Abstract
Mutations in sunlight-induced melanoma arise from cyclobutane pyrimidine dimers (CPDs), DNA photoproducts usually created picoseconds after an ultraviolet (UV) photon is absorbed at thymine or cytosine. Surprisingly we found that, in melanocytes, CPDs were generated for hours after UVA or UVB exposure. These “dark CPDs” constituted the majority of CPDs in cultured human and murine melanocytes and in mouse skin, and they were most prominent in skin containing pheomelanin, the melanin responsible for blonde and red hair.
The mechanism was also a surprise. Dark CPDs arise when UV-induced superoxide and nitric oxide combine to form peroxynitrite, one of the few biological molecules capable of exciting an electron. This process, termed “chemiexcitation”, is the source of bioluminescence in lower organisms. Excitation occurred in fragments of melanin, creating a quantum triplet state that had the energy of a UV photon but which induced CPDs by radiationless energy transfer to DNA. UVA and peroxynitrite also solubilized melanin and permeabilized the nuclear membrane, allowing melanin to enter. Melanin is evidently carcinogenic as well as protective. Chemiexcitation may also trigger pathogenesis in internal tissues because the same chemistry should arise wherever superoxide and nitric oxide arise near cells that contain melanin.
Keywords: ultraviolet, sunlight, melanoma, melanin, chemiexcitation, cyclobutane pyrimidine dimer, neurodegeneration
Disease is often traceable to chemistry. The ordinary chemistry familiar from undergraduate courses stems from molecular collisions that excite a molecule's vibrational or rotational states to an energy that allows its bonds to rearrange. Enzymes lower the energy barrier to these reactions. Disease pathogenesis follows from such chemistry, or from ligands that stimulate or inhibit the enzymes, or from foreign cells that introduce ectopic ligands and enzymes. In stark contrast to these chemical reactions, which involve energies of ~3 kcal/mol (0.1 eV), our recent findings call attention to excited-state chemistry, in which an electron is excited to a higher orbital at energies of ~100 kcal/mol (4.5 eV), the same realm reached when a TNT molecule splits or a blue photon strikes a chloroplast.
Excited states have unusual properties (Turro et al., 2010). Not only are the energies high, but the shapes of the molecule's electron orbitals change drastically, altering the spatial constraints on reactions and allowing reactions that cannot take place at room temperature. In addition, only one of the two oppositely-spinning electrons in an electron pair is excited; the now-unpaired electrons resemble two free radicals, which are chemically reactive.
In biology, excited states are typically reached when physics intrudes: a trip to the beach allows an ultraviolet photon to strike the DNA in your arm, be absorbed where two pyrimidines (thymine or cytosine) lie next to each other, and excite an electron to create a “cyclobutane pyrimidine dimer” (CPD). This DNA photoproduct links two adjacent bases and distorts the DNA helix, leading to mutations or cell death. Bioluminescent lower organisms can excite electrons chemically – termed “chemiexcitation” – by using ATP; they then use the excited-state energy to release visible light. Several chemical and enzymatic reactions have been found capable of chemiexcitation without ATP (Adam and Cilento, 1982), but chemiexcitation in mammals had never been found. Recently, we reported that chemiexcitation indeed occurs in the melanocytes of mammals – the cells that give skin and hair their color. It is followed by biophysical transfer of the energy to DNA to make CPDs, and is linked to melanoma (Premi et al., 2015).
Sunlight-induced melanomas contain UV-signature mutations, C→T base substitutions at sites of two adjacent pyrimidines (Krauthammer et al., 2012, Brash, 2015). This location is a sign that the mutations were caused primarily by CPDs in DNA. Typically, CPDs are created after a UVB photon is absorbed, exciting an electron to a “singlet” quantum state in which the electron and its former partner retain their opposite spins. This process takes picoseconds. Alternatively, CPDs can be created after UVA exposure, via a lower-energy “triplet” excited state in which the two electrons have the same spins. This process takes microseconds.
Surprisingly, while studying murine and human melanocytes exposed to UVB or UVA, we discovered that melanocytes generated CPDs for >3 hours after exposure (Premi et al., 2015). This occurred only if the cells contained the pigment melanin. In albino melanocytes, CPDs were made only by the expected picosecond route. The 1016 fold slower timescale with melanin implicated an unusual chemistry. Inhibiting concurrent repair of these “dark CPDs” revealed that 1/2 to 2/3 of the CPDs arise after UV exposure ends. The implication for people is that most of the harmful DNA damage from UV occurs in the car on the way home from the beach. Our initial experiments using an anti-CPD antibody were confirmed by mass spectrometry; this more precise method also revealed that UVA-irradiated cells contained an excess of cytosine-containing CPDs, again indicating that the CPDs were created by an unusual chemistry. In mouse skin, dark CPDs were created in melanocytes and in the keratinocytes to which they donated melanosomes. Dark CPDs were most prominent in skin containing pheomelanin, the melanin responsible for blonde and red hair; people with blonde or red hair are particularly susceptible to melanoma and non-melanoma skin cancer.
The mechanism was also a surprise. Four decades ago, it had been shown that a dioxetane, a strained ring containing two C atoms and two O atoms, could induce CPDs in the dark by transferring excited-state energy directly to DNA (Lamola, 1971). The molecule spontaneously cleaves between the two Os, creating two carbonyls, one of which ends in a high-energy triplet state having the same energy as a UVA photon. While the triplet-state carbonyl does not have enough energy to make CPDs by the usual singlet path, it can transfer its UVA-like energy directly to the lower triplet DNA energy levels – without using a photon. A diagnostic for chemiexcitation is the generation of ultraweak blue chemiluminescence, one of several ways the excited-state energy can be dissipated. Using a photon-counting photomultiplier tube, we found that ultraweak chemiluminescence was indeed generated after UV exposure of melanin-containing melanocytes, but not with albino melanocytes. A research tool developed by the excited-state field is the compound sorbate, which quenches the energy from triplet states without quenching singlet states or acting as an antioxidant. Sorbate blocked dark CPD production.
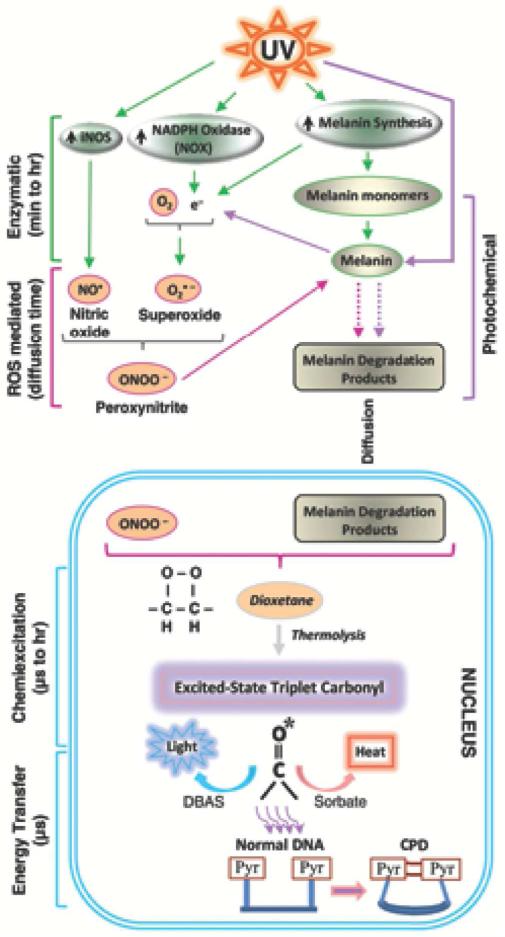
How did the dioxetane arise, and on what molecule? Researchers had encountered two biological molecules capable of exciting an electron to make a triplet-state carbonyl: the powerful oxidant peroxynitrite, and the combination of a peroxidase enzyme plus hydrogen peroxide. Peroxynitrite is formed spontaneously when nitric oxide and superoxide are co-present. Correspondingly, we found that dark CPDs could be prevented by inhibiting iNOS, the enzyme that synthesizes nitric oxide, or by inhibiting NADPH oxidase, which makes superoxide. To identify the molecule carrying the dioxetane, we constructed a cell-free system containing only peroxynitrite (or peroxidase and hydrogen peroxide), DNA, and melanin or a melanin precursor monomer. This system was able to generate both ultraweak chemiluminescence and dark CPDs – with no UV involvement at all. Mass spectrometry revealed a melanin oxidation product consistent with a dioxetane intermediate. Finally, to address the fact that melanin is in the cytoplasm and DNA is in the nucleus, we showed that either UVA or peroxynitrite can solubilize melanin and permeabilize the nuclear membrane, allowing melanin to enter. These steps are summarized in Figure 1.
Figure 1.
Dark CPDs are generated in melanocytes by chemiexcitation after UV exposure ends, with melanin being an active participant. Exposing cells to UV radiation up-regulates iNOS, NADPH oxidase (NOX), and enzymes of melanin synthesis, causing sustained generation of nitric oxide (NO•) and superoxide (O2•−). NOS and NOX isoforms are located in both cytoplasm and nucleus. The two radicals produce the powerful oxidant peroxynitrite (ONOO−), which degrades melanin polymer to fragments that appear in the nucleus. Peroxynitrite is also one of the few biological molecules capable of exciting an electron to a triplet state. On a fast time scale, peroxynitrite excites an electron in a melanin fragment to a triplet state that has the high energy of a UV photon. The typical triplet-state reaction intermediate, not yet demonstrated (hence indicated in italics), is a cycloaddition of –O–O– to create an unstable dioxetane; dioxetanes undergo spontaneous thermolysis to yield two carbonyls, one of which acquires most of the energy and finishes in a high-energy triplet state (denoted by *). For the melanin-related triplet, the half-life of the reaction intermediate appeared to be minutes, and a carbonyl consistent with a dioxetane precursor was identified by mass spectrometry. Triplet energy then discharges on a microsecond time scale to generate visible luminescence, or discharges in a radiation-independent manner to an acceptor (“DBAS”) which re-emits it as fluorescence, to a triplet quencher (“sorbate”) which dissipates it via isomerization and heat, or to DNA bases where it makes CPDs. Figure from Premi et al., 2015.
Therefore, melanin is evidently carcinogenic as well as protective. The process begins when UV induces enzymes that synthesize superoxide and nitric oxide; this is the slow and persistent step. These molecules combine instantly to form peroxynitrite, one of the few biological molecules capable of exciting an electron. Excitation creates a quantum triplet state in the skin pigment melanin that has the energy of a UV photon but induces CPDs by transferring its energy to DNA in a radiation-independent manner. These findings may underlie the dependence of UV-induced and spontaneous skin cancers on melanin type. This carcinogenic effect of melanin seems counter-intuitive because we think of melanin as protecting us from sunlight. It does indeed block sunlight, but at a cost. Melanin may be the best solution that evolution could find: it is beneficial at young ages and the detrimental effects of the mutations it creates appear only at post-reproductive ages that are not a strong factor in evolutionary selection. Moreover, postponing the appearance of CPDs from a bolus at the time of UV exposure to a trickle over the course of hours may allow DNA excision repair systems to keep abreast of the damage.
From a practical point of view, the prolonged time course offers new control points for preventing dark CPDs: blocking enzyme activation, scavenging reactive oxygen and nitrogen species, and quenching triplet-state energy before it can transfer to DNA. Blockers could be administered after sun exposure as well as during it. Chemiexcitation also suggests novel origins for personal differences in melanoma risk, beyond skin darkness and DNA repair: polymorphisms in photoinduced signaling, enzyme induction, scavenging systems, chemical reactivity of different melanin types, and innate triplet-state quenchers.
A pathologist will now ask: “If chemiexcitation is an entirely new mode of tissue damage and disease pathogenesis, can melanoma be the only disease it causes?” The same chemistry should arise wherever superoxide and nitric oxide arise near cells that contain melanin. UV is not needed if superoxide and nitric oxide arise in some other way in an internal tissue, and inflammation does generate both radical species. A new research direction would then be to ask whether inflammation, combined with the melanin present in the substantia nigra, locus ceruleus, cochlea, or macula, leads to chemiexcitation and neurodegeneration in Parkinson's Disease, Alzheimer's Disease, noise-induced deafness, or macular degeneration.
Abbreviations
- CPD
cyclobutane pyrimidine dimer
- iNOS
inducible nitric oxide synthase
- NOX
NADPH oxidase
- UVA
ultraviolet radiation (315-400 nm)
- UVB
ultraviolet radiation (280-315 nm)
References
- Adam W, Cilento G, editors. Chemical and Biological Generation of Excited States. Academic Press; New York: 1982. [Google Scholar]
- Brash DE. UV signature mutations. Photochem. Photobiol. 2015:15–26. doi: 10.1111/php.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, Cheng E, Davis MJ, Goh G, Choi M, Ariyan S, Narayan D, Dutton-Regester K, Capatana A, Holman EC, Bosenberg M, Sznol M, Kluger HM, Brash DE, Stern DF, Materin MA, Lo RS, Mane S, Ma S, Kidd KK, Hayward NK, Lifton RP, Schlessinger J, Boggon TJ, Halaban R. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamola AA. Production of pyrimidine dimers in DNA in the dark. Biochem Biophys Res Commun. 1971;43:893–898. doi: 10.1016/0006-291x(71)90701-7. [DOI] [PubMed] [Google Scholar]
- Premi S, Wallisch S, Mano CM, Weiner AB, Bacchiocchi A, Wakamatsu K, Bechara EJ, Halaban R, Douki T, Brash DE. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science. 2015;347:842–847. doi: 10.1126/science.1256022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turro NJ, Ramamurthy V, Scaiano JC. Modern molecular photochemistry of organic molecules. University Science Books; Sausalito, CA.: 2010. [Google Scholar]