Abstract
Osteoarthritis (OA) is a progressive, degenerative disease of articulating joints that not only affects the elderly, but also involves younger, more active individuals with prolonged participation in high physical-demand activities. Thus, effective therapies that are easy to adopt clinically are critical in limiting the societal burden associated with OA. This review is focused on intra-articular injectable regimens and provides a comprehensive look at existing in vivo models of OA that might be suitable for developing, testing, and finding a cure for OA by intra-articular injections. We first discuss the pathology, molecular mechanisms responsible for the initiation and progression of OA, and challenges associated with disease-specific targeting of OA. We proceed to discuss available animal models of OA and provide a detailed perspective on the use of mouse models in studies of experimental OA. We finally provide a closer look at intra-articular injectable treatments for OA, focusing on biomaterials-based nanoparticles, and provide a comprehensive overview of the various nanometer-size ranges studied.
Keywords: nanoparticles, in vivo models, intra-articular, interleukins, proteases
Challenges in Disease-Specific Targeting of OA
Osteoarthritis (OA) is a progressive, degenerative, and disabling disease of articulating joints that not only affects the elderly, but also involves younger, more active individuals with prolonged participation in high physical-demand activities. OA is the leading cause of disability in adults with more than 50 million adults reporting arthritis 1. As the population ages and obesity rates rise, the prevalence of OA will increase, and by 2030, approximately 25% of the adult population is expected to suffer from OA 2.
OA is characterized by articular cartilage degeneration, subchondral bone sclerosis and osteophyte formation. In addition to these three hallmarks, changes also occur in the menisci, synovium, ligaments, and peri-articular muscle. As OA progresses and changes in the joint become more severe, gradual loss of function occurs due to pain and stiffness 3. OA can occur in multiple sites, primarily affecting the knees, hips, hands, and spine. A large number of risk factors for OA are known, including increased age, obesity, previous joint damage, and joint malalignment 4. Because of the wide range of symptoms and risk factors, effective non-operative treatments and preventative measures for OA are limited prior to surgical intervention.
One of the most substantial challenges in developing OA treatments is optimally targeting the disease. During the evolution of the osteoarthritic process, inflammatory factors released by synovial macrophages and fibroblasts induce changes in chondrocytes within the cartilage matrix that normally serve to benefit the structural integrity of collagen and aggrecan. Under OA conditions, however, chondrocytes up-regulate the production of proteases, including matrix metalloproteinases (MMPs) and aggrecanases. These proteases degrade the cartilage matrix, releasing matrix degradation products and protein fragments. These fragments then initiate further inflammatory responses, leading to a vicious cycle of cytokine production and cartilage destruction 5. Additionally, inflammatory cytokines and alarmins act on the adjacent synovium and bone to stimulate synovial inflammation and de-regulate peri-articular bone remodeling. In this section, we will briefly review some of the main inflammatory and tissue-specific targets for OA treatment.
Inflammatory factors, including tumor necrosis factor (TNF)-α and interleukins (ILs), play a crucial role in OA pathogenesis (Figure 1). TNF-α plays a major catabolic role in OA and downregulates production of major cartilage extracellular components, including aggrecan and collagen type II 6. In addition to its direct effects on cartilage matrix synthesis, TNF-α also promotes the production of other cytokines, including IL-1, IL-6 and IL-8. Lastly, TNF-α interacts with the receptors TNFR1 and TNFR2 to activate sensory neurons, leading to pain associated with OA 7. Similar to TNF-α, IL-1 directly affects cartilage by decreasing levels of matrix components and inhibiting anabolic chondrocyte activity 8. IL-1 also induces production of collagenases and aggrecanases in both synovial fibroblasts and chondrocytes, leading to further cartilage destruction 9. IL-6 is another interleukin that is elevated in the synovial fluid of individuals with OA. The role of IL-6 in disease onset and progression is confounding, involving both proinflammatory joint destruction 10 and anti-inflammatory mediation 11. Other interleukins involved in OA include IL-17 and IL-18, which both aid in the production of other interleukins, reactive oxygen species, and collagenases 12, 13. In addition to TNF-α and interleukins, other inflammatory targets play an important role in OA progression. The inducible nitric oxide synthase (iNOS) is upregulated in OA chondrocytes, resulting in excess amounts of nitric oxide (NO) in the OA state. In turn, NO promotes the release of other inflammatory cytokines and inhibits proteoglycan and collagen II synthesis 14.
Figure 1. Osteoarthritis pathology.
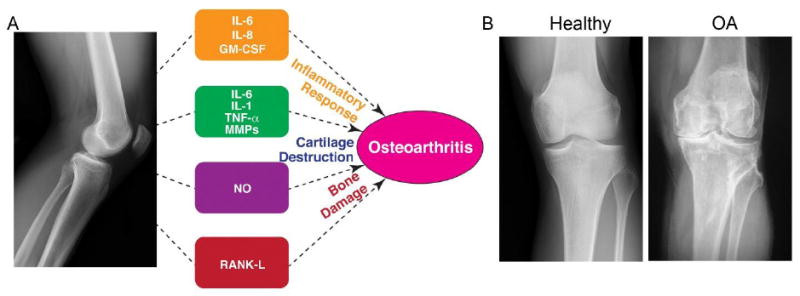
A) Schematic of OA pathology with potential targeting molecules for OA therapy. B) Human knee joints depicting healthy and OA conditions (Image courtesy: Dr. Mathias P. Bostrom at Hospital for Special Surgery, New York).
Immune cells, such as neutrophils and macrophages, are present in the joints of OA patients and release reactive oxygen species (ROS). When levels of ROS are elevated above normal, an oxidative stress state occurs in the cartilage and can lead to cell death and matrix degradation 15, 16. In addition, another oxidative factor, myeloperoxidase (MPO), is higher in both early 17 and late-stage 18 OA patients, further suggesting that oxidative stress plays a key role in both disease onset and progression. Thus, preventing oxidative stress associated with ROS may be important in treating OA. In addition to oxidative stress, granulocyte macrophage colony-stimulating factor (GM-CSF) is another potential key inflammatory factor in OA. Specifically, therapeutically neutralizing GM-CSF in a collagenase-induced mouse model of OA abolished OA-associated pain and significantly reduced cartilage degradation 19.
Structural damage of the cartilage matrix occurs from a variety of proteolytic enzymes that break down aggrecan, collagen type II, and other key matrix components. Preventing these enzymes from harmfully degrading the cartilage matrix without affecting other parts of the body will be a major step in OA therapeutics. The major aggrecanases involved in OA include ADAMTS (A Disintegrin and Metalloproteinase with ThromboSpondin type I motifs)-4 and -5, and MMP-3. In general, aggrecanases cleave the aggrecan protein between the G1 and G2 globular domains, creating aggrecan fragments that do not have the same compressive properties as the intact aggrecan proteins 20. The major collagenases in OA are MMP-1 and MMP-13, which cleave collagen proteins at specific locations, resulting in two or more collagen fragments 21, 22. Again, these collagen fragments do not provide the structural support or tensile strength that intact collagen fibrils provide to the articular cartilage matrix, resulting in compromised load-bearing properties. Importantly, the presence of matrix fragments in the synovial joint space initiates further inflammation, perpetuating the vicious cycle between inflammation and matrix destruction. In addition to aggrecanases and collagenases, other enzymes can degrade additional key components of the cartilage matrix and alter the underlying bone. One example of the former is hyaluronidase, which degrades hyaluronic acid, a molecule that provides lubrication in joints. An example of the latter is the RANKL (receptor activator of NF-κβ ligand)-to-OPG (osteoprotegerin) ratio, which regulates osteoblast and osteoclast activity. With OA, this ratio is altered in osteoblasts, suggesting that regulation of RANKL and OPG may be useful in treating OA 23.
The bottom line is that a large number of factors can be targeted to prevent the symptoms of OA, but the most effective method to treat the disease still remains unclear. A recent review discusses individual studies that have shown efficacy in preventing the activity of aggrecanases and collagenases, and others that have analyzed the effects of preventing inflammatory cytokines 24. Further approaches for treating OA are being studied, including apoptosis prevention, matrix replacement, and the addition of growth factors. Most likely, a combinatorial approach will be the most successful clinical therapy, but further research is required to understand how to best combine and deliver these drugs.
Animal models to mimic OA progression
To better understand OA pathology and the effectiveness of novel treatments, numerous preclinical animal models that mimic human OA progression are available. Although animal models are generally the fastest and most reliable way to study OA, careful consideration must be taken when choosing the appropriate model for any research question. Each model has its own benefits and limitations, and each has its unique pathology. Thus, studies analyzing specific biological questions or the efficacy of a targeted treatment may benefit from using one model over another. This portion of the review will discuss the various animal models available to study OA progression and provide insight on the appropriateness for using these models to test novel treatments.
Preclinical OA models include mice, rats, guinea pigs, rabbits, dogs, pigs, sheep, goats and horses (Figure 2). In general, initial investigations of disease mechanism or therapy effectiveness use small animal models. The primary advantages of small animals include rapid disease progression, ease of handling and housing, and lower costs. Large animal models, on the other hand, are often used to verify the effectiveness of therapies based on either previous in vitro results or in vivo data from small animals. The joints of large animals more closely resemble human joints, and therefore more accurately predict performance of OA therapies in humans. Because of slow disease progression and difficulty in obtaining large sample numbers due to cost and housing requirements, however, large animals are generally used for late stage development and validation of therapies. Importantly, cartilage thickness increases with animal size and is critical to the intra-articular retention of drugs, because diffusion binding kinetics depends on the square of cartilage thickness 25. Therefore, smaller animal models may be limited for evaluating and predicting drug retention times in humans. In addition to choosing between small and large animals, the mode of OA induction is also a critical factor in OA studies using animal models. Models can be spontaneous or induced, invasive or non-invasive, and chemically, surgically or mechanically-induced. Because thorough comparisons of small and large animal models have been recently published 26, we will primarily focus on mouse models due to the wide variety of biological and therapeutic questions they can help to answer. We will review the most commonly used in vivo methods to induce OA, concluding with newer techniques that are becoming more accepted for understanding the response of joints to mechanical loading.
Figure 2. Comparative in vivo models of OA.
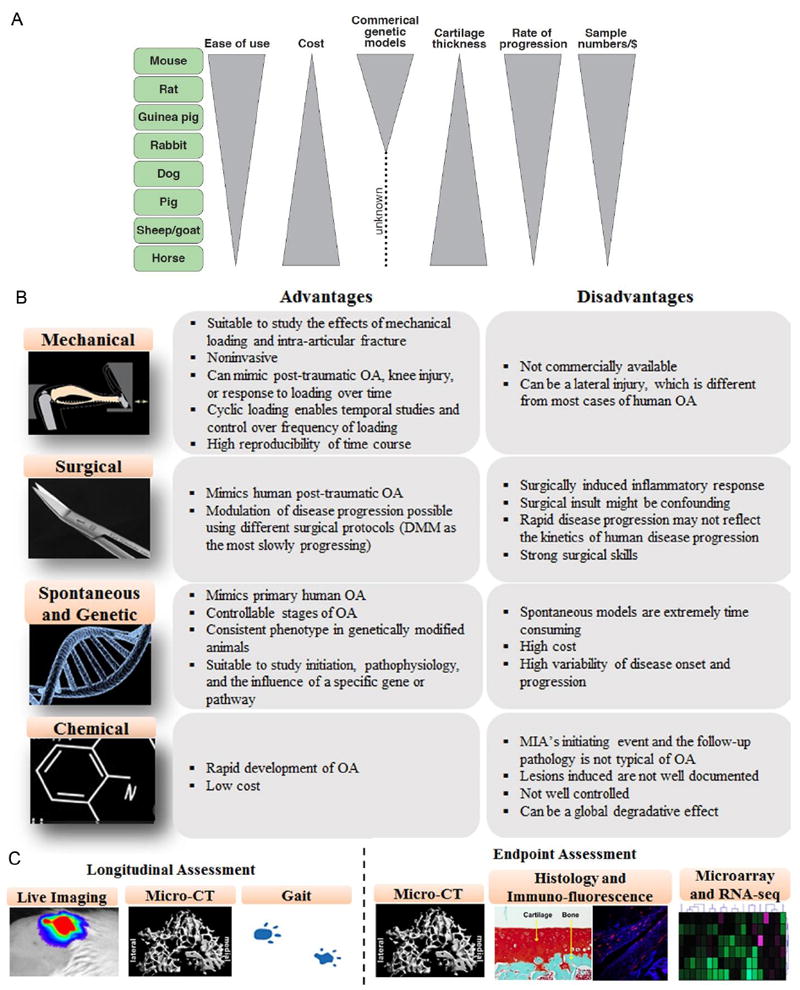
A) Comparison between existing small and large animal models with focus on cost, ease of use, onset of OA, cartilage thickness, and sample size. B-C) Existing mouse models of OA with specific advantages, risk factors, and outcome assessments. Notably, apart from the assessment included here, mouse models are amenable to several other cutting-edge assays such as RNA-seq, proteomics and metabolic assays, genome sequencing, and can be manipulated with reporter gene system to longitudinally measure the expression level of key proteins.
Spontaneous OA
Spontaneous models of OA come in multiple forms. One method of achieving spontaneous OA is to track an animal’s joints throughout the entire lifespan. For example, guinea pigs and certain strains of mice are prone to developing OA with age 26, 27. A second method involves the use of knockout, knock-in, or transgenic mice. In these animals, the genetic makeup is altered by removing, adding, or mutating specific genes. Altered genetics allow for direct investigation of specific molecular and genetic markers in the disease. Overall, spontaneous OA models reflect naturally-occurring articular cartilage degradation, and therefore, accurately resemble disease progression from early to late stages. However, because a significant portion of an animal’s life span is required for OA symptoms to develop, spontaneous OA models are extremely time-consuming. Thus, these naturally-occurring OA models are probably not the best choice to test novel therapies, unless the goal is to determine long-term effectiveness.
Chemically-induced OA
Chemically-induced OA is another common model used to investigate OA. A variety of chemicals can be used to induce OA in animals. The most commonly used are monosodium iodoacetate (MIA), papain, and collagenases. MIA induces OA by causing chondrocyte death via inhibition of cellular aerobic glycolosis. Following chondrocyte death, OA-like symptoms progress, osteophytes form, and changes in gait occur 28. Papain degrades proteoglycans in the cartilage matrix to produce OA. Lastly, collagenases enzymatically break down structures containing collagen. Because each chemically-induced OA model damages the joint via a different mechanism, this approach is useful for studying the role of a specific biological mechanism in disease progression, but does not closely mimic natural joint degradation. For example, the initiating event and resulting pathology induced by MIA are not typical of human OA, particularly the cartilage transcriptome 29. While chondrocytes are affected, this agent also acts on several other cell types present within the joint 27. If the mechanism of a therapeutic acts to inhibit a specific pathway associated with a chemically-induced OA model, then that particular model may be a suitable choice.
Surgically-induced OA
The most common OA models involve surgical disruption of the joint, simulating post-traumatic OA (PTOA). Approximately 12% of OA cases are attributed to PTOA 30, and this type of OA is the primary source of disability in active duty soldiers. To better understand PTOA, the anterior cruciate ligament transection (ACLT) model and the destabilized medial meniscus (DMM) model were developed decades ago. ACLT was first introduced in 1973 to study the effects of ACL injuries in the dog 31. Since that time, the model has been used in both large and small animals. The DMM is the most common model used in mice. In this model, transection of the mediomeniscal tibial ligament results in an unstable medial meniscus and altered joint kinematics. Both the ACLT and DMM models lead to OA progression through biomechanical mechanisms induced by joint instability. These models accurately represent human injury and the events that lead to joint degradation post-injury. However, a confounding inflammatory response occurs in these models due to the surgery. Thus, the progression of joint degradation may be altered from an increased level of inflammation surrounding the joint. Surgically-induced OA models are appropriate to test therapeutics if the goal of the therapeutic is to inhibit PTOA.
Mechanically-induced OA
Recently-developed models of OA, specifically in mice, use mechanical forces to non-invasively alter the mechanics of the joint. These models were thoroughly explained recently 32, and will be briefly presented here to discuss their potential uses with biomaterials. These models are noninvasive and range from loading on intact knees to loading to create traumatic injuries.
Cyclic loading applied to the tibia of mice noninvasively without traumatic injury induces OA-like changes over time (Figure 2, 3) 33, 34. This in vivo loading model allows the user to specify the load magnitude, number of cycles, frequency of each cycle, and duration of loading. In general, higher loads lasting for longer durations lead to more cartilage degeneration and subchondral bone changes. Importantly, unlike other OA animal models, cartilage degeneration in this model is most likely not due to joint instability from an injury, but is caused by direct mechanical overload of the articular cartilage. Because of the variety of OA severities that the tibial cyclic compression model can produce, therapeutics can be tested for efficacy under moderate and severe OA conditions with the model. Furthermore, although changes in gait may occur in the non-loaded contralateral limb in this model, gait in loaded limbs remains consistent over time 34, suggesting therapeutics injected into loaded joints may behave similarly to how they would under OA conditions with normal gait.
Figure 3. Mouse tibial cyclic compression model and localized cartilage thickness after cyclic compressive loading in adult mice.
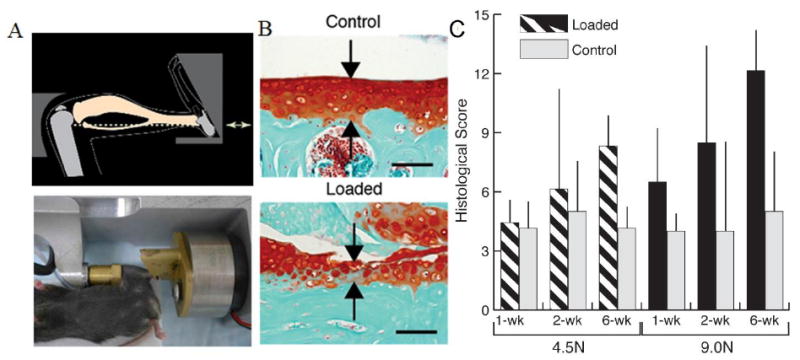
A) Mouse positioned in loading device, ready for in vivo axial loading to be applied to the left tibia. B) Safranin O–fast green staining of the nonloaded contralateral limb and loaded limb (peak load 9.0N) show the thickness of the cartilage (red, arrows) after 6 wks of mechanical loading in the posterior aspects of the lateral tibial plateau. Bars = 100 μm. C) Loading increased histological scores of cartilage degeneration in adult mice compared to controls, and the load-induced damage increases depending on the load level and duration. Bars show the mean ± SD of 42 adult mice. P < 0.05 by repeated-measures two-way analysis of variance (young mice) or three-way analysis of variance (adult mice) for comparisons of the effects of loading, duration, loading × duration, and loading × load level. Adapted with permission from Ko et al. 33.
Mechanical overload to rupture the ACL is a traumatic mechanically-induced OA model. Non-invasive ACL rupture can be achieved by applying a single compressive load 32 or by applying high-magnitude cyclic compressive load until the ACL ruptures 35. This model has been used in both mice 32 and rabbits 36. The model accurately models ACL rupture in humans, as the injury generally occurs due to a single traumatic overload event. However, ACL rupture causes severe instability in the joint, resulting in changes in the position of the femur relative to the tibia during normal gait. Severe articular degeneration occurs only after 8 weeks post-injury due to the resultant abnormal articular surfaces, and thus, the model may be better for studies analyzing acute activity early on in disease progression rather than later events. Taken together, the non-invasive ACL rupture model may be appropriate for analyzing early-stage activity of therapeutics immediately after injury to prevent PTOA, but may not be suitable for long-term studies due to the severity of altered articulations.
Summary of Animal OA Models
Animal models are integral to studying OA pathology and determining the effectiveness of novel therapeutics in vivo to treat OA. Many different animals are used as models, ranging from as small as the mouse to as large as the horse. Mouse models offer a unique advantage over other models because of their low cost, ease of housing, and wide range of genetic and mechanical methods to induce OA. However, the joints of larger animals more closely resemble human joints, and therefore may be more suitable than mice for verifying the efficacy of novel therapeutics. In addition to choice of animal, choice of induction method is also a crucial factor in any study. Being aware of each model’s advantages and disadvantages is important when answering any question about OA. Specifically, when therapeutics are aimed at inhibiting or altering a particular aspect of OA progression, choosing a model that accurately mimics that aspect of progression is necessary. Ultimately, understanding and appropriately using animal models of OA will be a critical factor as novel therapeutics continue to emerge, as will be discussed in the final sections of the review.
Treatments for OA
We will now redirect our focus to current treatments for OA and will conclude with a review of novel nanoparticle-based treatments with an emphasis on how they are being tested. For patients with early stage OA, non-pharmacological, non-operative treatments are the first option for intervention37-39. Non-pharmacological treatments include assistive devices (knee braces, foot wear and lateral wedged insoles, splints) and encouraging patients to improve life-style, control body weight and undertake moderate intensity physical exercises. For patients with moderate stage OA, pharmacological treatments are recommended, including analgesics, nutritional supplements, orally- or topically-administered non-steroidal anti-inflammatory drugs (NSAIDs) and intra-articular (IA) injectable hyaluronic acid (HA) and cortical steroids. Surgical intervention is required when both non-pharmacological and pharmacological treatments become ineffective in relieving pain. While joint replacement is the most effective surgical procedure, arthroscopy, osteotomy and joint fusion are also recommended depending on age, OA severity, and other factors.
A Closer Look at Injectable Treatments for OA
Because OA pathology is restricted to the affected joint, IA administration of biomolecules targeting chondrocyte activity or synovial inflammation is a promising strategy for therapeutic intervention 40. This review will therefore focus on IA injectable regimens. These treatments are of particular interest owing to their low doses for local administration, high potency and minimal side effects compared to intravenously- or orally-administered drugs and supplements. In comparison, intravenous injections of drugs result in poor localization to diseased joints and often require continuous administration of large doses. Orally administered drugs often are associated with complications to the gastrointestinal tract 41.
Corticosteroids and hyaluronic acid (HA) are both widely used IA injectable regimens, and their efficacy in disease treatment has been extensively reviewed 42-48. Corticosteroids function as anti-inflammatory and immunosuppressive agents. Although the mechanism of action is not fully understood, corticosteroids suppress local inflammation by down-regulating arachidonic acids and prostaglandins 43, leading to vasoconstriction 49. The clinical effects of corticosteroids have been reviewed extensively 50. In general, IA injection of corticosteroids effectively reduces pain up to 3 weeks post injection, while the effect diminishes after longer periods 51. Yet, no conclusion can be drawn to confirm the improvement in joint function, measured in terms of walking distance, range of motion and several scoring systems.
IA injected HA has been proposed to alleviate OA in many aspects. Physically, it acts as a viscoelastic lubricant at low shear rates (resting and walking) and an energy absorber at high shear rates (exercising) 52. Exogenous HA also mediates inflammation, regulates nerve sensitivity and enhances synthesis of proteoglycans and HA.53 The outcome from clinical trials of IA injection of hyaluronic acid has been systematically reviewed.48 When combining the results from multiple clinical trials, the injection of HA did not improve function of joints, at any time point. Like corticosteroids, the benefits of HA injection were limited to pain reduction in patients after exercise, but not pain at rest. In general, the pain-relieving effects of HA are not significant during the first 5 weeks post-injection, but become significant after 10 weeks. Therefore, it is widely believed that the effect of HA has a delayed initiation and prolonged duration, compared with corticosteroids. Although HA of higher molecular weight (MW) was suggested to be more effective in alleviating OA from in vitro and in vivo models 54, no difference between high and low MW HA was observed in clinical trials 48, 54.
Another current IA injectable medication for OA that has recently emerged is Platelet-Rich Plasma (PRP) 55. PRP can be separated from a patient’s blood via centrifugation and injected back into the affected site and potentially increase the rate of healing because of its high concentration of growth factors. In the past, PRP was primarily used to treat chronic tendon injuries, but more recently, it is being used to treat acute tendon and ligament injuries as well as chronic knee OA. A recent review discussed how PRP reduces pain and improves function significantly better than HA in OA patients 56. Further research is still required to fully understand PRP’s effects in treating OA.
Improving the efficacy of injectable treatments with biomaterials
Although IA injections of drugs offer high potency and minimal systemic side effects compared to oral administration, a clear limitation of IA injections is the lack of retention that leads to a decrease in efficacy. Corticosteroids and hyaluronic acid, for example have half lives in suspension of 1 to 2 hours and 22-26 hours, respectively 57. These problems partly arise because of the physical and electrical barriers that the articular cartilage extracellular matrix provides to the chondrocytes. Specifically, the dense collagen II network, the avascularity, and the negatively charged aggrecan molecules may prevent entrance of therapeutic agents into the cartilage matrix 58. In addition, the synovial membrane surrounding the intra-articular joint space creates a compartment that can retain molecules larger than 100 kDa 59 , and therefore many small molecules such as NSAIDs and other therapeutics can rapidly leak out of the joint space. Therefore, a large research area has emerged in creating biomaterials to improve the delivery to and retention times in the articular cartilage or synovial joint space, and thus increasing the efficacy of drugs delivered via IA injections 60. While biomaterials at multiple length scales (nm – cm) have been considered for this application, this review particularly focuses on nanoparticles because the nanoscale dimension: (i) provides for increased penetration and retention of the carrier in the joint (ii) is smaller than micron-sized particles that are easily phagocytosed by resident cells and elicit inflammation; and (iii) allows simple delivery via IA injections for local administration.
Nanoscale delivery systems for OA
At present, nanoparticles (NPs) are the most common form of new biomaterials being tested for IA delivery of drugs for OA treatment. A major advantage of NPs is the ability to design and target delivery locally to the cells and tissues of the joint. Furthermore, drugs and proteins loaded onto NPs can be used to slow disease progression. A wide range of NPs have been implemented to treat OA via IA injection (Table 1). The following sections will summarize modifications to NPs to enhance their retention in the joint, and the proteins and drugs being loaded onto NPs to treat OA.
Table 1.
Nanoparticle size range used for intra-articular injection to treat OA.
| Material description | Size | Refs. |
|---|---|---|
| Chitosan, berberine chloride (BBR) | 50 - 400 nm | (Zhou et al., 2015) |
| Hyaluronic acid, bovine serum albumin, brucine | 150 nm | (Chen et al., 2013) |
| Neutral core, cationic surface (e.g. ethyl cellulose, Eudagrit RL100) | 100 - 150 nm | (Morgen et al., 2013) |
| Tristearin, hydrogenated soya phosphatidylcholine, Aceclofenac, chondroitin sulfate (CS) | 140 - 160nm | (Bishnoi, Jain, Hurkat, & Jain, 2014) |
| Chitosan, Kartogenin | 150 nm and 1800 nm | (Kang, Ko, Kim, & Im, 2014) |
| Chitosan chloride, hyaluronic acid, salmon-calcitonin | 160 - 230 nm | (Ryan et al., 2013) |
| Tetraethylene glycol methacrylate, cyclohexyl methacrylate | 270 nm | (Whitmire et al., 2012) |
| PLGA, brucine | 250 – 260 nm and 1250 nm | (Chen et al., 2014) |
| Soya lechitin, diacerein, Chondoritin sulfate | 390 - 400 nm | (Jain et al., 2014) |
| Pyridine - Poly(2-hydroxyethyl methacrylate), IL-1Ra/model protein | 300, 500, and 900 nm (tunable) | (Singh et al., 2014) (Agarwal et al., 2015) |
Articular Cartilage Targeting
A major benefit of NPs is their ability to attach and bind to specific constituents of articular cartilage and thus, improve retention time within the joint. However, many proteins have the capability of binding to articular cartilage, and each protein interacts with cartilage differently. For example, some proteins may bind to ligands on chondrocytes directly, while others may bind to collagen, aggrecan, or hyaluronic acid. Ultimately, the targeting should be geared toward the aspect of cartilage that the drug will modify.
Cartilage-specific targeting has been used in only a few studies analyzing the efficacy of NPs in IA delivery to articular cartilage. One molecule that has been used multiple times as a homing mechanism is chondroitin sulfate (CS) 61, 62. This polysaccharide not only serves to target articular cartilage via certain binding motifs, but may also serve as a potential therapeutic agent. A recent review explains the variety of beneficial in vitro effects of CS, including increased collagen II and proteoglycan synthesis and reductions in pro-inflammatory cytokines 16. Furthermore, CS slightly reduced joint space narrowing and is recommended as an OA therapeutic by the Osteoarthritis Research Society International (OARSI)38 and the European League Against Rheumatism (EULAR) 63. However, CS also binds certain aspects of cells within cartilage, a key feature for NP delivery. For example, evidence suggests that CS binds cell-surface molecules including L-selectin, P-selectin, and CD44 64. CS also binds to annexin 6 65, a binding protein found on the surface of a variety of cells. Overall, CS has potential to improve IA NP delivery to the articular cartilage because of its ability to bind to chondrocytes.
Another potential articular cartilage targeting technique involves phage display biopanning. Recently, a chondrocyte-affinity peptide (CAP), was identified via affinity peptide biopanning 66. Gene vehicles can be modified by this peptide to allow for specific transfection into chondrocytes. Although CAP has not been extensively studied like CS has, its ability to aid in NP delivery to prevent OA progression has recently been investigated 67. CAP has shown superior cartilage transfection efficiency compared to randomly selected peptides 66. NPs combining CAP and a gene therapeutic were tested for treatment of OA in a surgically induced mouse OA model 67. CAP nanoparticles helped to inhibit cartilage matrix breakdown via silencing of catabolic factors and reduced non-specific toxicity of the gene therapeutic because of its ability to target articular cartilage. Based on the first few studies examining CAP, the peptide seems to be a promising agent in delivering gene therapies to articular cartilage.
A third method to improve articular cartilage targeting of NPs involves forming links with articular cartilage based on its electric charge. Because hyaluronate in the synovial cavity is negatively charged, a cationic polymeric NP was fabricated and injected into healthy rat knee joints to investigate whether the opposite charges would lead to longer retention of NPs 68. Indeed, the anionic hyaluronate and cationic NP interacted to form an “ionically cross-linked hydrogel.” Compared to a bolus drug mimic, targeted NP delivery greatly improved retention time of fluorescently labeled drug mimics. Future work with this method of targeting will need to investigate how the hydrogel affects the biomechanics of the knee and how drugs will behave in such conditions.
Treatments that prevent OA progression used in NP systems
A wide variety of OA drugs have been delivered via NPs in preclinical animal models. The following sections will briefly review the potential therapeutic effects of combining these drugs with NP delivery. To date, the primary categories of drugs delivered via NP systems are those that inhibit inflammation, those that protect chondrocytes from apoptosis or promote chondrocyte differentiation, and those that benefit from NP delivery to limit toxic systemic side effects. Although numerous drugs are being considered for IA delivery by NPs, in general their ability to prevent and/or treat OA-like symptoms is promising.
Drugs that inhibit inflammation
Hyaluronic acid (HA) is a primary candidate for delivery via NPs, because of its known clinical efficacy, but limited retention times, as previously discussed. Indeed, HA has been used with NPs, in combination with another drug, salmon calcitonin 69. Salmon calcitonin benefits cartilage metabolism and inhibits collagen breakdown via inhibition of MMP 70. NPs with both HA and salmon calcitonin were IA injected into mouse knee joints subjected to inflammatory arthritis using a chemically-induced model (K/BxN) 71. This model, however, causes induction of rapid acute inflammation rather than chronic inflammation associated with OA. Therefore, this model is suitable for determining whether an acute inflammatory response could be prevented.The NPs loaded with both salmon calcitonin and HA successfully reduced joint inflammation, preserved bone architecture, and decreased inflammatory gene expression, highlighting the powerful anti-inflammatory effects of these drugs with NP delivery. A similar in vivo NP study showed that HA increased chondrocyte targeting and retention time of NPs in healthy rat knees 72, suggesting that HA can potentially be used both for targeting and therapy, similar to CS. Future work with HA-loaded NPs should focus on its efficacy in treating and/or targeting OA-affected joints using a validated OA animal model.
IL-1 inhibitors are another category of drugs delivered by NPs to reduce inflammation. Interleukin-1 receptor antagonist (IL-1Ra, 17 kDa) is a natural and powerful anti-inflammatory mediator and reduces inflammation associated with OA 73. Many initial studies targeted interleukin-1β (IL-1β) based on its potent catabolic and pro-inflammatory activities 73. Several approaches were employed, including the use of IL-1 specific antibodies or the administration of the IL-1 receptor antagonist (IL-1Ra) that blocks IL-1 receptor signaling. In addition, IL-1Ra was injected into mice subjected to PTOA using an intra-articular fracture model 74, 75. The injections were administered both systemically and IA. In this case, IA injections of IL-1Ra were successful in reducing cartilage degeneration and synovial inflammation after IA fracture. In clinical trials, IA injection of IL-1Ra into the osteoarthritic knee was well-tolerated without any acute inflammatory reaction 76; however, improvements in OA symptoms were not observed between experimental and placebo subjects. The variable results with IL-1Ra in both human and animal studies have been attributed in part to the short half-life of IL-1Ra in the joint and the challenging kinetics of IL-1 receptor interactions that may require sustained IL-1Ra receptor occupancy 77.
Strategies to improve the residence lifetime of IL-1Ra (and other drugs) in the joint are critical to the clinical effectiveness of these therapeutics 78-80. Gene therapy-based approaches for expression of IL-1Ra in the joint reduce inflammation and OA progression 81-85. Whitmire et al 40 recently reported self-assembling NPs presenting IL-1Ra for enhanced delivery, retention and bioactivity in the knee joint of healthy rats (Figure 4). The study reported RAFT-chemistry-based self-assembled NPs (300 nm) that efficiently bound IL-1Ra, targeted synoviocytes that were located inside joints in the synovium, and inhibited IL-1β mediated signaling 40. IL-1Ra delivered bound to 300-nm nanoparticles was retained significantly longer in the rat stifle joint compared to soluble IL-1Ra, without any adverse effect on the cartilage structure in the joint (Figure 4A). This study showed that IL-1Ra NPs are indeed capable of blocking IL-1β activity in response to NF-κβ signaling activation in vitro. In another study, Singh et al 86 engineered nanoparticles made of PHEMA modified with hydrophobic pyridine (Figure 4B). The unique hydrophilic/hydrophobic balance of properties of the polymer allowed for precise control of the size of NPs in the 300-900 nm range and demonstrated a size dependent retention of protein-nanoparticle in a healthy rat knee joint with 900 nm protein particles localized to the knee for at least 14 days compared to depletion of bolus protein within hours of injection. These studies highlight the need for localized delivery of NPs to prolong the retention of IL-1Ra in the joint. Furthermore, IL-1Ra-based NPs need to be evaluated in clinically-relevant OA animal models to determine their effectiveness in preventing and/or treating OA.
Figure 4. Injectable, protein tethering nano-biomaterials.
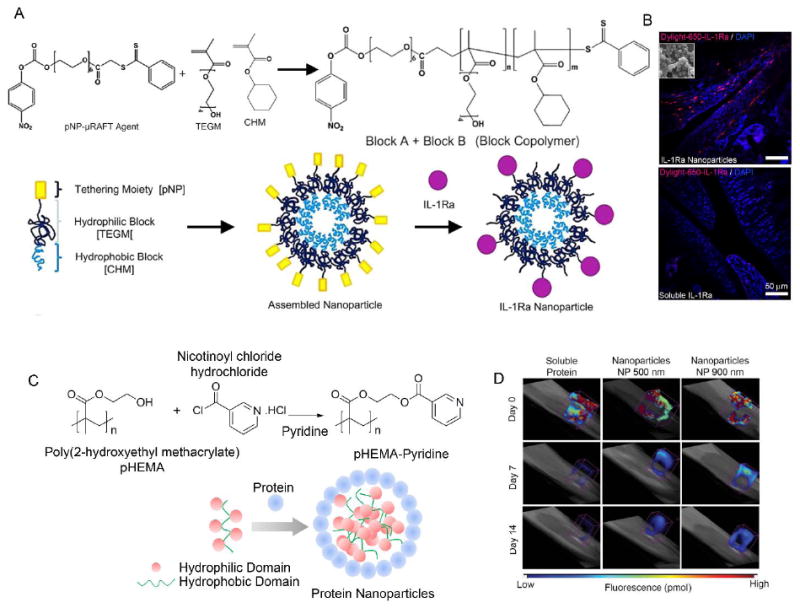
A) Protein tethering block copolymer. A modified commercial RAFT agent (m-RAFT) was used for polymerization. Schematic of self-assembly of the nanoparticles and protein conjugation. B) IL-1Ra-tethered particles are distributed throughout the intra-articular joint space. IL-1Ra was tagged with a Dylight-IR-650 dye prior to tethering IL-1Ra to particles. Tagged IL-1Ra-tethered particles or soluble IL-1Ra was injected into the right stifle joint of 8–10 wk old rats while the left stifle joints received saline. Cryosectioned samples were counterstained with DAPI to localize dye tagged protein. Scale bar = 50 μm. C) Protein complexed pHEMA–pyridine nanoparticles. pHEMA–pyridine was synthesized by reacting pHEMA with nicotinoyl chloride hydrochloride in tetrahydrofuran and pyridine. Schematic of nanoparticle self-assembly based on protein/polymer complexation. D) Nanoparticle size controls retention in the intra-articular space in rat joints as shown in fluorescence molecular tomography of rat stifle joint injected with bolus VivoTag-S 750-BSA protein and nanoparticle-complexed protein. VivoTag-S 750-BSA-particles with 900 nm size show sustained signal compared to 500 nm nanoparticles and soluble BSA. Reproduced with permission 86,40.
The final category of drugs that inhibit inflammation used in NP studies is NSAIDs. In general, NSAIDs act as analgesics by inhibiting prostaglandin synthesis and cyclo-oxygenase enzyme (COX) activity, substances that cause pain and lead to other inflammatory processes 87. To investigate whether NSAID-loaded NPs could reduce OA, solid lipid NPs with an NSAID (aceclofenac) with and without CS to target articular cartilage were injected intravenously (IV) into rats with chemically-induced OA using the MIA model 61. The NPs with CS specifically targeted the inflamed knee joints inhibited edema to a greater degree than plain drug alone. This approach may further be improved by using IA injection rather than IV to deliver the NPs to the articular cartilage.
Drugs that protect chondrocytes
Preserving chondrocyte health is a major objective of OA treatment, as chondrocytes are responsible for producing and maintaining matrix components for the structural integrity of cartilage. At the onset of disease, chondrocytes begin releasing degradative and/or inflammatory enzymes and eventually undergo apoptosis. Therefore, either preventing chondrocyte apoptosis or producing more chondrocytes in later disease stages are excellent options for OA prevention and/or treatment.
Anti-apoptotic drugs may be useful in the treatment of OA. For example, Brucine is an anti-apoptotic alkaloid. Brucine may aid in cartilage regeneration by inhibiting apoptosis and acting as an antagonist to nitric oxide, an inhibitor of chondrocyte proliferation 72. However, brucine is severely toxic to the central nervous system and, therefore, requires local delivery to the joint. To deliver locally, brucine has been applied to multiple NP systems and injected into healthy rat knees 72, 88. NPs consistently increased IA retention times of the drug. However, the effect of the brucine-NP system on disease progression was not investigated. On the other hand, studies involving berberine-NP systems have shown efficacy in the drug’s anti-apoptotic effects when properly delivered to the joint 89. Specifically, berberine chloride was cross-linked with chitosan to form NPs. The NPs were injected into rat knees with surgically-induced OA using combined ACL transection and medial meniscus resection. Berberine-loaded NPs significantly reduced apoptosis and prevented OA progression after surgery and were more effective than berberine chloride alone. Thus, specific prevention of apoptosis in articular chondrocytes may be useful in OA treatment. However, the OA model used here induces severe instability in the knee joint due to removal of both the ACL and medial meniscus and most likely represents severe PTOA. Thus, further studies need to be performed to determine whether anti-apoptotic effects are useful in slower OA progression models.
In addition to preventing chondrocyte apoptosis, promoting chondrocyte proliferation may also be a viable option for OA treatment. Kartogenin selectively differentiates mesenchymal stem cells (MSCs) into chondrocytes and may benefit from local delivery to the joint. To test this principle, kartogenin was conjugated to chitosan to make NPs and microparticles (MPs) 90. In vitro kartogenin differentiated MSCs into chondrocytes better in NPs than MPs. However, the MPs showed longer retention times in rat knee joints with surgically-induced OA using ACL transection. Both NPs and MPs successfully prevented articular cartilage degeneration in the ACL-transected rat knee joints and were remarkably more successful than unconjugated kartogenin. Therefore, drugs that have anti-apoptotic and pro-proliferative effects may also aid in PTOA prevention and/or OA treatment.
Drugs that require local delivery to limit toxicity
Many medications with systemic toxicity could benefit from local IA injection with NPs. Of the drugs already discussed, brucine can be severely toxic to the central nervous system, and prolonged use of NSAIDs can irritate the gastrointestinal tract and adversely affect the cardiovascular system. Therefore, local delivery to the joint may be helpful in limiting the harmful systemic effects for these drugs. Additionally, certain gene therapies may benefit strongly from local delivery to overcome the cytotoxic side effects of systemic delivery. For example, Hif-2α is known to directly induce chondrocytes to release harmful collagenases, aggrecanases, and reactive oxygen species 91. Biomaterials-based small-interfering RNA (siRNA) delivery systems targeting Hif-2α can slow down OA progression. A recent study with cationic, chondrocyte-homing NPs of polyethylenimine and Hif-2α siRNA effectively slowed down OA progression and reduced IL-1β levels 67 in a surgically-induced mouse knee joint. The surgery involved dissection of the ACL, MCL, and the anterior horn of the medial meniscus. Although the protective effects of anti- Hif-2α siRNA were evident in this study, limitations included the high severity of the OA model and the cytotoxicity of polyethylenimine, both of which limit the application of this approach. In addition, gene therapy by itself may have detrimental effects and more work is needed before gene therapy can be safely translated for OA treatment.
Summary of Treatments and Targets used in NP delivery systems
Preclinical studies clearly demonstrate that NP delivery systems can be effective for OA treatments. By combining both NPs and cartilage targeting techniques, drugs for OA are able to perform effectively, remain in the joint longer, and can potentially avoid systemic side effects. A variety of drugs are being tested with NPs and multiple targeting techniques exist, but the most effective methods remain to be determined and may depend on the specific therapeutic. In addition, the best techniques may be patient-dependent and vary with disease stage. Overall, drugs, tissue-targeting techniques, and biomaterials must continue to be developed and tested in vivo using appropriate animal models to determine the most effective, safest and easiest-to-adopt treatments.
Conclusions
Biomaterials have the ability to better target the articular cartilage by incorporating targeting ligands and improve retention in joints by means of their size, chemical properties and bio-functionalities. A wide variety of therapeutics can be incorporated into nanoparticles and often multiple drugs can be delivered simultaneously. Many nanoparticle systems have shown efficacy in inhibiting OA progression by locally delivering drugs that primarily prevent chondrocyte apoptosis or inhibit inflammation. Despite the success, a better understanding of OA pathology and appropriate preclinical testing is necessary to understand the behavior of biomaterials in vivo. Many studies to date have tested biomaterials-based delivery platforms in healthy joints; however, it is critical to perform this testing in diseased joints using clinically relevant animal models. It is possible that the release kinetics and degradation process of biomaterials may change under pathological conditions, for example with the release of inflammatory cytokines and proteases or with infiltration of immune cells. Many animal models for OA exist and careful consideration must be taken to match the preclinical model with the mechanism whereby the biomaterial inhibits joint degradation or relieves pain. While this review covered several popular mouse models of OA, there are others that may be of importance. For example, a mouse intra-articular fracture model of post-traumatic OA uses an indenting tool to create a fracture in the lateral tibial plateau that closely mimics clinically observed tibial plateau fractures in humans 74. Because this model causes an intra-articular fracture, the geometry of the knee joint changes drastically, and therefore, biomaterials may function differently in the altered joint geometry than they would under normal OA conditions. Although there are certain limitations associated with mouse models of OA as compared to large animal models, increased use of mouse models, in conjunction with bioengineered platforms, will undoubtedly contribute substantially to our understanding of OA pathology, initiation, and progression, possibly enable development of disease-modifying drugs for OA, as well as pave the path for development of biomaterials-based treatment options with minimal intervention of the diseased joints.
Acknowledgments
The authors would like to thank the following funding agencies for supporting their research that was discussed in the review: NIH. Grant Numbers: R21-AR064034, R01-AG-022021, RC4-AR-060546, R01-AG-028664, P30-AR-046121. The authors would like to thank Dr. Mathias P. Bostrom at Hospital for Special Surgery, New York, for providing a de-identified knee joint image.
References
- 1.Lawrence RC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 3.Hunter DJ, Felson DT. Osteoarthritis. Bmj. 2006;332:639–642. doi: 10.1136/bmj.332.7542.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felson DT. Risk factors for osteoarthritis: understanding joint vulnerability. Clin Orthop Relat Res. 2004:S16–21. doi: 10.1097/01.blo.0000144971.12731.a2. [DOI] [PubMed] [Google Scholar]
- 5.Yasuda T. Cartilage destruction by matrix degradation products. Mod Rheumatol. 2006;16:197–205. doi: 10.1007/s10165-006-0490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986;322:547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Goldring MB, et al. Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J Clin Invest. 1994;94:2307–2316. doi: 10.1172/JCI117595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ismail HM, et al. JNK2 controls aggrecan degradation in murine articular cartilage and the development of experimental osteoarthritis. Arthritis Rheumatol. 2015 doi: 10.1002/art.39547. [DOI] [PubMed] [Google Scholar]
- 10.Fonseca JE, Santos MJ, Canhao H, Choy E. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev. 2009;8:538–542. doi: 10.1016/j.autrev.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 12.Imamura M, et al. Concentration of cytokines in patients with osteoarthritis of the knee and fibromyalgia. Clin Interv Aging. 2014;9:939–944. doi: 10.2147/CIA.S60330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loeser RF. Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 2006;54:1357–1360. doi: 10.1002/art.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abramson SB. Osteoarthritis and nitric oxide. Osteoarthritis Cartilage. 2008;16(Suppl 2):S15–20. doi: 10.1016/S1063-4584(08)60008-4. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg M, Chevalier X, Henrotin Y, Hunter DJ, Uebelhart D. Symptom and structure modification in osteoarthritis with pharmaceutical-grade chondroitin sulfate: what’s the evidence? Curr Med Res Opin. 2013;29:259–267. doi: 10.1185/03007995.2012.753430. [DOI] [PubMed] [Google Scholar]
- 16.Henrotin Y, Marty M, Mobasheri A. What is the current status of chondroitin sulfate and glucosamine for the treatment of knee osteoarthritis? Maturitas. 2014;78:184–187. doi: 10.1016/j.maturitas.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Steinbeck MJ, Nesti LJ, Sharkey PF, Parvizi J. Myeloperoxidase and chlorinated peptides in osteoarthritis: potential biomarkers of the disease. J Orthop Res. 2007;25:1128–1135. doi: 10.1002/jor.20400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deberg M, et al. One-year follow-up of Coll2-1, Coll2-1NO2 and myeloperoxydase serum levels in osteoarthritis patients after hip or knee replacement. Ann Rheum Dis. 2008;67:168–174. doi: 10.1136/ard.2007.073452. [DOI] [PubMed] [Google Scholar]
- 19.Cook AD, et al. Granulocyte-macrophage colony-stimulating factor is a key mediator in experimental osteoarthritis pain and disease development. Arthritis Res Ther. 2012;14:R199. doi: 10.1186/ar4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang K, Wu LD. Aggrecanase and aggrecan degradation in osteoarthritis: a review. J Int Med Res. 2008;36:1149–1160. doi: 10.1177/147323000803600601. [DOI] [PubMed] [Google Scholar]
- 21.Yang CC, Lin CY, Wang HS, Lyu SR. Matrix Metalloproteases and Tissue Inhibitors of Metalloproteinases in Medial Plica and Pannus-like Tissue Contribute to Knee Osteoarthritis Progression. PLoS One. 2013;8:e79662. doi: 10.1371/journal.pone.0079662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little CB, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyce BF, Xing L. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep. 2007;5:98–104. doi: 10.1007/s11914-007-0024-y. [DOI] [PubMed] [Google Scholar]
- 24.Rousseau J, Garnero P. Biological markers in osteoarthritis. Bone. 2012;51:265–277. doi: 10.1016/j.bone.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Bajpayee AG, Scheu M, Grodzinsky AJ, Porter RM. A rabbit model demonstrates the influence of cartilage thickness on intra-articular drug delivery and retention within cartilage. J Orthop Res. 2015;33:660–667. doi: 10.1002/jor.22841. [DOI] [PubMed] [Google Scholar]
- 26.McCoy AM. Animal Models of Osteoarthritis: Comparisons and Key Considerations. Vet Pathol. 2015;52:803–818. doi: 10.1177/0300985815588611. [DOI] [PubMed] [Google Scholar]
- 27.Fang H, Beier F. Mouse models of osteoarthritis: modelling risk factors and assessing outcomes. Nat Rev Rheumatol. 2014;10:413–421. doi: 10.1038/nrrheum.2014.46. [DOI] [PubMed] [Google Scholar]
- 28.Bove SE, et al. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage. 2003;11:821–830. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 29.Barve RA, et al. Transcriptional profiling and pathway analysis of monosodium iodoacetate-induced experimental osteoarthritis in rats: relevance to human disease. Osteoarthritis Cartilage. 2007;15:1190–1198. doi: 10.1016/j.joca.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 31.Pond MJ, Nuki G. Experimentally-induced osteoarthritis in the dog. Ann Rheum Dis. 1973;32:387–388. doi: 10.1136/ard.32.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christiansen BA, et al. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2012;20:773–782. doi: 10.1016/j.joca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Ko FC, et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 2013;65:1569–1578. doi: 10.1002/art.37906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poulet B, et al. Intermittent applied mechanical loading induces subchondral bone thickening that may be intensified locally by contiguous articular cartilage lesions. Osteoarthritis Cartilage. 2015;23:940–948. doi: 10.1016/j.joca.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onur TS, et al. Joint instability and cartilage compression in a mouse model of posttraumatic osteoarthritis. J Orthop Res. 2014;32:318–323. doi: 10.1002/jor.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Killian ML, et al. Traumatic anterior cruciate ligament tear and its implications on meniscal degradation: a preliminary novel lapine osteoarthritis model. J Surg Res. 2010;164:234–241. doi: 10.1016/j.jss.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Hochberg MC, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthrit Care Res. 2012;64:465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Jevsevar DS. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline, 2nd Edition. J Am Acad Orthop Sur. 2013;21:571–576. doi: 10.5435/JAAOS-21-09-571. [DOI] [PubMed] [Google Scholar]
- 40.Whitmire RE, et al. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials. 2012;33:7665–7675. doi: 10.1016/j.biomaterials.2012.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta M, Eisen GM. NSAIDs and the gastrointestinal tract. Current gastroenterology reports. 2009;11:345–353. doi: 10.1007/s11894-009-0053-z. [DOI] [PubMed] [Google Scholar]
- 42.Bannuru R, Natov N, Dasi U, Schmid C, McAlindon T. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis–meta-analysis. Osteoarthritis and cartilage. 2011;19:611–619. doi: 10.1016/j.joca.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Hong SL, Levine L. Inhibition of arachidonic acid release from cells as the biochemical action of anti-inflammatory corticosteroids. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:1730–1734. doi: 10.1073/pnas.73.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang F, He X. Intra-articular hyaluronic acid and corticosteroids in the treatment of knee osteoarthritis: A meta-analysis. Exp Ther Med. 2015;9:493–500. doi: 10.3892/etm.2014.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monticone M, et al. Hyaluronic acid intra-articular Injection and exercise therapy: effects on pain and disability in subjects affected by lower limb joints osteoarthritis. The Italian Society of Physical and Rehabilitation Medicine (SIMFER) systematic review. Eur J Phys Rehabil Med. 2015 [PubMed] [Google Scholar]
- 46.Miller LE, Block JE. US-Approved Intra-Articular Hyaluronic Acid Injections are Safe and Effective in Patients with Knee Osteoarthritis: Systematic Review and Meta-Analysis of Randomized, Saline-Controlled Trials. Clin Med Insights Arthritis Musculoskelet Disord. 2013;6:57–63. doi: 10.4137/CMAMD.S12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang KV, Hsiao MY, Chen WS, Wang TG, Chien KL. Effectiveness of intra-articular hyaluronic acid for ankle osteoarthritis treatment: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2013;94:951–960. doi: 10.1016/j.apmr.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 48.Arrich J, et al. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. Cmaj. 2005;172:1039–1043. doi: 10.1503/cmaj.1041203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams TJ, Peck MJ. Role of Prostaglandin-Mediated Vasodilatation in Inflammation. Nature. 1977;270:530–532. doi: 10.1038/270530a0. [DOI] [PubMed] [Google Scholar]
- 50.Bellamy N, et al. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Db Syst Rev. 2005 doi: 10.1002/14651858.CD005328. [DOI] [PubMed] [Google Scholar]
- 51.Kruse DW. Intraarticular cortisone injection for osteoarthritis of the hip. Is it effective? Is it safe? Curr Rev Musculoskelet Med. 2008;1:227–233. doi: 10.1007/s12178-008-9029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balazs EA, Denlinger JL. Viscosupplementation - a New Concept in the Treatment of Osteoarthritis. J Rheumatol. 1993;20:3–9. [PubMed] [Google Scholar]
- 53.Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5:54–67. doi: 10.1186/ar623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Seminars in arthritis and rheumatism. 2002;32:10–37. doi: 10.1053/sarh.2002.33720. [DOI] [PubMed] [Google Scholar]
- 55.Kon E, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy. 2011;27:1490–1501. doi: 10.1016/j.arthro.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of Intra-articular Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Systematic Review. Arthroscopy. 2016;32:495–505. doi: 10.1016/j.arthro.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Coleman PJ, Scott D, Ray J, Mason RM, Levick JR. Hyaluronan secretion into the synovial cavity of rabbit knees and comparison with albumin turnover. J Physiol. 1997;503(Pt 3):645–656. doi: 10.1111/j.1469-7793.1997.645bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothenfluh DA, Bermudez H, O’Neil CP, Hubbell JA. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat Mater. 2008;7:248–254. doi: 10.1038/nmat2116. [DOI] [PubMed] [Google Scholar]
- 59.Perman V. Clinical biochemistry of domestic animals. 3. Academic Press; 1980. [Google Scholar]
- 60.Kang ML, Im GI. Drug delivery systems for intra-articular treatment of osteoarthritis. Expert Opin Drug Deliv. 2014;11:269–282. doi: 10.1517/17425247.2014.867325. [DOI] [PubMed] [Google Scholar]
- 61.Bishnoi M, Jain A, Hurkat P, Jain SK. Aceclofenac-loaded chondroitin sulfate conjugated SLNs for effective management of osteoarthritis. J Drug Target. 2014;22:805–812. doi: 10.3109/1061186X.2014.928714. [DOI] [PubMed] [Google Scholar]
- 62.Jain A, et al. Targeting of diacerein loaded lipid nanoparticles to intra-articular cartilage using chondroitin sulfate as homing carrier for treatment of osteoarthritis in rats. Nanomedicine. 2014;10:1031–1040. doi: 10.1016/j.nano.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 63.Zhang W, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Annals of the rheumatic diseases. 2005;64:669–681. doi: 10.1136/ard.2004.028886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawashima H, et al. Binding of a large chondroitin sulfate/dermatan sulfate proteoglycan, versican, to L-selectin, P-selectin, and CD44. J Biol Chem. 2000;275:35448–35456. doi: 10.1074/jbc.M003387200. [DOI] [PubMed] [Google Scholar]
- 65.Takagi H, Asano Y, Yamakawa N, Matsumoto I, Kimata K. Annexin 6 is a putative cell surface receptor for chondroitin sulfate chains. J Cell Sci. 2002;115:3309–3318. doi: 10.1242/jcs.115.16.3309. [DOI] [PubMed] [Google Scholar]
- 66.Pi Y, et al. Targeted delivery of non-viral vectors to cartilage in vivo using a chondrocyte-homing peptide identified by phage display. Biomaterials. 2011;32:6324–6332. doi: 10.1016/j.biomaterials.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 67.Pi Y, et al. Intra-articular delivery of anti-Hif-2alpha siRNA by chondrocyte-homing nanoparticles to prevent cartilage degeneration in arthritic mice. Gene Ther. 2015;22:439–448. doi: 10.1038/gt.2015.16. [DOI] [PubMed] [Google Scholar]
- 68.Morgen M, et al. Nanoparticles for improved local retention after intra-articular injection into the knee joint. Pharm Res. 2013;30:257–268. doi: 10.1007/s11095-012-0870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ryan SM, et al. An intra-articular salmon calcitonin-based nanocomplex reduces experimental inflammatory arthritis. J Control Release. 2013;167:120–129. doi: 10.1016/j.jconrel.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 70.Esenyel M, Icagasioglu A, Esenyel CZ. Effects of calcitonin on knee osteoarthritis and quality of life. Rheumatol Int. 2013;33:423–427. doi: 10.1007/s00296-012-2399-z. [DOI] [PubMed] [Google Scholar]
- 71.Rampersad RR, et al. S100A9 is not essential for disease expression in an acute (K/BxN) or chronic (CIA) model of inflammatory arthritis. Scand J Rheumatol. 2009;38:445–449. doi: 10.3109/03009740902895743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Z, et al. Hyaluronic acid-coated bovine serum albumin nanoparticles loaded with brucine as selective nanovectors for intra-articular injection. Int J Nanomedicine. 2013;8:3843–3853. doi: 10.2147/IJN.S50721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cunnane G, Madigan A, Murphy E, FitzGerald O, Bresnihan B. The effects of treatment with interleukin-1 receptor antagonist on the inflamed synovial membrane in rheumatoid arthritis. Rheumatology (Oxford) 2001;40:62–69. doi: 10.1093/rheumatology/40.1.62. [DOI] [PubMed] [Google Scholar]
- 74.Furman BD, et al. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res. 2007;25:578–592. doi: 10.1002/jor.20331. [DOI] [PubMed] [Google Scholar]
- 75.Furman BD, et al. Targeting pro-inflammatory cytokines following joint injury: acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res Ther. 2014;16:R134. doi: 10.1186/ar4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chevalier X, et al. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J Rheumatol. 2005;32:1317–1323. [PubMed] [Google Scholar]
- 77.Martel-Pelletier J, Wildi LM, Pelletier JP. Future therapeutics for osteoarthritis. Bone. 2012;51:297–311. doi: 10.1016/j.bone.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 78.Chevalier X. Intraarticular treatments for osteoarthritis: new perspectives. Curr Drug Targets. 2010;11:546–560. doi: 10.2174/138945010791011866. [DOI] [PubMed] [Google Scholar]
- 79.Iqbal I, Fleischmann R. Treatment of osteoarthritis with anakinra. Curr Rheumatol Rep. 2007;9:31–35. doi: 10.1007/s11926-007-0019-9. [DOI] [PubMed] [Google Scholar]
- 80.Malemud CJ. Anticytokine therapy for osteoarthritis: evidence to date. Drugs Aging. 2010;27:95–115. doi: 10.2165/11319950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 81.Bakker AC, et al. Prevention of murine collagen-induced arthritis in the knee and ipsilateral paw by local expression of human interleukin-1 receptor antagonist protein in the knee. Arthritis Rheum. 1997;40:893–900. doi: 10.1002/art.1780400517. [DOI] [PubMed] [Google Scholar]
- 82.Pelletier JP, et al. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997;40:1012–1019. doi: 10.1002/art.1780400604. [DOI] [PubMed] [Google Scholar]
- 83.Kay JD, et al. Intra-articular gene delivery and expression of interleukin-1Ra mediated by self-complementary adeno-associated virus. J Gene Med. 2009;11:605–614. doi: 10.1002/jgm.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gouze E, et al. Lentiviral-mediated gene delivery to synovium: potent intra-articular expression with amplification by inflammation. Mol Ther. 2003;7:460–466. doi: 10.1016/s1525-0016(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 85.Fernandes J, et al. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: prevention of osteoarthritis progression. Am J Pathol. 1999;154:1159–1169. doi: 10.1016/S0002-9440(10)65368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh A, et al. Nanoengineered Particles for Enhanced Intra-Articular Retention and Delivery of Proteins. Advanced healthcare materials. 2014;3:1562–1567. doi: 10.1002/adhm.201400051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cashman JN. The mechanisms of action of NSAIDs in analgesia. Drugs. 1996;52(Suppl 5):13–23. doi: 10.2165/00003495-199600525-00004. [DOI] [PubMed] [Google Scholar]
- 88.Chen Z, et al. Development of nanoparticles-in-microparticles system for improved local retention after intra-articular injection. Drug Deliv. 2014;21:342–350. doi: 10.3109/10717544.2013.848495. [DOI] [PubMed] [Google Scholar]
- 89.Zhou Y, et al. In vivo anti-apoptosis activity of novel berberine-loaded chitosan nanoparticles effectively ameliorates osteoarthritis. Int Immunopharmacol. 2015;28:34–43. doi: 10.1016/j.intimp.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 90.Kang ML, Ko JY, Kim JE, Im GI. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials. 2014;35:9984–9994. doi: 10.1016/j.biomaterials.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 91.Yang S, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]


