Cancer Treat Rep. 1979 Jun;63(6):1115–1121. [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Mistry P., Kelland L. R., Abel G., Sidhar S., Harrap K. R. The relationships between glutathione, glutathione-S-transferase and cytotoxicity of platinum drugs and melphalan in eight human ovarian carcinoma cell lines. Br J Cancer. 1991 Aug;64(2):215–220. doi: 10.1038/bjc.1991.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. R., Anderson M. E., Meister A., Murata K., Kimura A. Increased capacity for glutathione synthesis enhances resistance to radiation in Escherichia coli: a possible model for mammalian cell protection. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1461–1464. doi: 10.1073/pnas.86.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEISH W. J., DAVIES H. M., REEVE P. M. CARCINOGENIC AZO DYES, DYE-BINDING AND LIVER GLUTATHIONE. Biochem Pharmacol. 1964 Sep;13:1291–1303. doi: 10.1016/0006-2952(64)90230-8. [DOI] [PubMed] [Google Scholar]
- NEISH W. J., RYLETT A. AZO DYES AND RAT LIVER GLUTATHIONE. Biochem Pharmacol. 1963 Aug;12:893–903. doi: 10.1016/0006-2952(63)90120-5. [DOI] [PubMed] [Google Scholar]
- O'Dwyer P. J., Hamilton T. C., Young R. C., LaCreta F. P., Carp N., Tew K. D., Padavic K., Comis R. L., Ozols R. F. Depletion of glutathione in normal and malignant human cells in vivo by buthionine sulfoximine: clinical and biochemical results. J Natl Cancer Inst. 1992 Feb 19;84(4):264–267. doi: 10.1093/jnci/84.4.264. [DOI] [PubMed] [Google Scholar]
- Ozols R. F., Masuda H., Hamilton T. C. Mechanisms of cross-resistance between radiation and antineoplastic drugs. NCI Monogr. 1988;(6):159–165. [PubMed] [Google Scholar]
- Ozols R. F., Young R. C. Chemotherapy of ovarian cancer. Semin Oncol. 1991 Jun;18(3):222–232. [PubMed] [Google Scholar]
- Perez R. P., Godwin A. K., Hamilton T. C., Ozols R. F. Ovarian cancer biology. Semin Oncol. 1991 Jun;18(3):186–204. [PubMed] [Google Scholar]
- Perez R. P., Hamilton T. C., Ozols R. F. Resistance to alkylating agents and cisplatin: insights from ovarian carcinoma model systems. Pharmacol Ther. 1990;48(1):19–27. doi: 10.1016/0163-7258(90)90015-t. [DOI] [PubMed] [Google Scholar]
- Richman P. G., Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975 Feb 25;250(4):1422–1426. [PubMed] [Google Scholar]
- Seelig G. F., Simondsen R. P., Meister A. Reversible dissociation of gamma-glutamylcysteine synthetase into two subunits. J Biol Chem. 1984 Aug 10;259(15):9345–9347. [PubMed] [Google Scholar]
- Suzukake K., Vistica B. P., Vistica D. T. Dechlorination of L-phenylalanine mustard by sensitive and resistant tumor cells and its relationship to intracellular glutathione content. Biochem Pharmacol. 1983 Jan 1;32(1):165–167. doi: 10.1016/0006-2952(83)90671-8. [DOI] [PubMed] [Google Scholar]
- Wolf C. R., Hayward I. P., Lawrie S. S., Buckton K., McIntyre M. A., Adams D. J., Lewis A. D., Scott A. R., Smyth J. F. Cellular heterogeneity and drug resistance in two ovarian adenocarcinoma cell lines derived from a single patient. Int J Cancer. 1987 Jun 15;39(6):695–702. doi: 10.1002/ijc.2910390607. [DOI] [PubMed] [Google Scholar]
- Wolf C. R., Hayward I. P., Lawrie S. S., Buckton K., McIntyre M. A., Adams D. J., Lewis A. D., Scott A. R., Smyth J. F. Cellular heterogeneity and drug resistance in two ovarian adenocarcinoma cell lines derived from a single patient. Int J Cancer. 1987 Jun 15;39(6):695–702. doi: 10.1002/ijc.2910390607. [DOI] [PubMed] [Google Scholar]
- Yan N., Meister A. Amino acid sequence of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem. 1990 Jan 25;265(3):1588–1593. [PubMed] [Google Scholar]