Abstract
Background
Rate of nicotine metabolism is an important factor influencing cigarette smoking behavior, dependence, and efficacy of nicotine replacement therapy. The current study examined the hypothesis that chronic alcohol abuse can accelerate the rate of nicotine metabolism. Nicotine metabolite ratio (NMR, a biomarker for rate of nicotine metabolism) and patterns of nicotine metabolites were assessed at three time points after alcohol cessation.
Methods
Participants were 22 Caucasian men randomly selected from a sample of 165 smokers entering a 7-week alcohol dependence treatment program in Poland. Data were collected at three time points: baseline (week 1, after acute alcohol detoxification), week 4, and week 7. Urine was analyzed for nicotine and metabolites and used to determine the nicotine metabolite ratio (NMR, a biomarker for rate of nicotine metabolism), and total nicotine equivalents (TNE, a biomarker for total daily nicotine exposure).
Results and conclusions
There was a significant decrease in urine NMR over the 7 weeks after alcohol abstinence (F(2,42)=18.83, p<0.001), indicating a decrease in rate of nicotine metabolism. On average NMR decreased 50.0% from baseline to week 7 (9.6 ± 1.3 vs. 4.1 ± 0.6). There was no change in urine TNE across the three sessions, indicating no change daily nicotine intake. The results support the idea that chronic alcohol abuse may increases the rate of nicotine metabolism, which then decreases over time after alcohol cessation. This information may help to inform future smoking cessation interventions in this population.
Keywords: CYP2A6, alcoholism, alcohol abuse, ethanol, tobacco, smoking, cigarettes
1. Introduction
Cigarette smoking and excessive alcohol consumption remain two of the leading preventable causes of premature death in the U.S. and around the world (Danaei et al., 2009). These are highly co-morbid behaviors and the prevalence of cigarette smoking in individuals with alcohol use disorders is high (see McKee and Weinberger, 2013 for review). Results from a national sample found the prevalence of any alcohol use disorders was higher among individuals with nicotine dependence compared to the overall population (22.8% vs 8.5%; Grant et al., 2004); and around half of all adults in the US with an alcohol use disorder also smoke cigarettes (McKee and Weinberger, 2013). Individuals with alcohol use disorders have also been found to be heavier smokers, report greater nicotine dependence, and have poorer smoking cessation rates (Burling et al., 1997; Cook et al., 2012; Friend et al., 2005; Keenan et al., 1990; Hughes and Kalman, 2006; Hurt et al., 1995; John et al., 2003a-b; Marks et al., 1997; York and Hirsh, 1995).
Underlying factors that contribute to the differences in prevalence of cigarette smoking and nicotine dependence among individuals with alcohol use disorders remain unclear. Research has largely focused on the hypothesis that nicotine and alcohol in combination may have combined pharmacological effects that support their co-use (e.g., increased reward and/or decreased withdrawal/aversive effects). An additional mechanism that could contribute to this difference in cigarette smoking behavior and dependence in this population is a change in the pharmacokinetics of nicotine induced by chronic alcohol consumption.
Nicotine is primarily metabolized into cotinine by the liver enzyme CYP2A6 (C-oxidation), which accounts for approximately 75% of nicotine metabolism (Benowitz et al., 2009). Cotinine is further metabolized to trans-3′-hydroxycotine (3HC) in a process mediated exclusively or nearly exclusively by the same enzyme, CYP2A6. The ratio of 3HC/ cotinine, termed the nicotine metabolite ratio (NMR) is a validated biomarker for CYP2A6 activity and the rate of nicotine metabolism (Dempsey et al., 2004). A higher NMR indicates greater CYP2A6 enzyme activity and faster rate of nicotine metabolism.
In addition to cotinine and 3HC, nicotine is metabolized to a number of other metabolites via the CYP2A6 and other metabolic pathways (see Benowitz et al., 2009 for review). In the current study we also assessed a number of different metabolites of nicotine in addition to products from the C-oxidation pathway. For example nicotine is also metabolized by glucuronidation (primarily by UDP glucuronosyltransferase 2 family, polypeptide B10; UGT2B10) and N-oxidation (by flavin containing monooxygenase 3; FMO3), although these pathways contribute less to the overall metabolism of nicotine compared to CYP2A6 (Hukkanen et al., 2005). If metabolism of nicotine via the C-oxidation pathway is slower, higher levels of non-C-oxidation products (e.g., nicotine glucuronide and nicotine-N-oxide) would be expected.
A faster rate of nicotine metabolism (greater CYP2A6 enzyme activity) was previously found to be associated with smoking more cigarettes per day (Benowitz et al., 2003; Strasser et al., 2011; Tanner et al., 2015), greater nicotine withdrawal symptoms (Rubinstein et al., 2008; Sofuoglu et al., 2012), and decreased efficacy of nicotine replacement therapy (NRT) for smoking cessation (Lerman et al., 2006; 2015). Tobacco use characteristics associated with faster rate of nicotine metabolism are similar to what has been found in individuals with alcohol use disorders, as previously discussed. Both genetic (Ray et al., 2009) and environmental factors have been found to influence CYP2A6 enzyme activity. For example estrogen (Benowitz et al., 2006; Dempsey et al., 2002), and certain medications such as phenobarbital (Benowitz et al., 2009) and rifampicin (Xia et al., 2002) have been found to induce CYP2A6 enzyme activity, resulting in accelerated rate of nicotine metabolism.
Previous research suggests that chronic alcohol may induce CYP2A6 enzyme activity. Protein levels of CYP2A6 were found to be higher among patients who abuse alcohol (Niemelä et al., 2000) and alcohol was found to induce CYP2A6 activity in the U937 macrophage cell line (Jin et al., 2011; 2012). In mice chronic alcohol consumption induced CYP2A5, the mouse orthologue of human CYP2A6 (Lu et al., 2011; 2012). The current study examined the hypothesis that chronic alcohol abuse accelerates the rate of nicotine metabolism and is associated with higher NMR and altered patterns of nicotine metabolism. We examined this hypothesis by looking at reversal of postulated metabolic induction after cessation of alcohol abuse, testing subjects at three time points over the course of 7 weeks of inpatient treatment for alcohol dependence. It was hypothesized that rate of nicotine metabolism assessed by NMR would decrease over the 7 weeks of alcohol abstinence, reflecting normalization after prior enzyme induction.
Total Nicotine Equivalents (TNE), the molar sum of nicotine and its metabolites measured in urine, is a highly reliable biomarker of total nicotine exposure (Scherer., 2007) that is unaffected by differences in CYP2A6 enzyme activity (Benowitz et al., 2010; Derby et al., 2008; Feng et al., 2007). TNE was used to determine if there was a change in nicotine exposure at the three assessments after alcohol cessation. In addition, biomarkers of liver function were also assessed to verify subjects did not have severe liver function impairments and to verify that the expected decrease in these liver function metabolites occurred after cessation of alcohol.
Understanding changes in nicotine metabolism associated with chronic alcohol abuse and recovery (during alcohol abstinence) could have important implications for understanding smoking behavior and improving smoking cessation interventions for current and former heavy alcohol drinkers.
2. Methods
2.1. Setting
The study was conducted from September, 2011 to May, 2012 at the Center for Addiction Treatment (Ośrodek Terapii Uzależnień, OTU), an inpatient program dedicated to the treatment of alcohol dependence located in Parzymiechy, Poland. The center treats approximately 1,200 individuals per year for alcohol dependence with an average treatment duration per patient of 8 weeks. Patients entering the program were first treated for acute alcohol withdrawal for up to 2 weeks in an alcohol detoxification program. During detoxification, patients were administered benzodiazepines as needed, titrated down over subsequent days. Only intoxicated individuals or those with acute withdrawal symptoms were admitted to the alcohol detoxification program. Once patients were stabilized they entered a 7 week inpatient treatment program for alcohol dependence. The 7 week alcohol treatment program consisted of only psychotherapy. No pharmacotherapy was administered for alcohol dependence during the 7 week treatment program. Breath alcohol was monitored daily, up to 2 times/ day. Any individual testing positive for alcohol was discharged and removed from the treatment program. At the end of the 7 week program patients were given the option to stay longer if needed. Inpatients were free to smoke cigarettes outside of the facility during their stay at the OTU.
The current study was approved by the Institutional Review Board of the Medical University of Silesia in Katowice Poland and conducted according to the Declaration of Helsinki, the European Guidelines on Good Clinical Practice, and relevant national and regional authority requirements and ethics committees. The current study was a secondary analysis of the dataset.
2.2. Participants
Men and women from the OTU alcohol addiction treatment center were recruited before entering the 7 week treatment program (after detoxification) for a study to examine the effects of vitamin supplementation during treatment of alcohol dependence. Inclusion criterion for the current analyses were: age 30-60, a diagnosis of alcohol dependence based on the ICD F10.2 criteria (WHO, 2010), and self-reported daily smoking for at least 5 years. Smoking status was self-reported and verified with a Fagerstrom Test for Cigarette Dependence (FTCD) score of 3 or greater, and expired carbon monoxide (CO) of 5 ppm or greater. Exclusion criteria were: a diagnosis of liver cirrhosis or kidney disease, recent myocardial infarction, high blood pressure, cancer diagnosis, pregnancy, diagnosis of major psychiatric disorder, and those taking inhibitors of renin-angiotensin system (RAS), statins, hormone replacement therapy (including estrogen and progesterone), aspirin, and erythropoietin (EPO). Participants were also excluded if they reported taking any vitamin supplements in the prior 2 months before entering the OTU program. A total of 318 participants were screened for eligibility and 270 consented; 165 smokers completed all three sessions and were used in the current analyses. Nicotine biomarkers were assessed in 22 participants randomly selected from male smokers who completed all three assessments. Urine samples were randomly selected in Poland and shipped to the United States for analysis. Only a subset of the sample was analyzed for nicotine metabolites as it would have been cost prohibitive to analyze the full sample. Only male participants were included in this analysis as the overall sample was 83% male, and there are known sex differences in the metabolism of nicotine (see Benowitz et al., 2009 for review) that could complicate interpreting the effects of alcohol cessation on rate of nicotine metabolism.
2.3. Study protocol
All data collection occurred after cessation of alcohol consumption. The current study collected data at three time points: baseline (after remission of acute withdrawal symptoms from alcohol during the first week of the 7 week treatment program); week 4 (after completing 3 full weeks of treatment); and week 7 (after completing 6 full weeks of treatment). Assessment of nicotine withdrawal symptoms, smoking measures and urine samples were collected in the morning before the first cigarette of the day at each of the three time points. Patients were told not to smoke for at least 6 hrs (after midnight) the day before each assessment session which occurred between 6:00-7:00 AM. Urine samples were frozen and stored at -20°C. Urine samples were shipped frozen to the University of California, San Francisco for analysis of concentrations of nicotine and metabolites.
2.4. Measures
Demographic variables included age, sex, and body mass index (BMI). Usual cigarettes/day and cigarettes smoked during the past 24 hours were self-reported. All individuals were diagnosed with alcohol dependence. Amount of alcohol consumption (e.g., usual drinks/ day) was not assessed. Instead, years of heavy drinking before entering the treatment program was estimated by the participant. Participants entered screening after alcohol detoxification and the time since the last drink, before the baseline assessment session was calculated for each individual. Because there could be differences in duration of detoxification and assessments were performed after acute detoxification, there could be slight differences in number of days since individuals had last consumed alcohol. Expired breath carbon monoxide level (CO) and number of cigarettes smoked in the previous day were used to determine cigarette smoking behavior. Baseline nicotine dependence was assessed using the 7-item version of the Fagerstrom Test for Cigarette Dependence (FTCD; Heatherton et al., 1991).
2.5. Analytical chemistry
Urine samples were analyzed using liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) for concentrations of nicotine, cotinine, trans-3′-hydroxycotinine (3-HC), nicotine N′-oxide (NNO), cotinine N-oxide (CNO), nornicotine (NNIC), norcotinine (NCOT) as previously described (Benowitz et al., 2010; Jacobs et al., 2011). Samples were assayed with and without enzymatic deconjugation, and the differences between assays taken to be the conjugated metabolite concentration, as described previously. Urine creatinine was measured by LC-MS/MS with a limit quantitation of 0.05 mg/ mL, using a method developed at the Clinical Pharmacology Research Laboratories at the University of California, San Francisco. Plasma levels of liver function enzymes aspartate transaminase (AST), alanine transaminase (ALT), and gamma-glutamyl transpeptidase (GGTP) were measured at the OTU in-house clinical laboratory in Poland.
2.6. Data analysis
NMR was calculated as the ratio of total 3HC over free cotinine {(free 3HC + 3HC glucuronide)/free cotinine}. This calculation provides the best estimate of CYP2A6 activity as it includes all cotinine that is not metabolized via glucuronidation (free cotinine), which is the precursor chemical, while accounting for all 3HC metabolized via the C-oxidation pathway (free 3HC + glucuronide 3HC), which is the product of CYP2A6 metabolism.
TNE was used a measure of total nicotine intake. TNE was used instead of cotinine because cotinine levels with a given level of nicotine intake are influenced by CYP2A6 enzyme activity (Zhu et al., 2013). Using cotinine as a biomarker of daily nicotine intake underestimates daily exposure in individuals with high CYP2A6 enzyme activity. TNE as a measure of daily nicotine intake is independent of individual differences in patterns of nicotine metabolism (Benowitz et al., 2010; Zhu et al., 2013). TNE was calculated as the molar sum of urine nicotine, and nicotine metabolites (cotinine, 3HC, NNO, CNO, NNIC, NCOT and their glucuronide metabolites). Nicotine biomarkers in urine and TNE were corrected for creatinine values. In addition, we examined the molar sum of all metabolites formed by C-oxidation (cotinine + 3HC + their respective glucuronides, cotinine N-oxide, nornicotine, and norcotinine) and the sum of all metabolites generated via pathways other than by C-oxidation (nicotine + nicotine glucuronide, and nicotine N-oxide) as a fraction of the total of all nicotine and metabolites excreted in urine. To examine minor pathways of nicotine metabolism we also computed urine ratios of nicotine glucuronide/nicotine, cotinine glucuronide/cotinine, 3HC glucuronide/3HC and nicotine N-oxide/nicotine.
For each measure, data from the three assessment time points were analyzed by ANOVA with repeated measures (RM-ANOVA). The current research was a secondary analysis of a vitamin supplementation study (participants received either vitamin [B6, B12 and Folic acid] or placebo). We are aware of no evidence that supplementation with any of these vitamins affects CYP2A6 activity or alters the rate of nicotine metabolism. Half of the sample analyzed for nicotine metabolites were from participants that received the vitamin supplementation and half were from the placebo group. Initial analyses included treatment condition (vitamin supplementation or placebo) in the model. Because there was no significant difference on the effects of alcohol abstinence on rate of nicotine metabolism between the vitamin and placebo treated participants, all analyses are presented collapsed on treatment condition. Significant RM-ANOVAs were interpreted using pairwise comparisons with Bonferroni corrections. Effects were considered significant at an alpha level of 0.05 or less. Statistical analyses were performed using SPSS 22 (IBM Corporation, Armonk, NY, USA).
3. Results
3.1. Baseline characteristics
The baseline demographic, smoking, and alcohol drinking variables for the entire group of male and female smokers who completed the study (n=165) are shown in Table 1. As discussed in methods, 22 male individuals were randomly selected for analysis of nicotine metabolites. Shown in Table 1 is a comparison of the subsample that was included in the metabolite assay compared to the overall sample population. The subsample and overall sample were comparable with the exception of a slightly higher average CPD (p=0.05; t-test), and fewer days since last drink of alcohol (p<0.001; t-test) for the subsample.
Table 1. Baseline (week 1) tobacco, alcohol, and demographic characteristics.
| % or Mean ± SEM | Total Sample (N=165) | Subsample for nicotine metabolite analysis (N=22) | t-statistic (p-value) |
|---|---|---|---|
| % Male | 83.0% | 100% | --- |
| % Caucasian | 100% | 100% | --- |
| Age | 44.6 ± 0.6 | 44.5 ± 1.4 | 0.08 (0.94) |
| BMI | 23.2 ± 0.3 | 23.2 ± 0.6 | 0.06 (0.95) |
| Years of heavy alcohol drinking | 15.2 ± 0.7 | 14.4 ± 1.8 | 0.49 (0.63) |
| Days not drinking alcohol | 6.7 ± 0.2 | 4.3 ± 0.5 | 4.13 (<0.001) |
| Usual CPD | 19.6 ± 0.6 | 22.5 ± 1.9 | -1.97 (0.05) |
| Smoking years | 25.9 ± 0.7 | 27.0 ±1.6 | -0.64 (0.52) |
| Expired breath CO (ppm) | 30.7 ± 1.2 | 31.7 ± 3.9 | -0.37 (0.72) |
| FTCD at baseline | 6.6 ± 0.2 | 7.0 ± 0.5 | -1.00 (0.32) |
BMI: body mass index; FTCD: Fagerstrom Test for Cigarette Dependence; CO: carbon monoxide; CPD: cigarettes per day.
3.2. Smoking behavior over the course of treatment for alcohol dependence
For the full sample, there was a slight but significant increase in the number of self-reported past day cigarettes smoked (F(2, 414)=7.34, p=0.001) at week 4 (17.7 ±0.6) and week 7 (18.0 ±0.5), compared to baseline (16.5 ±0.6). However, there was no significant change in smoking in the subsample (fig 1A). There was no significant change in expired breath CO level between the 3 assessments in either the full sample or the subsample (fig. 1B). There was also no significant difference in urine TNE over the three sessions assessed in the subsample (fig. 1C), indicating that there was no change in total nicotine exposure at each of the three assessments over 7 weeks of alcohol abstinence.
Figure 1.
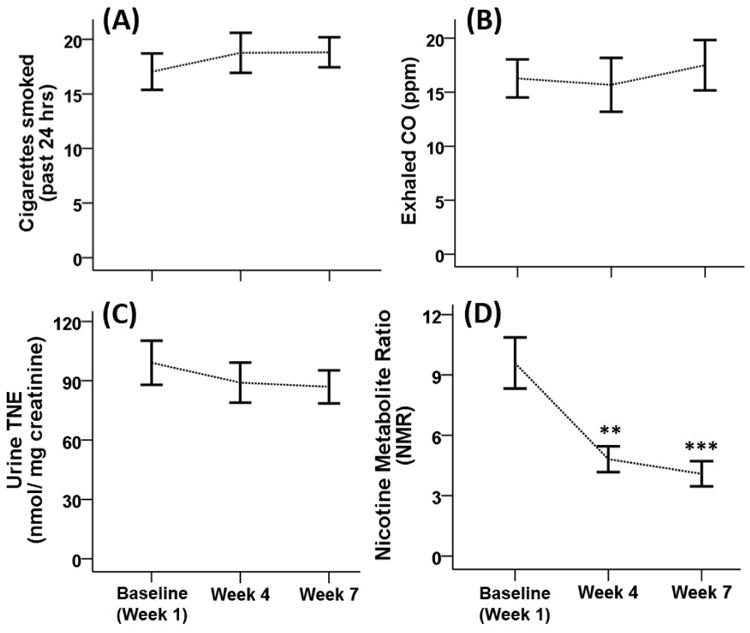
Tobacco use characteristics for the subsample (N=22) over 7 weeks of abstinence from alcohol. Individuals were assessed at three time points after cessation of alcohol baseline (week 1), week 4, and week 7. Shown are the mean (± SEM) for (A) cigarettes smoked in past 24 hrs; (B) expired breath CO; (C) urine total nicotine equivalents (TNE); and (D) Nicotine Metabolite Ratio (NMR). **: p < 0.01; ***: p < 0.001 for the comparison with the week 1 baseline.
3.3. Changes in nicotine metabolism over 7 weeks of alcohol abstinence
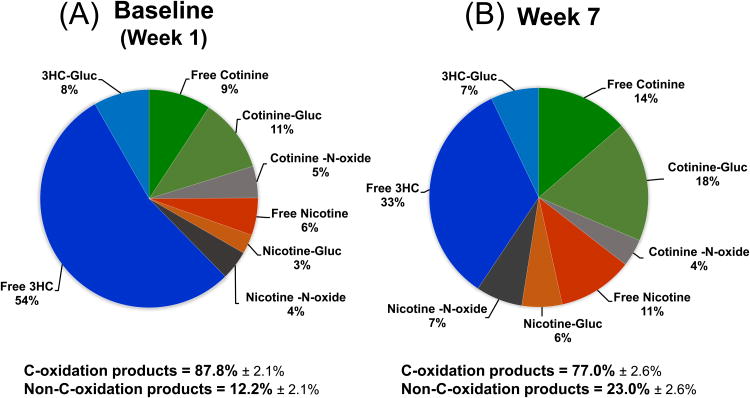
Cessation of alcohol consumption resulted in a significant change in nicotine metabolites measured in urine over the 7 weeks of abstinence from alcohol (Table 2). Higher levels of urine cotinine were found on week 4 and 7 compared to baseline as well as lower urine 3HC levels on week 7 compared to baseline (F(2, 42)=5.83, p=0.006). NMR decreased significantly (Fig. 1A) over the 7 weeks of alcohol cessation (F(2, 42)=18.83, p<0.001). Overall NMR decreased 50.0% from baseline to week 7. A full profile of nicotine metabolites in urine at baseline and at week 7 is presented in Fig. 2. There was a 12.3% decrease in nicotine C-oxidation products, and an 88.5% increase in non-C-oxidation products found in urine comparing baseline to week 7 (Fig. 2). No significant changes were observed in urine ratios of nicotine glucuronide/nicotine, cotinine glucuronide/cotinine, 3HC glucuronide/3HC or nicotine N-oxide/nicotine.
Table 2. Nicotine metabolite levels in urine (ng/ mg creatinine) over 7 weeks after cessation of alcohol.
| Analyte | Baseline (Week 1) | Week 4 | Week 7 | F, p-value |
|---|---|---|---|---|
| Cotinine | 1467 ± 206 | 2529 ± 316* | 2439 ± 325* | 6.66, p=0.003 |
| Cotinine glucuronide | 2048 ± 391 | 3214 ± 565 | 3172 ± 333* | 4.07, p=0.03 |
| Total cotinine | 3515 ± 555 | 5743 ± 786* | 5611 ± 517** | 7.64, p=0.004 |
| 3HC | 9919 ± 1272 | 8678 ± 1231** | 6454 ± 836** | 9.82, p<0.001 |
| 3HC glucuronide | 1669 ± 389 | 2048 ± 778 | 1282 ± 218 | NS |
| Total 3HC | 11589 ± 1594 | 10726 ± 1768 | 7736 ± 988** | 5.83, p=0.01 |
| Nicotine | 582 ± 159 | 1224 ± 264 | 2023 ± 550** | 6.13, p=0.009 |
| Nicotine glucuronide | 464 ± 93 | 914 ± 143* | 983 ± 163** | 7.33, p=0.002 |
| Total Nicotine | 1046 ± 223 | 2138 ± 341* | 3006 ± 591*** | 9.98, p<0.001 |
| Nicotine N'Oxide | 621 ± 164 | 803 ± 146 | 1155 ± 246* | 5.14, p=0.01 |
| Cotinine N'Oxide | 786 ± 90 | 794 ± 83 | 765 ± 78 | NS |
| Nornicotine | 102 ± 17 | 152 ± 26 | 143 ± 24 | NS |
| Norcotinine | 439 ± 60 | 447 ± 64 | 374 ± 51 | NS |
Nicotine metabolites measured in the subsample (N=22). Blood and urine samples were obtained before the first cigarette of the day at baseline (week 1), week 4 and week 7 after abstinence from alcohol. Shown are the mean metabolite levels in urine (ng/ mg creatinine ± SEM) and results from RM-ANOVAs across the three sessions. Significant differences from baseline are indicated as:
= p<0.05;
= p<0.01;
p<0.001.
3HC = trans-3′-hydroxycotinine; NS = nonsignificant.
Figure 2.
Change in nicotine metabolic profile from the week 1 baseline (A) to week 7 of alcohol abstinence (B). The metabolic profile of nicotine is shown as pie charts representing amount of each metabolite found in urine. Cotinine, nicotine, and 3HC (3′-hydroxycotinine) are shown as percent free and percent conjugated as glucuronide (gluc). Also shown are the molar sum of all metabolites formed by C-oxidation (cotinine + 3HC + their respective glucuronides, cotinine N-oxide, nornicotine and norcotinine) and the sum of all metabolites generated via pathways other than by C-oxidation (nicotine + nicotine glucuronide and nicotine N-oxide) as a fraction of the total of all nicotine and metabolites excreted in urine.
3.4. Biomarkers of liver function over 7 weeks of alcohol abstinence
There was no evidence of significant liver injury among the participants included in this study (supplemental figure 11). Among the 22 participants in whom nicotine metabolites were assessed there was a change in biomarkers of liver function over the three assessment sessions, as expected after cessation of alcohol. Plasma levels of both AST (supplemental fig. 1A2; F(2, 42)= 9.54, p<0.001) and ALT (supplemental fig. 1B3; F(2, 42)=10.39, p<0.001) were significantly lower at week 4 and week 7 compared to the first week of alcohol abstinence, however, there was no significant change in the ratio of AST/ ALT (supplemental fig. 1C4). Levels of GGTP were also found to decrease over the 7 weeks of alcohol abstinence (supplemental fig. 1D5; F(2, 42)= 8.43, p=0.001), with significantly lower levels on week 4 and 7 compared to baseline.
4. Discussion
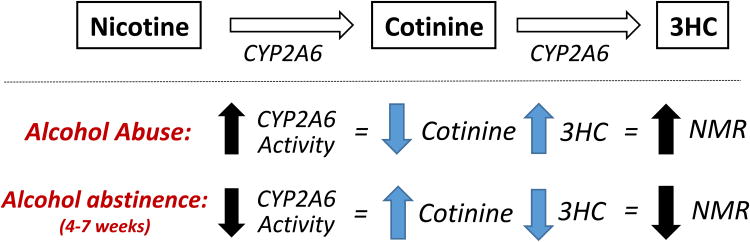
The present study among alcohol-dependent male Caucasian cigarette smokers is to the best of our knowledge the first to demonstrate that in alcohol dependent cigarette smokers the cessation of alcohol consumption results in a significant and substantial decrease in the rate of nicotine metabolism (lower NMR). This is consistent with previous work suggesting that chronic alcohol consumption may induce CYP2A6, the enzyme primarily responsible for the metabolism of nicotine. CYP2A6 protein levels were found to be elevated in the livers of individuals who abuse alcohol (Niemelä et al., 2000) and alcohol was found to induce CYP2A6 activity in the U937 macrophage cell line (Jin et al., 2011; 2012). This finding is consistent with work in mice showing that chronic alcohol consumption induced CYP2A5 (Lu et al., 2011; 2012), the mouse orthologue of human CYP2A6. Together these results suggest that chronic heavy alcohol consumption may lead to an increase in the rate of nicotine metabolism, which recovers with cessation of alcohol consumption (Fig. 3).
Figure 3.
A theoretical model summarizing the effects of alcohol abuse and cessation on rate of nicotine metabolism via the C-oxidation pathway as measured by the nicotine metabolite ratio (NMR). Alcohol abuse may induce the rate of nicotine metabolism via the CYP2A6 pathway. Our study finds that among alcohol abusers, abstinence from alcohol for 4 to 7 weeks results in a significant decrease in rate of nicotine metabolism suggesting a normalization of this effect.
It was previously reported that alcohol use was associated with higher NMR levels among individuals who drank <25 drinks/ week (Chenoweth et al., 2014). This finding suggests the idea that alcohol consumption may be associated with more rapid nicotine metabolism in non-alcohol-dependent individuals. However, not all studies have found this relationship (Ferguson et al., 2012; Mwenifumbo et al., 2007). These inconsistent findings highlight the need to better characterize the relationship between amount, frequency, and duration of alcohol consumption in relation to the induction of nicotine metabolism.
An important aspect of this work was characterizing the change in the profile of nicotine metabolites from baseline to week 7 (fig. 2). Overall we found a decrease in excretion of C-oxidation products and an increase in non-C-oxidation pathway products comparing week 1 to week 7 after alcohol abstinence. This shift in nicotine metabolite products is consistent with the interpretation that there was a decrease in CYP2A6 enzymatic activity at week 7 compared to baseline. Alcohol has also been found to induce the cytochrome p450 enzyme CYP2B6 (Hesse et al., 2004), which may be a minor pathway of nicotine metabolism. However induction of CYP2B6 would not affect the metabolism of cotinine to 3HC, and thus would not alter the NMR, suggesting the observed effects were due to a change in CYP2A6 enzymatic activity. In addition, we considered the possibility that alcohol abuse might alter nicotine metabolism by other enzymatic pathways. Our finding that the ratios of nicotine glucuronides/ total nicotine, cotinine glucuronides/ total cotinine, 3HC glucuronides/ total 3HC, and NNO/ total nicotine, did not significantly change during alcohol abstinence indicates that changes in minor nicotine pathways were not a significant factor influencing the nicotine metabolism or the observed changes in NMR.
The implications of our study are as follows. Lower smoking cessation rates are found in alcohol-dependent individuals, but not in former alcoholics (See Hughes and Kalman, 2006 for review). Higher NMR has been found to be associated with lower smoking cessation outcomes using nicotine replacement therapy (NRT; Lerman et al., 2006; 2015). Chronic heavy alcohol consumption may lead to an increases in the rate of nicotine metabolism, which could be one contributing factor to the poor smoking cessation rates in this population. Results from the current study suggest a normalization of the rate of nicotine metabolism had likely occurred by week 4 after alcohol cessation, as NMRs at week 4 were comparable to what has been measured in the general population (Benowitz et al., 2003). This could have implications for the timing or choice of smoking cessation treatments in recovering alcoholics. Future research should evaluate if NRT may be more effective as a smoking cessation intervention after a period of abstinence from heavy alcohol consumption or if individuals who are alcohol-dependent or heavy drinkers may require higher doses of nicotine replacement therapy or non-nicotine medications (such as varenicline or bupropion) for optimal smoking cessation efficacy.
Another implication of this work is that cotinine levels were significantly higher at 4 and 7 weeks after cessation of alcohol compared to the week 1 baseline, with no change in total nicotine exposure. Cotinine is often used as a biomarker for cigarette smoking. If chronic heavy drinking results in an induction of CYP2A6 metabolism, using cotinine as a biomarker of cigarette smoking would result in an underestimation of cigarette smoking in these individuals (Zhu et al., 2013). This has important implications for interpreting studies using cotinine as a biomarker tobacco exposure in this population.
Although there was a small but significant increase in self-reported number of cigarettes smoked for the full sample, there was no significant change in the subsample in which nicotine metabolites were assessed. In addition, there was no change in total nicotine exposure (TNE) or expired CO over the three assessment periods after alcohol cessation, indicating that nicotine or tobacco smoke intake did not change with the decrease in rate of nicotine metabolism. One might expect that as the rate of nicotine metabolism decreases, smokers would smoke fewer cigarettes and take in less nicotine per day, which was not observed. A lack of change, an increase, and a decrease in CPD have all been reported in individuals during treatment for alcohol dependence (Aubin et al., 1995; 1999). There are a number of other factors that influence smoking behavior in this population. Perhaps the effect of metabolic rate on smoking behavior would be observed over a longer period of time or when patients left the treatment facility.
Chronic heavy alcohol consumption is known to result in elevated levels of liver function biomarkers (ALT, AST, and GGTP). Results in the current study were consistent with a normalization of liver function biomarkers with cessation of alcohol consumption (Allen et al., 2001). These results suggest that the change in rate of nicotine metabolism corresponded with a normalization of liver function biomarkers seen at week 4 and week 7 compared to baseline. Minor elevations in liver enzymes are not likely to be causally associated with changes in drug metabolism. This correspondence was likely due to independent effects of alcohol cessation.
Consent for the current study occurred after participants were treated for acute alcohol withdrawal and the baseline sample occurred during the first week of alcohol abstinence. It is possible that the rate of nicotine metabolism would have been different when participants are actively drinking alcohol. For some drugs, acute alcohol consumption inhibits metabolism, while in the absence of alcohol, drug metabolism is accelerated due to underlying liver enzyme induction (Altomare et al., 1984). Also, in the first week of detoxification, before our baseline measurement, the rate of nicotine metabolism could have been even higher.
A limitation of the current study was that it is observational rather than experimental. All data was collected after alcohol abstinence. It would be difficult to prospectively study the effects of heavy alcohol use and withdrawal on nicotine metabolism for ethical reasons. An important future direction of this research is to examine rate of nicotine metabolism among current heavy drinkers and to better characterize the relationship between amount, frequency, and duration of alcohol consumption in relation to the induction of nicotine metabolism. Another limitation is that all of our subjects were male and Caucasian. Future research should examine if similar changes in nicotine metabolism are found in alcohol-dependent women smokers after cessation of alcohol consumption. In addition, there are known racial differences in rate of nicotine metabolism (Pérez-Stable et al., 1998; Shiffman et al., 2014; Tanner et al., 2015), and the possibility of racial differences in the effect of chronic alcohol consumption on the rate of nicotine metabolism needs further examination.
In conclusion, our results indicate that chronic alcohol abuse may increase the rate of nicotine metabolism, which then decreases after alcohol cessation. This research may help to inform future smoking cessation interventions in this population.
Supplementary Material
Highlights.
Nicotine metabolism was examined in alcoholics over 7 weeks of alcohol treatment
Rate of nicotine metabolism was assessed using the nicotine metabolite ratio (NMR)
NMR was significantly higher at week 1 compared to week 7 after alcohol cessation
Suggests that chronic alcohol abuse increased the rate of nicotine metabolism
Accelerated rate of nicotine metabolism decreased 4-7 weeks after alcohol cessation in alcoholics
Acknowledgments
We thank Faith Allen for data management and Trisha Mao, Lisa Yu and Lawrence Chan for analytical chemistry.
Author Disclosures: Role of funding source: The study was funded by grant N404 145539 from the Ministry of Science and Higher Education of Poland. Nicotine biomarker analysis was support by NIH (NIDA DA02277 and P30 DA012393). The preparation of this manuscript was supported by NIH NCI CA-113710. The funding sources had no involvement in the study design, in the collection, analysis and interpretation of the data, in the writing of the report, or in the decision to submit the article for publication.
AS received personal fees from the Smoking Institute in Poznan, Poland, and nonfinancial support from Chic Group LTD, a manufacturer of electronic cigarettes in Poland, outside of the submitted work. MLG received a research grant from Pfizer Inc., a manufacturer of smoking cessation medications. NLB serves as a paid consultant to pharmaceutical companies that are developing or that market smoking cessation medications. He also has been a paid expert witness in litigation against tobacco companies
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributions: N.R.G, N.L.B and M.L.G. wrote the manuscript. M.L.G and A.S designed the research. A.K-K., I.S-B., E.S-M., J.G. and M.L.G. performed the research and data collection. P.J. III supervised the laboratory analyses of nicotine metabolites. N.R.G, N.L.B, and M.L.G. analyzed the data. All authors read and approved the final manuscript.
Conflict of interest: All other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JP, Litten RZ, Strid N, Sillanaukee P. The role of biomarkers in alcoholism medication trials. Alcohol Clin Exp Res. 2001;25:1119–1125. [PubMed] [Google Scholar]
- Altomare E, Leo MA, Sato C, Vendemiale G, Lieber CS. Interaction of ethanol with acetaminophen metabolism in the baboon. Biochem Pharmacol. 1984;33:2207–2212. doi: 10.1016/0006-2952(84)90655-5. [DOI] [PubMed] [Google Scholar]
- Aubin H, Tilikete S, Laureaux C, Nguyen Hac H, Roullet-Volmi M, Troupel S, Barrucand D. Smoking and coffee intake following alcohol withdrawal in alcoholic inpatients. Eur Psychiatry. 1995;10:383–385. doi: 10.1016/0924-9338(96)80342-7. [DOI] [PubMed] [Google Scholar]
- Aubin HJ, Laureaux C, Tilikete S, Barrucand D. Changes in cigarette smoking and coffee drinking after alcohol detoxification in alcoholics. Addiction. 1999;94:411–416. doi: 10.1046/j.1360-0443.1999.94341110.x. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P., 3rd Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;5:621–624. doi: 10.1080/1462220031000158717. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Dempsey D, Yu L, Jacob P., 3rd Estimation of nicotine dose after low-level exposure using plasma and urine nicotine metabolites. Cancer Epidemiol Biomarkers Prev. 2010;19:1160–1166. doi: 10.1158/1055-9965.EPI-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burling TA, Ramsey TG, Seidner AL, Kondo CS. Issues related to smoking cessation among substance abusers. J Subst Abuse. 1997;9:27–40. doi: 10.1016/s0899-3289(97)90004-3. [DOI] [PubMed] [Google Scholar]
- Chenoweth MJ, Novalen M, Hawk LW, Jr, Schnoll RA, George TP, Cinciripini PM, Lerman C, Tyndale RF. Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment-seeking smokers. Cancer Epidemiol Biomarkers Prev. 2014;23:1773–1782. doi: 10.1158/1055-9965.EPI-14-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Fucito LM, Piasecki TM, Piper ME, Schlam TR, Berg KM, Baker TB. Relations of alcohol consumption with smoking cessation milestones and tobacco dependence. J Consult Clin Psychol. 2012;80:1075–1085. doi: 10.1037/a0029931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey D, Jacob P, 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301:594–598. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- Dempsey D, Tutka P, Jacob P, 3rd, Allen F, Schoedel K, Tyndale RF, Benowitz NL. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Derby KS, Cuthrell K, Caberto C, Carmella SG, Franke AA, Hecht SS, Murphy SE, Marchand L. Nicotine metabolism in three ethnic/racial groups with different risks of lung cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:3526–3535. doi: 10.1158/1055-9965.EPI-08-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Kapur S, Sarkar M, Muhammad R, Mendes P, Newland K, Roethig HJ. Respiratory retention of nicotine and urinary excretion of nicotine and its five major metabolites in adult male smokers. Toxicol Lett. 2007;173:101–106. doi: 10.1016/j.toxlet.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Ferguson CS, Miksys S, Palmour RM, Tyndale RF. Differential effects of nicotine treatment and ethanol self-administration on CYP2A6, CYP2B6 and nicotine pharmacokinetics in African green monkeys. J Pharmacol Exp Ther. 2012;343:628–637. doi: 10.1124/jpet.112.198564. [DOI] [PubMed] [Google Scholar]
- Friend KB, Pagano ME. Smoking cessation and alcohol consumption in individuals in treatment for alcohol use disorders. J Addict Dis. 2005;24:61–75. doi: 10.1300/J069v24n02_06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hesse LM, He P, Krishnaswamy S, Hao Q, Hogan K, von Moltke LL, Greenblatt DJ, Court MH. Pharmacogenetic determinants of interindividual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes. Pharmacogenetics. 2004;14:225–238. doi: 10.1097/00008571-200404000-00002. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Kalman D. Do smokers with alcohol problems have more difficulty quitting? Drug Alcohol Depend. 2006;82:91–102. doi: 10.1016/j.drugalcdep.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Dale LC, Offord KP, Croghan IT, Hays JT, Gomez-Dahl L. Nicotine patch therapy for smoking cessation in recovering alcoholics. Addiction. 1995;90:1541–1546. doi: 10.1046/j.1360-0443.1995.9011154112.x. [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:267–276. doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Arya P, Patel K, Singh B, Silverstein PS, Bhat HK, Kumar A, Kumar S. Effect of alcohol on drug efflux protein and drug metabolic enzymes in U937 macrophages. Alcohol Clin Exp Res. 2011;35:132–139. doi: 10.1111/j.1530-0277.2010.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Earla R, Shah A, Earla RL, Gupte R, Mitra AK, Kumar A, Kumar S. A LC-MS/MS method for concurrent determination of nicotine metabolites and role of CYP2A6 in nicotine metabolism in U937 macrophages: implications in oxidative stress in HIV + smokers. J Neuroimmune Pharmacol. 2012;7:289–299. doi: 10.1007/s11481-011-9283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Hapke U. Probabilities of alcohol high-risk drinking, abuse or dependence estimated on grounds of tobacco smoking and nicotine dependence. Addiction. 2003a;98:805–814. doi: 10.1046/j.1360-0443.2003.00381.x. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Schumann A, Thyrian JR, Hapke U. Strength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sample. Alcohol Alcohol. 2003b;38:606–612. doi: 10.1093/alcalc/agg122. [DOI] [PubMed] [Google Scholar]
- Keenan RM, Hatsukami DK, Pickens RW, Gust SW, Strelow LJ. The relationship between chronic ethanol exposure and cigarette smoking in the laboratory and the natural environment. Psychopharmacology (Berl) 1990;100:77–83. doi: 10.1007/BF02245794. [DOI] [PubMed] [Google Scholar]
- Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, Benowitz N. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79:600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Lerman C, Schnoll RA, Hawk LW, Jr, Cinciripini P, George TP, Wileyto EP, Swan GE, Benowitz NL, Heitjan DF, Tyndale RF PGRN-PNAT Research Group. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3:131–138. doi: 10.1016/S2213-2600(14)70294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhuge J, Wu D, Cederbaum AI. Ethanol induction of CYP2A5: permissive role for CYP2E1. Drug Metab Dispos. 2011;39:330–336. doi: 10.1124/dmd.110.035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhang XH, Cederbaum AI. Ethanol induction of CYP2A5: role of CYP2E1-ROS-Nrf2 pathway. Toxicol Sci. 2012;128:427–438. doi: 10.1093/toxsci/kfs164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks JL, Hill EM, Pomerleau CS, Mudd SA, Blow FC. Nicotine dependence and withdrawal in alcoholic and nonalcoholic ever-smokers. J Subst Abuse Treat. 1997;14:521–527. doi: 10.1016/s0740-5472(97)00049-4. [DOI] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH. How can we use our knowledge of alcohol-tobacco interactions to reduce alcohol use? Annu Rev Clin Psychol. 2013;9:649–674. doi: 10.1146/annurev-clinpsy-050212-185549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwenifumbo JC, Sellersm EM, Tyndale RF. Nicotine metabolism and CYP2A6 activity in a population of black African descent: impact of gender and light smoking. Drug Alcohol Depend. 2007;89:24–33. doi: 10.1016/j.drugalcdep.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Niemelä O, Parkkila S, Juvonen RO, Viitala K, Gelboin HV, Pasanen M. Cytochromes P450 2A6, 2E1, and 3A and production of protein-aldehyde adducts in the liver of patients with alcoholic and non-alcoholic liver diseases. J Hepatol. 2000;33:893–901. doi: 10.1016/s0168-8278(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Pérez-Stable EJ, Herrera B, Jacob IP, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280:152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J Neurogenet. 2009;23:252–261. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein ML, Benowitz NL, Auerback GM, Moscicki AB. Rate of nicotine metabolism and withdrawal symptoms in adolescent light smokers. Pediatrics. 2008;122:e643–647. doi: 10.1542/peds.2007-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul Toxicol Pharmacol. 2007;47:171–183. doi: 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Benowitz NL. A comparison of nicotine biomarkers and smoking patterns in daily and nondaily smokers. Cancer Epidemiol Biomarkers Prev. 2014;23:1264–1272. doi: 10.1158/1055-9965.EPI-13-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Herman AI, Nadim H, Jatlow P. Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology. 2012;37:1509–1516. doi: 10.1038/npp.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser AA, Benowitz NL, Pinto AG, Tang KZ, Hecht SS, Carmella SG, Tyndale RF, Lerman CE. Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev. 2011;20:234–238. doi: 10.1158/1055-9965.EPI-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JA, Chenoweth MJ, Tyndale RF. Pharmacogenetics of nicotine and associated smoking behaviors. Curr Top Behav Neurosci. 2015;23:37–86. doi: 10.1007/978-3-319-13665-3_3. [DOI] [PubMed] [Google Scholar]
- World Health Organization– WHO. International Statistical Classification Of Diseases And Related Health Problems, 10th Revision (ICD-10) WHO Library Cataloguing-in-Publication Data; Geneva, Switzerland: 2010. [Google Scholar]
- Xia XY, Peng RX, Yu JP, Wang H, Wang J. In vitro metabolic characteristics of cytochrome P-450 2A6 in Chinese liver microsomes. Acta Pharmacol Sin. 2002;23:471–476. [PubMed] [Google Scholar]
- York JL, Hirsch JA. Drinking patterns and health status in smoking and nonsmoking alcoholics. Alcohol Clin Exp Res. 1995;19:666–673. doi: 10.1111/j.1530-0277.1995.tb01565.x. [DOI] [PubMed] [Google Scholar]
- Zhu AZ, Renner CC, Hatsukami DK, Swan GE, Lerman C, Benowitz NL, Tyndale RF. The ability of plasma cotinine to predict nicotine and carcinogen exposure is altered by differences in CYP2A6: the influence of genetics, race, and sex. Cancer Epidemiol Biomarkers Prev. 2013;22:708–718. doi: 10.1158/1055-9965.EPI-12-1234-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.