Abstract
The onset of developmental stuttering typically occurs between 2 to 4 years of age, coinciding with a period of rapid development in speech, language, motor and cognitive domains. Previous studies have reported generally poorer performance and uneven, or “dissociated” development across speech and language domains in children who stutter (CWS) relative to children who do not stutter (CWNS)(Anderson, Pellowski, & Conture, 2005). The aim of this study was to replicate and expand previous findings by examining whether CWS exhibit dissociated development across speech-language, cognitive, and motor domains that are also reflected in measures of neuroanatomical development. Participants were 66 CWS (23 females) and 53 CWNS (26 females) ranging from 3 to 10 years. Standardized speech, language, cognitive, and motor skills measures, and fractional anisotropy (FA) values derived from diffusion tensor imaging from speech relevant “dorsal auditory” left perisylvian areas (Hickok & Poeppel, 2007) were analyzed using a correlation-based statistical procedure (Coulter, Anderson, & Conture, 2009) that quantified dissociations across domains. Overall, CWS scored consistently lower on speech, language, cognitive and motor measures, and exhibited dissociated development involving these same measures and white matter neuroanatomical indices relative to CWNS. Boys who stutter exhibited a greater number of dissociations compared to girls who stutter. Results suggest a subgroup of CWS may have incongruent development across multiple domains, and the resolution of this imbalance may be a factor in recovery from stuttering.
Keywords: Stuttering, dissociation, speech, language, Diffusion tensor imaging, White matter
1. Introduction
Stuttering is conventionally considered to be a disorder associated with deficient speech motor control, the final step in linguistic and speech processing preceding overt speech production (Caruso, Gracco, & Abbs, 1987; Forster & Webster, 2001; Ludlow & Loucks, 2004; Max, Guenther, Gracco, Ghosh, & Wallace, 2004). While stuttering does not seem to be directly related to apparent higher order cognitive or linguistic difficulties, there are numerous theories, models (see Bloodstein, 2002, 2006; Karniol, 1995; Ratner, 1995) and investigations that point to a relationship between language processing and stuttering (Smith, Goffman, Sasisekaran, & Weber-Fox, 2012; Smith, Sadagopan, Walsh, & Weber-Fox, 2010; Spencer, Packman, Onslow, & Ferguson, 2005; but see Nippold (2012) for opposing view). For example, stuttering is more likely to occur on initial sounds and words (Brown, 1945; Wingate, 1982), consonants (Taylor, 1966), unfamiliar words (Hubbard & Prins, 1994), and during longer utterances (Robb, Sargent, & O'Beirne, 2009).
The loci of stuttering do not appear to be consistent across development. With age, there is a shift from stuttering more on function words to content words (Au-Yeung, Gomez, & Howell, 2003; however, see Buhr & Zebrowski, 2009; Howell, Au-Yeung, & Sackin, 1999; Rommel, 2001). Research suggests a link between linguistic development, cognitive capacity, and stuttering frequency; children who stutter (CWS) exhibit higher rates of disfluencies with increasing utterance length (Sawyer, Chon, & Ambrose, 2008; Zackheim & Conture, 2003). The interaction between factors such as length of utterance, and grammatical and syntactic complexity may be salient particularly during early speech development in CWS (Logan & Conture, 1995; Ratner & Sih, 1987; Watson, Byrd, & Carlo, 2011; Weiss & Zebrowski, 1992; Yaruss, 1999).
Some studies have hinted at weaker language abilities in CWS relative to children who do not stutter (CWNS). At similar developmental stages, CWS, particularly those with persistent stuttering, tend to have lower scores - although still within the norm - on standardized expressive and receptive assessments compared to CWNS and children who recovered from stuttering (Anderson & Conture, 2000; Coulter et al., 2009; Yairi, Ambrose, Paden, & Throneburg, 1996). However, other studies have reported average or above-average language skills in CWS. Watkins, Yairi and Ambrose (1999) reported expressive language skills that were above developmental expectations for CWS with earlier stuttering onset relative to those with later stuttering onset. Additionally, children who recovered from stuttering performed better than children with persistent stuttering on all language measures.
Language deficits that may exist in CWS may be more conspicuous in some domains compared to others, with some studies reporting poorer expressive than receptive language skills in CWS (Byrd & Cooper, 1989; Ntourou, Conture, & Lipsey, 2011; Ryan, 1992), a pattern that is opposite to the developmental trend reported in CWNS (Ryan, 1992). These language deficits may also extend to lexical skills (see Hall, 2004, for review). Similar to adults who stutter, CWS display poorer lexical ability, including reduced lexical diversity, relative to CWNS (Anderson & Conture, 2000; Newman & Bernstein Ratner, 2007; Silverman & Bernstein Ratner, 2002; Wagovich & Bernstein Ratner, 2007). Children who stutter showed slower speech reaction times during picture naming tasks relative to CWNS even when semantically primed (Pellowski & Conture, 2005). Relative to CWNS, CWS also show greater syntactic priming effects (that is, greater difference between primed and non-primed conditions) (Anderson & Conture, 2004) and greater frequency of stuttering with increasing syntactic complexity (Kadi-Hanifi & Howell, 1992). Taken together, these findings suggest a speech motor system in CWS that may be more vulnerable to breakdown with increased language processing demands.
1.1. Studies examining dissociations among speech, motor, and linguistic domains in children who stutter
One explanation for stuttering in early development is related to the notion of dissociations across multiple linguistic domains (Bates, Appelbaum, Salcedo, Saygin, & Pizzamiglio, 2003). Dissociations may appear as uneven abilities in disparate tasks. In CWS, dissociations or imbalances in the speech language system may prompt greater allocation of resources in reconciling these mismatches, and subsequently, result in fewer resources available for fluent speech (Anderson et al., 2005; Coulter et al., 2009). Evidence for these imbalances has been reported by a number of studies (Anderson et al., 2005; Coulter et al., 2009).
In a study of 20 CWS between 3 and 5 years and their matched controls, Anderson and Conture (2000) reported greater incongruity between receptive/expressive skills and receptive vocabulary in CWS compared to CWNS, although no correlation was found between the magnitude of dissociation and stuttering frequency. In a subsequent study of similarly aged CWS (n = 45) and their matched peers (n = 45), Anderson, Pellowski and Conture (2005) found greater likelihood of dissociations across several speech language domains (e.g., vocabulary, oral communication, comprehension) in CWS relative to CWNS. A replication of this study by Coulter et al. (2009) with 85 CWS and their matched controls (n = 85) reported similar findings; CWS were more likely to exhibit dissociations involving receptive language and sound development skills relative to CWNS. It is essential to note that in both studies, some CWS did not present dissociations, while a small number of CWNS were found to exhibit dissociations, prompting the speculation that there may be subtypes in stuttering, and that incongruities in the speech language domain may not be the primary component in the manifestation of the disorder.
In their study, Anderson et al. (2005) found children with dissociations had lower receptive language skills compared to those without dissociations. However, Anderson et al. did not find any differences in the stuttering duration (time since stuttering onset) between CWS with and without dissociations, which in part may be related to the narrow range of ages (3 to 5 years) in their sample, a period when the trajectory of stuttering persistence versus recovery may not yet have been established. Accordingly, a longitudinal study that tracks the pattern of dissociation across a larger age range encompassing older age groups may uncover subtypes that are associated with persistence versus recovery from stuttering.
The idea that both language and motor components might be affected in stuttering (Peters & Starkweather, 1990; Van Riper, 1982), has prompted a number of studies to extend their evaluations beyond incongruities in the linguistic domain in CWS. A recent study by Hollister et al. (2012) reported dissociations within the motor domains, specifically in speech motor articulation (diadochokinesis [DDK]) and speech rate for CWS (n = 45) between 4 and 7 years compared to CWNS (n = 29). In addition, CWS with dissociations between expressive language and speech-motor abilities (as measured by DDK rate) exhibited more stuttering-like disfluencies (SLD). In another study examining sentence repetition performance in CWS between 4 and 6 years, MacPherson and Smith (2013) reported greater variability in lip aperture movement (a reflection of upper lip, lower lip, and jaw movement coordination as a function of time) for longer sentences than for shorter sentences. Even during syntactically simple sentences, CWS exhibited greater movement variability compared to CWNS. Smith et al. (2012) further found higher variability in lip aperture movement coordination for CWS relative to CWNS, even for non-word repetitions.
While some studies have found evidence of diminished performance in speech repetition tasks for CWS compared to CWNS (Hakim & Bernstein Ratner, 2004; Smith et al., 2012), others have not. An earlier study by Caruso and colleagues (1988), which required children to embed a word into a carrier phrase, did not find any differences between CWS and CWNS in the temporal sequence of the respiratory, laryngeal and supralaryngeal speech components, although CWS exhibited earlier onsets in each of these physiological events. These findings may suggest: first, the speech motor system of CWS may be more susceptible to breakdown than CWNS; second, some components of the speech motor system may be more vulnerable than others in the face of increasing processing demands; and third, deficits in the speech motor system in CWS may not be evident in the absence of overwhelming linguistic demands. The demands and capacities model of stuttering proposes that disfluencies result from mismatches between the capacity and demands for fluent speech in one or more areas of development (Adams, 1990; Starkweather & Gottwald, 1990); it is plausible that these mismatches may encompass both linguistic and motor domains.
Overall, these observations support the idea that stuttering is a dynamic, multifactorial disorder (Smith & Kelly, 1997), and incongruities that may be present in one developmental domain may interact with another in the manifestation of the disorder. While stuttering may not result from a language delay or disorder (Nippold, 2012), the capacity to produce fluent speech may be affected to a significant extent by language and/or other cognitive processing demands in CWS. In addition to linguistic ability, the capacity to produce fluent speech may be supported by cognitive, motoric or emotional components (Adams, 1990). Recent neuroimaging research examining CWS offer glimpses into some of these components that may affect the capacity for fluent speech (Beal et al., 2011; Chang & Zhu, 2013; Weber-Fox, Spruill, Spencer, & Smith, 2008).
1.2. White matter neuroanatomical development associated with cognitive and speech-language functions
In the healthy pediatric population, subtle changes in white matter structure are reported to coincide with cognitive development and function (see Craik & Bialystok, 2006 for review; Dubois et al., 2015; Skeide, Brauer, & Friederici, 2015). Cognitive functions that are dependent on widely distributed neural networks, such as executive control, show stronger correlations with white matter integrity (Gunning-Dixon & Raz, 2000; Schmithorst, Wilke, Dardzinski, & Holland, 2005). Fractional anisotropy (FA), a measure derived from Diffusion Tensor Imaging (DTI), reflects white matter integrity and has been shown to correlate with cognitive measures. Increased FA in the bilateral frontal and occipital-parietal regions, including the arcuate fasciculus (AF) and corpus callosum have been shown to be associated with increased IQ in both children and adults (Clayden et al., 2011; Schmithorst et al., 2005). Correlations between FA and cognitive function may be sex-specific. Dunst et al. (2014) found a correlation between FA in the corpus callosum and general intelligence in adult males but not in females. In older children and adolescents, higher FA values in the bilateral frontal and temporal lobes may be linked to better working memory capacity, as well as better reading ability and comprehension (Horowitz-Kraus, Wang, Plante, & Holland, 2014; Nagy, Westerberg, & Klingberg, 2004).
Language skills have also been linked to white matter maturation. In a study of more than 100 typically developing children, Pujol and colleagues (2006) reported vocabulary spurts that coincided with the end of peak white matter myelination in language-relevant regions, which occurred at ages 18 to 24 months. Notably, this study also reported concurrent white matter maturation in the frontal and temporal regions. Other studies have also demonstrated a link between linguistic ability and white matter structure. In a study of young adults, Gold et al. (2007) found that FA values in two language relevant regions --left frontal and parietal cortices-- were correlated with faster lexical processing.
A number of neurodevelopmental disorders including dyslexia, Tourette's Syndrome, attention deficit hyperactivity disorder (ADHD) and autism have been associated with white matter anomalies. Reading deficits in dyslexia may involve reduced FA in the frontoparietal regions (e.g., IFG, supramarginal gyrus) (Richards et al., 2008; Rimrodt, Peterson, Denckla, Kaufmann, & Cutting, 2010), which also exhibit aberrant activity during reading in affected children and adults (Grande, Meffert, Huber, Amunts, & Heim, 2011; Richlan et al., 2010). While left hemisphere regions exhibited reduced FA in dyslexia, FA values were increased in the right hemisphere including the putamen and superior temporal gyrus (STG) (Rimrodt et al., 2010). Increased FA in the right hemisphere parallels greater right hemisphere activation documented in fMRI studies, and may possibly reflect compensation for left hemisphere deficiencies (Pugh et al., 2000). Lower FA values in the SLF – a major white matter tract that interconnects speech-language relevant frontal motor and temporoparietal regions – have been found in children and adolescents with ADHD (Hamilton et al., 2008), who are reported to be at higher risk for language problems (42% of girls and 40% of boys with ADHD) relative to typically developing children (17%) (Sciberras et al., 2014).
White matter neuroanatomical differences have also been reported in CWS. A recent study found that relative to CWNS, CWS show reduced FA in white matter tracts connecting auditory and motor regions, corpus callosum, and tracts connecting cortical and subcortical regions; specifically, these included the left SLF, left IFG, left (pre)motor regions, and left pSTG/middle temporal gyrus (Chang, Zhu, Choo, & Angstadt, 2015). Similar to dyslexia (Marino et al., 2014), CWS showed lower FA values primarily in the left hemisphere. Although attenuated FA values in CWS were also observed in the right hemisphere, group differences here were much smaller than that found in the left hemisphere. An earlier study by Chang et al. (2008) also reported decreased FA in CWS in the left SLF underlying the rolandic operculum. These white matter differences may be associated with reduced capacity for rapid, efficient neural transmission among widely distributed networks including the left dorsal auditory-motor network that supports fluent speech production. Although scant in numbers, studies examining FA, and gray and white matter structural differences in CWS relative to CWNS provide strong evidence of potential neuroanatomical anomalies affecting regions that support efficient auditory-motor integration and speech motor control in stuttering. Such anomalies may be associated with expression of the disorder (Beal, Gracco, Brettschneider, Kroll, & De Nil, 2013; Choo, Chang, Zengin-Bolatkale, Ambrose, & Loucks, 2012).
1.3 The current study and hypotheses
In this study, we examined cognitive, language, speech, motor, and neuroanatomical measures in CWS and similarly-aged CWNS to ascertain whether group differences are present in language performance in general, and in the number of dissociations among speech-language, cognitive and motor domains that may coincide with neuroanatomical differences previously reported in CWS. The present study replicates and extends earlier investigations by Anderson and Conture (2000), Anderson et al. (2005), and Coulter et al. (2009); in addition to standardized tests administered by these studies, we included cognitive and motor non-speech tasks (see Section 2.2.1 Speech, Language, Cognitive, and Motor Skills Evaluation below), and measures reflecting white matter coherence (FA) in the left dorsal auditory-motor areas using DTI. We also included sex comparisons in a wider age range (3 to 10 years) compared to previous studies. Based on previous findings, we expected that CWS would exhibit weaker language performance, and would be more likely to exhibit dissociations among speech, language, cognitive and motor domains compared to CWNS. Given that boys are more likely to exhibit persistent stuttering than girls, we further hypothesized that higher rates of dissociations would be observed in boys who stutter relative to girls who stutter. Further, we predicted dissociations in CWS would coincide with aberrant neuroanatomical development in the left dorsal tracts that interconnect auditory and motor areas supporting fluent speech processing.
2. Methods
2.1. Participants
Participants were 66 children who stutter (CWS: 43 M, 23 F; mean age [years] = 5.55, SD = 2.02) and 53 fluent controls (CWNS: 27 M, 26 F; mean age = 5.99, SD = 2.00) ranging from 3 to 10 years of age (Table 1). Most children were strongly right-handed (51 CWS, 47 CWNS). The groups included nine who were left-handed (6 CWS, 3 CWNS), and nine ambidextrous (7 CWS, 2 CWNS) as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971). Handedness of two CWS and one CWNS could not be determined. CWS and CWNS did not differ in chronological age, handedness, or socioeconomic status (SES) based on mother's education level (Hollingshead, 1975). The children were recruited through the Speech Neurophysiology Lab at Michigan State University as part of a longitudinal study examining neurodevelopmental correlates of stuttering.
Table 1.
Participant demographics information for children who stutter (CWS) and children who do not stutter (CWNS) including the information for males and females within each group.
| CWS | CWNS | |||||
|---|---|---|---|---|---|---|
| Measure | Male | Female | Group | Male | Female | Group |
| Child's age (yrs) | 5.17 (1.74) | 6.27 (2.29) | 5.55 (2.02) | 5.77 (1.93) | 6.22 (2.05) | 5.99 (2.00) |
| Edinburgh Handedness Quotient | 66.51 (47.08) | 51.77 (64.00) | 61.21 (54.24) | 68.66 (45.40) | 78.76 (31.27) | 73.71 (39.31) |
| Mother's Education | 6.00 (2.61)** | 6.59 (1.54)** | 6.24 (2.43) | 6.22 (2.29) | 6.50 (2.42) | 6.36 (2.36) |
| PPVT-4 | 0.61 (0.86) | 0.72 (0.90) | 0.65 (0.87)*** | 1.22 (0.96) | 1.15 (0.80) | 1.18 (0.88)*** |
| EVT-2 | 0.49 (0.85) | 0.38 (0.63) | 0.45 (0.78)*** | 0.97 (0.89) | 1.23 (1.02) | 1.09 (0.96)*** |
| GFTA-2 | 0.35 (0.81) | 0.28 (0.56) | 0.33 (0.73) | 0.42 (0.55) | 0.27 (0.66) | 0.35 (0.61) |
| Receptive Language Quotient | −0.40 (0.70)* | 0.10 (0.81)* | −0.23 (0.78)*** | 0.32 (0.78) | 0.67 (0.70) | 0.50 (0.76)*** |
| FSIQ | 0.12 (0.85) | 0.53 (0.92) | 0.27 (0.89)*** | 0.88 (0.94) | 1.05 (0.88) | 0.96 (0.91)*** |
| VIQ | 0.22 (0.82) | 0.53 (1.06) | 0.33 (0.93)*** | 1.00 (0.89) | 1.18 (1.04) | 1.09 (0.97)*** |
| PIQ | 0.20 (0.84) | 0.42 (0.84) | 0.28 (0.85)** | 0.75 (1.11) | 0.78 (0.87) | 0.76 (1.00)** |
| PPT-D | −0.95 (1.15) | −0.93 (0.75) | −0.94 (1.03)* | −0.39 (1.14) | −0.54 (1.14) | −0.46 (1.14)* |
| PPT-ND | −0.72 (0.90) | −0.67 (0.99) | −0.70 (0.93) | −0.52 (1.01) | −0.22 (1.01) | −0.37 (1.02) |
| PPT-B | −0.87 (1.20)* | −0.48 (0.74)* | −0.73 (1.07)** | 0.07 (1.06) | −0.25 (1.06) | −0.09 (1.07)** |
| L IFG (FA value) | −0.54 (0.76)* | −0.28 (0.56)* | −0.43 (0.70)*** | 0.43 (0.88) | 0.36 (0.75) | 0.40 (0.82)*** |
| L BA6 (FA value) | −0.26 (0.70) | −0.61 (0.98) | −0.41 (0.82)*** | 0.12 (0.70)* | 0.64 (0.56)* | 0.38 (0.67)*** |
| L 4p (FA value) | −0.47 (0.71) | −0.29 (0.71) | −0.39 (0.71)** | 0.34 (0.90) | 0.39 (0.72) | 0.36 (0.82)** |
| L STG (FA value) | −0.53 (0.77) | −0.04 (0.91) | −0.32 (0.83)** | 0.22 (0.74) | 0.36 (0.64) | 0.29 (0.70)** |
| Percent of SLD | 7.46 (5.38) | 4.78 (4.39) | 6.51 (5.21)*** | 1.30 (0.89)* | 0.72 (0.70)* | 1.01 (0.85)*** |
| Percent of OD | 5.15 (2.26) | 5.85 (2.90) | 5.40 (2.53) | 5.59 (2.15)** | 3.81 (1.98)** | 4.70 (2.25) |
| SSI-4 | 21.74 (8.12) | 18.55 (5.02) | 20.64 (7.36) | N/A | N/A | N/A |
EVT = Expressive Vocabulary Test (2nd ed.), FA= fractional anisotropy FSIQ = Full Scale Intelligence Quotient, GFTA = Goldman-Fristoe Test of Articulation (2nd ed.), IFG = inferior frontal gyrus, PIQ = Performance Intelligence Quotient, PPT-B = Purdue Pegboard Test - bimanual, PPT-D = Purdue Pegboard Test - dominant hand, PPT-ND = Purdue Pegboard Test - nondominant hand, PPVT = Peabody Picture Vocabulary Test (4th ed), RL = receptive language, SLD = stuttering like disfluency, SSI = stuttering severity instrument, STG = superior temporal gyrus, VIQ = Verbal Intelligence Quotient.
Z scores, standard deviations of the mean (in parentheses) for each measure are shown. Italicized values indicate within group differences between males and females, bolded values indicate group differences between CWS and CWNS
p < 0.001
p < 0.010
p < 0.050.
All children underwent careful screening to ensure normal speech and language development and typical developmental history except for the presence of stuttering in the CWS group. All participants were monolingual speakers of North American English, with normal hearing, without concomitant developmental disorders such as dyslexia, ADHD, learning delay, or other confirmed developmental or psychiatric conditions and were not taking any medication affecting the central nervous system. All research procedures were approved by the Michigan State University Institutional Review Board, and both child and parents gave verbal assent and signed informed consents. All participants were given nominal remuneration and small rewards (e.g., stickers) for participation.
2.2. Procedure
2.2.1. Speech, Language, Cognitive, and Motor Skills Evaluation
A battery of standardized speech language, cognitive, and motor skills tests were administered to all participants. These consisted of the Peabody Picture Vocabulary Test (PPVT-4; Dunn & Dunn, 1997), Expressive Vocabulary Test (EVT-2; Williams, 2007), Goldman-Fristoe Test of Articulation (GFTA-2; Goldman & Fristoe, 2000), and subtests of Fluharty Preschool Speech and Language Screening Test (Fluharty-2; Fluharty, 2000), Test of Language Development, (TOLD-P:3, I-4; Newcomer & Hamill, 1997), Test for Auditory Comprehension of Language, (TACL-3; Carrow-Woolfolk, 1999), Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III; for children 2:6 to 7:3; Wechsler, 2002), Wechsler Abbreviated Scale of Intelligence (WASI; for children aged 7 and up; Wechsler, 1999) and the Purdue Pegboard Test (PPT; Tiffin, 1968).
Each child provided spontaneous speech samples including a conversation with a certified speech-language pathologist, and a story-telling monologue elicited with the wordless pictures book “Frog, where are you?” (Mayer, 1969). Children's interactions and speech samples were video recorded and transcribed by trained research assistants. Speech samples were also analyzed for stuttering-like disfluencies (SLD), other-disfluencies (OD), and physical concomitants based on the Stuttering Severity Instrument (SSI-4; Riley, 2009). These disfluency measures were based on the full speech samples (at least 10 minutes in length) collected during the conversation and narrative tasks. To determine measurement reliability of the SSI ratings, an intra-class correlation (ICC) coefficient was calculated based on two judges’ ratings of SSI from about 40% of randomly selected children's speech samples; the ICC of 0.98 for overall SSI measurements indicated high reliability. The number of SLDs and ODs were also determined for CWNS but no composite stuttering severity rating was calculated for this group.
2.2.2. MRI data acquisition
The MRI scans were acquired on a GE 3T Signa® HDx MR scanner (GE Healthcare) with an 8-channel head coil. The DTI data were acquired with a dual spin-echo echo-planar imaging sequence for 12 min and 6 s with the following parameters: 48 contiguous 2.4 mm axial slices in 65 an interleaved order, field of view = 22 cm × 22 cm, matrix size = 128 × 128, number of excitations (NEX) = 2, echo time = 77.5 ms, repetition time = 13.7 s, 25 diffusion-weighted volumes (one per gradient direction) with b = 1000 s/mm2, one volume with b = 0 and parallel imaging acceleration factor = 2. DTI was acquired as part of an existing study protocol that also included structural (IRFSPGR) and resting state fMRI data acquisition (Chang & Zhu, 2013). A staff member sat inside the scanner room next to the child at all times to monitor the child's comfort and to ensurecooperation during scanning. During acquisition of DTI scans, children viewed a movie to help them remain still.
2.3. Analyses
2.3.1. Data Analysis
Multivariate analysis of covariance (MANCOVA) was used to analyze the effects of group and sex on speech language, intelligence and motor task performance measures (PPVT-4, EVT-2, GFTA-2, receptive language [RL], FSIQ, and PPT-bimanual [PPT-B]); chronological age and mother's level of education were entered as covariates. Bivariate correlation coefficients were calculated to examine relationships among speech-language (PPVT-4, EVT-2, RL, GFTA-2), intelligence (FSIQ, VIQ, PIQ), motor (PPT), fractional anisotropy (FA), and disfluency measures (SSI score, %SLD). Receptive language scores were determined from select subtests from one of the following tests, depending on child's age at time of testing: the Repeating Sentences and Following Directives and Answering Questions subtests in the Fluharty-2 administered to 3 to 6:11 year-old children, which yielded a “Receptive Language Quotient”; Grammatic Understanding, Sentence Imitation and Grammatic Completion subtests in the TOLD-P:3 administered to 4 to 8:11 year-old children that yielded a “Syntax Quotient”; the Sentence Combining, Word Ordering and Morphological Comprehension subtests in the TOLD-I:4 administered to 9 year-old children and older, providing a “Receptive Language Quotient”; and the Grammatical Morphemes subtest in TACL-3 administered to 4 to 8:11 year-old children. Considering no one “receptive language” test could encompass the wide range of age of the children tested in this study, we utilized the above method to obtain at least one measure of receptive language from subtests within these standardized language tests to gauge the level of syntax development in each child.
To identify dissociations among the measures examined, we replicated the methods published by Anderson et al. (2005) and Coulter et al. (2009). Dissociation points were identified based on density ellipses representing extent of data, center of mass, linear fit, and correlation between two measures. Dissociations were defined as those scores that fell outside of the density ellipse in the space occupied by 95% of the CWNS population, and were required to also exhibit a one standard deviation difference between the two measures (Anderson et al., 2005; Sall, Creighton, & Lehman, 2004). The DTI-based measure of FA was previously reported in Chang, Zhu, Choo and Angstadt (2015). Here, we extracted the average FA values from regions of interest, which consisted of the IFG, 4p, BA6, and pSTG in the left hemisphere from each child's DTI data. These values were entered into a bivariate correlation analysis with the speech, language, cognitive and motor measures. Standard scores were converted to z-scores, and analyses were limited to scores falling between −2SD and +3SD, which was applied to all measures but the PPT, %SLD and SSI scores. Information was not available on some measures for some participants; for each planned analysis only participants where all measures were available were included.
3. Results
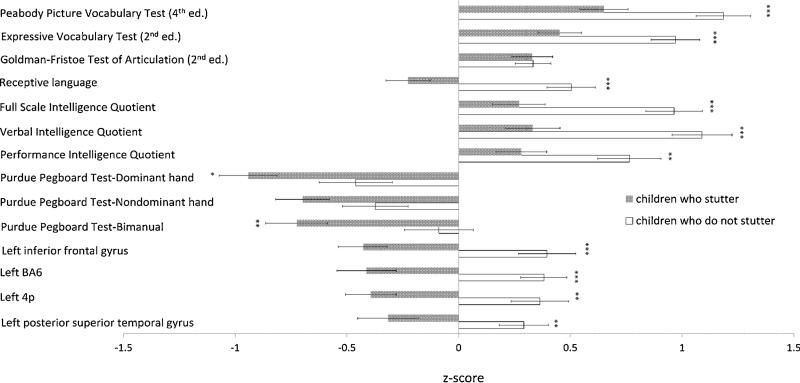
Overall, CWS scored significantly lower across most speech, language, intelligence and motor measures, as well as in FA values in ROIs examined relative to CWNS (Figure 1). In addition, across most domains, CWS were more likely than CWNS to meet the criteria for dissociation and fall outside the density ellipses; this trend was also more prominent in boys than girls for both groups. These results are discussed in detail below.
Figure 1.
Group differences in behavioral assessment scores and white matter integrity measures. Bars represent average z-scores for each measure ± standard error. Asterisks denote statistically significant differences between children who stutter and children who do not stutter; *** p < 0.001, ** p < 0.010, * p < 0.050.
3.1. CWS versus CWNS: Between-group comparisons of measures across speech-language, cognitive, motor and neuroanatomical domains
Between-group multivariate analysis (Bonferroni correction, p = .008) found significantly decreased performance in CWS relative to CWNS on the PPVT (F [1, 83] = 8.380, p = .005), EVT (F [1, 83] = 13.710, p < .001), and receptive language (F [1, 83] = 11.703, p = .001) (Figure 1). As was reported in Chang et al. (2015), when FA scores were included in the analysis, CWS showed significantly lower FA value in the left IFG in relative to CWNS (Bonferroni correction, p = .005; F [1, 53] = 10.340, p = .002).
Closer inspection of the data suggested left-handed children represented a greater proportion of outliers and dissociations cases relative to their numbers in the sample population. Together, left- handed CWS and left-handed CWNS represented about 7.6% (n = 9) of the experimental sample population but made up about 23% of the outliers (n = 103) and 17.3% (n = 53) of the dissociation cases. Left-handed CWS constituted about 19.7% (n = 88) of the outliers and 14% (n = 43) of the dissociation cases. In view of the relationship between the degree of handedness and hemispheric dominance for language (Knecht et al., 2000), and cerebral asymmetry of white matter distribution in language relevant regions and language ability during neurodevelopment (O'Muircheartaigh et al., 2013), an analysis limited to only right-handed participants was performed. The between group results held up when analysis was limited to only right-handed subjects, with the exception of FA value in the left IFG which neared significance (F [1, 46] = 8.085, p = .007).
3.2. Differences in dissociations rates across groups
Due to the non-normal distribution of the data, nonparametric analyses were used to compare the number of dissociations across groups. We entered the total number of dissociations occurring across the domains from each child as the dependent variable, and group (CWS versus CWNS) as the independent variable. The Mann Whitney U test revealed a higher number of dissociations across the behavioral (speech-language, cognitive and motor) and white matter structural (FA) domains for children who stutter (mean = 3.33, SD = 4.484) compared to children who do not stutter (mean = 1.72, SD = 3.336) (U = 2236.500, p = 0.005).
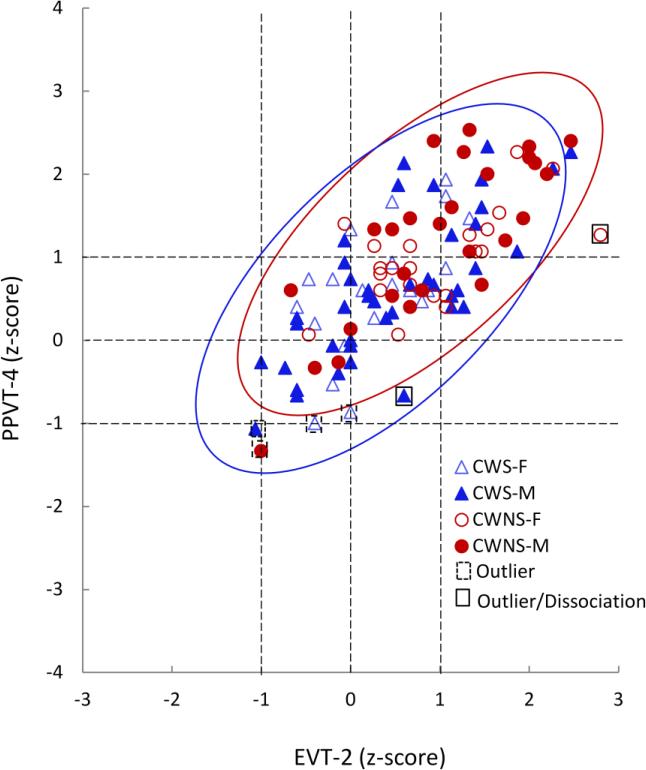
Both CWS (r = .702, p < .001) and CWNS (r = .728, p < .001) showed a strong positive correlation between the PPVT and EVT scores. This correlation was also found when the analysis was restricted to only right-handed subjects (CWS: r = .676, p = .000; CWNS: r =.663, p = .000) Four CWS (6.1%, one left-handed [LH]) and two CWNS (3.8%) fell outside the density ellipsis; of these, only one CWS (1.5%) and one CWNS (1.9%) met the criteria for dissociation (Figure 2). Both groups showed a positive correlation between expressive language (EVT) and articulation (GFTA) scores, albeit slightly weaker in CWS (r = .277, p = .027) compared to CWNS (r = .341, p = .016). Analysis of only right-handed subjects showed consistent results (CWS: r = .341, p =.019; CWNS: r = .465, p = .002). Five CWS (7.6%, three LH) and two CWNS (3.8%, both LH) fell outside the ellipsis and met the criteria for exhibited dissociation (Table 2). Results for the bivariate analysis on speech, language, IQ and speech motor measures are shown in Table 2, and results for non-speech motor measures are displayed in Table 3.
Figure 2.
Correlation between expressive (EVT) and receptive (PPVT) vocabulary test scores (converted to Z scores) for boys and girls who stutter (CWS-M and CWS-F, respectively) and control boys and girls (CWNS-M and CWNS-F, respectively). The density ellipsis is delineated in red and is based on space occupied by 95% of fluent controls.
Table 2.
Results of the bivariate correlations between speech-language and cognitive domains in children who do (CWS) and do not stutter (CWNS). The table displays the r values, number of participants included in each analysis, number of dissociations, number of outliers, and dissociation patterns.
| Speech-language-cognitive domains | Associations (r values) |
Number of cases |
Number of dissociations |
Number of outliers |
Dissociation pattern |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| CWSa | CWNSa | CWSa | CWNSa | CWSa | CWNSa | CWSa | CWNSa | CWSb | CWNSb | |
| Vocabulary | ||||||||||
| PPVT vs EVT | 0.702*** | 0.728*** | 64 (42) | 49 (27) | 1 (1) | 1 (0) | 4 (2) | 2 (1) | PPVT < EVT (1) | PPVT < EVT (1) |
| Articulation and vocabulary | ||||||||||
| PPVT vs GFTA | 0.154 | 0.378** | 63 (41) | 52 (27) | 5 (4) | 3 (2) | 5 (4) | 3 (2) | PPVT < GFTA (4) | PPVT > GFTA (2) |
| EVT vs GFTA | 0.277* | 0.341* | 64 (41) | 49 (27) | 5 (5) | 2 (1) | 5 (5) | 2 (1) | EVT < GFTA (4) | EVT > GFTA (2) |
| Vocabulary and language | ||||||||||
| PPVT vs RL | 0.178 | 0.409** | 62 (41) | 50 (25) | 5 (4) | 1 (0) | 6 (5) | 1 (0) | PPVT > RL (4) | PPVT < RL (1) |
| EVT vs RL | 0.234 | 0.438** | 63 (41) | 47 (25) | 6 (4) | 0 | 8 (6) | 0 | EVT > RL (4) | - |
| Articulation and language | ||||||||||
| GFTA vs RL | −0.065 | −0.026 | 62 (40) | 51 (25) | 5 (5) | 2 (1) | 5 (5) | 3 (1) | GFTA > RL (4) | GFTA < RL (2) |
| Vocabulary and IQ | ||||||||||
| PPVT vs PIQ | 0.369** | 0.395** | 55 (35) | 47 (24) | 1 (1) | 0 | 4 (2) | 0 | PPVT > PIQ (1) | - |
| PPVT vs VIQ | 0.645*** | 0.576*** | 57 (37) | 47 (25) | 3 (1) | 0 | 5 (2) | 0 | PPVT > VIQ (2) | - |
| PPVT vs FSIQ | 0.614*** | 0.499*** | 57 (37) | 48 (25) | 1 (1) | 1 (1) | 5 (3) | 1 (1) | PPVT > FSIQ (1) | PPVT < FSIQ (1) |
| EVT vs PIQ | 0.388** | 0.426** | 56 (35) | 45 (24) | 0 | 1 (0) | 1 (1) | 1 (0) | - | EVT < PIQ (1) |
| EVT vs VIQ | 0.616*** | 0.529*** | 58 (37) | 46 (25) | 1 (0) | 0 | 3 (2) | 0 | EVT > VIQ (1) | - |
| EVT vs FSIQ | 0.585*** | 0.525*** | 58 (37) | 46 (25) | 0 | 0 | 2 (2) | 0 | - | - |
| Articulation and IQ | ||||||||||
| GFTA vs PIQ | −0.059 | 0.232 | 56 (35) | 48 (24) | 3 (2) | 3 (2) | 3 (2) | 3 (2) | GFTA > PIQ (2) | GFTA < PIQ (2) |
| GFTA vs VIQ | 0.001 | 0.288* | 58 (37) | 48 (25) | 7 (6) | 3 (2) | 7 (6) | 3 (2) | GFTA > VIQ (5) | GFTA < VIQ (2) |
| GFTA vs FSIQ | −0.015 | 0.244 | 58 (37) | 49 (25) | 4 (4) | 3 (2) | 4 (4) | 3 (2) | GFTA > FSIQ (3) | GFTA < FSIQ (2) |
| Language and IQ | ||||||||||
| RL vs PIQ | 0.429** | 0.448** | 55 (35) | 48 (24) | 4 (3) | 1 (1) | 5 (4) | 1 (1) | RL < PIQ (4) | RL < PIQ (1) |
| RL vs VIQ | 0.388** | 0.455** | 57 (37) | 48 (25) | 3 (2) | 1 (0) | 6 (4) | 1 (0) | RL < VIQ (3) | RL > VIQ (1) |
| RL vs FSIQ | 0.480*** | 0.535*** | 57 (37) | 49 (25) | 4 (3) | 2 (1) | 6 (5) | 2 (1) | RL < FSIQ (4) | RL > FSIQ (1) |
| IQ | ||||||||||
| PIQ vs VIQ | 0.468*** | 0.446** | 56 (35) | 47 (24) | 0 | 0 | 3 (2) | 0 | - | - |
| Total | - | - | 1116 (717) | 914 (476) | 58 (46) | 24 (13) | 87 (66) | 26 (14) | - | - |
Table 3.
Results of the bivariate correlations between speech-language and (non)speech motor measures in children who do (CWS) and children who do not stutter (CWNS). The table displays the r values, number of cases included in each analysis, number of dissociations and number of outliers, and the dissociation patterns.
| Speech-language-motor domains | Associations (r values) |
Number of cases |
Number of dissociations |
Number of outliers |
Dissociation pattern |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| CWS | CWNS | CWSa | CWNSa | CWSa | CWNSa | CWSa | CWNSa | CWSb | CWNSb | |
| Vocabulary and nonspeech motor | ||||||||||
| PPVT vs PPT-D | 0.315* | 0.060 | 56 (36) | 48 (25) | 6 (4) | 0 | 7 (4) | 0 | PPVT > PPT-D (6) | - |
| PPVT vs PPT-ND | 0.050 | 0.263 | 56 (36) | 48 (25) | 1 (0) | 2 (1) | 5 (3) | 2 (1) | PPVT > PPT-ND (1) | PPVT > PPT-ND (1) |
| PPVT vs PPT-B | 0.106 | 0.138 | 56 (36) | 48 (25) | 4 (3) | 0 | 5 (3) | 0 | PPVT > PPT-B (3) | - |
| EVT vs PPT-D | 0.191 | 0.159 | 57 (36) | 46 (25) | 2 (2) | 0 | 2 (2) | 0 | EVT < PPT-D (1) | - |
| EVT vs PPT-ND | 0.057 | 0.216 | 57 (36) | 46 (25) | 2 (1) | 0 | 4 (3) | 0 | EVT > PPT-ND (1) | - |
| EVT vs PPT-B | 0.279* | 0.204 | 57 (36) | 46 (25) | 2 (2) | 1 (0) | 2 (2) | 1 (0) | EVT > PPT-B (2) | EVT > PPT-B (1) |
| Speech and nonspeech motor | ||||||||||
| GFTA vs PPT-D | 0.076 | 0.019 | 57 (36) | 49 (25) | 1 (0) | 1 (1) | 3 (2) | 2 (1) | GFTA > PPT-D (1) | GFTA < PPT-D (1) |
| GFTA vs PPT-ND | 0.073 | 0.183 | 57 (36) | 49 (25) | 1 (1) | 2 (2) | 2 (2) | 4 (3) | GFTA > PPT-ND (1) | GFTA > PPT-ND (2) |
| GFTA vs PPT-B | 0.028 | 0.123 | 57 (36) | 49 (25) | 2 (2) | 2 (1) | 3 (3) | 3 (1) | GFTA > PPT-B (2) | GFTA > PPT-B (1) |
| Language and nonspeech motor | ||||||||||
| RL vs PPT-D | 0.090 | 0.041 | 56 (36) | 49 (25) | 3 (3) | 2 (1) | 5 (5) | 2 (1) | RL < PPT-D (2) | RL > PPT-D (2) |
| RL vs PPT-ND | 0.144 | 0.238 | 56 (36) | 49 (25) | 2 (2) | 2 (2) | 2 (2) | 2 (2) | RL < PPT-ND (1) | RL > PPT-ND (2) |
| RL vs PPT-B | 0.053 | 0.135 | 56 (36) | 49 (25) | 3 (3) | 0 | 3 (3) | 0 | RL > PPT-B (2) | - |
| Intelligence and nonspeech motor | ||||||||||
| FSIQ vs PPT-D | 0.393* | 0.101 | 56 (35) | 49 (25) | 2 (2) | 0 | 5 (4) | 0 | FSIQ > PPT-D (2) | - |
| FSIQ vs PPT-ND | 0.219 | 0.301* | 56 (35) | 49 (25) | 4 (2) | 2 (1) | 5 (3) | 2 (1) | FSIQ > PPT-ND (3) | FSIQ > PPT-ND (1) |
| FSIQ vs PPT-B | 0.177 | 0.275 | 56 (35) | 49 (25) | 2 (2) | 1 (0) | 4 (4) | 1 (0) | FSIQ > PPT-B (2) | FSIQ > PPT-B (1) |
| Total | - | - | 846 (537) | 723 (375) | 37 (29) | 15 (9) | 57 (45) | 19 (10) | - | - |
Asterisk denotes significant results
p < 0.050
values in parentheses denote males
number of dissociation cases following the overall dissociation pattern
EVT = Expressive Vocabulary Test (2nd ed.), FSIQ = Full Scale Intelligence Quotient, GFTA = Goldman-Fristoe Test of Articulation (2nd ed.), IFG = inferior frontal gyrus, PIQ = Performance Intelligence Quotient, PPT-B = Purdue Pegboard Test - bimanual, PPT-D = Purdue Pegboard Test - dominant hand, PPT-ND = Purdue Pegboard Test - nondominant hand, PPVT = Peabody Picture Vocabulary Test (4th ed), RL = receptive language, STG = superior temporal gyrus, VIQ = Verbal Intelligence Quotient.
While there was a moderate positive correlation between receptive language and FA in the auditory region (left pSTG) in CWS (r = .377, p = .031), no such correlation was evident in CWNS (r = .073, p = .658). An analysis of only right-handed participants showed a similar pattern (CWS: r = .402, p = .042, CWNS: r = .048, p = .785). Six CWS (9.1%, one LH) fell outside the ellipsis; of these three (4.5%) met the criteria for dissociation; one CWNS (1.9%) fell outside the ellipsis but none met the criteria for dissociation. Results for the bivariate analysis on white matter (FA) measures are displayed in Table 4.
Table 4.
Results of the bivariate correlations between white matter (FA values) and speech, language, cognitive, and motor measures in children who do (CWS) and do not stutter (CWNS). The table displays the r values, number of cases included in each analysis, number of dissociations, number of outliers, and the dissociation patterns.
| Neuroanatomical and speech language domains | Associations (r value) | Number of cases | Number of dissociations | Number of outliers | Dissociation pattern | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CWS | CWNS | CWSa | CWNSa | CWSa | CWNSa | CWSa | CWNSa | CWSb | CWNSb | |
| Regions of interests | ||||||||||
| L IFG vs L 4p | 0.107 | 0.217 | 36 (20) | 40 (20) | 0 | 0 | 3 (3) | 0 | - | - |
| L IFG vs L BA6 | 0.077 | 0.198 | 31 (20) | 40 (20) | 0 | 1 (1) | 3 (3) | 2 (2) | - | L IFG > L BA6 (1) |
| L IFG vs L STG | 0.177 | 0.355* | 34 (19) | 39 (19) | 4 (3) | 0 | 6 (5) | 0 | L IFG > L STG (3) | - |
| L 4p vs L BA6 | 0.499** | −0.128 | 30 (19) | 40 (20) | 2 (2) | 2 (2) | 4 (4) | 2 (2) | L 4p < L BA6 (1) | L 4p > L BA6 (2) |
| L 4p vs L STG | 0.196 | 0.370* | 33 (18) | 39 (19) | 3 (1) | 0 | 4 (2) | 0 | L 4p > L STG (2) | - |
| L BA6 vs L STG | 0.115 | 0.075 | 29 (18) | 39 (19) | 6 (5) | 2 (2) | 6 (5) | 2 (2) | L BA6 > L STG (4) | L BA6 < L STG (2) |
| Left frontal and speech language | ||||||||||
| L IFG vs PPVT | −0.095 | −0.138 | 36 (21) | 39 (20) | 3 (1) | 0 | 4 (1) | 0 | L IFG < PPVT (2) | - |
| L IFG vs EVT | −0.108 | −0.215 | 37 (21) | 36 (20) | 1 (0) | 0 | 2 (1) | 0 | L IFG < EVT (1) | - |
| L IFG vs GFTA | 0.054 | −0.067 | 37 (21) | 40 (20) | 0 | 1 (0) | 1 (1) | 2 (1) | - | L IFG > GFTA (1) |
| L IFG vs RL | 0.048 | 0.051 | 36 (21) | 40 (20) | 2 (1) | 0 | 3 (2) | 0 | L IFG > RL (1) | - |
| L IFG vs FSIQ | −0.105 | 0.085 | 37 (21) | 39 (20) | 1 (1) | 0 | 3 (2) | 0 | L IFG < FSIQ (1) | - |
| L IFG vs PPT-B | −0.008 | −0.040 | 36 (20) | 39 (20) | 3 (3) | 0 | 5 (4) | 0 | L IFG > PPT-B (3) | - |
| Left auditory (pSTG) and speech language | ||||||||||
| L STG vs PPVT | −0.348* | −0.073 | 33 (19) | 38 (19) | 5 (3) | 0 | 7 (4) | 0 | L STG < PPVT (4) | - |
| L STG vs EVT | −0.525** | −0.164 | 34 (19) | 35 (19) | 5 (4) | 0 | 5 (4) | 0 | L STG < EVT (4) | - |
| L STG vs GFTA | −0.149 | 0.036 | 34 (19) | 39 (19) | 5 (4) | 1 (1) | 5 (4) | 2 (1) | L STG < GFTA (4) | L STG > GFTA (1) |
| L STG vs RL | 0.377* | 0.073 | 33 (19) | 39 (19) | 3 (2) | 0 | 6 (4) | 1 (1) | L STG < RL (3) | - |
| L STG vs FSIQ | −0.164 | 0.048 | 34 (19) | 38 (19) | 6 (5) | 0 | 8 (6) | 0 | L STG < FSIQ (5) | - |
| L STG vs PPT-B | −0.012 | 0.214 | 33 (18) | 38 (19) | 6 (5) | 0 | 7 (6) | 0 | L STG < PPT-B (3) | - |
| Left motor cortex (4p) and speech language | ||||||||||
| L 4p vs PPVT | −0.064 | 0.052 | 35 (20) | 39 (20) | 2 (1) | 0 | 3 (1) | 0 | L 4p < PPVT (1) | - |
| L 4p vs EVT | 0.123 | −0.046 | 36 (20) | 36 (20) | 2 (2) | 0 | 3 (3) | 0 | L 4p < EVT (2) | - |
| L 4p vs GFTA | −0.058 | −0.031 | 36 (20) | 40 (20) | 1 (1) | 1 (1) | 1 (1) | 1 (1) | L 4p > GFTA (1) | L 4p > GFTA (1) |
| L 4p vs RL | 0.297 | 0.289 | 35 (20) | 40 (20) | 1 (1) | 0 | 2 (2) | 1 (1) | L 4p > RL (1) | - |
| L 4p vs FSIQ | 0.144 | 0.060 | 36 (20) | 39 (20) | 1 (1) | 0 | 3 (2) | 0 | L 4p < FSIQ (1) | - |
| L 4p vs PPT-B | 0.106 | −0.064 | 35 (19) | 39 (20) | 3 (3) | 0 | 4 (4) | 0 | L 4p > PPT-B (2) | - |
| Left premotor (BA6) and speech language | ||||||||||
| L BA6 vs PPVT | 0.015 | −0.240 | 31 (20) | 39 (20) | 3 (2) | 2 (2) | 4 (3) | 2 (2) | L BA6 < PPVT (2) | L BA6 < PPVT (2) |
| L BA6 vs EVT | 0.137 | −0.203 | 31 (20) | 36 (20) | 3 (3) | 1 (1) | 5 (5) | 1 (1) | L BA6 < EVT (3) | L BA6 < EVT (1) |
| L BA6 vs GFTA | −0.026 | −0.093 | 31 (20) | 36 (20) | 3 (3) | 4 (3) | 3 (3) | 4 (3) | L BA6 < GFTA (2) | L BA6 < GFTA (2) |
| L BA6 vs RL | 0.109 | 0.118 | 31 (20) | 40 (20) | 3 (3) | 2 (2) | 4 (4) | 2 (2) | L BA6 < RL (2) | L BA6 < RL (2) |
| L BA6 vs FSIQ | −0.010 | −0.166 | 31 (20) | 39 (20) | 2 (2) | 3 (3) | 4 (4) | 3 (3) | L BA6 < FSIQ (2) | L BA6 < FSIQ (3) |
| L BA6 vs PPT-B | 0.214 | −0.141 | 30 (19) | 39 (20) | 3 (3) | 2 (2) | 4 (4) | 2 (2) | L BA6 > PPT-B (2) | L BA6 < PPT-B (2) |
| Total | - | - | 1011 (590) | 1159 (591) | 82 (65) | 22 (20) | 122 (97) | 27 (24) | - | - |
Asterisk denotes significant results
*** p < 0.001
p < 0.010
p < 0.050
values in parentheses denote males
value in parentheses denotes number of dissociation cases following the overall dissociation pattern
EVT = Expressive Vocabulary Test (2nd ed.), FSIQ = Full Scale Intelligence Quotient, GFTA = Goldman-Fristoe Test of Articulation (2nd ed.), IFG = inferior frontal gyrus, PIQ = Performance Intelligence Quotient, PPT-B = Purdue Pegboard Test - bimanual, PPT-D = Purdue Pegboard Test - dominant hand, PPT-ND = Purdue Pegboard Test – non-dominant hand, PPVT = Peabody Picture Vocabulary Test (4th ed), RL = receptive language, STG = superior temporal gyrus, VIQ = Verbal Intelligence Quotient.
Children who stutter showed correlations between age and some language measures that were not found for CWNS. There were negative correlations between PPVT and age for CWS (r = −.383, p = .002) but not for CWNS (r = −.117, p = .410), and between EVT and age for CWS (r = −.441, p = .000) but not for CWNS (r = −.147, p = .313). Further, CWS showed a positive correlation between receptive language and age (r = .294, p = .019) that was absent for CWNS (r = .184, p = .197).
3.3. Sex differences in dissociation rates
Boys in both groups showed a positive correlation between expressive (EVT) and receptive language scores (CWS r = .342, p = .029; CWNS: r = .587, p = .002); no such correlations were evident for girls in either group (CWS: r = .119, p = .599; CWNS: r = .256, p = .249). An analysis of right-handed only subjects did not find a correlation between EVT and receptive language in boys who stutter (r = .287, p = .111) although the positive correlation remained for CWNS-boys (r = .588, p = .005). No correlations were found for right-handed girls in both groups (CWS: r = −.050, p = .850; CWNS: r = .271, p = .235). Six boys who stutter (14.0%, two LH) fell outside the density ellipsis; of these four (9.3%) met the criteria for dissociation. Two girls who stutter (8.7%) fell outside the density ellipsis and met the criteria for dissociation. No CWNS fell outside the ellipsis. Dissociation rates between sexes for speech language, cognitive and motor measures are displayed in Supplementary Table 1.
Boys (r = −.550, p = .015) but not girls who stutter (r = −.469, p = .078) nor CWNS of either sex (r = −.164, p = .346) displayed a negative correlation between FA value in the left pSTG and expressive vocabulary (EVT). Analysis of only right-handed subjects found negative correlations between the left pSTG and EVT for both boys who stutter (r = −.525, p = .044) and girls who stutter (r = −.594, p = .042) but not for CWNS boys (r = −.230, p = .391) or CWNS girls (r = −.186, p = .506). Four boys (9.3%, one LH) and one girl (4.3%) who stutter fell outside the ellipse and all of these (one LH) met the criteria for dissociation. No CWNS fell outside the ellipsis. Boys who stutter (r = .505, p = .027) showed a positive correlation between FA value in motor regions, left 4p and left BA6 that was absent in girls who stutter (r = .501, p = .117) and controls (r = −.128, p = .433).
Analysis of only right-handed participants did not find correlations between left 4p and left BA6 for boys who stutter (r = .420, p = .106), girls who stutter (r = .408, p = .276), CWNS-boys (r = −.250, p = .332) or CWNS-girls (r = −.032, p = .896). Four boys who stutter (9.3%) fell outside the density ellipsis; of these two (4.7%) met the criteria for dissociation. Two CWNS-boys (7.4%) fell outside the ellipsis and met the criteria for dissociation. No girls from either group fell outside the ellipsis. Dissociation rates for regions of interests and other measures are shown in Supplementary Table 2.
Children who do not stutter showed a positive correlation between FA values in the left pSTG and left 4p (r = .370, p = .020), but not in boys or girls who stutter (r = .115, p = .275) (Figure 3). Analysis of only right-handed subjects showed a similar pattern. Children who do not stutter showed a positive correlation between left pSTG and left 4p (r = .420, p = .012) that was absent in boys who stutter (r = .296, p = .304) and girls who stutter (r = .123, p = .704). Two boys who stutter (4.7%) fell outside the ellipsis; of these, one (2.3%) met the criteria for dissociation. Two girls who stutter (8.7%) fell outside the ellipsis and both met the criteria for dissociation. No CWNS fell outside the ellipsis.
Figure 3.
Correlation between left superior temporal gyrus (L STG) and left primary motor cortex (L 4p) FA values (converted to Z scores) for boys and girls who stutter (CWS-M and CWS-F, respectively) and control boys and girls (CWNS-M and CWNS-F, respectively). The density ellipsis is delineated in red and is based on space occupied by 95% of fluent controls.
Girls who stutter showed a strong correlation between receptive vocabulary (PPVT) and gross motor skill (PPT-dominant hand) (r = .635, p = .003) that was absent in boys who stutter (r = .216, p = .205) and CWNS (r = .060, p = .684). This pattern of correlation was also found when only right-handed participants were analyzed (CWS-girls: r = .717, p = .002; CWS-boys: r = .139, p = .488; CWNS: r = −.063, p = .690). Four boys who stutter (9.3%, two LH) fell outside the density ellipsis and met the criteria for dissociation (two LH). Three girls who stutter (13%) fell outside the density ellipsis; of these, two (8.7%) met the criteria for dissociation. No CWNS- boys or girls - fell outside the ellipse.
There was also a strong positive correlation between verbal language intelligence (VIQ) and gross motor skill (PPT-dominant hand) for girls (r = .556, p = .008) but not boys who stutter (r = .246, p = .155) or CWNS of either sex (r = .157, p = .288). This pattern of correlation was found for right-handed subjects (CWS-girls: r = .513, p = .035, CWS-boys: r = .263, p = .185; CWNS-boys: r = −.071, p = .759; CWNS-girls: r = .371, p = .089). Four boys who stutter (9.3%, two LH) fell outside the density ellipsis; of these three (7%, one LH) met the criteria for dissociation. One girl who stutters (4.3%) fell outside the ellipsis but did not meet the criteria for dissociation. No CWNS of either sex met the dissociation criteria or fell outside the ellipsis. Further, analysis of only right-handed subjects found a positive correlation between GFTA and PPT-D for girls who stutter (r = .597, p =.011) but not for boys who stutter (r = −.155, p = .441) or CWNS of either sex (r = .092, p = .552).
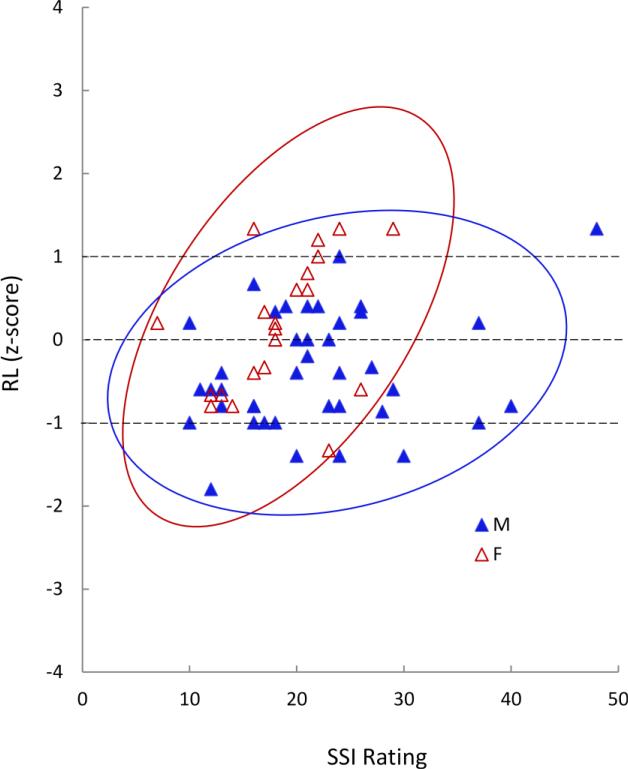
There was a strong positive correlation between stuttering severity (SSI) and receptive language for girls (r = .479, p = .032) but not boys who stutter (r = .256, p = .111) (Figure 4). Analysis of only right-handed CWS found a similar correlation (CWS-girls: r = .616, p = .011; CWS-boys: r = .196, p = .291). Boys who stutter showed a strong negative correlation between %SLD and gross motor skill (PPT-non-dominant hand: r = −.442, p = .007; PPT-bimanual: r = −.453, p = .006); no such correlations were found in girls who stutter (PPT-non-dominant hand: r = −.002, p = .995; and PPT-bimanual: r = −.077, p = .741). Analysis of only-right-handed participants found negative correlations between %SLD and PPT-non-dominant hand for boys who stutter (r = −.410, p = .033) but not for girls who stutter (r = −.051, p = .847). Similarly, analysis of only right-handed participants found a negative correlation between %SLD and FSIQ for boys (r = −.444, p = .016) but not girls who stutter (r = .095, p = .717). No other correlations were found between SSI or %SLD, and other speech, IQ, cognitive, motor or FA measures (Supplementary Table 3).
Figure 4.
Correlation between stuttering severity instrument (SSI-4) rating and z-scores of receptive language (RL) scores for girls (F) and boys (M) who stutter shown with density ellipses based on space occupied by 95% of each population (red = females, blue = males).
4. Discussion
The present study examined whether CWS exhibit incongruent development across speech, language, cognitive and motor domains, and whether uneven development among these domains is associated with stuttering and atypical neuroanatomical development. We sought to replicate and extend the dissociation paradigm utilized in previous studies (Anderson & Conture, 2000; Anderson et al., 2005; Coulter et al., 2009; Hollister et al., 2012). Our findings are consistent with previous reports that showed greater dissociations as well as generally lower language performance in CWS relative to CWNS. New findings from our study included a greater number of dissociations for boys who stutter compared to girls who stutter, greater number of dissociations for left-handed CWS relative to right-handed CWS, and atypical correlations between white matter coherence measures (FA) in the left dorsal language pathways and speech language and motor performance measures in CWS.
4.1. Dissociation rates are higher among CWS relative to CWNS
Children who stutter showed lower performance and higher rates of dissociations across multiple domains, encompassing speech language, motor skills and neuroanatomy, which may indicate a tenuous speech motor system that may be susceptible to increased disruptions. A higher number of dissociations in children who stutter suggest atypical and incoherent maturational processes across the speech motor and language systems that are associated with stuttering. Generally, maturation of skills across domains occurs in a coordinated manner. For example, typically developing children show concurrent improvements in motor and language skills (Iverson, 2010). A higher number of dissociations in children who stutter may point to maturational processes that are less coordinated compared to children who do not stutter. For example, children who do not stutter show a significant correlation between speech articulation (GFTA) and receptive vocabulary (PPVT) scores that was absent in children who stutter, which may indicate reduced coordination between speech motor and language development in stuttering.
The demands and capacities model (DCM) of stuttering (Adams, 1990; Starkweather & Gottwald, 1990) lends itself to explaining deficits and dissociations in CWS. First, it is worth noting that in the demands and capacities model, the difference in magnitude between demands and capacities need not be immense; even modest demands may perturb the system beyond available resources. Second, incongruity between demands and capacities across any speech development domains may impact speech motor production (Adams, 1990; Starkweather & Gottwald, 1990). According to DCM, “it is possible that the stuttering child does not develop the capacities for fluency fast enough to catch up to the increasing demands of his communicative environment “ (Starkweather & Gottwald, 1990; p. 145). The present finding of greater number of dissociations in CWS across developmental domains may reflect a developmental trend whereby some skills or abilities may be established earlier, while some are delayed. It is beyond the scope of the present study to examine individual demands and resources that impact the capacity for fluent speech. However, results of the present study suggest that coherent development across speech-language, cognitive and motor development may affect the capacity for fluent speech in CWS. Future studies that examine the rate and types of dissociations in children who recover before the onset of stuttering and after recovery may better elucidate the role of dissociations in the risk for developing stuttering, and further, the risk of continuing to stutter.
4.2. Neuroanatomical correlates of behavioral dissociations
The presence of dissociations across all domains examined in the current study suggests dissociations may be modulated by developmental imbalances across brain regions. We found dissociations in FA values (a measure reflecting white matter coherence) among regions along the dorsal language pathway, including the left IFG (n = 19), left BA6 (n = 34), left 4p (n = 18) and left pSTG (n = 55). In a previous study, it was found that CWS presented lower FA values relative to CWNS in these same regions (Chang et al., 2015).
In CWNS, improvement in language performance is associated with maturation of these speech relevant regions (Lu et al., 2007; Sowell et al., 2004; Sowell, Thompson, Tessner, & Toga, 2001). These systems may be differentially engaged in the case of CWS, as a consequence of subtle deficits or maturational delay. Results from the present showed that there was a negative correlation between the FA value in left pSTG and expressive and receptive vocabulary measures for CWS that was absent in CWNS. Further, CWS showed a positive correlation between the left pSTG and receptive language (that included assessments for receptive syntax, morphology, and grammar) that was not found in CWNS. Broadly, these atypical correlational patterns involving the left pSTG and language measures (i.e., vocabulary, syntax) may suggest deficiencies in how neural structures support language processing and performance in CWS. While stuttering is not associated with an apparent language disorder, the atypical pattern of correlations between the left pSTG and language measures suggest that CWS may engage this area in a different manner to support expressive and receptive language compared to CWNS. Additionally, CWS showed a positive correlation between receptive language and age that was absent in CWNS, which suggests that receptive language skills may still be actively developing in CWS in the age range captured in the study. In contrast, CWS exhibited negative correlations between age and EVT (as well as for age and PPVT), which was not the case in CWNS. CWS also exhibited a negative correlation between the left pSTG FA value and the EVT scores, not shown to be the case in CWNS. In addition, there was a positive correlation of FA scores in the left pSTG (auditory) and left 4p (motor) in CWNS but not in CWS. These results point to a disjointed relationship between the left pSTG and different language modalities in CWS. Namely, these findings suggest incoherent development of the dorsal and ventral networks that both interface with the left pSTG in stuttering, where the dorsal stream connectivity (between auditory to motor) may be decreased, while ventral stream connectivity (between auditory to language) may be aberrantly increased with age in CWS relative to controls.
These findings from the present study are consistent with past reports related to anomalous structure and function in the left auditory region in stuttering. In terms of structure, children who stutter exhibit decreased connectivity between the left pSTG, and left frontal motor and subcortical areas relevant to speech motor control (Chang & Zhu, 2013). In adults who stutter, the pSTG was found to have aberrant asymmetry (attenuated left laterality) (Foundas, Bollich, Corey, Hurley, & Heilman, 2001; Foundas et al., 2004). In terms of function, the left pSTG is consistently reported to be deactivated or underactivated during speech, which tends to normalize during “induced fluency” conditions when people who stutter are fluent (Braun et al., 1997; Chang, Kenney, Loucks, & Ludlow, 2009; De Nil, Kroll, Kapur, & Houle, 2000; Fox et al., 1996; Toyomura, Fujii, & Kuriki, 2011; Van Borsel, Achten, Santens, Lahorte, & Voet, 2003). Functional connectivity (i.e., correlated activity patterns of spatially distant areas) between the left pSTG and the frontal motor areas (inferior frontal gyrus, premotor cortex, motor cortex) was found to be attenuated in children who stutter compared to controls, and this was especially so for boys who stutter, who are also more likely to experience persistent stuttering (Chang & Zhu, 2013). Further, in both boys and girls who stutter, there was attenuated functional connectivity between the left pSTG and the putamen, a neural circuit thought to support internal timing of movements (Chang & Zhu, 3013). These findings suggest an atypical sensorimotor integration circuit that includes aberrant structure and function in the left pSTG in CWS. Thus, findings related to the left pSTG in the present study is consistent with the past reports which point to atypical connectivity of this region that is associated with anomalous function in related speech-language behavior.
Further, the absence of a positive correlation in FA between the auditory (left pSTG) and motor (4p) regions in CWS may indicate incongruity between auditory and motor area maturation. Auditory feedback can modulate speech motor control in updating speech motor commands to coincide with auditory targets (Guenther, Ghosh, & Tourville, 2006). In the healthy speech system, rapid compensatory motor responses are evident in the presence of altered auditory feedback, suggesting a critical link between the auditory and motor domains, and the role of sensorimotor adaptation in speech motor control. Children who do not stutter around 4 years exhibit adaptive speech motor changes that are comparable to adults in the presence of altered auditory feedback, however, younger children around 2 years do not display these compensatory adaptations (Macdonald, Johnson, Forsythe, Plante, & Munhall, 2012; Shiller, Gracco, & Rvachew, 2010). Age-related differences in motor adaptation between older and younger children are likely related to neurodevelopment (Barnea-Goraly et al., 2005). In the stuttering population, adults feature slower and smaller articulatory adjustments compared to fluent controls when given altered spatial and temporal feedback (Cai, Beal, Ghosh, Guenther, & Perkell, 2014; Cai et al., 2012).
Collectively, these findings indicating weaker motor adaptation in response to auditory perturbations in adults who stutter, and lower FA values and dissociations involving the auditory and motor behavioral domains in CWS in the present study, provide support for possible disruption in auditory-motor integration that is also associated with subtle decreases in white matter coherence in the speech motor and auditory regions. It is possible that maturational imbalances between the auditory and motor system constrain the ability of the speech motor system, particularly in the presence of higher demands and disruptions, in people who stutter.
4.3. Sex differences in dissociation rates
In the present study, there was a clear difference in dissociation rates between boys and girls who stutter. Overall, boys who stutter were nearly 4 times more likely than girls who stutter to exhibit dissociations. Specifically, boys who stutter presented dissociations at higher rates involving speech motor articulation scores compared to girls who stutter. Further, right-handed girls who stutter showed a positive correlation between speech motor articulation and motor skills that was absent in right-handed boys who stutter, which may suggest more congruent motor development across these domains in girls who stutter relative to boys. At the time of the study about 50% of the right handed girls were within 3 years of stuttering onset. Since children who stutter typically recover within 2 to 3 years of stuttering onset, it is plausible that some of the girls in this cohort may recover at a later time, and the correlation between speech articulation and gross motor skills for right handed girls who stutter may be driven by this cohort of girls who may recover. A longitudinal study that examines the correlation between speech articulation and gross motor skills prior to the onset of stuttering to the time of recovery or persistent stuttering may be able to elucidate this pattern of correlation.
In other disorders with greater male than female prevalence such as autism (Fombonne, 2003) and developmental language disorder (Tomblin et al., 1997), males typically demonstrate poorer cognitive and motor performance when compared to similarly affected females. For example, girls with autism exhibit better fine motor competency than their male counterparts, a gap which widens with age (Mandy et al., 2012). Relative to the healthy population, females with autism show equivalent motor skills while males with autism show depressed motor skills (Lai et al., 2011). Likewise, in DLD, boys with mild expressive language deficits showed lower performance in functional motor ability (e.g., ball catching, balance) compared to control boys, while girls with a similar diagnosis performed comparably to control girls (Müürsepp, Ereline, Gapeyeva, & Pääsuke, 2009).
This sex-based difference is also observed in the healthy population: boys exhibit slower and later occurring speech-language development relative to girls (Zubrick, Taylor, Rice, & Slegers, 2007), and are more likely to present language and motor delays (Whitehouse et al., 2012). This pattern of female advantage is apparent early on. By age 2, girls demonstrate better language competency including higher lexical diversity compared to boys (Lutchmaya, Baron-Cohen, & Raggatt, 2002). Prenatal females (mean gestational age of 24 weeks) show significantly earlier speech motor development (e.g., complex oro-motor and upper respiratory skills), relative to boys (Miller, Macedonia, & Sonies, 2006).
In stuttering, a developmental disadvantage in males compared to females that includes later maturation in the speech language domains, may translate into higher dissociation rates and/or comparatively diminished performance in boys relative to girls who stutter at the same chronological age. The finding that dissociation rates differ between the sexes in both groups also prompts the following hypothesis: higher rates of dissociation in boys across both stuttering and control groups may signal a speech motor system that is more susceptible to perturbations and demands relative to girls. In stuttering, this may be compounded by neuroanatomical deficits. Two recent findings related to white matter development are relevant to the present study (Chang et al., 2015). First, a negative correlation between FA values along major white matter tracts (including the IFG) and stuttering severity was mainly driven by boys who stutter. Second, the more severe cases of stuttering (which tended to be boys) were associated with lower FA values in the left hemisphere speech motor regions (which included the IFG and primary motor area reported in this study). Namely, less white matter coherence in speech relevant regions was correlated with more severe stuttering symptoms particularly in boys. In the present study, boys who stutter showed a negative correlation between %SLD and PPT (dominant hand) scores that were not found in girls who stutter. In other words, boys who presented with more severe stuttering also had poorer motor skills which may point to a higher risk for persistent stuttering. Further, right-handed girls who stutter showed a positive correlation between speech articulation and gross motor skills, which may indicate more congruent development of these skills that was absent in right-handed boys who stutter. Manual and oral motor skills have been shown to be predictive of later expressive and receptive language development (Hellendoorn et al., 2015), and speech skills (e.g., ability to produce long utterances, mispronunciations) (Gernsbacher, Sauer, Geye, Schweigert, & Hill Goldsmith, 2008). Although the present study suggests a link between motor ability and severity of symptoms, without a longitudinal study that tracks these developmental trajectories, it is unknown at this time whether motor skills are predictive of recovery or persistent stuttering.
Neuroanatomical findings, along with behavioral assessments that point to differences between boys and girls who stutter likely reflect sexual dimorphism in stuttering and sex-specific mechanisms that may underlie manifestation and eventual outcome of the disorder (e.g., persistence versus recovery). However, the heterogeneity of stuttering symptoms across individuals and situations, and the ambiguity by which various components across different domains may interact to influence expression of the disorder, warrants a longitudinal study that tracks developmental trajectories in children of both sexes. Such a study would elucidate whether dissociations across specific domains signal an underlying neurodevelopmental immaturity that is transitory or a marker of persistent stuttering.
4.4. Relationship between handedness and dissociations
The notion that stuttering is related to handedness has been proposed since the early 20th century (Travis, 1929). In the present study, left-handed CWS were more likely to present outliers compared to right-handed CWS, which may indicate a higher rate of incongruent development across domains in left handed CWS. In the healthy population, right-handed children have been shown to perform better than left-handed children in a number of cognitive tasks including spatial and speech language abilities (e.g., vocabulary, comprehension) (Somers, Shields, Boks, Kahn, & Sommer, 2015), and visuomotor organization as evaluated using the Rey-Osterrieth Complex Figure (Karapetsas & Vlachos, 1997), suggesting potentially earlier and more congruent development across (the language, motor and visual) domains in right-handed children. A right-handed advantage in cognitive ability is thought to be a consequence of heightened hemispheric laterality, one that favors the left cerebral hemisphere for speech and motor processing (Langel, Hakun, Zhu, & Ravizza, 2014; Serrien & Sovijärvi-Spapé, 2015; Yelle & Grimshaw, 2009).
Neuroimaging studies examining resting state functional connectivity and white matter development may provide support for a right-handed advantage. Typically, right-handed individuals show stronger resting state functional connectivity in the left hemisphere, compared to that of left-handed individuals (Saenger, Barrios, Martínez-Gudiño, & Alcauter, 2012). Greater left-sided functional connectivity has been shown to be associated with better cognitive (e.g., reading, comprehension) (Koyama et al., 2011) and motor performance (e.g., hand movements) (Barber et al., 2011; Pool, Rehme, Eickhoff, Fink, & Grefkes, 2015; Pool, Rehme, Fink, Eickhoff, & Grefkes, 2014). O'Muircheartaigh et al. (2013) reported that greater leftward asymmetry of myelin content in caudate and anterior frontal cortex was associated with better receptive language skills in healthy children between 1 to 6 years. Right-handed CWS in the present study may potentially have a similar developmental advantage (including greater resting state functional connectivity on the left hemisphere, and earlier and more congruent maturation across domains) compared to left-handed CWS that translates into proportionally lower rates of dissociations.
Some studies have suggested handedness as a factor in white matter development. Buchël et al. (2004) reported greater FA in the left precentral gyrus relative to the right precentral gyrus in right-handed adults, whereas the reverse was found for left-handed adults. White matter organization may also differ between right- and left-handers. Li et al. (2014) reported more prominent asymmetries in white matter structural networks in right-handers relative to left handers. Right-handed subjects showed a greater number of asymmetries in local clustering and distance between nodes between the left and right hemispheres relative to left-handed subjects. However, other studies have reported a left hemisphere bias in white matter development regardless of handedness. Both right- and left-handers show greater white matter volume and fiber density in the left hemisphere speech relevant regions/tracts including the inferior frontal and temporal regions, and arcuate fasciculus (Hervé, Crivello, Perchey, Mazoyer, & Tzourio-Mazoyer, 2006; Vernooij et al., 2007).
The relationship between handedness and functional laterality has also been equivocal. Sequeira et al. (2006) found an interaction between handedness and speech lateralization in males but not females. Further, asymmetry of the planum temporale was only evident in subjects with right ear advantage. Right-handed males with a right ear advantage showed larger leftward asymmetry of the planum temporale relative to right-handed females with a right ear advantage. Although a similar pattern of asymmetry was found in left-handed males and females with right ear advantage, the magnitude of difference was smaller relative to right handers. In the stuttering population, the relationship between handedness and functional lateralization including speech language may be compounded by the presence of stuttering. Further studies will need to be conducted to determine the relationship between handedness, functional and structural asymmetries, and the development of stuttering across age and sex.
5. Conclusions
Our results are consistent with previous studies that showed decreased performance on speech-language measures in CWS compared to typically developing controls. The present study expands on these results to show that CWS exhibit attenuated performance on cognitive and motor assessments and lower white matter coherence in speech relevant left hemisphere perisylvian regions of interest relative to CWNS. Strong evidence pointing to potentially uneven development across multiple domains in CWS was found, particularly in boys who stutter, and more so for left-handed versus right-handed children. These results may have clinical implications for the management of stuttering, including assessment, treatment, and prediction of outcomes. Higher dissociation rates in CWS may reflect a speech-language system that is developmentally delayed in certain components, rendering it more vulnerable to perturbations. If so, the presence or absence of dissociations could potentially distinguish between children at risk for persistent stuttering from those who are more likely to recover. Future studies are needed to ascertain interactions among additional factors that may predict persistent stuttering, including those that examine dissociations among neuroanatomical measures and temperament/emotional reactivity; such research should provide a comprehensive understanding of the nature of persistent stuttering as it relates to other developmental factors in childhood stuttering.
Supplementary Material
Highlights.
Children who stutter exhibited uneven development across multiple domains.
Dissociated white matter development in the left dorsal auditory stream was found in stuttering.
Boys, compared to girls who stutter exhibited more dissociated development.
Acknowledgements
The authors wish to thank all the children and parents who have participated in this study. In addition, we wish to thank Ashley Larva and the members of the Speech Neurophysiology Lab at Michigan State University for their assistance in scoring and transcribing the speech samples. This study was supported by Award Number #R01DC011277 (PI: Chang) from the National Institute on Deafness and other Communication Disorders (NIDCD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MR. The demands and capacities model I: Theoretical elaborations. Journal of Fluency Disorders. 1990;15(3):135–141. [Google Scholar]
- Anderson JD, Conture EG. Language abilities of children who stutter: A preliminary study. Journal of Fluency Disorders. 2000;25(4):283–304. [Google Scholar]
- Anderson JD, Conture EG. Sentence-structure priming in young children who do and do not stutter. Journal of Speech, Language, and Hearing Research. 2004;47(3):552–571. doi: 10.1044/1092-4388(2004/043). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JD, Pellowski MW, Conture EG. Childhood stuttering and dissociations across linguistic domains. Journal of Fluency Disorders. 2005;30(3):219–253. doi: 10.1016/j.jfludis.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Au-Yeung J, Gomez IV, Howell P. Exchange of disfluency with age from function words to content words in Spanish speakers who stutter. Journal of Speech, Language, and Hearing Research. 2003;46(3):754–765. doi: 10.1044/1092-4388(2003/060). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AD, Srinivasan P, Joel SE, Caffo BS, Pekar JJ, Mostofsky SH. Motor “dexterity”?: evidence that left hemisphere lateralization of motor circuit connectivity is associated with better motor performance in children. Cerebral Cortex. 2011:bhr062. doi: 10.1093/cercor/bhr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Bates E, Appelbaum M, Salcedo J, Saygin AP, Pizzamiglio L. Quantifying dissociations in neuropsychological research. Journal of Clinical and Experimental Neuropsychology. 2003;25(8):1128–1153. doi: 10.1076/jcen.25.8.1128.16724. [DOI] [PubMed] [Google Scholar]
- Beal DS, Gracco VL, Brettschneider J, Kroll RM, De Nil LF. A voxel-based morphometry (VBM) analysis of regional grey and white matter volume abnormalities within the speech production network of children who stutter. Cortex. 2013;49(8):2151–2161. doi: 10.1016/j.cortex.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal DS, Quraan MA, Cheyne DO, Taylor MJ, Gracco VL, Luc F. Speech-induced suppression of evoked auditory fields in children who stutter. Neuroimage. 2011;54(4):2994–3003. doi: 10.1016/j.neuroimage.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodstein O. Early stuttering as a type of language difficulty. Journal of Fluency Disorders. 2002;27(2):163–167. doi: 10.1016/s0094-730x(02)00111-0. [DOI] [PubMed] [Google Scholar]
- Bloodstein O. Some empirical observations about early stuttering: A possible link to language development. Journal of communication disorders. 2006;39(3):185–191. doi: 10.1016/j.jcomdis.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Braun A, Varga M, Stager S, Schulz G, Selbie S, Maisog J, Ludlow C. Altered patterns of cerebral activity during speech and language production in developmental stuttering. An H2 (15) O positron emission tomography study. Brain. 1997;120(5):761–784. doi: 10.1093/brain/120.5.761. [DOI] [PubMed] [Google Scholar]
- Brown SF. The loci of stutterings in the speech sequence. Journal of Speech Disorders. 1945;10(3):181–192. [Google Scholar]
- Büchel C, Raedler T, Sommer M, Sach M, Weiller C, Koch M. White matter asymmetry in the human brain: a diffusion tensor MRI study. Cerebral Cortex. 2004;14(9):945–951. doi: 10.1093/cercor/bhh055. [DOI] [PubMed] [Google Scholar]
- Buhr A, Zebrowski P. Sentence position and syntactic complexity of stuttering in early childhood: A longitudinal study. Journal of Fluency Disorders. 2009;34(3):155–172. doi: 10.1016/j.jfludis.2009.08.001. doi: http://dx.doi.org/10.1016/j.jfludis.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd K, Cooper EB. Expressive and receptive language skills in stuttering children. Journal of Fluency Disorders. 1989;14(2):121–126. [Google Scholar]
- Cai S, Beal DS, Ghosh SS, Guenther FH, Perkell JS. Impaired timing adjustments in response to time-varying auditory perturbation during connected speech production in persons who stutter. Brain and language. 2014;129:24–29. doi: 10.1016/j.bandl.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Beal DS, Ghosh SS, Tiede MK, Guenther FH, Perkell JS. Weak responses to auditory feedback perturbation during articulation in persons who stutter: evidence for abnormal auditory-motor transformation. PloS one. 2012;7(7):e41830. doi: 10.1371/journal.pone.0041830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrow-Woolfolk E. Test for Auditory Comprehension of Language: TACL-3: Pro-Ed. 1999 [Google Scholar]
- Caruso AJ, Conture EG, Colton RH. Selected temporal parameters of coordination associated with stuttering in children. Journal of Fluency Disorders. 1988;13(1):57–82. [Google Scholar]
- Caruso AJ, Gracco VL, Abbs JH. A Speech Motor Control Perspective on Stuttering: Preliminary Observations. In: Peters HM, Hulstijn W, editors. Speech Motor Dynamics in Stuttering. Springer; Vienna: 1987. pp. 245–258. [Google Scholar]
- Chang S-E, Kenney MK, Loucks TM, Ludlow CL. Brain activation abnormalities during speech and non-speech in stuttering speakers. Neuroimage. 2009;46(1):201–212. doi: 10.1016/j.neuroimage.2009.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. Neuroimage. 2008;39(3):1333–1344. doi: 10.1016/j.neuroimage.2007.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Zhu DC. Neural network connectivity differences in children who stutter. Brain. 2013:awt275. doi: 10.1093/brain/awt275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Zhu DC, Choo AL, Angstadt MK. White matter differences in young children who stutter. Brain. 2015;138(Pt 3):694–711. doi: 10.1093/brain/awu400. Chang et al., 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo AL, Chang S-E, Zengin-Bolatkale H, Ambrose NG, Loucks TM. Corpus callosum morphology in children who stutter. Journal of communication disorders. 2012;45(4):279–289. doi: 10.1016/j.jcomdis.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayden JD, Jentschke S, Muñoz M, Cooper JM, Chadwick MJ, Banks T, Vargha-Khadem F. Normative development of white matter tracts: similarities and differences in relation to age, gender, and intelligence. Cerebral Cortex. 2011:bhr243. doi: 10.1093/cercor/bhr243. [DOI] [PubMed] [Google Scholar]
- Coulter CE, Anderson JD, Conture EG. Childhood stuttering and dissociations across linguistic domains: A replication and extension. Journal of Fluency Disorders. 2009;34(4):257–278. doi: 10.1016/j.jfludis.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FI, Bialystok E. Cognition through the lifespan: mechanisms of change. Trends in cognitive sciences. 2006;10(3):131–138. doi: 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- De Nil L, Kroll RM, Kapur S, Houle S. A positron emission tomography study of silent and oral single word reading in stuttering and nonstuttering adults. Journal of Speech, Language, and Hearing Research. 2000;43(4):1038–1053. doi: 10.1044/jslhr.4304.1038. [DOI] [PubMed] [Google Scholar]
- Dubois J, Poupon C, Thirion B, Simonnet H, Kulikova S, Leroy F, Dehaene-Lambertz G. Exploring the Early Organization and Maturation of Linguistic Pathways in the Human Infant Brain. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv082. doi: 10.1093/cercor/bhv082. [DOI] [PubMed] [Google Scholar]
- Dunn L, Dunn D. Peabody Picture Vocabulary Test. American Guidance Service. Inc. Publishing; Circle Pines, MN: 1997. [Google Scholar]
- Dunst B, Benedek M, Koschutnig K, Jauk E, Neubauer AC. Sex differences in the IQ-white matter microstructure relationship: A DTI study. Brain and cognition. 2014;91:71–78. doi: 10.1016/j.bandc.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluharty NB. Fluharty Preschool Speech and Language Screening Test. 2nd ed. Pro-Ed; Teaching Resources Corporation; Austin, TX: 2000. [Google Scholar]
- Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. Journal of autism and developmental disorders. 2003;33(4):365–382. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- Forster DC, Webster WG. Speech-motor control and interhemispheric relations in recovered and persistent stuttering. Developmental neuropsychology. 2001;19(2):125–145. doi: 10.1207/S15326942DN1902_1. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Bollich A, Corey D, Hurley M, Heilman K. Anomalous anatomy of speech–language areas in adults with persistent developmental stuttering. Neurology. 2001;57(2):207–215. doi: 10.1212/wnl.57.2.207. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Bollich AM, Feldman J, Corey DM, Hurley M, Lemen LC, Heilman KM. Aberrant auditory processing and atypical planum temporale in developmental stuttering. Neurology. 2004;63(9):1640–1646. doi: 10.1212/01.wnl.0000142993.33158.2a. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Hirsch TB, Downs JH, Martin C, Lancaster JL. A PET study of the neural systems of stuttering. 1996 doi: 10.1038/382158a0. [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA, Sauer EA, Geye HM, Schweigert EK, Hill Goldsmith H. Infant and toddler oral - and manual - motor skills predict later speech fluency in autism. Journal of Child Psychology and Psychiatry. 2008;49(1):43–50. doi: 10.1111/j.1469-7610.2007.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: evidence from diffusion tensor imaging. Neuropsychologia. 2007;45(11):2439–2446. doi: 10.1016/j.neuropsychologia.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R, Fristoe M. Goldman Fristoe 2: Test of Articulation American Guidance Service. Circle Pines; MN.: 2000. [Google Scholar]
- Grande M, Meffert E, Huber W, Amunts K, Heim S. Word frequency effects in the left IFG in dyslexic and normally reading children during picture naming and reading. Neuroimage. 2011;57(3):1212–1220. doi: 10.1016/j.neuroimage.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain and language. 2006;96(3):280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14(2):224. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Hakim HB, Bernstein Ratner N. Nonword repetition abilities of children who stutter: An exploratory study. Journal of Fluency Disorders. 2004;29(3):179–199. doi: 10.1016/j.jfludis.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Hall NE. Lexical development and retrieval in treating children who stutter. Language, Speech, and Hearing Services in Schools. 2004;35(1):57–69. doi: 10.1044/0161-1461(2004/007). [DOI] [PubMed] [Google Scholar]
- Hamilton LS, Levitt JG, O'Neill J, Alger JR, Luders E, Phillips OR, Narr KL. Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport. 2008;19(17):1705. doi: 10.1097/WNR.0b013e3283174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellendoorn A, Wijnroks L, van Daalen E, Dietz C, Buitelaar JK, Leseman P. Motor functioning, exploration, visuospatial cognition and language development in preschool children with autism. Research in developmental disabilities. 2015;39:32–42. doi: 10.1016/j.ridd.2014.12.033. [DOI] [PubMed] [Google Scholar]
- Hervé P-Y, Crivello F, Perchey G, Mazoyer B, Tzourio-Mazoyer N. Handedness and cerebral anatomical asymmetries in young adult males. Neuroimage. 2006;29(4):1066–1079. doi: 10.1016/j.neuroimage.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. 1975 [Google Scholar]
- Hollister J, Alpermann A, Zebrowski P. Dissociations within and across linguistic motor domains in children who stutter.. Paper presented at the American Speech-Language Hearing Associaiton; Atlanta, GA.. 2012. [Google Scholar]
- Horowitz-Kraus T, Wang Y, Plante E, Holland SK. Involvement of the right hemisphere in reading comprehension: A DTI study. Brain research. 2014;1582(0):34–44. doi: 10.1016/j.brainres.2014.05.034. doi: http://dx.doi.org/10.1016/j.brainres.2014.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell P, Au-Yeung J, Sackin S. Exchange of stuttering from function words to content words with age. Journal of Speech, Language, and Hearing Research. 1999;42(2):345–354. doi: 10.1044/jslhr.4202.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard CP, Prins D. Word familiarity, syllabic stress pattern, and stuttering. Journal of Speech, Language, and Hearing Research. 1994;37(3):564–571. doi: 10.1044/jshr.3703.564. [DOI] [PubMed] [Google Scholar]
- Iverson JM. Developing language in a developing body: The relationship between motor development and language development. Journal of child language. 2010;37(02):229–261. doi: 10.1017/S0305000909990432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi-Hanifi K, Howell P. Syntactic analysis of the spontaneous speech of normally fluent and stuttering children. Journal of Fluency Disorders. 1992;17(3):151–170. [Google Scholar]
- Karapetsas AB, Vlachos FM. Sex and handedness in development of visuomotor skills. Perceptual and motor skills. 1997;85(1):131–140. [PubMed] [Google Scholar]
- Karniol R. Stuttering, language, and cognition: a review and a model of stuttering as suprasegmental sentence plan alignment (SPA). Psychological bulletin. 1995;117(1):104. doi: 10.1037/0033-2909.117.1.104. [DOI] [PubMed] [Google Scholar]
- Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Lai M-C, Lombardo MV, Pasco G, Ruigrok AN, Wheelwright SJ, Sadek SA, Consortium MA. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PloS one. 2011;6(6):e20835. doi: 10.1371/journal.pone.0020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langel J, Hakun J, Zhu DC, Ravizza SM. Functional specialization of the left ventral parietal cortex in working memory. Front Hum Neurosci. 2014;8:440. doi: 10.3389/fnhum.2014.00440. doi: 10.3389/fnhum.2014.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chen H, Wang J, Liu F, Long Z, Wang Y, Chen H. Handedness-and hemisphere-related differences in small-world brain networks: a diffusion tensor imaging tractography study. Brain connectivity. 2014;4(2):145–156. doi: 10.1089/brain.2013.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan KJ, Conture EG. Length, grammatical complexity, and rate differences in stuttered and fluent conversational utterances of children who stutter. Journal of Fluency Disorders. 1995;20(1):35–61. [Google Scholar]
- Lu L, Leonard C, Thompson P, Kan E, Jolley J, Welcome S, Sowell E. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cerebral Cortex. 2007;17(5):1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- Ludlow CL, Loucks T. Stuttering: a dynamic motor control disorder. Journal of Fluency Disorders. 2004;28(4):273–295. doi: 10.1016/j.jfludis.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P. Foetal testosterone and eye contact in 12-month-old human infants. Infant Behavior and Development. 2002;25(3):327–335. doi: http://dx.doi.org/10.1016/S0163-6383(02)00094-2. [Google Scholar]
- Macdonald, Johnson EK, Forsythe J, Plante P, Munhall KG. Children's development of self-regulation in speech production. Current Biology. 2012;22(2):113–117. doi: 10.1016/j.cub.2011.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson MK, Smith A. Influences of sentence length and syntactic complexity on the speech motor control of children who stutter. Journal of Speech, Language, and Hearing Research. 2013;56(1):89–102. doi: 10.1044/1092-4388(2012/11-0184). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, Skuse D. Sex differences in autism spectrum disorder: evidence from a large sample of children and adolescents. Journal of autism and developmental disorders. 2012;42(7):1304–1313. doi: 10.1007/s10803-011-1356-0. [DOI] [PubMed] [Google Scholar]
- Marino C, Scifo P, Della Rosa PA, Mascheretti S, Facoetti A, Lorusso ML, Molteni M. The DCDC2/intron 2 deletion and white matter disorganization: focus on developmental dyslexia. Cortex. 2014;57:227–243. doi: 10.1016/j.cortex.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max L, Guenther FH, Gracco VL, Ghosh SS, Wallace ME. Unstable or insufficiently activated internal models and feedback-biased motor control as sources of dysfluency: A theoretical model of stuttering. Contemporary issues in communication science and disorders. 2004;31:105–122. [Google Scholar]
- Mayer M. Frog, where are you? Dial Press New York. 1969 [Google Scholar]
- Miller J, Macedonia C, Sonies B. Sex differences in prenatal oral-motor function and development. Developmental Medicine & Child Neurology. 2006;48(06):465–470. doi: 10.1017/S0012162206001009. [DOI] [PubMed] [Google Scholar]
- Müürsepp I, Ereline J, Gapeyeva H, Pääsuke M. Motor performance in 5 - year - old preschool children with developmental speech and language disorders. Acta Paediatrica. 2009;98(8):1334–1338. doi: 10.1111/j.1651-2227.2009.01294.x. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Newcomer P, Hamill D. Test of language development PRO-ED. Austin; TX.: 1997. [Google Scholar]
- Newman RS, Bernstein Ratner N. The role of selected lexical factors on confrontation naming accuracy, speed, and fluency in adults who do and do not stutter. Journal of Speech, Language, and Hearing Research. 2007;50(1):196–213. doi: 10.1044/1092-4388(2007/016). [DOI] [PubMed] [Google Scholar]
- Nippold MA. Stuttering and language ability in children: Questioning the connection. American Journal of Speech-Language Pathology. 2012;21(3):183–196. doi: 10.1044/1058-0360(2012/11-0078). [DOI] [PubMed] [Google Scholar]
- Ntourou K, Conture EG, Lipsey MW. Language abilities of children who stutter: A meta-analytical review. American Journal of Speech-Language Pathology. 2011;20(3):163–179. doi: 10.1044/1058-0360(2011/09-0102). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Muircheartaigh J, Dean DC, Dirks H, Waskiewicz N, Lehman K, Jerskey BA, Deoni SC. Interactions between white matter asymmetry and language during neurodevelopment. The Journal of neuroscience. 2013;33(41):16170–16177. doi: 10.1523/JNEUROSCI.1463-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pellowski MW, Conture EG. Lexical priming in picture naming of young children who do and do not stutter. Journal of Speech, Language, and Hearing Research. 2005;48(2):278–294. doi: 10.1044/1092-4388(2005/019). [DOI] [PubMed] [Google Scholar]
- Peters HF, Starkweather CW. The interaction between speech motor coordination and language processes in the development of stuttering: Hypotheses and suggestions for research. Journal of Fluency Disorders. 1990;15(2):115–125. [Google Scholar]
- Pool E-M, Rehme AK, Eickhoff SB, Fink GR, Grefkes C. Functional resting-state connectivity of the human motor network: Differences between right- and left-handers. Neuroimage. 2015;109(0):298–306. doi: 10.1016/j.neuroimage.2015.01.034. doi: http://dx.doi.org/10.1016/j.neuroimage.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool E-M, Rehme AK, Fink GR, Eickhoff SB, Grefkes C. Handedness and effective connectivity of the motor system. Neuroimage. 2014;99:451–460. doi: 10.1016/j.neuroimage.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz BA. Functional neuroimaging studies of reading and reading disability(developmental dyslexia). Mental retardation and developmental disabilities research reviews. 2000;6(3):207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Ortiz H, Sebastian-Galles N, Losilla J, Deus J. Myelination of language-related areas in the developing brain. Neurology. 2006;66(3):339–343. doi: 10.1212/01.wnl.0000201049.66073.8d. [DOI] [PubMed] [Google Scholar]
- Ratner NB. Language complexity and stuttering in children. Topics in Language Disorders. 1995;15(3):32–47. [Google Scholar]
- Ratner NB, Sih CC. Effects of gradual increases in sentence length and complexity on children's dysfluency. Journal of Speech and Hearing Disorders. 1987;52(3):278–287. doi: 10.1044/jshd.5203.278. [DOI] [PubMed] [Google Scholar]
- Richards T, Stevenson J, Crouch J, Johnson L, Maravilla K, Stock P, Berninger V. Tract-based spatial statistics of diffusion tensor imaging in adults with dyslexia. American Journal of Neuroradiology. 2008;29(6):1134–1139. doi: 10.3174/ajnr.A1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Sturm D, Schurz M, Kronbichler M, Ladurner G, Wimmer H. A common left occipito-temporal dysfunction in developmental dyslexia and acquired letter-by-letter reading? PloS one. 2010;5(8):e12073. doi: 10.1371/journal.pone.0012073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley GD. Stuttering Severity Instrument: SSI-4. Pro-Ed; 2009. [Google Scholar]
- Rimrodt SL, Peterson DJ, Denckla MB, Kaufmann WE, Cutting LE. White matter microstructural differences linked to left perisylvian language network in children with dyslexia. Cortex. 2010;46(6):739–749. doi: 10.1016/j.cortex.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb MP, Sargent A, O'Beirne GA. Characteristics of disfluency clusters in adults who stutter. Logoped Phoniatr Vocol. 2009;34(1):36–42. doi: 10.1080/14015430902748519. doi: 10.1080/14015430902748519. [DOI] [PubMed] [Google Scholar]
- Rommel D. The influence of psycholinguistic variables on stuttering in childhood. Journal of Fluency Disorders. 2001;25(3):212. [Google Scholar]
- Ryan BP. Articulation, Language, Rate, and Fluency Characteristics of Stuttering and Nonstuttering Preschool Children. Journal of Speech, Language, and Hearing Research. 1992;35(2):333–342. doi: 10.1044/jshr.3502.333. doi: 10.1044/jshr.3502.333. [DOI] [PubMed] [Google Scholar]
- Saenger VM, Barrios FA, Martínez-Gudiño ML, Alcauter S. Hemispheric asymmetries of functional connectivity and grey matter volume in the default mode network. Neuropsychologia. 2012;50(7):1308–1315. doi: 10.1016/j.neuropsychologia.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Sall J, Creighton L, Lehman A. JMP start statistics: A guide to statistics and data analysis using JMP and JMP IN software Thomson Learning. Belmont; CA.: 2004. [Google Scholar]
- Sawyer J, Chon H, Ambrose NG. Influences of rate, length, and complexity on speech disfluency in a single-speech sample in preschool children who stutter. Journal of Fluency Disorders. 2008;33(3):220–240. doi: 10.1016/j.jfludis.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Human brain mapping. 2005;26(2):139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciberras E, Mueller KL, Efron D, Bisset M, Anderson V, Schilpzand EJ, Nicholson JM. Language Problems in Children With ADHD: A Community-Based Study. Pediatrics. 2014;133(5):793–800. doi: 10.1542/peds.2013-3355. [DOI] [PubMed] [Google Scholar]
- Sequeira SDS, Woerner W, Walter C, Kreuder F, Lueken U, Westerhausen R, Wittling W. Handedness, dichotic-listening ear advantage, and gender effects on planum temporale asymmetry—a volumetric investigation using structural magnetic resonance imaging. Neuropsychologia. 2006;44(4):622–636. doi: 10.1016/j.neuropsychologia.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Sovijärvi-Spapé MM. Hemispheric asymmetries and the control of motor sequences. Behavioural Brain Research. 2015 doi: 10.1016/j.bbr.2015.01.021. [DOI] [PubMed] [Google Scholar]
- Shiller DM, Gracco VL, Rvachew S. Auditory-motor learning during speech production in 9-11-year-old children. PloS one. 2010;5(9):e12975. doi: 10.1371/journal.pone.0012975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman S, Bernstein Ratner N. Measuring lexical diversity in children who stutter: application of< i> vocd</i>. Journal of Fluency Disorders. 2002;27(4):289–304. doi: 10.1016/s0094-730x(02)00162-6. [DOI] [PubMed] [Google Scholar]
- Skeide MA, Brauer J, Friederici AD. Brain Functional and Structural Predictors of Language Performance. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv042. doi: 10.1093/cercor/bhv042. [DOI] [PubMed] [Google Scholar]
- Smith A, Goffman L, Sasisekaran J, Weber-Fox C. Language and motor abilities of preschool children who stutter: Evidence from behavioral and kinematic indices of nonword repetition performance. Journal of Fluency Disorders. 2012;37(4):344–358. doi: 10.1016/j.jfludis.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Kelly E. Stuttering: A dynamic, multifactorial model. Nature and treatment of stuttering: New directions. 1997;2:204–217. [Google Scholar]
- Smith A, Sadagopan N, Walsh B, Weber-Fox C. Increasing phonological complexity reveals heightened instability in inter-articulatory coordination in adults who stutter. Journal of Fluency Disorders. 2010;35(1):1–18. doi: 10.1016/j.jfludis.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers M, Shields LS, Boks MP, Kahn RS, Sommer IE. Cognitive benefits of right-handedness: A meta-analysis. Neuroscience & Biobehavioral Reviews. 2015;51(0):48–63. doi: 10.1016/j.neubiorev.2015.01.003. doi: http://dx.doi.org/10.1016/j.neubiorev.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. The Journal of neuroscience. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer E, Packman A, Onslow M, Ferguson A. A preliminary investigation of the impact of stuttering on language use. Clinical linguistics & phonetics. 2005;19(3):191–201. doi: 10.1080/02699200410001698625. [DOI] [PubMed] [Google Scholar]
- Starkweather CW, Gottwald SR. The demands and capacities model II: Clinical applications. Journal of Fluency Disorders. 1990;15(3):143–157. [Google Scholar]
- Taylor IK. What words are stuttered? Psychological bulletin. 1966;65(4):233. doi: 10.1037/h0023180. [DOI] [PubMed] [Google Scholar]
- Tiffin J. Purdue pegboard examiner manual: Science Research Associates. 1968 [Google Scholar]
- Tomblin JB, Records NL, Buckwalter P, Zhang X, Smith E, O'Brien M. Prevalence of specific language impairment in kindergarten children. Journal of Speech, Language, and Hearing Research. 1997;40(6):1245–1260. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomura A, Fujii T, Kuriki S. Effect of external auditory pacing on the neural activity of stuttering speakers. Neuroimage. 2011;57(4):1507–1516. doi: 10.1016/j.neuroimage.2011.05.039. [DOI] [PubMed] [Google Scholar]
- Travis LE. Recurrence of stuttering following shift from normal to mirror writing. Archives of Neurology & Psychiatry. 1929;21(2):386–391. [Google Scholar]
- Van Borsel J, Achten E, Santens P, Lahorte P, Voet T. fMRI of developmental stuttering: a pilot study. Brain and language. 2003;85(3):369–376. doi: 10.1016/s0093-934x(02)00588-6. [DOI] [PubMed] [Google Scholar]
- Van Riper C. The nature of stuttering , 1982. Prentice-Hall; Englewood Cliffs, NJ.: 1982. [Google Scholar]
- Vernooij M, Smits M, Wielopolski P, Houston G, Krestin G, van der Lugt A. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right-and left-handed healthy subjects: a combined fMRI and DTI study. Neuroimage. 2007;35(3):1064–1076. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Wagovich SA, Bernstein Ratner N. Frequency of verb use in young children who stutter. Journal of Fluency Disorders. 2007;32(2):79–94. doi: 10.1016/j.jfludis.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Watkins RV, Yairi E, Ambrose NG. Early Childhood Stuttering IIIInitial Status of Expressive Language Abilities. Journal of Speech, Language, and Hearing Research. 1999;42(5):1125–1135. doi: 10.1044/jslhr.4205.1125. [DOI] [PubMed] [Google Scholar]
- Watson JB, Byrd CT, Carlo EJ. Effects of length, complexity, and grammatical correctness on stuttering in Spanish-speaking preschool children. American Journal of Speech-Language Pathology. 2011;20(3):209–220. doi: 10.1044/1058-0360(2011/10-0019). [DOI] [PubMed] [Google Scholar]
- Weber-Fox C, Spruill JE, Spencer R, Smith A. Atypical neural functions underlying phonological processing and silent rehearsal in children who stutter. Developmental Science. 2008;11(2):321–337. doi: 10.1111/j.1467-7687.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. Psychological Corporation; 1999. [Google Scholar]
- Wechsler D. Wechsler preschool and primary scale of intelligence. The Psychological Corporation; San Antonio, TX.: 2002. [Google Scholar]
- Weiss AL, Zebrowski PM. Disfluencies in the Conversations of Young Children Who StutterSome Answers About Questions. Journal of Speech, Language, and Hearing Research. 1992;35(6):1230–1238. doi: 10.1044/jshr.3506.1230. [DOI] [PubMed] [Google Scholar]
- Whitehouse AJO, Mattes E, Maybery MT, Sawyer MG, Jacoby P, Keelan JA, Hickey M. Sex-specific associations between umbilical cord blood testosterone levels and language delay in early childhood. Journal of Child Psychology and Psychiatry. 2012;53(7):726–734. doi: 10.1111/j.1469-7610.2011.02523.x. [DOI] [PubMed] [Google Scholar]
- Williams KT. EVT-2: Expressive Vocabulary Test: Pearson Assessments. 2007 [Google Scholar]
- Wingate ME. Early position and stuttering occurrence. Journal of Fluency Disorders. 1982;7(2):243–258. [Google Scholar]
- Yairi E, Ambrose NG, Paden EP, Throneburg RN. Predictive factors of persistence and recovery: Pathways of childhood stuttering. Journal of communication disorders. 1996;29(1):51–77. doi: 10.1016/0021-9924(95)00051-8. [DOI] [PubMed] [Google Scholar]
- Yaruss JS. Utterance length, syntactic complexity, and childhood stuttering. Journal of Speech, Language, and Hearing Research. 1999;42(2):329–344. doi: 10.1044/jslhr.4202.329. [DOI] [PubMed] [Google Scholar]
- Yelle SK, Grimshaw GM. Hemispheric specialization for linguistic processing of sung speech. Percept Mot Skills. 2009;108(1):219–228. doi: 10.2466/PMS.108.1.219-228. doi: 10.2466/pms.108.1.219-228. [DOI] [PubMed] [Google Scholar]
- Zackheim CT, Conture EG. Childhood stuttering and speech disfluencies in relation to children's mean length of utterance: A preliminary study. Journal of Fluency Disorders. 2003;28(2):115–142. doi: 10.1016/s0094-730x(03)00007-x. [DOI] [PubMed] [Google Scholar]
- Zubrick SR, Taylor CL, Rice ML, Slegers DW. Late language emergence at 24 months: An epidemiological study of prevalence, predictors, and covariates. Journal of Speech, Language, and Hearing Research. 2007;50(6):1562–1592. doi: 10.1044/1092-4388(2007/106). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.