Abstract
A group of transcription factors (TF) that are master developmental regulators of the establishment and maintenance of pluripotency during embryogenesis play additional roles to control tissue homeostasis and regeneration in adults. Among these TFs, members of the Octamer-binding transcription factor (OCT) gene family are well documented as major regulators controlling the self-renewal and pluripotency of stem cells isolated from different adult organs including the brain. In the last few years a large number of studies show the aberrant expression and dysfunction of OCT in different types of cancers including glioblastoma multiforme (GBM). GBM is the most common malignant primary brain tumor, and contains a subpopulation of undifferentiated stem cells (GSCs), with self-renewal and tumorigenic potential that contribute to tumor initiation, invasion, recurrence, and therapeutic resistance. In this review, we have summarized the current knowledge about OCT family in GBM and their crucial role in the initiation, maintenance and drug resistance properties of GSCs.
Keywords: Octamer-binding transcription factor, cancer stem cell, glioblastoma multiforme, tumor microenvironment
Introduction
TFs related to pluripotency and stemness are developmental regulators of self-renewal and cell differentiation. The dissection of transcriptional networks has provided invaluable information regarding the nature of the master regulators that control entire gene expression signatures that drive the phenotype of normal and malignant cells of the brain. Understanding how such transcriptional networks regulate transitions into physiological or pathological cellular states remains a central challenge in systems biology. Stemness is a hallmark of tumor aggressiveness in GBM, but the regulatory programs responsible for implementing the molecular signature associated with this phenotype are largely unknown [1]. Members of the OCT gene family (HUGO nomenclature: POU class homeoboxes and pseudogenes) are recognized as being among the master TFs controlling the expression of genes responsible for pluripotency and embryogenesis during the early stages of development. Recently, multiple studies have implicated the altered expression and function of OCT family members in the pathogenesis of several types of cancer, including GBM. In this review, we discussed current knowledge on the effects of OCT deregulation in GBM and the role of OCT in the maintenance of GSCs. Targeting of pluripotency TFs, including OCT4, represents a promising therapeutic approach that may improve overall survival and reduce tumor relapse in GBM patients [2]. OCT4 and OCT7 [3–5], together with other master regulators, SOX2, SALL2, OLIG2 and NANOG, has the most established anti-differentiation, pro-stemness and pro-tumorigenic function of all the OCT TF family members in stem cells, including GSCs. Among genes regulated by these TFs are genes encoding key signaling pathways that control pluripotency and self-renewal. At the same time, they repress genes that promote differentiation [5, 6]. These TFs are also involved in an auto-regulatory loop controlling their own expression, and targeting one of these TFs may have a broad effect on pluripotency [7]. Although these studies add additional layers of complexity and underscore the importance of network-based rearrangements in the heterogeneous subpopulation of GBM cells; however their global functions remain poorly understood in the context of multifaceted phenotype of this disease.
Glioblastoma multiforme: characteristics, subtypes and cancer stem cells
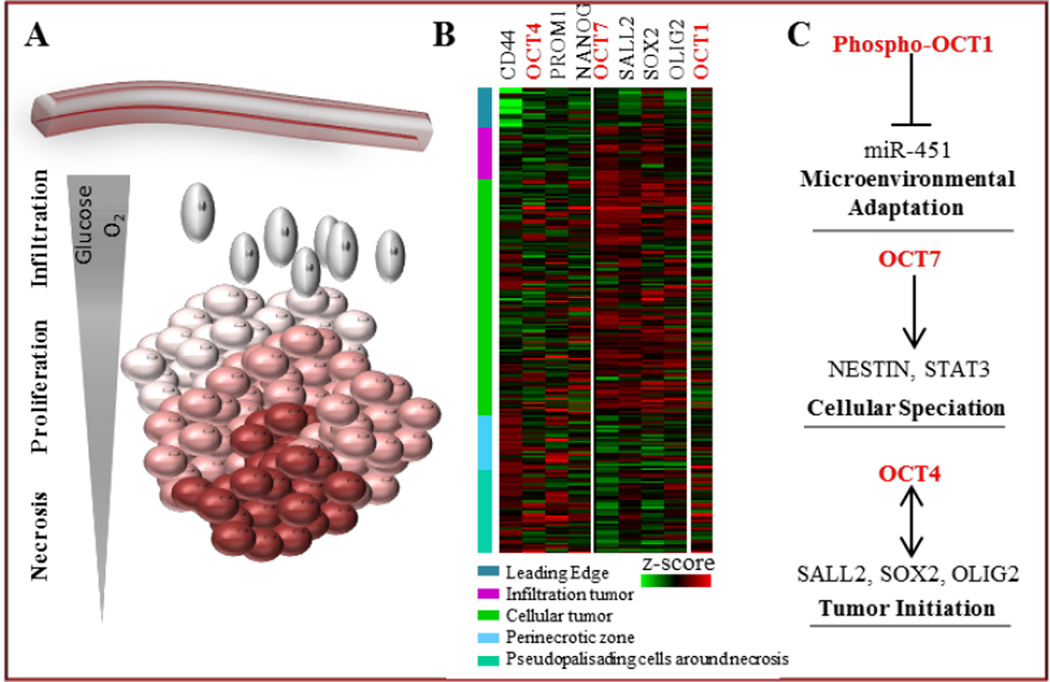
GBM is the most frequent and aggressive primary brain tumor in adults, and despite improvements in therapy and progress in understanding of GBM pathophysiology, the prognosis of GBM patients remains poor, with a median overall survival of only 14.2 months [8–10]. The treatment modalities for GBM include maximal safe surgical resection, followed by irradiation and chemotherapy with temozolomide (TMZ) [8]. GBM is an extremely aggressive, complex and heterogeneous tumor composed of distinct cellular components, including tumor cells with different phenotypes, genotypes and epigenetic characteristics, associated astrocytes, infiltrating immune cells and microglia, abnormal vasculature and extensive necrotic and hypoxic zones (Figure 1A).
Figure 1. Schematic representation of OCT family expression and function in GBM microenvironment.
A. Cellular heterogeneity reflects the complexity of the GBM ecosystem. Subpopulations of GBM cells grow in divergent intra-tumoral anatomic sites determined by microenvironmental cues (e.g. hypoxia, nutrient availability) which may contribute to tumor cell speciation, growth and invasion.
B. OCTs are differentially expressed in intra-tumoral anatomic niches. Expression of OCT4 is prevalent in hypoxic, necrotic zones, while OCT 7 is expressed almost exclusively in proliferative, cellular areas. Expression of OCT1 is not specific to any distinct, intra-tumoral anatomic site. Ivy GAP database-based expression of OCTs signature in different areas of GBM for annotated genes is shown.
C. OCT TFs determine GBM cell fate. Overexpression of OCT7 and OCT4, along with their partner TFs in neural stem cells leads to cellular speciation and tumor initiation, while AMPK-dependent, phospho-OCT1-mediated de-activation of miR-451 signaling allows microenvironmental adaptation which results in decreased proliferation and increased migratory phenotype.
WHO classification of gliomas into grades I–IV is based mainly on the histological features of the tumor [11], and frequently does not reflect the molecular heterogeneity of the disease. Our knowledge of GBM biology had been enriched immensely by the advent of molecular characterization and cancer genomics. Large-scale, high-throughput characterization of GBM has clearly identified a combination of genetic, epigenetic, and transcriptome modifications defining four GBM subtypes. Despite intra- and inter-tumoral heterogeneity at the molecular and histopathological levels, GBMs can be divided into four major subtypes based on distinct transcriptional signatures as well as particular genetic aberrations: the proneural, neural, classical and mesenchymal subtypes [12–14]. The classical subtype is characterized by extreme EGFR amplification, homozygous deletion of CDKN2A, and wild-type TP53. Unlike what is observed in classical GBM tumors, TP53 is frequently mutated in proneural GBM (in 54% cases), and this subtype is also characterized by frequent mutations in the IDH1 and PDGFR-α genes, as well as a G-CIMP+ (GBM-CpG island methylator) phenotype. This subgroup is most prevalent in younger patients, and these tumors demonstrate global hyper-methylation, associated with IDH1 mutations and better survival [13, 15]. The mesenchymal subtype is defined by frequent mutations in the NF1 (in 37% cases), PTEN and TP53 tumor suppressor genes, whereas no distinctive mutations have been demonstrated in the neural subtype of GBMs [16]. Apart from these several gene mutation in GBM subtypes, adjuvant therapy with TMZ undoubtedly leaves an imprint in the genome evolution in low grade glioma. TMZ-treated patients have been frequently related to have tumor recurrence with high rate of mutation in the genes associated with inactivation of the mismatch repair pathway and subsequently underwent to malignant progression to GBM [17]. Recently, single-cell RNA analysis of cells from several GBM patients revealed a mixture of cells with different subtypes in each individual patient, adding an additional level of complexity to the pathobiology of GBM [7]. The impact of such complex heterogeneity will not be fully understood without describing the self-renewal and drug resistance properties of subpopulation of GBM cells, having stem like properties.
To understand the critical role and origin of cancer stem cells (CSC) in the pathogenesis of GBM, we first must consider earlier stochastic models. These models theorized that all cells in a tumor, after clonal evolution of acquired genetic mutations, are responsible for tumor growth, and that most clones are highly proliferative and tumorigenic, with the capacity to develop tumors after transplantation. In the last two decades, however, the stochastic model has been largely supplanted by the concept of CSC, which acknowledges that cancers are heterogeneous entities with cellular hierarchies and small subpopulations of self-renewing cells capable of driving tumor initiation, growth, propagation, and resistance to therapy [18]. The CSC concept was first described in acute leukemia models, where a subset of tumor cells could self-renew and propagate leukemia in vivo after xenotransplantation into athymic mice [19, 20]. Compelling evidence exists that supports the presence of CSCs in numerous solid tumors, including GBM [21].
Because of inherent characteristics of GBM, such as invasiveness that prevents total resection and resistance to radio- and chemotherapy, almost all GBMs recur after treatment. A relatively small subpopulation of GSCs, is highly tumorigenic [21–23] and therapy-resistant [24]. This subpopulation has the ability, upon intracranial transplantation, to generate a tumor that recapitulates the cellular heterogeneity and molecular characteristics of the parental tumor, indicative of its crucial role in the tumorigenicity, progression and maintenance of GBM [22, 23]. GSCs also have the ability to differentiate along neural lineages when placed in a differentiating medium with the appropriate combination of growth factors. Exploring the molecular signaling cascade through which GSCs maintain stemness and pluripotency is thus crucial to understanding the pathophysiology of GBM and for the development of novel anti-GBM therapies.
Transcription factors regulating pluripotency and stemness
During prenatal development, the brain is one of the organs derived from the ectodermal lineage, which includes the neuronal stem cells (NSCs) regulated by pluripotency genes and TFs. These NSCs are also self-renewing and have the capacity to differentiate into several neural cell lineages, such as neurons, astrocytes, and oligodendrocytes [25]. These cells are found in specific regions of the brain, such as the sub-ventricular zone of the lateral ventricle or the sub-granular zone in the hippocampus, where they reside in a restricted, specialized microenvironment appropriate for stem cell self-renewal and differentiation [26]. Along with the appropriate microenvironmental niche, the fate of NSCs towards maintenance or differentiation is determined by many signaling pathways, such as Hedgehog [27], Wnt [28], and NOTCH [29]. In addition to the microenvironmental niche and signaling pathways, stem cell properties are also regulated by several TFs and pluripotency genes. Among these TFs controlling the pluripotency of both NSCs and GSCs are OCT family TFs, SOX2, and the homeobox protein NANOG [5, 30, 31]. In addition to these TFs, other genes such as KLF4, REX1, Olig2, SALL2 and c-Myc are also pivotal in regulating the complex pluripotency circuitry in NSCs [32–36] and GSCs [4, 22, 23].
The OCT family of transcription factors
The octamer-binding proteins are a family of highly conserved TFs which specifically bind to the octamer motif (consensus sequence ATGCAAAT) and closely related sequences, using a bipartite DNA-binding domain known as a POU (Pit-Oct-Unc) domain that consists of an N-terminal specific subdomain (POUS) and a C-terminal homeo-subdomain (POUH) [37]. These domains are crucial for high-affinity, site specific binding of the octamer and other closely related sequences. Both of these POU domains also facilitate protein-protein interaction with other TFs and co-factors. Target octamers and closely related sequences are found in numerous promoters and enhancers of cell type-specific genes. Sequence differences in the transcription-activating domain sequence regulate the OCT family members’ capacity to activate or inhibit specific target genes. These proteins are transcribed from highly conserved OCT genes; eleven OCT proteins have been described and eight encoding genes, OCT1 [38], OCT2 [39], OCT3/4 [40], OCT6 [41], OCT7 [42], OCT8 [43], OCT9 [44], and OCT11 [45], have been cloned and characterized thus far from the human genome. Apart from the ubiquitously expressed OCT1, most of the members exhibit a developmental and tissue specific pattern of expression. OCT proteins are thus present in multiple organs, including the brain [46]. A role for OCT proteins in neurogenesis was also postulated after their expression in neural tube and adult brain was identified [43]. In embryonic stem cells (ESCs), the genes responsible for pluripotency remain activated, whereas most developmental genes are in a poised state in other tissue types. During early brain development, the ability to differentiate into the neuronal or astrocytic lineage is suppressed until the appropriate TFs, such as OCT4, initiate the differentiation processes [3]. On the other hand, the neurogenesis process in adults is mainly responsible for the replacement of neurons and glia in response to cellular injury and during cellular replenishment and remodeling [47]. Recently, it has been shown in a zebrafish model that CNS injury upregulated the expression of OCT4, along with SOX2, to initiate the process of regeneration by activating neural progenitor cells [48].
The fact that adult fibroblasts can be reprogrammed to have a phenotype identical to pluripotent ESCs raises the possibility that the combined expression of stem cell-related factors and selected oncogenes could also maintain a non-differentiated stemness state in cancer cells [49]. Multiple studies have documented the aberrant expression of OCT TFs in various types of cancer [50–58] including GBM [30, 59–63], and the enrichment of this group of TFs in a subpopulation of undifferentiated cancer stem cells indicated their critical role in tumor initiation, metastasis and resistance to chemotherapy. Expression of OCT4 was significantly higher in several malignancies and positively correlated with poor prognosis [64–66]. Moreover, the pivotal role of OCT family members in cancer has been demonstrated in several studies of tumor initiation and propagation, either by knocking down endogenous OCT or overexpressing ectopic OCT proteins. Hochedlinger et al. [67] first showed that overexpression of OCT4 inhibited progenitor cell differentiation and triggered dysplasia in epithelial tissues. Importantly, OCT4 is highly expressed in GBM (Figure 1B) and correlates with tumor grade. It also promotes colony formation and inhibits differentiation in GBM cells, and is specifically increased in GSCs, promoting their tumorigenic activity, as validated by neurosphere formation and intracerebral tumor formation [62, 68, 69].
OCT Family and GBM
Due to high phenotypic and behavioral similarities between NSC and GSCs, GBM is postulated to be derived from transformed NSCs that undergo oncogenic transformation. The process of GBM initiation varies among the subtypes, as the genetic footprint of the cells that give rise to tumors differs among the subtypes. As GSCs are multipotent and capable of self-renewal by expressing OCT family proteins, it is possible that the aberrant expression of members of this family is responsible for the development and maintenance of primary brain tumors. However, it is not always true that the gene expression profile of a GBM will necessarily follow the same pattern as its cell of origin. For example, though OCT4 is crucial for self-renewal and pluripotency of ESCs and can be detected in high grade glioma, it is not expressed in adult brain [59]. OCT4 was found only in cultured rat C6 glioma cells and neural stem cells but not in differentiated rat brain cells. In this study, reducing the level of endogenous OCT4 in C6 cells by RNAi attenuated cell proliferation and colony formation. OCT4 also can upregulate the signal transducer and activator of transcription 3 (STAT3) phosphorylation to promote tumor cell proliferation [59]. STAT3 activates a number of proteins that are known to promote tumor growth, such as cellular proto-oncogenes, cell cycle regulating proteins, and anti-apoptotic proteins [70–72]. It was reported that STAT3 was constitutively activated in nearly all major human malignancies occupying a key position in mediating neoplastic cellular transformation [73]. Overexpression of OCT4 resulted in an elevated level of activated STAT3 but not on its expression, thus it remains to be determined whether how OCT4 activates STAT3 and whether the function of OCT4 in GBM carcinogenesis is STAT3 dependent. Furthermore, the downstream signaling targets of OCT4 are more frequently upregulated in GBM than in more differentiated types of brain tumors [74]. Thus, the overexpression of stem cell factors such as OCT4 may contribute to the enhanced malignancy and stemness of GBM by upregulating the expression of other stemness factors, such as Nestin or STAT3 [59]. Apart from the findings that OCT4 is highly expressed in GBM and that its expression level is positively correlated with pathological grade, the methylation levels of the OCT4 gene promoter were significantly lower than that observed in normal cells [75].
Although the expression of OCT family members has been explored in several germ cell models, few studies have examined their expression and function in GBM tumorigenesis. OCT4 is overexpressed in human GBMs and GBM cell lines compared with low grade gliomas or normal brain. The expression of OCT4 correlates with the grade of glioma [59], supporting the concept that the malignancy of glioma is related to the abundance of stem-like cells in the tumor. In another study involving patient samples obtained from both low grade (II) and high grade (II) glioma, OCT4 was expressed at significantly higher levels in grade III and IV tumors compared with grade II tumors [30]. Co-expression of OCT4 with other pluripotent stem cell markers, such as Nanog, Sox2, CD133, and Nestin, was also demonstrated in high grade glioma tissue, suggesting the importance of ESC-associated proteins for the transcriptional regulatory network that maintains the self-renewal capacity of GSCs. Suppression of neurosphere formation in vitro and tumor formation in vivo by siRNA-mediated knockdown of OCT4 was demonstrated in GSCs, supporting the notion of a crucial role for OCT4 as a transcriptional regulator in GBM initiation [62].
Comprehensive comparative analysis of TFs differentially expressed in cells derived from the same tumors, but cultured either as GSCs (serum-free) or as differentiated GBM cells (with serum), yielded a set of 19 TFs with significantly higher expression in GSCs. Of these, the most effective transition of differentiated GBM cells into GSCs, surveyed by neurosphere formation in serum-free conditions, surface marker induction, and tumor propagation by orthotopic xenotransplantation into athymic mice, was achieved by co-expression of OCT7, SOX2, SALL2 and OLIG2 [4]. This 4TF cocktail appears highly specific, as any other combination failed to initiate tumors. Thus, all four core TFs are required to reprogram the epigenetic landscape of GBM cells, which is consistent with their requirement for the functional GSC phenotype. Moreover, by performing shRNA-mediated knockdown in GSCs, authors showed that OCT7 is required for sphere formation in vitro and for tumor propagation in vivo. This analysis identified OCT7 as a component of TF cocktail sufficient to reprogram serum-derived differentiated GBM cells into GSCs capable of unlimited self-renewal and tumor propagation. Of interest, such induced GSCs displayed molecular characteristics of proneural subtype and it remains to be investigated which TF combinations are capable of inducing other GSC subtypes. To investigate the clinical relevance of authors’ findings, they analyzed whether the core TFs and corresponding regulatory elements are active in human GBM tumors. Detailed analysis identified a small subset of cells in tumors (~2%–7%) that coordinately express all four TFs. Remarkably more than 50% of the 4TF-positive cells also express CD133, a striking enrichment over 4TF-negative cells, which almost entirely lack this stem cell marker [4].
Several microRNAs with epigenetic reprogramming potential, such as miR-302/367 or miR-145, can exert an indirect effect on the expression of OCT3 [76] or OCT4 [77] in GBM. Reciprocally, OCT4-mediated reprogramming of GBM cells led to DNA methyl transferase (DNMT)-mediated suppression of multiple microRNAs [78]. These data link cancer stem cell-specific TF signaling to a microRNA regulatory network that is altered in GBM and can be targeted to attenuate CSC self-renewal.
Drug Resistance of GSC and OCT
Cancer stem-like cells represent poorly differentiated, multipotent, tumor-propagating cells that contribute disproportionately to therapeutic resistance and tumor recurrence. Transcriptional mechanisms that control the phenotypic conversion of therapy-sensitive tumor cells lacking tumor-propagating potential to tumor-propagating, stem-like, therapy resistant cells remain obscure. Interestingly, the reprogramming of TFs including OCT4 was shown to induce GBM cells to become stem-like and tumor-propagating via a mechanism involving direct DNMT promoter transactivation, resulting in global DNA methylation [78]. In this study, one such downregulated miRNA, miRNA-148a, inhibits GBM cell stem-like properties and tumor-propagating potential, indicating a novel and targetable molecular circuit by which GBM cell stemness and tumor-propagating capacity are regulated.
The current standard-of-care therapy for GBM consists of surgery, radiotherapy, and chemotherapy with TMZ, an oral alkylating agent [8], though acquired drug resistance remains a major obstacle for efficient chemotherapeutic treatment of GBM. The complex mechanisms leading to acquisition of resistance are multifactorial, involving multiple genes. Often GSCs acquire drug resistance through overexpression or aberrant activity of intrinsic regulators of chemo-resistance such as O6-methylguanine-DNA-methyltransferase (MGMT) [3] and plasma membrane transporters such as the ATP-binding cassette transporters (ABC superfamily), which extrude anti-tumor drugs from GSCs [48]. The chemo-resistance of GSCs may also be regulated by extrinsic factors, such as the hypoxic tumor microenvironment or aberrant activation (by mutation, autocrine loop or genomic amplification) of signaling pathways (e.g., EGFR, PI3 Kinase/mTOR/AKT or mitogen activated kinases Ras/Raf/MAPK) [47, 66]. Upregulation of OCT4 in high grade GBM also contributes to chemo-resistance, as OCT4 knockdown enhances sensitivity to TMZ in GBM-initiating cells [62]. In another study, increased resistance to doxorubicin, an anthracycline antitumor antibiotic, was linked to up-regulated co-expression of ABCG2 and OCT3/4 in established GBM cell lines, resulting in increased drug efflux from these cells [79]. This observation suggests a contribution of OCT3/4 to the development of a multi-drug resistance phenotype in GBM. In contrast, numerous studies have shown that prolonged treatment with TMZ or other chemotherapeutic drugs promotes the evolution of stem cell-like phenotypes from differentiated cells. Long-term treatment with sub-toxic concentrations of TMZ significantly increased tumor initiation and growth in a murine model of GBM [66]. Similarly, using clinically relevant doses of TMZ, differentiated GBM cells acquired stem cell properties, with high levels of OCT4 and other stemness markers [80]. These newly dedifferentiated GSCs were highly invasive when implanted intra-cranially. These studies suggest that re-expression of OCT4 is important for TMZ-mediated dedifferentiation and also supports cellular plasticity, which can stimulate the dedifferentiation of non-GSCs and increase the overall OCT4 associated ‘stemness' of tumors.
GBM Metabolism and OCT
Most primary and metastatic tumors exhibit aberrant glucose metabolism with increased glycolysis, even in normoxic conditions, a feature known as ‘the Warburg effect’ [81]. In rapidly proliferating GBM cells, metabolic alterations are intrinsically involved in tumor growth, allowing cancer cells to survive in low glucose/ATP conditions and hypoxic microenvironments. Several studies describe a relationship between OCT protein expression and the altered metabolism of cancer cells [61, 82, 83]. In a knockout mouse model, OCT1-deficient cells were resistant to glucose deprivation due to reduced metabolism. This low metabolic rate inhibits oncogenic transformation and tumorigenesis both in vitro and in vivo [83]. In this OCT1-deficient background, there is a shift in metabolism from glycolysis in favor of mitochondrial oxidative metabolism.
With aberrant glucose metabolism and a higher rate of glycolysis, cancer cells adapt to metabolic stress by cellular alterations regulated by changes in transcriptional activity [84]. Understanding the molecular signaling pathways by which changes in glucose metabolism allow for cancer cell adaptation may provide new insights into GBM pathogenesis. AMP-activated kinase (AMPK) is a crucial cellular energy sensor. Metabolic stress or low energy conditions result in a conformational change triggered by AMP, resulting in activation of AMPK by the LKB1 complex. Once activated, AMPK facilitates cell survival by increasing catabolic processes while conserving ATP by switching off anabolic metabolism [85]. In a recent study from our group, we demonstrated that OCT1 acts as transcriptional regulator of miR-451 transcription, and endogenous OCT1 was recruited to the miR-451 gene promoter in a glucose-dependent manner [61]. In sufficient glucose environments, miR-451 inhibits AMPK signaling and pro-migratory behavior in GBM cells by targeting components of the LKB1 kinase complex, as well as numerous downstream effectors [86, 87]. In low glucose conditions, active AMPK complex phosphorylates OCT1 at Ser335, which is sufficient in rapidly proliferating GBM cells to drastically reduce OCT1 transcriptional activity. As a result, lowered level of miR-451 allows further activation of AMPK and increased migration. These results revealed the presence in GBM cells of an AMPK/OCT1/miR-451/LKB1 reciprocal negative feedback loop, allowing tumor cell adaptation to variations in nutrient availability in the tumor microenvironment. Low glucose-dependent AMPK activation leading to shut off of the OCT1/miR-451 axis turns on energy-conserving and migratory behavior. Both the energy-conserving metabolic shift and resource-seeking behavioral change require GBM cell to shut down miR-451, while forced expression of miR-451 during stress leads to cytotoxicity [61, 86, 87].
Metabolic alterations are intrinsically involved in tumor growth and metastasis beyond mere energy production, through many different pathways that provide an advantage to tumors under rapid growing or hypoxic situations. Pyruvate kinase isoform 2 (PKM2) is an important regulator of embryonic and cancer cell metabolism and tumor growth. In a rat glioma model, the non-metabolic role of PKM2 was revealed by showing that interaction of PKM2 with OCT4 induces differentiation by inhibiting OCT4 transcriptional activity [82]. Apart from altered glucose metabolism, increased glucose uptake is also a characteristic feature of cancer cells, as they require higher glucose flux due to reduced generation of ATP per glucose molecule through anaerobic glycolysis. The expression and activity of glucose transporter 1 (GLUT1) and GLUT3 are pivotal for the maintenance and self-renewal of cancer stem cells, including GSCs [88, 89]. Expression of GLUT3 is associated with GBM initiation and is strongly correlated with patient outcome. More strikingly, non-stem GBM cells grown in restricted glucose conditions increase expression of the ESC master TFs and show functional enrichment for stem-like cells, indicating adaptation and reprogramming to a more stem-like state [88]. The exact epigenetic mechanisms underlying these adaptations are thus far unknown. However, the genomic locus of GLUT3 is part of a conserved, 200-kb gene cluster that is highly enriched for genes associated with pluripotency, including the master ESC transcription factor NANOG [90]. This region is under the control of OCT4 [91]. It is possible that cancer cells gain GLUT3 expression and stem cell properties simultaneously by epigenetically de-repressing this region of chromatin during stem cell reprogramming.
Another characteristic of solid tumors, including GBMs, is hypoxia [92], which not only increases the fraction of CD133-positive GBM cells, but also enhances the stem-like phenotype of cell lines, and is accompanied by overexpression of OCT4 [93]. Under hypoxic conditions, the hypoxia inducible factor (HIF) family of TFs activates the transcription of several downstream genes, such as GLUT1 and OCT4, to regulate the cellular metabolism and stemness of GSCs [94]. GSCs have higher expression of GLUT3, which allows preferential glucose uptake in the harsh microenvironment at the tumor core. OCT4 is upregulated along with other stemness markers in glucose-deprived conditions, suggesting that uptake of glucose through GLUT3 is directly associated with acquiring stemness [88]. In GBM cells grown in either adherent or neurosphere-promoting conditions, microenvironmental conditions associated with low glucose and oxygen, mimicking a poor vascular supply, increase glucose metabolism. All these microenvironmental niches induce GBM stemness by regulating the activity and expression of OCT and other pluripotency-regulating factors.
Conclusions
Efforts to identify TFs that are master regulators of specific cancer signatures, driving specific phenotypes on the basis of cellular-network models, have not yet produced experimentally validated discoveries. These networks remain only partly mapped, especially within specific mammalian cellular contexts [95]. Notwithstanding, recent developments in genome-wide reverse engineering successfully identified some of these interactions [96, 97] and showed promise in the identification of dysregulated genes within specific developmental and tumor-related pathways [98, 99]. Developmental fate decisions are dictated by master TFs that interact with cis-regulatory elements to direct transcriptional programs. Certain malignant tumors, such as GBM, may also depend on cellular hierarchies reminiscent of normal development but overlaid with genetic aberrations that also can be modulated by the microenvironmental niche. In GBM, a subset of GSCs appears to drive tumor progression and underlie the acquisition of therapeutic resistance, yet these GSCs remain poorly understood.
Four core TFs were found to be sufficient to reprogram differentiated GBM cells into “induced” GSCs that faithfully recapitulate in vitro and in vivo properties of GSCs established directly from human tumors [4]. Among these SOX2 and OCT7 can each partially reprogram the epigenetic landscape of differentiated GBM cells on their own, which is consistent with their partial ability to induce neurosphere growth and their established roles in direct conversion to neural lineages [100]. The clinical relevance of these findings is supported by the identification of GSCs that coordinately express all four factors in GBM tumors, and by the requirement of all four factors for in vivo tumorigenicity in xenotransplanted mice.
Characterizing the epigenetic states of phenotypically distinct cells and identifying TFs, including OCT4 and OCT7, that are sufficient to reprogram differentiated cells into a tumorigenic stem-like state suggest a plastic developmental hierarchy in GBM cell populations [7]. The recent observation that individual tumors contain a spectrum of GBM subtypes and hybrid cellular states [7], which is reflected in the diverse expression of ncRNAs [101–103] and range of environmental influences [104, 105], complicates such a view. To fully reconstruct a network model that highlights critical machinery sufficient to fully reprogram differentiated GBM cells, a comprehensive analysis of TFs and their downstream effectors (both protein-coding and non-coding RNAs) in the context of the tumor microenvironment is needed.
Current understanding of the role of TFs belonging to the OCT family is that OCT7 and its partner TFs such as SOX2, OLIG2 and SALL2, is indispensable in cellular speciation and reprogramming of the tumor-propagating potential of GSCs. OCT4, along with other TFs involved in maintaining pluripotency, plays a crucial role as a transcriptional regulator in GBM initiation. Finally, OCT1 is a target of the AMPK kinase complex allowing dynamic adaptation to fluctuating microenvironmental conditions. Interestingly, these OCT factors are differentially expressed in distinct intra-tumoral anatomic sites. Expression of OCT4 is the most prevalent in highly hypoxic necrotic and perinecrotic zones underlining the critical role of hypoxia in the maintenance of GSCs [106]. On the other hand, expression of OCT7 is restricted to proliferative, cellular regions (Figure 1B); highly correlating with its partner TFs which were shown to coordinately bind and activate GSC-specific regulatory elements. This suggests that their coordinated action indeed drives tumor progression and thus has a strong impact on GBM propagation. The expression of OCT1 is not correlated with any distinct intra-tumoral compartment highlighting its role in the localized, context-specific and dynamic microenvironmental adaptations (Figure 1B).
Identification of the key factors involved in GBM oncogenesis is a necessary precursor to the development of efficient and personalized targeted therapy. Combining data from high-throughput genetic screening, bioinformatic analysis and functional studies may help to identify new driving master regulators in GBM, including novel tumor regulatory networks controlled by ncRNAs, providing a fuller understanding to the role of the OCT family in GBM (Figure 1C).
Highlights.
Members of OCT family play important yet undefined role in biology of GBM.
OCT4 and OCT7, along with other transcription factors, control GBM stemness.
OCT1 is a part of a feedback loop controlling GBM metabolism and invasion.
Acknowledgments
The authors are grateful to Accixx Biomedical Consulting and Dr. Sean E. Lawler for their suggestions. This work was supported by NCI 1R01CA176203-01A1 (JG).
Abbreviations
- TF
transcription factors
- GBM
glioblastoma multiforme
- OCT
Octamer binding transcription factor
- TMZ
temozolomide
- CSC
cancer stem cells
- GSC
GBM stem cell
- NSC
neuronal stem cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors declare no conflict of interest.
References
- 1.Carro MS, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463(7279):318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seymour T, Nowak A, Kakulas F. Targeting Aggressive Cancer Stem Cells in Glioblastoma. Front Oncol. 2015;5:159. doi: 10.3389/fonc.2015.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JB, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136(3):411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Suva ML, et al. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell. 2014;157(3):580–594. doi: 10.1016/j.cell.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer LA, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122(6):947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loh YH, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38(4):431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 7.Patel AP, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stupp R, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 9.Ostrom QT, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(Suppl 4):iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson DR, O'Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012;107(2):359–364. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 11.Louis DN, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhaak RG, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan CW, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips HS, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Noushmehr H, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crespo I, et al. Molecular and Genomic Alterations in Glioblastoma Multiforme. Am J Pathol. 2015;185(7):1820–1833. doi: 10.1016/j.ajpath.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Johnson BE, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17(3):313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 20.Lapidot T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 21.Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 22.Galli R, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 23.Yuan X, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23(58):9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 24.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 25.Stuckey DW, Shah K. Stem cell-based therapies for cancer treatment: separating hope from hype. Nat Rev Cancer. 2014;14(10):683–691. doi: 10.1038/nrc3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13(5):543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci. 2007;27(22):5936–5947. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 29.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Y, et al. Expression profile of embryonic stem cell-associated genes Oct4, Sox2 and Nanog in human gliomas. Histopathology. 2011;59(4):763–775. doi: 10.1111/j.1365-2559.2011.03993.x. [DOI] [PubMed] [Google Scholar]
- 31.Gangemi RM, et al. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27(1):40–48. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 32.Qin S, Zhang CL. Role of Kruppel-like factor 4 in neurogenesis and radial neuronal migration in the developing cerebral cortex. Mol Cell Biol. 2012;32(21):4297–4305. doi: 10.1128/MCB.00838-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corti S, et al. Direct reprogramming of human astrocytes into neural stem cells and neurons. Exp Cell Res. 2012;318(13):1528–1541. doi: 10.1016/j.yexcr.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng H, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455(7216):1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, et al. c-Myc is required for maintenance of glioma cancer stem cells. PLoS One. 2008;3(11):e3769. doi: 10.1371/journal.pone.0003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ligon KL, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53(4):503–517. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tantin D. Oct transcription factors in development and stem cells: insights and mechanisms. Development. 2013;140(14):2857–2866. doi: 10.1242/dev.095927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sturm RA, Das G, Herr W. The ubiquitous octamer-binding protein Oct-1 contains a POU domain with a homeo box subdomain. Genes Dev. 1988;2(12A):1582–1599. doi: 10.1101/gad.2.12a.1582. [DOI] [PubMed] [Google Scholar]
- 39.Clerc RG, et al. The B-cell-specific Oct-2 protein contains POU box- and homeo box-type domains. Genes Dev. 1988;2(12A):1570–1581. doi: 10.1101/gad.2.12a.1570. [DOI] [PubMed] [Google Scholar]
- 40.Rosner MH, et al. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990;345(6277):686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- 41.Meijer D, et al. The octamer binding factor Oct6: cDNA cloning and expression in early embryonic cells. Nucleic Acids Res. 1990;18(24):7357–7365. doi: 10.1093/nar/18.24.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schreiber E, et al. cDNA cloning of human N-Oct3, a nervous-system specific POU domain transcription factor binding to the octamer DNA motif. Nucleic Acids Res. 1993;21(2):253–258. doi: 10.1093/nar/21.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He X, et al. Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature. 1989;340(6228):35–41. doi: 10.1038/340035a0. [DOI] [PubMed] [Google Scholar]
- 44.Douville PJ, et al. The brain-specific POU-box gene Brn4 is a sex-linked transcription factor located on the human and mouse X chromosomes. Mamm Genome. 1994;5(3):180–182. doi: 10.1007/BF00352353. [DOI] [PubMed] [Google Scholar]
- 45.Goldsborough AS, et al. Cloning, chromosomal localization and expression pattern of the POU domain gene Oct-11. Nucleic Acids Res. 1993;21(1):127–134. doi: 10.1093/nar/21.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scholer HR, et al. Octamer binding proteins confer transcriptional activity in early mouse embryogenesis. EMBO J. 1989;8(9):2551–2557. doi: 10.1002/j.1460-2075.1989.tb08393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fuentealba LC, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10(6):698–708. doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hui SP, Nag TC, Ghosh S. Characterization of Proliferating Neural Progenitors after Spinal Cord Injury in Adult Zebrafish. PLoS One. 2015;10(12):e0143595. doi: 10.1371/journal.pone.0143595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 50.Chang CC, et al. Oct-3/4 expression reflects tumor progression and regulates motility of bladder cancer cells. Cancer Res. 2008;68(15):6281–6291. doi: 10.1158/0008-5472.CAN-08-0094. [DOI] [PubMed] [Google Scholar]
- 51.Chen Z, et al. Oct4, a novel marker for human gastric cancer. J Surg Oncol. 2009;99(7):414–419. doi: 10.1002/jso.21270. [DOI] [PubMed] [Google Scholar]
- 52.Dai X, et al. OCT4 regulates epithelial-mesenchymal transition and its knockdown inhibits colorectal cancer cell migration and invasion. Oncol Rep. 2013;29(1):155–160. doi: 10.3892/or.2012.2086. [DOI] [PubMed] [Google Scholar]
- 53.Iida H, et al. Hypoxia induces CD133 expression in human lung cancer cells by up-regulation of OCT3/4 and SOX2. Int J Oncol. 2012;40(1):71–79. doi: 10.3892/ijo.2011.1207. [DOI] [PubMed] [Google Scholar]
- 54.Jones TD, et al. OCT4: A Sensitive and Specific Biomarker for Intratubular Germ Cell Neoplasia of the Testis. Clinical Cancer Research. 2004;10(24):8544–8547. doi: 10.1158/1078-0432.CCR-04-0688. [DOI] [PubMed] [Google Scholar]
- 55.Kim RJ, Nam JS. OCT4 Expression Enhances Features of Cancer Stem Cells in a Mouse Model of Breast Cancer. Lab Anim Res. 2011;27(2):147–152. doi: 10.5625/lar.2011.27.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodini CO, et al. Expression analysis of stem cell-related genes reveal OCT4 as a predictor of poor clinical outcome in medulloblastoma. J Neurooncol. 2012;106(1):71–79. doi: 10.1007/s11060-011-0647-9. [DOI] [PubMed] [Google Scholar]
- 57.Wang Q, et al. Oct3/4 and Sox2 are significantly associated with an unfavorable clinical outcome in human esophageal squamous cell carcinoma. Anticancer Res. 2009;29(4):1233–1241. [PubMed] [Google Scholar]
- 58.Yang L, et al. Downregulation of OCT4 promotes differentiation and inhibits growth of BE (2)-C human neuroblastoma I-type cells. Oncol Rep. 2013;29(6):2191–2196. doi: 10.3892/or.2013.2356. [DOI] [PubMed] [Google Scholar]
- 59.Du Z, et al. Oct4 is expressed in human gliomas and promotes colony formation in glioma cells. Glia. 2009;57(7):724–733. doi: 10.1002/glia.20800. [DOI] [PubMed] [Google Scholar]
- 60.Schreiber E, et al. Astrocytes and glioblastoma cells express novel octamer-DNA binding proteins distinct from the ubiquitous Oct-1 and B cell type Oct-2 proteins. Nucleic Acids Res. 1990;18(18):5495–5503. doi: 10.1093/nar/18.18.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ansari KI, et al. Glucose-based regulation of miR-451/AMPK signaling depends on the OCT1 transcription factor. Cell Rep. 2015;11(6):902–909. doi: 10.1016/j.celrep.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ikushima H, et al. Glioma-initiating cells retain their tumorigenicity through integration of the Sox axis and Oct4 protein. J Biol Chem. 2011;286(48):41434–41441. doi: 10.1074/jbc.M111.300863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holmberg J, et al. Activation of neural and pluripotent stem cell signatures correlates with increased malignancy in human glioma. PLoS One. 2011;6(3):e18454. doi: 10.1371/journal.pone.0018454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ge N, et al. Prognostic significance of Oct4 and Sox2 expression in hypopharyngeal squamous cell carcinoma. J Transl Med. 2010;8:94. doi: 10.1186/1479-5876-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Monajemzadeh M, et al. Expression and prognostic significance of Oct4 and Nanog in neuroblastoma. APMIS. 2014;122(9):734–741. doi: 10.1111/apm.12207. [DOI] [PubMed] [Google Scholar]
- 66.Zhang X, et al. Prognostic significance of OCT4 expression in adenocarcinoma of the lung. Jpn J Clin Oncol. 2010;40(10):961–966. doi: 10.1093/jjco/hyq066. [DOI] [PubMed] [Google Scholar]
- 67.Hochedlinger K, et al. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121(3):465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 68.Du Z, et al. Oct4 is expressed in human gliomas and promotes colony formation in glioma cells. Glia. 2009;57(7):724–733. doi: 10.1002/glia.20800. [DOI] [PubMed] [Google Scholar]
- 69.Ikushima H, et al. Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell. 2009;5(5):504–514. doi: 10.1016/j.stem.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 70.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101(4):1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 71.Kanda N, et al. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene. 2004;23(28):4921–4929. doi: 10.1038/sj.onc.1207606. [DOI] [PubMed] [Google Scholar]
- 72.Masuda M, et al. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62(12):3351–3355. [PubMed] [Google Scholar]
- 73.Frank DA. STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett. 2007;251(2):199–210. doi: 10.1016/j.canlet.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 74.Ben-Porath I, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40(5):499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi J, et al. OCT4 is epigenetically regulated by DNA hypomethylation of promoter and exon in primary gliomas. Oncol Rep. 2013;30(1):201–206. doi: 10.3892/or.2013.2456. [DOI] [PubMed] [Google Scholar]
- 76.Yang CM, et al. Expression of the miR-302/367 cluster in glioblastoma cells suppresses tumorigenic gene expression patterns and abolishes transformation related phenotypes. Int J Cancer. 2015;137(10):2296–2309. doi: 10.1002/ijc.29606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alvarado AG, et al. Coordination of self-renewal in glioblastoma by integration of adhesion and microRNA signaling. Neuro Oncol. 2015 doi: 10.1093/neuonc/nov196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lopez-Bertoni H, et al. DNMT-dependent suppression of microRNA regulates the induction of GBM tumor-propagating phenotype by Oct4 and Sox2. Oncogene. 2015;34(30):3994–4004. doi: 10.1038/onc.2014.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hosokawa Y, et al. Oct-3/4 modulates the drug-resistant phenotype of glioblastoma cells through expression of ATP binding cassette transporter G2. Biochim Biophys Acta. 2015;1850(6):1197–1205. doi: 10.1016/j.bbagen.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 80.Auffinger B, et al. Conversion of differentiated cancer cells into cancer stem-like cells in a glioblastoma model after primary chemotherapy. Cell Death Differ. 2014;21(7):1119–1131. doi: 10.1038/cdd.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 82.Morfouace M, et al. Control of glioma cell death and differentiation by PKM2-Oct4 interaction. Cell Death Dis. 2014;5:e1036. doi: 10.1038/cddis.2013.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shakya A, et al. Oct1 loss of function induces a coordinate metabolic shift that opposes tumorigenicity. Nat Cell Biol. 2009;11(3):320–327. doi: 10.1038/ncb1840. [DOI] [PubMed] [Google Scholar]
- 84.Dhruv HD, et al. Reciprocal activation of transcription factors underlies the dichotomy between proliferation and invasion of glioma cells. PLoS One. 2013;8(8):e72134. doi: 10.1371/journal.pone.0072134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Godlewski J, et al. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell. 2010;37(5):620–632. doi: 10.1016/j.molcel.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Godlewski J, et al. microRNA-451: A conditional switch controlling glioma cell proliferation and migration. Cell Cycle. 2010;9(14):2742–2748. [PubMed] [Google Scholar]
- 88.Flavahan WA, et al. Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat Neurosci. 2013;16(10):1373–1382. doi: 10.1038/nn.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shibuya K, et al. Targeting the facilitative glucose transporter GLUT1 inhibits the self-renewal and tumor-initiating capacity of cancer stem cells. Oncotarget. 2015;6(2):651–661. doi: 10.18632/oncotarget.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Korkola JE, et al. Down-regulation of stem cell genes, including those in a 200-kb gene cluster at 12p13.31, is associated with in vivo differentiation of human male germ cell tumors. Cancer Res. 2006;66(2):820–827. doi: 10.1158/0008-5472.CAN-05-2445. [DOI] [PubMed] [Google Scholar]
- 91.Levasseur DN, et al. Oct4 dependence of chromatin structure within the extended Nanog locus in ES cells. Genes Dev. 2008;22(5):575–580. doi: 10.1101/gad.1606308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heddleston JM, et al. Hypoxia inducible factors in cancer stem cells. Br J Cancer. 2010;102(5):789–795. doi: 10.1038/sj.bjc.6605551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCord AM, et al. Physiologic oxygen concentration enhances the stem-like properties of CD133+ human glioblastoma cells in vitro. Mol Cancer Res. 2009;7(4):489–497. doi: 10.1158/1541-7786.MCR-08-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Z, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15(6):501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rhodes DR, Chinnaiyan AM. Integrative analysis of the cancer transcriptome. Nat Genet. 2005;37(Suppl):S31–S37. doi: 10.1038/ng1570. [DOI] [PubMed] [Google Scholar]
- 96.Basso K, et al. Reverse engineering of regulatory networks in human B cells. Nat Genet. 2005;37(4):382–390. doi: 10.1038/ng1532. [DOI] [PubMed] [Google Scholar]
- 97.Chen Y, et al. Variations in DNA elucidate molecular networks that cause disease. Nature. 2008;452(7186):429–435. doi: 10.1038/nature06757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao X, et al. The N-Myc-DLL3 cascade is suppressed by the ubiquitin ligase Huwe1 to inhibit proliferation and promote neurogenesis in the developing brain. Dev Cell. 2009;17(2):210–221. doi: 10.1016/j.devcel.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Palomero T, et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc Natl Acad Sci U S A. 2006;103(48):18261–18266. doi: 10.1073/pnas.0606108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lodato MA, et al. SOX2 co-occupies distal enhancer elements with distinct POU factors in ESCs and NPCs to specify cell state. PLoS Genet. 2013;9(2):e1003288. doi: 10.1371/journal.pgen.1003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Du Z, et al. Integrative genomic analyses reveal clinically relevant long noncoding RNAs in human cancer. Nat Struct Mol Biol. 2013;20(7):908–913. doi: 10.1038/nsmb.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Godlewski J, et al. MicroRNAs and glioblastoma; the stem cell connection. Cell Death Differ. 2010;17(2):221–228. doi: 10.1038/cdd.2009.71. [DOI] [PubMed] [Google Scholar]
- 103.Peruzzi P, et al. MicroRNA-128 coordinately targets Polycomb Repressor Complexes in glioma stem cells. Neuro Oncol. 2013;15(9):1212–1224. doi: 10.1093/neuonc/not055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Godlewski J, et al. Belonging to a network--microRNAs, extracellular vesicles, and the glioblastoma microenvironment. Neuro Oncol. 2015;17(5):652–662. doi: 10.1093/neuonc/nou292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mack SC, et al. An epigenetic gateway to brain tumor cell identity. Nat Neurosci. 2015;19(1):10–19. doi: 10.1038/nn.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bar EE. Glioblastoma, cancer stem cells and hypoxia. Brain Pathol. 2011;21(2):119–129. doi: 10.1111/j.1750-3639.2010.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]