Abstract
Objectives
Varenicline (2mg/day) has been shown to be efficacious in reducing alcohol consumption. A lower dose of varenicline may be effective in reducing alcohol use while minimizing the potential for side effects.
Methods
This double-blind, placebo-controlled investigation examined the effect of varenicline (0, 1, 2 mg/day) on alcohol consumption in non-treatment seeking adults meeting DSM-IV criteria for alcohol use disorders (n = 60). Following 7-days of medication pretreatment, participants were administered a low fixed dose of alcohol (0.3 g/dL), and subjective and physiologic responses were assessed. A 2-hour ad-libitum alcohol self-administration period followed. We also explored relationships between plasma varenicline levels and consumption.
Results
Overall, frequency and severity of adverse events were minimal. The 1mg/day dose reduced the frequency of insomnia compared to the 2mg/day dose. The 2 mg/day varenicline dose versus placebo reduced alcohol craving and showed limited effect on reduced alcohol consumption. Alcohol craving and consumption did not differ between the 1 mg/day varenicline dose versus placebo. Trough varenicline plasma levels ≥ 3 ng/ml were associated with reduced drinking and levels ≥ 5ng/ml were associated with reduced heavy drinking.
Conclusions
Overall, we found no evidence supporting an effect of 1 mg/day varenicline on craving or consumption, suggesting that doses of varenicline less than 2mg/day may not be effective in reducing alcohol-related outcomes. Importantly, results suggest that higher plasma levels of varenicline may be needed in order to maximize the effect of varenicline on alcohol consumption and should be investigated in drinkers meeting criteria for alcohol use disorders.
Keywords: varenicline, alcohol, dose-response, nAchR, self-administration, laboratory, alcohol use disorders
INTRODUCTION
Alcohol use disorders (AUDs) continue to be a critical public health concern, affecting as many as 17 million adult Americans (National Institute on Alcohol Abuse and Alcoholism, 2014). Alcohol consumption has been found to increase risk for developing hemorrhagic stroke, cirrhosis of the liver, coronary heart disease, and cancer amongst an extensive list of other medical conditions (Rehm et al., 2003), and imposes an economic cost of $223.5 billion dollars in the United States due to lost productivity, increased healthcare, criminal justice expenses, and motor vehicular crashes (Bouchery et al., 2011). With the extensive global burden of disease associated with excessive alcohol use (Rehm, 2014) and the modest efficacy of current FDA-approved medications for AUDs (Zindel and Kranzler, 2014), the identification of novel pharmacotherapeutic treatment strategies for AUDs remains a high priority.
Varenicline (Chantix) (Pfizer, New York), a Food and Drug Administration (FDA)-approved medication for the treatment of nicotine dependence, is a partial agonist of α4β2 nicotinic acetylcholine receptor subtypes (nAchRs), and although varenicline for AUDs is investigational, evidence to date suggests that it is an effective target for the treatment of AUDs (McKee et al., 2009, Litten et al., 2013). All studies investigating varenicline for heavy drinking in humans have focused on assessing the 2 mg/day dose, demonstrating decreases in alcohol consumption and alcohol craving in heavy drinking smokers and in individuals meeting criteria for alcohol dependence (McKee et al., 2009, Mitchell et al., 2012, Plebani et al., 2013). A multi-site randomized, placebo-controlled clinical trial of 2 mg/day varenicline in smokers and non-smokers meeting criteria for alcohol dependence found that varenicline reduced the percent of heavy drinking days, drinks per day, and alcohol craving compared with placebo (Litten et al., 2013).
Using a well-validated human laboratory paradigm that evaluates medication effects on reactivity to a priming drink and subsequent alcohol self-administration behaviors, the primary purpose of the present investigation was to determine if 1 and 2 mg/day varenicline are both efficacious for reducing alcohol drinking and well-tolerated in smokers and non-smokers meeting criteria for AUDs. Previous studies demonstrate that 2 mg/day varenicline decreases alcohol craving and consumption, but, to our knowledge, 1 mg/day varenicline has not yet been tested for the treatment of AUDs in a human laboratory paradigm. Preclinical findings suggest that doses as low as 1 mg/kg reduce alcohol intake and seeking behavior (Steensland et al., 2007). We hypothesized that both 1 and 2 mg/day varenicline, would reduce alcohol craving and alcohol self-administration following a low dose priming drink of alcohol compared with placebo. We also hypothesized that 1 mg/day varenicline would minimize side effects often associated with 2 mg/day varenicline. Litten and colleagues demonstrated that individuals receiving 2 mg/day varenicline in a clinical trial for alcohol dependence reported significantly higher rates of nausea (37.1% vs. 17.8%), unusual dreams (27.8% vs. 11.9%), and constipation (9.3% vs. 2.0%) compared with placebo (Litten et al., 2013). Rates of certain side effects are dose-dependent in studies of varenicline for smoking. Nausea was reported in 30% of smokers treated with the 2 mg/day dose, but reported in only 16% of those receiving 1mg/day varenicline (Burstein et al., 2005). Lower dosing of varenicline may be effective in reducing alcohol use while minimizing the potential for side effects.
Finally, we explored relationships between plasma varenicline levels, alcohol craving, and consumption to further elucidate the effects of 1 and 2 mg/day varenicline on alcohol-related outcomes. Pharmacokinetic studies of varenicline indicate that there are dose-proportional increases in maximum observed plasma varenicline concentrations between 1 and 2 mg/day varenicline (Faessel et al., 2006a, Faessel et al., 2006b). However, few studies have examined the relationship between plasma varenicline levels and drug-related outcomes. In a recent investigation on pharmacokinetic modeling of varenicline on nicotine craving, 2 mg/day varenicline decreased craving and the magnitude of this response was related to higher plasma varenicline levels in smokers, though this response was highly variable (Ravva et al., 2015). To date, there are no studies examining the relationship between plasma varenicline levels and drinking behavior. We hypothesized that higher plasma varenicline levels be associated with reduced alcohol consumption.
MATERIALS AND METHODS
Participants
Participants were eligible if they were ≥ 21 years of age and were able to read and speak English. All participants met DSM-IV criteria for past 6 months alcohol abuse or alcohol dependence, and met criteria for heavy drinking (binge drinking at least once per week defined as +4/+5 per episode for females/males, respectively). Exclusion criteria included illicit drug use (except for occasional cannabis use), past 30-day use of psychoactive drugs, treatment-seeking for alcohol or smoking, current Axis I disorders (except for nicotine dependence or alcohol abuse), current suicidal or homicidal ideation, pregnancy or nursing, or medical conditions contraindicating alcohol use (e.g., liver enzymes ≥3× normal) or varenicline administration (e.g., known allergy to varenicline), or subjects likely to exhibit clinically significant alcohol withdrawal during the study.
Design
The study was a double-blind, placebo-controlled, parallel group design. Randomization to varenicline (1 mg/day or 2 mg/day) or a matching placebo (0 mg/day) was stratified by sex and smoking status. Participants were classified as smokers if they smoked ≥ 5 cigarettes per day, and had breath carbon monoxide (CO) levels > 10 ppm and urine cotinine > 150 ng/ml. Non-smokers reported no tobacco use for at least the past year, and CO < 10 ppm and urine cotinine < 150 ng/ml.
Procedures
Eligibility Screening
The Human Investigation Committee of Yale University approved this study and written informed consent was obtained. In addition to a physical examination, eligibility screening included: electrocardiogram, urine toxicology, pregnancy test, and basic blood chemistries. Of the 147 potential participants who were screened, 71 did not meet eligibility criteria related to illicit drug use (n=7), smoking (n=5) or drinking criteria (n=18), had medical contraindications (n=14), did not complete eligibility screening (n=22) or had psychiatric issues (n=5). Seventy-six met eligibility criteria; 9 lost interest in the study, and 7 were dismissed due to illicit drug use. Sixty participants completed the study.
Medication
Varenicline and matching placebo were provided by Pfizer (New York), and were over-encapsulated with riboflavin added to monitor compliance. Varenicline was titrated to steady-state levels over 7 days (for 1 mg/day varenicline: 0.5 mg daily for Days 1 – 5 and 0.5 mg twice daily for Days 6 and 7; for 2 mg/day varenicline: 0.5 mg daily for Days 1 and 2, 0.5 mg twice daily for Days 3 – 5, and 1.0 mg twice daily on Days 6 and 7). Medication compliance was monitored with pill counts and riboflavin marker on Days 5 and 8 (Del Boca et al., 1996). Trough plasma levels were collected at the start of the laboratory session on Day 8.
Laboratory Session
On Day 8, each subject completed a 14-hour laboratory session conducted at the Yale Center for Clinical Investigation, New Haven, Connecticut. The laboratory procedures were similar to those used in our previous alcohol self-administration studies (McKee et al., 2008, McKee et al., 2009), which conform to guidelines for alcohol administration (National Advisory Council on Alcohol Abuse and Alcoholism, 2005). Laboratory sessions started at 8:00am, and baseline assessments of breath alcohol, urine drug screen, and urine pregnancy screen were obtained. The final dose of medication was provided at 9:00am. Subjects were instructed not to consume alcohol the night before, and smokers were free to smoke as they normally would prior to entering the research unit. To ensure that participants who smoked were not nicotine deprived during the session, 15-min smoke breaks were provided at 10:00am, 12:00pm, and 2:00pm on the research unit in a negative pressure room.
Priming Dose
The alcohol priming drink, designed to raise blood alcohol levels to 0.3 g/dL (Watson, 1989) was administered from 3:00 – 3:05pm and consisted of 1 part 80-proof liquor of the subject’s choosing to 3 parts mixer chosen from a selection of equicaloric, non-caffeinated, non-carbonated drinks.
Alcohol Self-Administration
Starting at 50 min and 120 min following the priming drink, participants were exposed to two, 1-hour ad libitum drinking periods during which they were permitted to drink up to four alcoholic drinks (eight drinks total over the entire 2-hour self-administration session; each 0.15 g/kg), or to receive monetary reinforcement ($3 per drink) for each drink not consumed. Participants were discharged at 10:00pm at which time their breath alcohol levels had fallen below .02 g%.
Assessments
Alcohol craving was measured using the Alcohol Urge Questionnaire (AUQ) (Bohn et al., 1995), subjective intoxication effects were measured using the Alcohol Effects Scale (AES) (Schuckit, 1984), and, for smokers, DSM-IV symptoms of nicotine withdrawal was assessed using the Minnesota Nicotine Withdrawal Scale (MNWS; range 0 – 32) (Hughes and Hatsukami, 1986) and tobacco craving was assessed using the Tiffany Questionnaire of Smoking Urges (QSU-Brief) (Cox et al., 2001). AUQ, AES, and QSU-Brief all used the Visual Analogue Scale (VAS) with a range of 1 – 100. Systolic and diastolic blood pressure and heart rate were also assessed.
Timing of Assessments
Breath alcohol concentrations (BACs), alcohol craving, tobacco craving, and physiologic measures (systolic and diastolic blood pressure, heart rate, and skin temperature) were assessed 15 min before and 10, 20, 30, 40, 80, 110, 150, and 180 min following the consumption of the priming drink. Subjective effects of alcohol (intoxication), nicotine withdrawal, and potential adverse effects (nauseous, dizzy, jittery) were assessed at baseline, 20, 40, 110, and 180 min following priming drink consumption.
Plasma varenicline levels
Human plasma containing varenicline and the internal standard, varenicline-15N2D2, was extracted using a solid phase extraction method for the determination of varenicline in human K2-EDTA plasma by Worldwide Clinical Trials, Austin, TX. The range of quantitation was 0.0500 to 10.0 ng/mL based on the analysis of 0.500 mL of plasma. An aliquot of the extract was injected onto a Sciex API 4000 or 5000 LC-MS-MS equipped with an HPLC column. The peak area of the m/z 212®169 varenicline product ion was measured against the peak area of the m/z 216®173 varenicline-15N2D2 internal standard product ion. Quantitation was performed using a weighted linear least squares regression analysis generated from fortified plasma calibration standards prepared fresh daily.
Adverse Events
Adverse effects were assessed in person on Days 1, 5, and 8 (at the times outlined above) and by phone on Day 2 (Levine and Schooler, 1986). Common varenicline side effects (> 5% and twice the rate seen in placebo-treated patients for smoking cessation) included nausea, abnormal dreams, insomnia, constipation, flatulence, and vomiting, in addition to cardiac and neuropsychiatric adverse events (Pfizer Inc, 2014). Severity ratings were rated on a 4-point scale (1=mild, 2=minimal, 3=moderate, 4=severe).
Statistical Analysis
Baseline characteristics, frequency counts of total side effects, and severity ratings of total side effects, were compared across medication (1 or 2 mg/day varenicline vs. 0 mg placebo) with either t-tests or chi-square tests. Separate repeated measures analyses of variance [ANOVAs using General Linear Models (GLM)] models, with medication (0, 1, and 2 mg/day varenicline) as a between subjects factor and time as a within-subjects factor, were conducted for the priming drink and self-administration periods to examine BALs, subjective measures, and physiologic reactivity across medication and within time. For the self-administration period, a repeated measures ANOVA across all medications was conducted to examine the number of drinks consumed by medication and hour. A priori contrasts, based on our hypothesis for our primary outcome, compared 1 and 2mg/day varenicline to placebo for drinks consumed. All other contrasts were calculated as post-hoc tests within the GLM analysis, comparing 1 mg/day versus placebo, 2 mg/day versus placebo, and 1 mg/day versus 2 mg/day as planned contrasts. Subjective and physiologic measures are not presented for the self-administration period as outcomes were simply a reflection of drinking behavior (all medication effects were not significant for alcohol craving, subjective intoxication, physiologic reactivity; data not shown). Smoking status and sex were assessed as between-subjects variables and subsequently as covariates in all models and were removed from all final models (except drinks consumed) as no significant effects on outcomes were demonstrated. Exploratory analysis examined linear associations (Pearson r) between alcohol craving and drinks consumed and varenicline plasma levels and drinks consumed. Binge drinking, as an outcome, was defined as women consuming 4 or more drinks and men consuming 5 or more drinks over the 2-hour self-administration period.
RESULTS
Baseline Characteristics
Varenicline (1 or 2 mg/day) and placebo groups were well matched for baseline demographic variables, and smoking and drinking behavior (Table 1).
Table 1.
Baseline Characteristics by Medication Condition, Mean (SD) or n (%)
| Placebo (n=20) |
1 mg/day Varenicline (n=20) |
2 mg/day Varenicline (n=20) |
|
|---|---|---|---|
| Age | 34.2 (9.52) | 33.35 (8.51) | 34.15 (11.6) |
| Sex (% male) | 70% | 60% | 75% |
| Race (% White) | |||
| White | 11 (55) | 10 (50) | 15 (75) |
| Other | 9 (45) | 10 (50) | 5 (25) |
| Education | |||
| ≤ High school | 10 (50) | 8 (40) | 3 (15) |
| ≥ College | 10 (50) | 12 (60) | 17 (85) |
| Marital Status | |||
| Not married | 15 (75) | 16 (80) | 19 (95) |
| Married | 5 (25) | 4 (20) | 1 (5) |
| Tobacco Use (smokers only) | |||
| Cigarettes per day | 14.2 (6.63) | 12.6 (5.00) | 16.0 (6.26) |
| FTND scorea* | 4.25 (2.14) | 4.92 (1.80) | 4.91 (1.38) |
| Alcohol Use | |||
| Weekly frequency (days)b | 4.84 (1.64) | 4.41 (1.48) | 4.43 (1.51) |
| Drinks per episodec | 6.67 (3.18) | 7.28 (2.86) | 6.36 (3.25) |
| AUDIT scores | 13.95 (6.79) | 13.45 (5.01) | 10.75 (3.92) |
AUDIT, Alcohol Use Disorders Identification Test; FTND, Fagerström Test for Nicotine Dependence.
Range: scores ≥ 4 for nicotine dependence.
Means calculated over 30 days before intake.
Scores ≥ 8 for alcohol misuse.
n= 12, 13, 11 for Placebo, 1 mg, and 2 mg, respectively
Note: no differences between groups using chi-square or ANOVA where appropriate.
Titration Period
Adverse Events
During the medication titration period, the placebo group had the highest rates of dry mouth (p=.03), and the 1 mg/day group had the lowest rates of insomnia (p=.05; Table 2). All subjects completed their assigned doses, no subject discontinued the study due to adverse events, and no subject required a dose adjustment. Mean severity ratings for each adverse event were in the minimal to mild range and did not differ across medication groups.
Table 2.
Frequency Counts (n, %) of Treatment Emergent Symptomsa
During the 1-week Medication Titration Period (Days 1 – 8).
| Adverse Event | Placebo (n=20) |
1 mg/day Varenicline (n=20) |
2 mg/day Varenicline (n=20) |
|---|---|---|---|
| Constipation | 0, 0% | 1, 5% | 2, 10% |
| Nausea/Vomiting | 6, 30% | 4, 20% | 5, 25% |
| Dry Mouth* | 7, 35% | 2, 10% | 2, 10% |
| Insomnia* | 2, 10% | 0, 0% | 5, 25% |
| Flatulence | 1, 5% | 0, 0% | 1, 5% |
| Difficulty Breathing | 0, 0% | 1, 5% | 1, 5% |
| Shortness of Breath | 1, 5% | 0, 0% | 1, 5% |
| Tightness in Chest | 0, 0% | 1, 5% | 0, 0% |
| Abnormal Dreams | 2, 10% | 1, 5% | 3, 15% |
| Fast Heartbeat | 1, 5% | 1, 5% | 3, 15% |
| Suicidal Thoughts | 0, 0% | 0, 0% | 0, 0% |
| Erratic Behavior | 0, 0% | 0, 0% | 0, 0% |
More than 5% and twice the rate seen in placebo-treated subjects.
Note: Frequency counts differed for dry mouth and insomnia (*p<.05).
Severity ratings wereall minimal or mild and didnot differ across medication groups.
Changes in Drinking and Smoking Behavior
Medication did not change drinking behavior (quantity, frequency, drinks per episode) during the titration week, compared to baseline values. There was a main effect of medication on smoking during the titration week (F (2, 33) = 3.81, p=.03); 2mg/day varenicline decreased cigarettes per day by 4.69 cigarettes (SE = 1.14) versus placebo (mean = −.39, SE = 1.14) and 1mg/day varenicline decreased cigarettes per day by 1.54 cigarettes (SE = 1.10).
Medication Compliance
All subjects were at least 80% compliant with pill counts and urine florescence. Mean trough plasma varenicline levels were; 1mg/day varenicline = 2.36 ng/ml (SE = 0.29); and 2mg/day varenicline = 4.60 ng/ml (SE = 0.28).
Priming Drink Period
Breath Alcohol Concentrations
BACs demonstrated a significant quadratic effect of time (F(1,57) = 79.64, p < .0001) but did not differ by or interact with medication. Peak mean BACs were achieved at the +10 time point (grand mean = 0.028 g%, SE = 0.002).
Subjective Measures
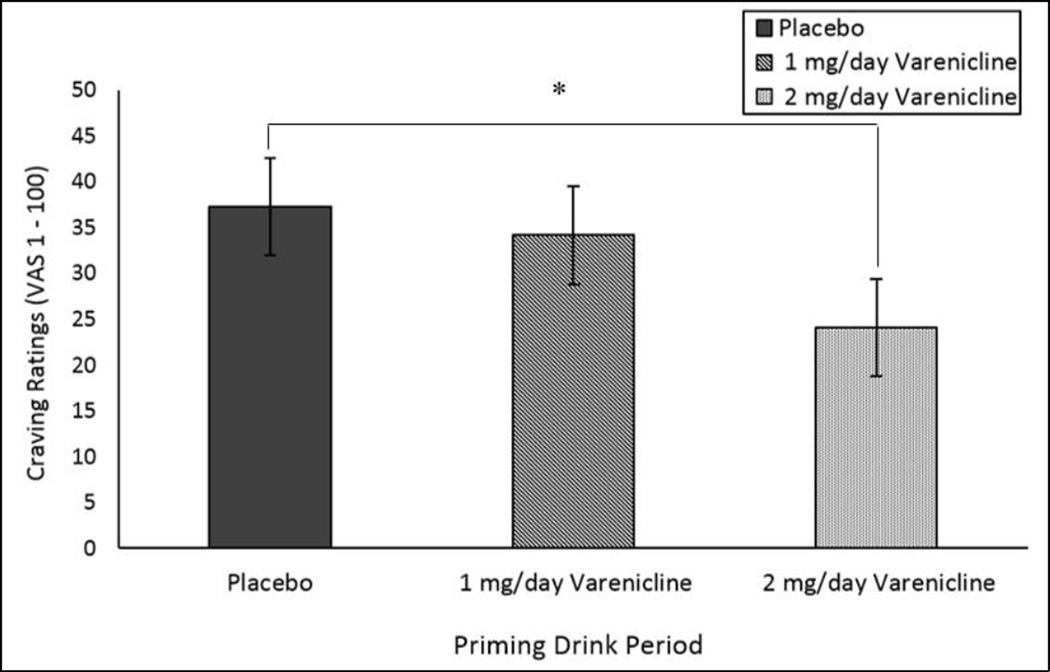
Alcohol craving demonstrated a significant main effect of medication on baseline scores (F (2,52) = 3.18, p=.05; see Figure 1). 2mg/day varenicline demonstrated significantly lower values versus placebo, but not differ from 1mg/day varenicline. When controlling for this baseline difference as a covariate in the GLM, there are no significant effects of time or medication on alcohol craving (p>.05). Subjective intoxication ratings demonstrated a significant effect of time (p<.0001), but there were no main or interactive effects of medication. In smokers, tobacco craving increased over the priming drink (p<.0001) period but there was no main or interactive effects with medication. Adverse events (nausea, dizziness) and nicotine withdrawal (in smokers only) were minimal (all mean ratings were less than 5 on a 100 point scale) and did not differ by medication.
Figure 1.
Mean subjective alcohol craving ratings at baseline by medication. *p<.05 contrasting 2mg/day varenicline versus placebo.
Physiologic Measures
Systolic and diastolic blood pressure and heart rate demonstrated significant effects of time (p<.0001, p<.01, and p=.01, respectively) such that systolic and diastolic blood pressure decreased over the course of the priming drink period, and heart rate increased over time. There were no significant effects of medication or medication by time on the physiologic measures.
Smoking Behavior
For smokers, there was no effect of medication on cigarettes smoked during the three smoking breaks. On average, subjects smoked 1 cigarette per smoke break.
Ad-Libitum Drinking Period
Drinking Behavior
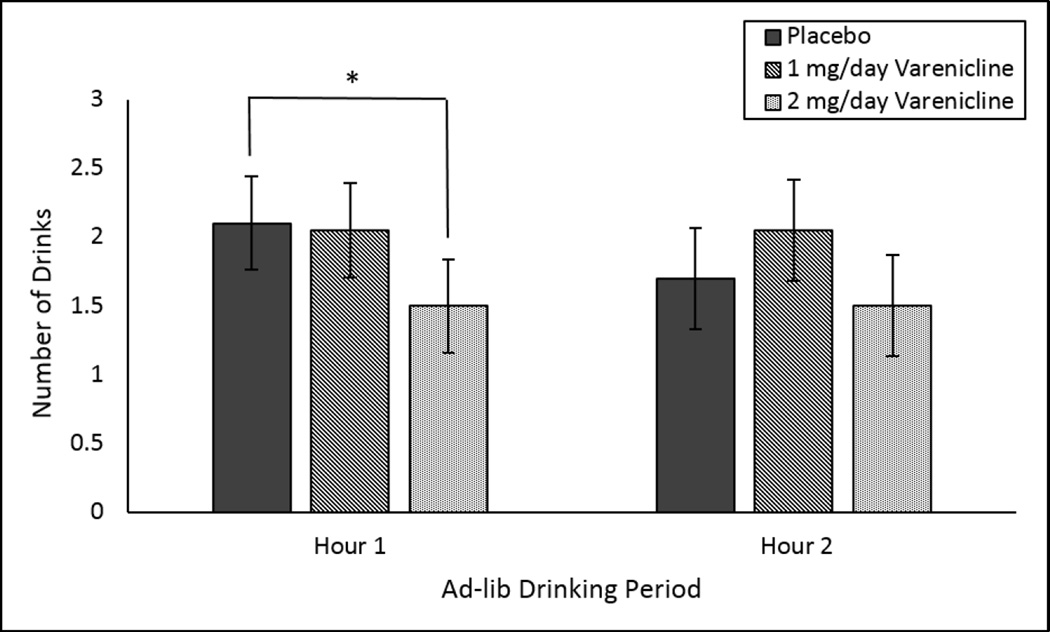
Overall, drinks consumed during the 2-hour self-administration period did not differ by medication. During the first hour, a priori contrasts comparing the placebo group to the 2 mg/day varenicline groups identify that mean drinks consumed during the first hour was lower in the 2 mg/day varenicline group versus the placebo group (t (38) = 2.60, p<.05). This difference did not persist during the second hour as drinking was reduced in the placebo group (see Figure 2). The a-priori contrast comparing the placebo group to the 1 mg/day varenicline group was not significant. There was a main effect of smoking status on drinks consumed (F(1,54) = 4.36, p= 0.04) but no interactive effects with medication. Smokers consumed more drinks during the 2-hour ad-lib period (smoker mean = 4.27, SE=0.48; non-smoker mean = 2.67, SE=0.61).
Figure 2.
Mean drinks consumed during the first and second hour of the ad-lib drinking period. *p<.05 a priori contrast of 2mg/day varenicline versus placebo.
Exploratory Analyses
Binge Drinking
Forty-three percent of subjects engaged in binge drinking during the 2-hour ad-lib period. Differences contrasting the 2 mg/day varenicline (30% binge drinking) with the placebo (50%) and 1 mg/day (50%) groups were not statistically different (2 mg/day varenicline versus placebo; z = 1.30, p<.10). Participants classified as ‘binge-drinkers’ reached a mean BAC of 0.085 g% (SE=.006) by the end of the ad-lib period (versus a mean BAC of 0.01 g% (SE=.005) in participants that did not binge drink.
Associations between Craving and Drinking
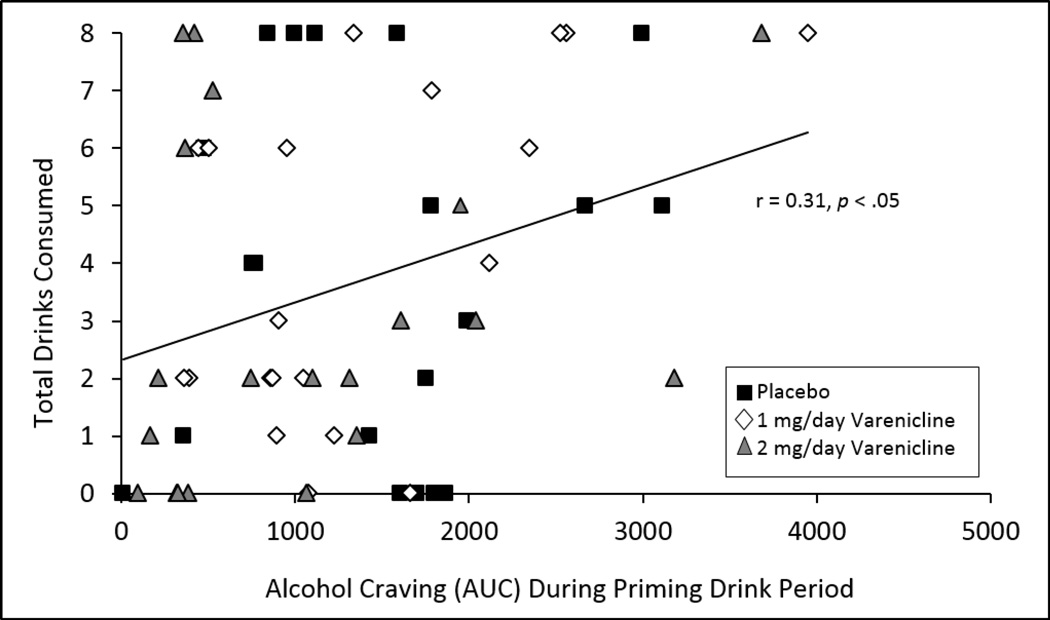
Alcohol craving (assessed using area under the curve (AUC)) during the priming drink period was significantly associated with drinks consumed during the ad-lib drinking period over all medication conditions (r = 0.31, p= 0.02 see Figure 3).
Figure 3.
Scatterplot with line of best fit for alcohol craving during the priming drink (assessed as area under the curve [AUC] by total drinks consumed during the 2-hour self-administration period.
Exploratory Associations between Varenicline Plasma Levels and Drinking
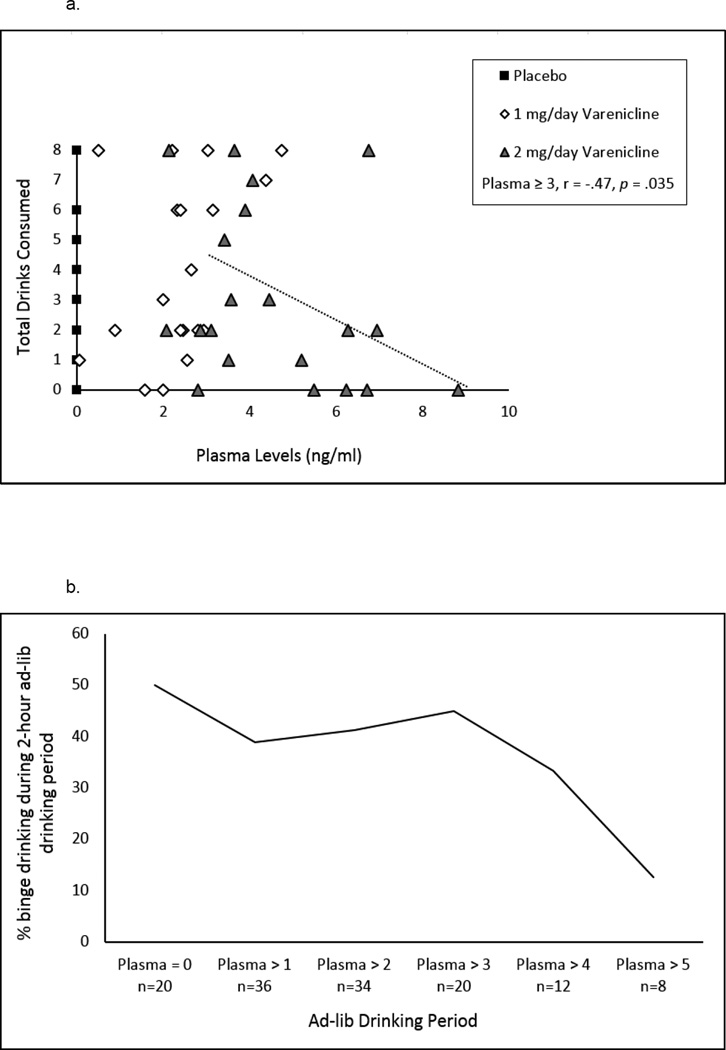
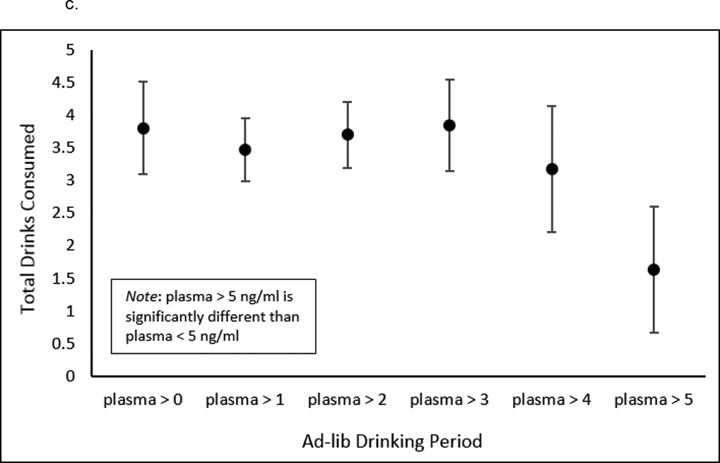
Overall correlations between plasma levels and drinking were not significant. However, when we examined increasing cut points of varenicline plasma levels the strength of the relationship with drinks consumed was increasingly negative up to plasma levels greater than or equal to 3 ng/ml (see Figure 4a). We also examined the relationship between varenicline plasma levels and rates of binge drinking during the ad-lib session. Increasing cut points of plasma levels were associated with reduced rates of binge drinking (see Figure 4b). The BAC achieved by the end of the ad-lib period was negatively associated with plasma levels, with plasma levels ≥ 3 ng/ml being correlated with BAC at r = −0.55, p=.01. At plasma levels greater than 5 ng/ml (mean=1.63, SE=1.02) versus less than 5 ng/ml (mean=3.94; SE=0.40), there was a significant difference in total drinks consumed (F (1,58) = 4.44, p=.04; see Figure 4c).
Figure 4.
Associations between varenicline plasma levels and drinking during the 2-hour self-administration period. (A) The correlation between increasing cut points of varenicline plasma levels and total drinks consumed was increasingly negative up to plasma levels ≥ 4 ng/ml [plasma ≥ 0, r = −.11, p = .4; plasma ≥ 1, r = −.16, p = .36; plasma ≥ 2, r = −.26, p = .14; plasma ≥ 3, r = −.47, p = .04; plasma ≥ 4, r = −.51, p = .09]. (B) The relationship between varenicline plasma levels and rates of heavy drinking. Heavy drinking is defined at 4 or more drinks for women and 5 or more drinks for men during the 2-hour self-administration period. (C) Total drinks consumed for each plasma level reached. At plasma levels > 5 ng/ml (mean=1.63, SE=1.02) versus < 5 ng/ml (mean=3.94, SE=0.4), there was a significant difference in total drinks consumed (F (1, 58) = 4.44, p = .04).
DISCUSSION
To our knowledge, this is the first investigation of the effects of a lower dose of varenicline on human alcohol self-administration and craving in adults meeting criteria for AUDs. Overall, we found few differences between 1 and 2 mg/day varenicline with regard to side effects, and no evidence supporting an effect of 1 mg/day varenicline on alcohol-related outcomes. The 1 mg/day dose did not decrease subjective effects of alcohol or ad-lib drinking behavior in the human laboratory, which is consistent with some preclinical findings demonstrating non-significant effects of lower varenicline doses on ethanol-motivated behaviors (Wouda et al., 2011, Randall et al., 2015). Our results regarding the 2 mg/day dose also confirm prior findings (McKee et al., 2009, Mitchell et al., 2012, Plebani et al., 2013), in that 2 mg/day varenicline reduced tonic alcohol craving in the priming drink period compared with placebo. Across the doses tested, we continue to demonstrate a significant association between alcohol craving and consumption, supporting prior work (McKee et al., 2009). Further, a priori comparisons showed a modest effect of the 2 mg/day dose on alcohol drinking in the ad-lib drinking period compared with placebo. Our ability to demonstrate effects associated with the 2 mg/day dose was complicated by decreased drinking in the placebo group during the second hour of the self-administration period.
Varenicline was well-tolerated in our sample of drinkers meeting criteria for AUDs. Mean severity ratings for each adverse event were reported as minimal to mild, and ratings did not differ across medication groups. Overall, there were few differences in the frequency ratings of adverse events during the titration period. Rates of dry mouth were greatest in the placebo group (35% placebo; 10% 1 mg/day varenicline; 10% 2 mg/day varenicline), whereas rates of insomnia were lowest in the 1 mg/day group (10% placebo; 0% 1 mg/day varenicline; 25% 2 mg/day varenicline). Further, no subject discontinued medication or required a dose adjustment as a result of adverse events. When varenicline was combined with alcohol following the priming drink, there were no effects of medication on ratings of nausea or dizziness. Overall, our findings suggest that varenicline appears to be safe and well-tolerated in individuals meeting criteria for AUDs.
The current investigation was also the first to explore the relationship between plasma varenicline and drinking behavior. Previous pharmacokinetic studies of varenicline suggest that there are dose-proportional increases in maximum observed plasma varenicline concentrations between the 1 and 2 mg/day doses (Faessel et al., 2006a, Faessel et al., 2006b), with steady state levels achieved within 4 days of repeat dosing (Faessel et al., 2010). In our study, mean plasma trough levels were 2.36 (SD=1.30) ng/ml and 4.60 (SD=1.25) ng/ml, for the 1 and 2 mg/day groups respectively. These values, obtained after a 7 day titration in participants with alcohol use disorders, are somewhat lower than the mean trough values demonstrated by Faessel and colleagues in smokers. That study found mean plasma trough levels of 6.17 ng/ml (SD=1.18) for 2 mg/day varenicline (1 mg twice daily) on Day 14 of treatment (Faessel et al., 2006a). In the Faessel study, there wasn’t a 1 mg/day twice daily dosing group for direct comparison to our findings. However, their 1 mg/day once daily dosing group obtained a plasma trough level of 3.65 (SD=0.89) on Day 17 of treatment. Once daily dosing compared to twice daily of the same total dose will result in higher plasma values. While the standard deviations for mean plasma trough levels are highly consistent between studies, our plasma trough levels are lower in value. This discrepancy could be due to length of dosing in each study. We collected plasma on Day 8 in the present study whereas Faessel and colleagues collected plasma on Day 14 or 17, a nearly one week difference. However, if steady state values of varenicline are truly obtained after 4 days (Faessel et al., 2010), it is unlikely this factor is completely accounting for the differences in findings. In our study we over-encapsulated tablets to include a riboflavin marker in order to monitor medication compliance. It is possible that the over-encapsulation process may have changed the absorption of varenicline and subsequent trough levels. Medication compliance is another potential factor contributing to the lower plasma trough levels found in the present investigation. Although all subjects were at least 80% compliant with pill counts and urine florescence, the compliance in the Faessel study was 100%. Finally, the Faessel study took place in a highly controlled inpatient setting, and additional factors unaccounted for in our outpatient design may have contributed to the lower plasma values obtained.
When examining increasing cut points of varenicline plasma levels, the strength of the relationship with drinks consumed was increasingly negative up to plasma levels of greater than 4 ng/ml, with a significant difference at ≥ 3 ng/ml. That is, there was evidence that ≥ 3 ng/ml plasma varenicline was associated with a reduction in alcohol drinking, and was negatively associated with breath alcohol levels achieved by the end of the self-administration session. Similarly, levels greater than 5 ng/ml plasma varenicline were associated with reduced rates of heavy drinking, though only 8 participants achieved plasma varenicline levels of ≥ 5. This is consistent with findings indicating that 2 mg/day varenicline decreased nicotine craving in smokers, and that this response was related to increased plasma varenicline levels (Ravva et al., 2015), although the pharmacokinetic data were also highly variable in that study. Together, our exploratory findings suggest that plasma varenicline levels may need to be greater than 5 ng/ml to be efficacious in reducing consumption in heavy drinkers. We caution that our plasma varenicline results are exploratory, are presented for the purposes of hypothesis generating, and need to be replicated.
Overall, results suggest that it may be worthwhile to push the varenicline dose higher to maximize effects on alcohol drinking. Our findings indicate that there was no advantage to lowering the dose of varenicline to 1 mg/day, and 2 mg/day varenicline had modest effects on alcohol consumption and craving. However, an investigation on the pharmacokinetics of varenicline found that a 3 mg/day dose was well-tolerated in healthy smokers (Faessel et al., 2006b). This finding may warrant further investigation into the efficacy and clinical utility of high dose varenicline in heavy drinkers. Although varenicline has high affinity for the α4β2 nAchR, it could be hypothesized that higher doses may have greater affinity for the α6 nAchR subtype. This nAchR subtype is of interest for several reasons. It has been identified in genetic investigations of alcohol initiation and consumption behavior (Hoft et al., 2009), α6 antagonists reduce ethanol self-administration and modulate sedative effects of ethanol in preclinical models (Kuzmin et al., 2009, Kamens et al., 2012), and α6 is one subunit included in over 70% of the nAchRs involved in nicotine-related dopamine release (Grady et al., 2007). Future work examining the mechanisms and efficacy of higher varenicline doses would further clarify the role of varenicline on alcohol-motivated behaviors.
There are some study limitations. The present study sample consisted of non-treatment seeking adults meeting criteria for alcohol abuse or dependence. The results may not generalize to treatment seeking adults with AUDs or drinkers without AUDs. Another important limitation is that drinking was only examined during the laboratory session. Thus, we do not know how drinking would be affected by a longer duration of treatment or outside of the laboratory. Fourth, only a single priming drink was investigated. However, our previous work using a single priming dose designed to raise BALs by 0.03 g/dl was effective in priming drinking behavior (McKee et al., 2009). Finally, no alternative beverage was provided during the ad-lib drinking period, and it is unknown whether reductions in alcohol consumption by 2 mg/day varenicline were specific to alcohol.
CONCLUSIONS
In summary, our results suggest that the nAchR system continues to hold promise as a pharmacotherapeutic treatment strategy for AUDs. In drinkers with AUDs, we found that 2 mg/day varenicline reduced tonic craving and moderately decreased the number of drinks consumed in the first hour of the ad-lib drinking period, with no effects found at the 1 mg/day dose. In an exploratory analysis, reductions in drinking and heavy drinking were associated with plasma varenicline levels greater than 3 ng/ml and 5 ng/ml, respectively. Given that lower doses of varenicline had no effect on drinking outcomes and higher plasma varenicline levels were associated with decreased drinking, future work examining higher doses of varenicline as a treatment for AUDs should be pursued.
Acknowledgments
This work was supported by NIH grants R01AA017976 (SAM) and NIAAA T32AA015496 (TLV). SAM had an investigator-initiated grant from Pfizer for this project which provided medication and support for varenicline plasma levels.
REFERENCES
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. American journal of preventive medicine. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Burstein AH, Fullerton T, Clark D, Faessel H. Safety, tolerability, and multipledose pharmacokinetics of varenicline in elderly smokers (poster). Presented at the 11th Annual Meeting and 7th European Conference of the Society for Research on Nicotine and Tobacco; March 12–20, 2005; Prague, Czech Republic. [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Faessel HM, Gibbs MA, Clark DJ, Rohrbacher K, Stolar M, Burstein AH. Multiple-dose pharmacokinetics of the selective nicotinic receptor partial agonist, varenicline, in healthy smokers. Journal of clinical pharmacology. 2006a;46:1439–1448. doi: 10.1177/0091270006292624. [DOI] [PubMed] [Google Scholar]
- Faessel HM, Obach RS, Rollema H, Ravva P, Williams KE, Burstein AH. A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. Clinical pharmacokinetics. 2010;49:799–816. doi: 10.2165/11537850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Faessel HM, Smith BJ, Gibbs MA, Gobey JS, Clark DJ, Burstein AH. Single-dose pharmacokinetics of varenicline, a selective nicotinic receptor partial agonist, in healthy smokers and nonsmokers. Journal of clinical pharmacology. 2006b;46:991–998. doi: 10.1177/0091270006290669. [DOI] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochemical pharmacology. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoft NR, Corley RP, McQueen MB, Huizinga D, Menard S, Ehringer MA. SNPs in CHRNA6 and CHRNB3 are associated with alcohol consumption in a nationally representative sample. Genes, brain, and behavior. 2009;8:631–637. doi: 10.1111/j.1601-183X.2009.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of general psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Hoft NR, Cox RJ, Miyamoto JH, Ehringer MA. The alpha6 nicotinic acetylcholine receptor subunit influences ethanol-induced sedation. Alcohol (Fayetteville, N.Y.) 2012;46:463–471. doi: 10.1016/j.alcohol.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Jerlhag E, Liljequist S, Engel J. Effects of subunit selective nACh receptors on operant ethanol self-administration and relapse-like ethanol-drinking behavior. Psychopharmacology. 2009;203:99–108. doi: 10.1007/s00213-008-1375-5. [DOI] [PubMed] [Google Scholar]
- Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side-effects in clinical trials. Psychopharmacology Bulletin. 1986;22:343-&. [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. Journal of addiction medicine. 2013;7:277–286. doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biological psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, O'Malley SS, Shi J, Mase T, Krishnan-Sarin S. Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacology. 2008;196:189–200. doi: 10.1007/s00213-007-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology. 2012;223:299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Advisory Council on Alcohol Abuse and Alcoholism. NIAAA guidelines (National Advisory Council on Alcohol Abuse and Alcoholism's Recommended Guidelines on Ethyl Alcohol Administration. [Accessed April 9, 2015]; Available at: http://www.niaaa.nih.gov/Resources/ResearchResources/job22.htm#dependent.
- National Institute on Alcohol Abuse and Alcoholism. Alcohol Facts & Statistics [NIAAA Web Site] [Accessed March 26, 2015];2014 Jul; Available at: http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics.
- Pfizer Inc. Chantix. [Accessed March 30, 2015]; Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=557. [Google Scholar]
- Plebani JG, Lynch KG, Rennert L, Pettinati HM, O'Brien CP, Kampman KM. Results from a pilot clinical trial of varenicline for the treatment of alcohol dependence. Drug and alcohol dependence. 2013;133:754–758. doi: 10.1016/j.drugalcdep.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Jaramillo AA, Frisbee S, Besheer J. The role of varenicline on alcohol-primed self-administration and seeking behavior in rats. Psychopharmacology. 2015 doi: 10.1007/s00213-015-3878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravva P, Gastonguay MR, Faessel HM, Lee TC, Niaura R. Pharmacokinetic-pharmacodynamic modeling of the effect of varenicline on nicotine craving in adult smokers. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2015;17:106–113. doi: 10.1093/ntr/ntu154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J. Burden of Disease Associated with Alcohol Use Disorders in the United States. Alcoholism, clinical and experimental research. 2014;38:1068–1077. doi: 10.1111/acer.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Room R, Graham K, Monteiro M, Gmel G, Sempos CT. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: an overview. Addiction (Abingdon, England) 2003;98:1209–1228. doi: 10.1046/j.1360-0443.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Archives of general psychiatry. 1984;41:879–884. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P. Total body water and blood alcohol levels: Updating the fundamentals. Human metabolism of alcohol. 1989;1:41–58. [Google Scholar]
- Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, Schoffelmeer AN, Pattij T, De Vries TJ. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology. 2011;216:267–277. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindel LR, Kranzler HR. Pharmacotherapy of alcohol use disorders: seventy-five years of progress. Journal of studies on alcohol and drugs. 2014;(Supplement 75 Suppl 17):79–88. doi: 10.15288/jsads.2014.s17.79. [DOI] [PMC free article] [PubMed] [Google Scholar]