Abstract
Due to the lack of cell-adhesive moieties in traditional synthetic hydrogels, the present work investigated the use of degradable gelatin microparticles (GMPs) as temporary adherent substrates for anchorage-dependent mesenchymal stem cells (MSCs). MSCs were seeded onto GMPs of varying crosslinking densities and sizes to investigate their role on influencing MSC differentiation and aggregation. The MSC-seeded GMPs were then encapsulated in poly(ethylene glycol)-based hydrogels and cultured in serum-free, growth factor-free osteochondral medium. Non-seeded MSCs co-encapsulated with GMPs in the hydrogels were used as a control for comparison. Over the course of 35 days, MSC-seeded GMPs exhibited more cell-cell contacts, greater chondrogenic potential, and a down-regulation of osteogenic markers compared to the controls. Although the factors of GMP crosslinking and size had nominal influence on MSC differentiation and aggregation, GMPs demonstrate potential as an adherent-substrate for improving cell delivery from hydrogel scaffold by facilitating cell-cell contacts and improving MSC differentiation.
Keywords: Cell delivery, Hydrogel encapsulation, Mesenchymal stem cell differentiation
Introduction
Articular cartilage has a limited endogenous ability for self-repair, and since current clinical treatments for damaged or diseased cartilage tissue fall short of holistic repair, tissue engineering strategies have emerged as an alternative for physiological cartilage regeneration. In particular, synthetic hydrogels are appealing as scaffolding structures due to their viscoelastic properties, ability to mimic the high water content of native tissues, and tunability for greater control over physical properties.28 Various biomaterials have consequently been developed into hydrogel structures13 and are attractive candidates as scaffolds for cell encapsulation and growth.21
In the field of cartilage tissue engineering, stem cells and/or chondrocytes have typically been incorporated in such synthetic hydrogels to guide cell and tissue growth. Specifically, cell delivery from hydrogels traditionally involves the homogenous suspension of cells within a liquid precursor solution and subsequent curing to form a cell-laden scaffold. However, as the field evolves, hydrogels have transitioned from merely delivery vehicles to dynamic, bioactive intermediaries of neo-tissue formation.21 In particular, synthetic hydrogels often lack cell-adhesion moieties, and cell-cell contacts mediated by N-cadherin cell adhesion molecules have increasingly been shown to influence mesenchymal stem cell (MSC) differentiation and cartilage tissue formation through condensation.5, 26 Condensation, a developmental process during skeletogenesis, is characterized by MSC aggregation following the establishment of cell-cell contacts, which in turn can improve chondrogenic differentiation and cartilaginous extracellular matrix production.2, 3 Therefore, the current study seeks to improve the differentiation potential of MSCs through the use of enzymatically-degradable gelatin microparticles (GMPs) embedded within a poly(ethylene glycol) (PEG)-based hydrogel matrix.
Synthesized from denatured collagen, gelatin naturally exhibits cell-adhesion moieties and has seen numerous applications in cell delivery in a variety of scaffold forms.7, 19, 25 Thus, gelatin will be fabricated into microparticles as a medium for cell seeding and cell-cell contacts. Specifically, MSCs will first be seeded onto the surface of GMPs, subsequently followed by the encapsulation of MSC-seeded GMPs (MSC-GMPs) within a hydrogel scaffold. It is hypothesized that initially seeding GMPs with MSCs can improve their differentiation potential, and that the GMPs will serve as enzymatically-digestible porogens as well as a temporary adherent-substrate for the MSCs. Additionally, it is hypothesized that the degradation of the GMPs will result in open space for MSC aggregation within the created pores. MSCs are known secretors of a number of matrix metalloproteinases (MMPs),17 and direct extracellular matrix contact can modulate MMP activity through specific substrate-protease responses.20 Moreover, the aggregation of MSCs is modulated by the ratio of cadherin to integrin expression; the lower the availability for substrate adhesion, the greater the affinity for cell-cell aggregation.1 Thus, it may be possible to form MSC aggregates following degradation of the GMP substrate within the macroporous hydrogel.
To investigate the previously stated hypotheses, this study 1) fabricates GMPs of varying sizes and degrees of crosslinking, 2) investigates GMP size, GMP crosslinking, and seeding method on the activity of MSCs encapsulated within PEG-based hydrogels, and 3) cultures the cell-laden hydrogel composites in a serum-free, growth factor-free medium to elucidate the sole effect of the aforementioned three variables on MSC chondrogenesis, osteogenesis, and condensation.
Materials and Methods
Experimental design
The main design criteria set for fabricating GMPs was a fast-degrading, temporary adhesive-substrate for MSC delivery. Thus, GMPs with a very low crosslinking density that would still allow for MSC seeding were used. In order to determine which GMPs to use for MSC seeding, GMPs with different crosslinking densities were degraded in phosphate-buffered saline (PBS) with a range of collagenase 1A concentrations (Table 1). From this study, select GMPs of different crosslinking density and diameter sizes were then co-encapsulated or seeded with MSCs in hydrogel composites to yield six different experimental groups as outlined in Table 2. Cell-laden hydrogel composites were then cultured for 35 days in serum- and growth factor-free osteochondral medium to assess MSC activity, condensation, and differentiation.
Table 1.
Gelatin microparticles (GMPs) of varying crosslinking and collagenase-containing phosphate-buffered saline (CC-PBS) concentrations used to model GMP degradation.
GMPs were crosslinked in a solution of glutaraldehyde at the concentrations indicated above at 4°C for 15 h
20 ng/mL CC-PBS concentration was only tested for 2 mM and 3 mM GMPs
Table 2.
Experimental groups tested for the encapsulation of MSCs and GMPs in a hydrogel composite.
| Experimental Group | Mode of MSC Encapsulation | GMP | Size of Dry GMP |
|---|---|---|---|
| 1 | Gel Phase | 4 mM | 50 – 100 μm |
| 2 | Gel Phase | 5 mM | 50 – 100 μm |
| 3 | GMP-Seeded | 4 mM | 50 – 100 μm |
| 4 | GMP-Seeded | 5 mM | 50 – 100 μm |
| 5 | GMP-Seeded | 4 mM | 100 – 150 μm |
| 6 | GMP-Seeded | 5 mM | 100 – 150 μm |
Oligo(poly(ethylene glycol) fumarate) synthesis
Oligo(poly(ethylene glycol) fumarate) (OPF) was synthesized from PEG (Sigma-Aldrich) with a nominal molecular weight of 3,350 according to methods previously developed in our laboratory.9 Gel permeation chromatography was used to characterize the OPF macromer to give a number average molecular weight of 7,500 ± 200 Da and a weight average molecular weight of 36,300 ± 600 Da. Purified OPF was stored at −20°C and sterilized with ethylene oxide for 12 h prior to use following established methods.22
Gelatin microparticle fabrication
GMPs were fabricated using acidic gelatin (Nitta Gelatin) with an isoelectric point of 5.0 using a modified process of established methods.16, 29 Briefly, 30 mL of a 10 wt% gelatin solution in distilled, deionized water (ddH2O), preheated to 45°C, was added drop-wise to 250 mL olive oil (Sigma-Aldrich) (containing 0.5 wt% Span 80) stirring at 400 RPM at room temperature. After 10 min, the water-in-oil emulsion was chilled on ice and stirred for 30 more min. Following the emulsion step, 100 mL chilled acetone (4°C) were added and stirred an additional 60 min. The resulting microparticles were then collected by vacuum filtration and washed with acetone. The collected GMPs were then placed in a 0.1 wt% Tween 80 aqueous solution containing different concentrations of glutaraldehyde (1, 2, 3, 4, and 5 mM) and stirred at 4°C for 15 h. After crosslinking, glycine was added to a concentration of 25 mM to block residual aldehyde groups of unreacted glutaraldehyde and allowed to stir for 1 h. Crosslinked microparticles were then vacuum-filtered, washed with ddH2O and acetone, dried under low-vacuum overnight, and sieved to different size ranges. GMPs of 50-100 μm and 100-150 μm in diameter were sterilized with ethylene oxide for 12 h prior to MSC culture and encapsulation.
Gelatin microparticle degradation
For the degradation study, GMPs of varying crosslinking were placed in PBS with different concentrations of collagenase 1A (Sigma-Aldrich) to facilitate enzymatic digestion (Table 1). The collagenase concentrations used were based on estimations of MMP-1 expression from MSCs,8 as well as observed degradation of GMPs when incubated with MSCs. 5 mg of 100-150 μm diameter GMPs were added to the bottom of cell strainers with a 40 μm mesh size. The cell strainers with GMPs were then placed in 6-well plates, with each well containing 10 mL of collagenase-containing PBS (CC-PBS), and incubated at 37°C for up to 35 days (n=3). The CC-PBS was collected and changed at day 1 and every third day thereafter. To measure the amount of gelatin in solution, a bicinchoninic acid assay (Micro BCA Protein Assay Kit, Thermo Scientific) for total protein determination was used with dissolved, uncrosslinked GMPs prepared as standards. Briefly, 150 μL of standard/sample were combined with 150 μL of the working reagent in a clear 96-well plate and incubated for 1 h at 37°C. The absorbance at 562 nm was then measured with a plate reader (PowerWave ×340 Microplate Reader). The GMPs were considered completely degraded when the amount of gelatin detected was within the standard error of the lower detection limit. This was verified via visual observation of the absence of GMPs in the cell strainer.
Rabbit marrow mesenchymal stem cell harvest and culture
Rabbit marrow MSCs were isolated from the tibias of 6-month-old New Zealand white rabbits as previously described.10 All surgical procedures were approved by the Institutional Animal Care and Use Committee of Rice University. Briefly, after anesthesia, bone marrow was collected into 10 mL syringes containing 3,000 units of heparin. The bone marrow was then plated and cultured in general medium (GM + FBS) containing Low Glucose-Dulbecco's modified Eagle's medium (LG-DMEM), 10% v/v fetal bovine serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin. GM + FBS was changed every 3 days and after 2 weeks of culture, the adherent fraction of cells was pooled from 6 rabbits to minimize inter-animal variability and cryopreserved in freezing medium containing LG-DMEM, 20% v/v FBS, 10% v/v dimethyl sulfoxide, 100 units/mL penicillin, and 100 μg/mL streptomycin. Prior to use, MSCs were thawed and expanded with GM + FBS up to passage three.
Mesenchymal stem cell seeding on gelatin microparticles
Prior to MSC seeding, 50 mg dry GMPs (50-100 or 100-150 μm diameter) were swollen in 2 mL GM without FBS (GM – FBS) for 1 h at 37°C. 6 × 106 MSCs and 50 mg GMPs in suspension were then split evenly between two 100 mm ultra-low attachment dishes (Corning) containing 20 mL GM – FBS each. The dish was gently swirled at 120 RPM for 2 min before incubation for 12 h at 37°C. After 12 h culture, aggregates of MSCs and GMPs were broken up with a 1000 μL positive displacement pipette, swirled at 120 RPM for 2 min, and incubated for 24 h at 37°C. For GMPs without seeded MSCs, GMPs were swollen in GM – FBS for 36 h at 37°C in static conditions. After a total of 36 h, the suspension of GMPs or MSC-seeded GMPs (MSC-GMP) was filtered through a 70 μm cell strainer to remove any non-adherent cells and washed with PBS to remove residual media. The GMPs or MSC-GMPs were then loaded in a 1 mL syringe shortly before encapsulation.
Hydrogel composite fabrication
Hydrogel composites were fabricated similar to methods previously described.16, 31 To prepare the OPF precursor solution, 50 mg of OPF and 25 mg of PEG-diacrylate (3,400 Da, Laysan Bio) were dissolved in 112.5 μL PBS and set at room temperature for 45 min to eliminate air bubbles. 23 μL each of radical initiators, 0.3 M ammonium persulfate (APS) and 0.3 M N,N,N’,N’-tetramethylethylenediamine (TEMED), were then mixed into the polymer solution and vortexed. The concentrations of APS and TEMED used were expected to have minimal cytotoxic effects and allow for high viability of encapsulated cells in OPF-based hydrogels following fabrication.10, 31 After 30 seconds, 100 μL PBS or cell suspension (6 × 106 MSCs) were added followed by the MSC-GMPs or blank GMPs, respectively. The solution was mixed carefully to create a homogenous distribution of GMPs and to avoid the formation of bubbles. Lastly, the polymer-GMP solution was injected into Teflon molds (6 mm diameter × 1 mm thickness) and crosslinked at 37°C for 20 min. Teflon molds were flipped at 5 min to prevent GMPs from settling.
Hydrogel composite culture
The resulting hydrogel constructs were transferred to ultra-low attachment 24-well plates (Corning) with 1 mL serum- and growth factor-free osteochondral medium (OM), which contained LG-DMEM supplemented with ITS + Premix (6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 μg/mL selenous acid, 5.35 μg/mL linoleic acid and 1.25 μg/mL bovine serum albumin) (BD Biosciences), 100 units/mL penicillin, and 100 μg/mL streptomycin, 50 mg/L ascorbic acid, 10mM β-glycerophosphate, 10−7 M dexamethasone, and 1 mM sodium pyruvate.6 OM was changed every two days and cell-laden composites were cultured up to 35 days.
Confocal fluorescence microscopy
Confocal fluorescence microscopy was used to visualize MSCs encapsulated in the hydrogel composites at day 0 (D0). To assess cell viability, constructs from D0 were rinsed in PBS to remove media and then incubated in a dye solution containing 4 μM ethidium homodimer-1 and 2 μM calcein acetoxymethyl ester (Invitrogen) for 30 min as reported previously.6 MSCs complexed with the Live/Dead reagent were then imaged using a Zeiss LSM 510.
To assess the morphology of MSCs either seeded on GMPs or encapsulated in the gel phase, select samples at D7 were stained for nuclei and F-actin. Briefly, samples were fixed in 10% neutral buffered formalin for 10 min at room temperature, washed with PBS, and stored at 4°C until staining. Hydrogels were immersed in 0.3% Triton X-100 for 10 min, 2×, to permeabilize cells, then incubated with DAPI (5 μg/mL) and phalloidin (1:20 dilution, Alexa Fluor 488 Phalloidin, Life Technologies) in 3% BSA diluted in Triton X-100 for 20 min at room temperature. Samples were then washed in PBS for 10 min and imaged with a Nikos A1-Rsi.
Biochemical assays
At each time point (D0, D7, D21, and D35), hydrogel composites were collected (n=4), rinsed in PBS to remove media, cut in half, and stored in −20°C until used for biochemical analysis. To determine DNA, glycosaminoglycan (GAG), and hydroxyproline (HYP) content, 4 hydrogel halves were each digested in 500 μL of proteinase K solution (1 mg/mL proteinase K, 10 μg/mL pepstatin A, and 185 μg/mL iodoacetamide in tris-EDTA solution (6.055 mg/mL tris(hydroxymethyl aminomethane), 0.372 mg/mL EDTA, pH 7.6 adjusted by HCl) at 56°C for 16 h. To determine alkaline phosphatase (ALP) activity and calcium content, 4 hydrogel halves were each thawed in 500 μL of ddH2O. All samples were then subjected to homogenization with a syringe and needle, three freeze-thaw cycles, and probe sonication.
DNA content was quantified using a Quant-iT PicoGreen dsDNA Assay Kit (Molecular Probes) according to the manufacturer's instructions. Sample supernatant, assay buffer, and dye solution were combined in a black, opaque 96-well plate, and incubated for 10 min at room temperature. Fluorescence was measured using excitation and emission wavelengths of 485 nm and 528 nm (FL ×800 Fluorescence Microplate Reader), respectively. DNA concentrations were determined relative to a lambda DNA standard curve.
GAG content was determined with a dimethylmethylene blue colorimetric assay as previously described.10 Sample supernatant and color reagent were combined in a clear 96-well plate and the absorbance at 520 nm was measured (PowerWave ×340 Microplate Reader). GAG concentrations were determined relative to a chondroitin sulfate standard curve. Synthetic GAG activity was determined by normalizing total GAG content to the DNA content for each sample.
HYP content, an indicator of total collagen amount, was determined via a colorimetric assay as previously described.18 An aliquot of sample supernatant was combined with an equal volume of 4 N NaOH and hydrolyzed by autoclaving for 15 min, 121°C (approximately 50 min total processing time). The solution was neutralized with HCl and acetic acid to pH 5.5-7.0 and divided into duplicate reactions. Chloramine-T and p-dimethylaminobenzaldehyde solutions were then added sequentially, incubated at 60°C for 30 min, and read at an absorbance of 570 nm with a plate reader. HYP concentrations were determined relative to a trans-4-hydroxy-L-proline standard curve.
ALP enzymatic activity was determined using alkaline buffer solution and phosphatase substrate tablets (Sigma-Aldrich). Sample supernatant and reagents were combined and incubated for 1 h at 37°C. The reaction was stopped by adding 1 N NaOH, and the absorbance at 405 nm was measured using a plate reader. ALP activity was determined relative to a p-nitrophenol standard curve. Enzymatic activity was normalized to the DNA content for each respective hydrogel composite half.
Calcium content was measured using a colorimetric assay by adding acetic acid to an aliquot of sample supernatant with a final concentration of 0.5 M and incubating at room temperature overnight to dissolve the calcium. Samples were combined with calcium arsenazo III reagent (Genzyme), and the absorbance at 650 nm was measured using a plate reader. Calcium concentrations were determined relative to a CaCl2 standard curve and normalized to the DNA content for each respective hydrogel composite half.
Real-time reverse transcription polymerase chain reaction
At D7, D21, and D35, hydrogel samples were collected (n=4), rinsed in PBS to remove media, and stored in lysis buffer at −20°C until used for real-time reverse transcription polymerase chain reaction (RT-PCR) analysis as previously described.22 Additionally, D0 samples were collected from experimental groups containing 4 mM GMPs. Briefly, RNA was isolated from hydrogel samples using the RNeasy Mini Kit (Qiagen), reverse transcribed to cDNA using superscript III transcriptase (Invitrogen) and Oligo dT primers (Promega), and subjected to real-time PCR (Applied Biosystems 7300 Real-Time PCR System) using SYBR Green detection (PerfeCTa SYBR Green FastMix, Rox; Quanta Biosciences) with custom designed primers (Integrated DNA Technologies). Primer sequences used are listed in Table 3. All target gene expression were normalized to the housekeeping gene GAPDH and expressed as the fold change relative to the baseline expression of the Group 1 control at D0. All gene expression data were calculated using the 2−ΔΔCt method.27
Table 3.
Forward (F) and reverse (R) primers used for real-time RT-PCR analysis.
| Gene | Primer sequence | Product length | Reference* |
|---|---|---|---|
| Type II Collagen (COL2A1) | F: 5′-AACACTGCCAACGTCCAGAT-3′ R: 5′-CTGCAGCACGGTATAGGTGA-3′ |
201 | 10 |
| Aggrecan (ACAN) | F: 5′-GCTACGGAGACAAGGATGAGT-3′ R: 5′-CGTAAAAGACCTCACCCTCCA-3′ |
114 | 10 |
| (Sex determining region Y)-box 9 (SOX9) | F: 5′-GAGCGAAGAGGACAAGTTCC-3′ R: 5′-GTCCAGTCGTAGCCCTTGAG-3′ |
72 | - |
| Type I Collagen (COL1A2) | F: 5′-CCCAGAATGGAGCAGTGGTTA-3′ R: 5′-AGCAGACGCATGAAGGCAAG-3′ |
270 | 10 |
| Runt-related transcription factor 2 (RUNX2) | F: 5′-CCTTCCACTCTCAGTAAGAAGA-3′ R: 5′-TAAGTAAAGGTGGCTGGATAGT-3′ |
143 | 4 |
| N-cadherin (CDH2) | F: 5′-CTGCTATTGATGCGGATGAC-3′ R: 5′-TGAACATGTTGGGAGAAGGA-3′ |
96 | - |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | F: 5′-TCACCATCTTCCAGGAGCGA-3′ R: 5′-CACAATGCCGAAGTGGTCGT-3′ |
292 | 10 |
Primers without a reference were custom designed and validated
Statistical analysis
A significance level of p<0.05 was used for all statistical analysis through JMP Pro v11.0.0. All results are presented as means ± standard deviation. Biochemical assay data were analyzed using one-way ANOVA to test variance followed by Tukey's HSD All Pairs test, whereas RT-PCR data were analyzed using the Kruskal-Wallis test followed by the Wilcoxon Each Pair test to determine significant differences between groups at each time point and between time points for each group.
Results
Gelatin microparticle degradation
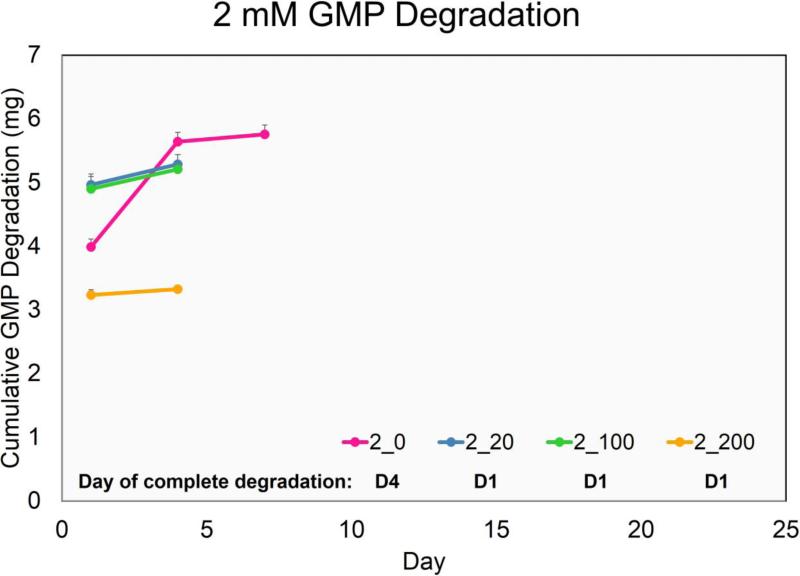
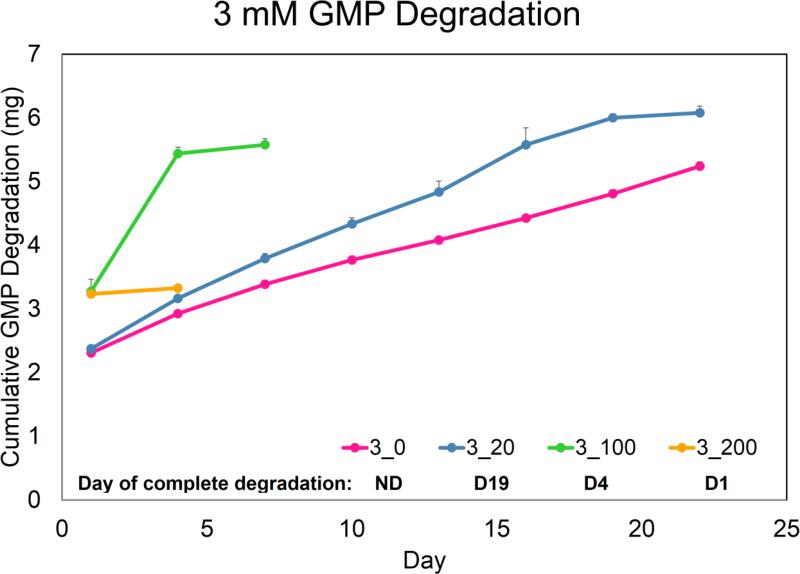
The main design criterion set for fabricating GMPs was a fast-degrading, temporary adhesive-substrate for MSC delivery. Thus, GMPs with a very low crosslinking density that would still allow for MSC seeding were used. Of the five crosslinking-densities tested, GMPs crosslinked in 1mM glutaraldehyde solution (1mM GMPs) dissolved in 37°C PBS within 24 h while 2, 3, 4, and 5mM GMPs remained stable. In order to model the degradation of GMPs due to MMPs secreted from MSCs, 2mM and 3mM GMPs were initially tested in varying CC-PBS concentrations (Fig 1a,b). However, 2mM and 3mM GMPs completely degraded when cultured with MSCs during the seeding period (36 h), whereas 4mM and 5mM GMPs allowed for cell adhesion. As a result, 4mM and 5mM GMPs were only tested at 0, 100, and 200 ng/mL CC-PBS concentrations for up to 35 days. Both 4mM and 5mM GMPs held their spherical morphology in PBS without collagenase during the culture period, and while 4mM GMPs degraded in CC-PBS within 13 and 16 days, 5mM GMPs degraded within 19 and 28 days at the 100 and 200 ng/mL CC-PBS concentrations, respectively.
Figure 1.
Degradation profile of GMPs crosslinked with a 2mM (A), 3mM (B), 4mM (C), and 5mM (D) glutaraldehyde solution. Cumulative degradation of GMPs was measured up to 35 days in collagenase-containing PBS. 2_0, 2_20, 2_100, and 2_200 designate 2 mM GMPs incubated with PBS containing 0, 20, 100, or 200 ng/mL collagenase 1A, respectively. Similar shorthand designations apply for 3mM, 4mM, and 5mM GMPs. The day of complete degradation is stated and is indicated by a slight plateau in the cumulative GMP degradation graph. GMPs that did not degrade within the culture period are denoted with an ND. Error bars correspond to standard deviation for n=3 samples.
Mesenchymal stem cell seeding on gelatin microparticles
After MSCs were seeded onto 4mM and 5mM GMPs, the MSC-GMPs were encapsulated in OPF hydrogels, and the viability and morphology of the MSCs were qualitatively examined with confocal fluorescence microscopy. At D0 post-fabrication, the cell-laden hydrogel composites were evaluated with a Live/Dead assay kit, with live cells and dead cells fluorescing green and red, respectively. As seen in Figure 2, MSCs are viable following hydrogel encapsulation, whether through direct encapsulation or initial GMP seeding. The minimal cell death seen in the MSC-GMP groups also points to the MSC seeding procedure on the GMPs as cell-friendly. In order to visualize cell morphology in the hydrogel composites, MSCs were stained with DAPI (blue) and phalloidin (red) for cell nuclei and F-actin, respectively (Fig 3). As shown in Figure 3a, the MSCs exhibit a round morphology, while in Figures 3b and 3c, MSCs are attached and spread on the surface of GMPs within the hydrogel scaffold.
Figure 2.
Confocal fluorescence microscopy images of MSCs, complexed with live/dead reagents, encapsulated in hydrogel composites at day 0. Green fluorescence designates live cells, whereas red fluorescence indicates dead cells. Round cells can be seen in Groups 1 and 2 (A and B) while flattened cells can be seen in Group 3-6 (C-F). (Scale bar: 200 μm)
Figure 3.
Confocal fluorescence microscopy images of MSCs, stained with DAPI and phalloidin, encapsulated in hydrogel composites at day 7. Blue fluorescence designates nuclei, whereas green fluorescence indicates F-actin staining of MSCs. Round MSCs encapsulated in the gel phase can be seen in a representative sample from Group 2 (A), while elongated MSCs can be seen in a representative sample from Group 4 (B). A z-axial projection stack of 30 images (10 μm apart) set against differential interference contrast images shows the spread morphology of MSCs seeded on the GMPs (C). (Scale bar: 100 μm)
Biochemical assays
OPF hydrogels containing MSCs + GMPs (Groups 1 and 2) or MSC-GMPs (Groups 3-6) were cultured in a serum-free, growth factor-free OM for 35 days and evaluated for cellularity, synthetic GAG activity, GMP degradation, ALP activity, and mineralization potential (Fig 4). Cellularity, as assessed by DNA content, is depicted in Figure 4a for each group and time point. At D0, Groups 1 and 2 had significantly greater total DNA content than Groups 3 and 4 (MSC-GMPs, 50-100 μm), which had greater DNA content than Groups 5 and 6 (MSC-GMPs, 100-150 μm). All groups displayed a decrease in total DNA content from D0 to D7. However, the DNA amount remained relatively stable over the course of 35 days following Day 7.
Figure 4.
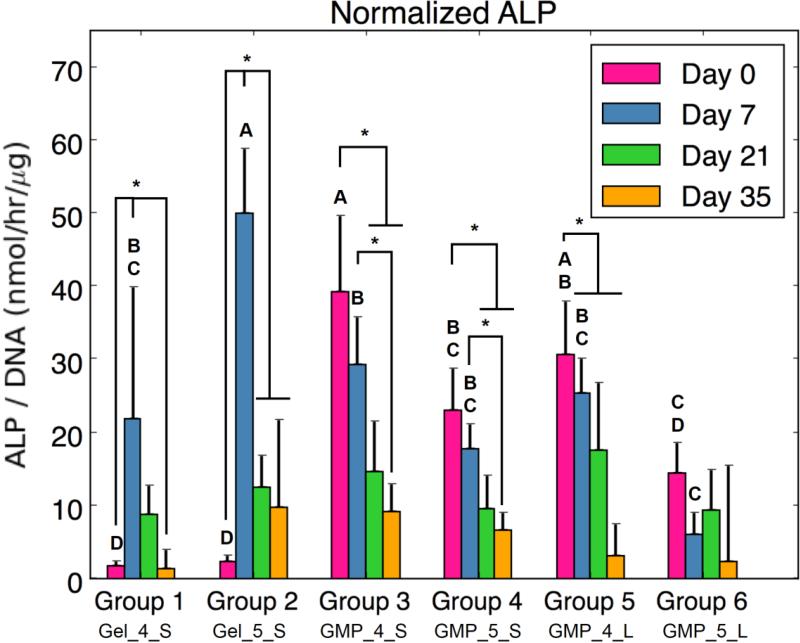
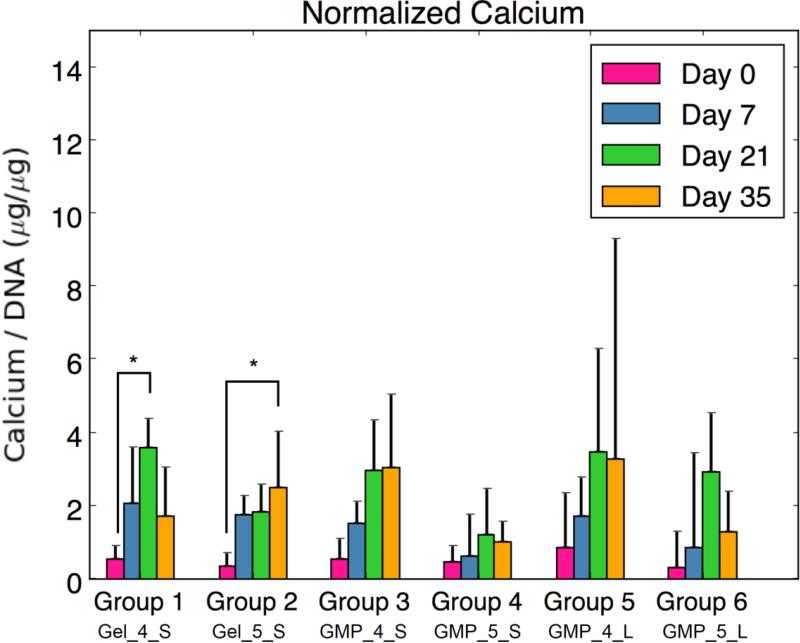
(A) DNA content, (B) GAG content normalized to DNA content, (C) HYP content, (D) ALP activity normalized to DNA content, and (E) calcium content normalized to DNA content for each experimental group at various time points. At each time point, groups connected with different letters are significantly different (p < 0.05). Within each group, time points connected by lines and noted with (*) are significantly different. The x-axis labels are denoted by Gel or GMP, representing MSCs co-encapsulated with GMPs vs seeded on GMPs; 4 or 5, representing the crosslinking extent of GMPs at either 4mM vs 5mM; and S or L, representing smaller 50-100μm GMPs vs larger 100-150μm GMPs. Error bars correspond to standard deviation for n=4 samples.
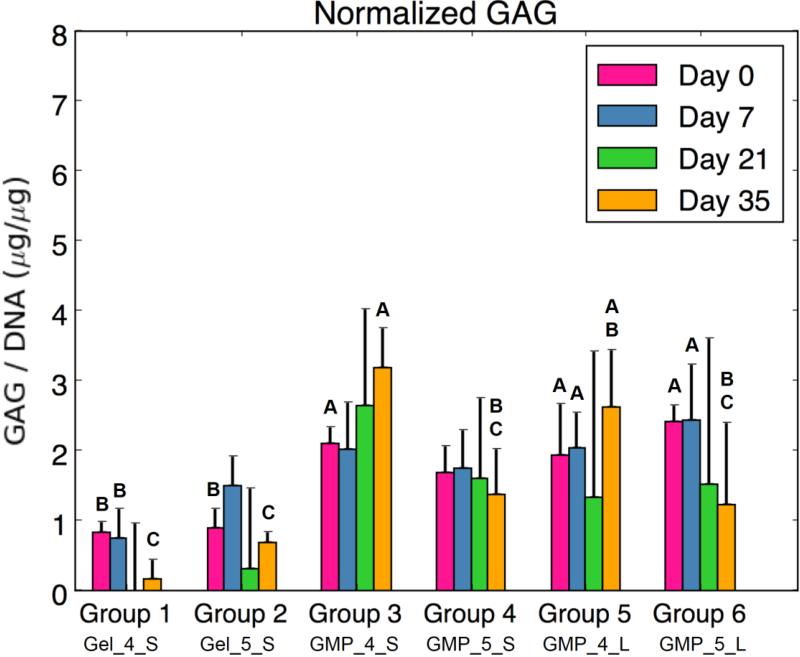
Figure 4b represents the synthetic GAG activity as produced by encapsulated MSCs. Groups 3, 5, and 6 (MSC-GMPs) showed a significantly larger normalized GAG amount at D0 compared to the co-encapsulated MSC + GMPs. Additionally, groups with MSCs seeded on 4 mM GMPs showed the greatest GAG synthetic activity at Day 35: Groups 3 and 5 were significantly greater than the control groups and Group 3 was significantly greater than the 5 mM GMP groups. No direct differences were seen between larger and smaller sized GMP groups.
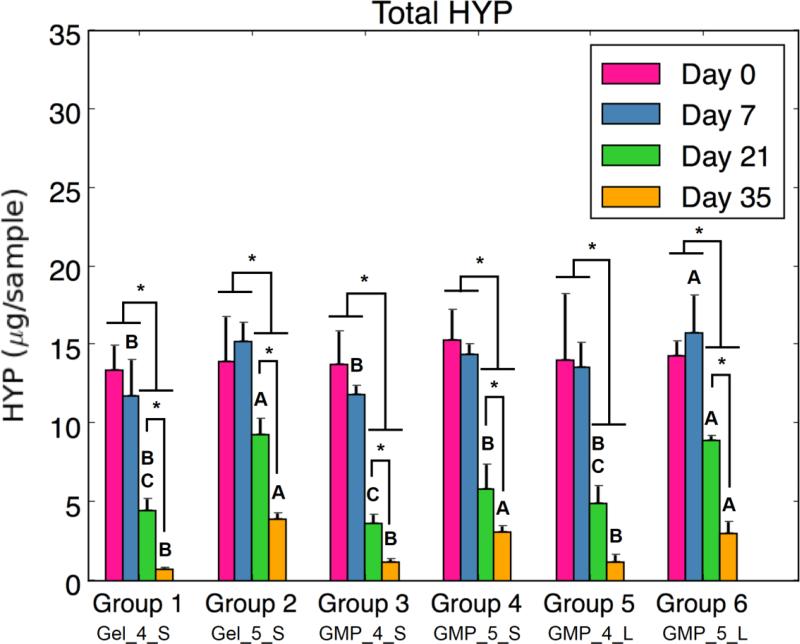
In order to examine the change in collagen content as an estimation of GMP degradation, total HYP content of the hydrogel constructs was determined over time. As seen in Figure 4c, total HYP amounts dropped overtime with D21 and D35 values significantly lower than D0 and D7 values for all groups. A difference between groups with 4mM and 5mM GMP groups can be seen at D21 and especially at D35, with 4mM groups having less HYP content than 5mM groups. However, the GMPs were still present and had a spherical morphology within the hydrogels after the culture period (as verified by light microscopy), which can be seen by remaining HYP content in each group at D35.
Since MSCs were cultured in an OM without factors for specific chondrogenic or osteogenic directed differentiation, ALP enzymatic activity and calcium content were also examined as markers of osteogenic differentiation. Figure 4d shows a peak in normalized ALP enzymatic activity at D7 for Groups 1 and 2, while the groups with encapsulated MSC-GMPs decreased in ALP activity over time from D0. MSCs co-encapsulated with 5mM GMPs (Group 2) specifically exhibited the highest normalized ALP activity at D7 among groups. Interestingly, the MSC-GMPs groups with 4mM GMPs (Groups 3 and 5) had greater initial ALP activity at D0 compared to their 5mM GMP counterparts (Groups 4 and 6). Looking at normalized calcium content (Fig 4e), no significant differences were observed between groups, and calcium levels remained relatively low over the course of 35 days. However, a significant increase in normalized calcium content was seen from D0 to D21 for Group 1 and from D0 to D35 for Group 2.
Real-time RT-PCR gene expression
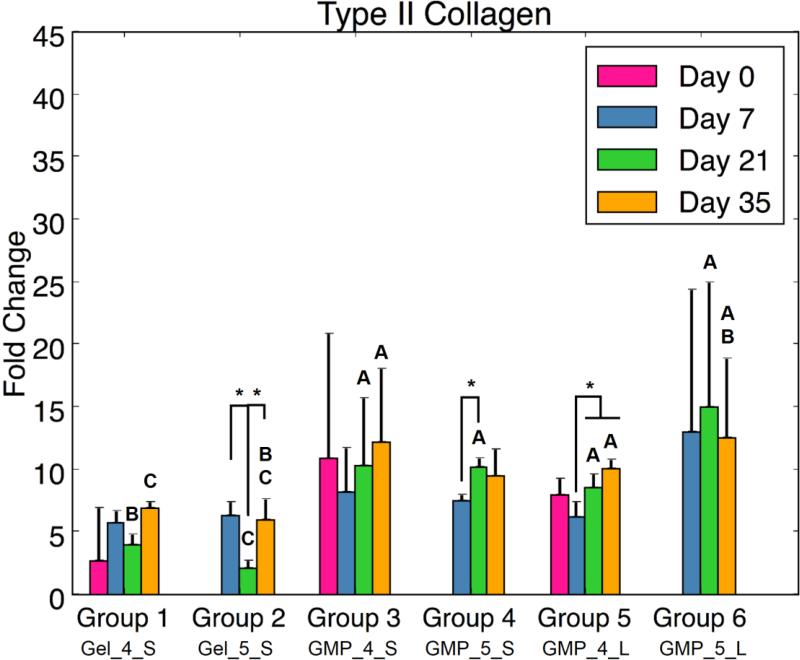
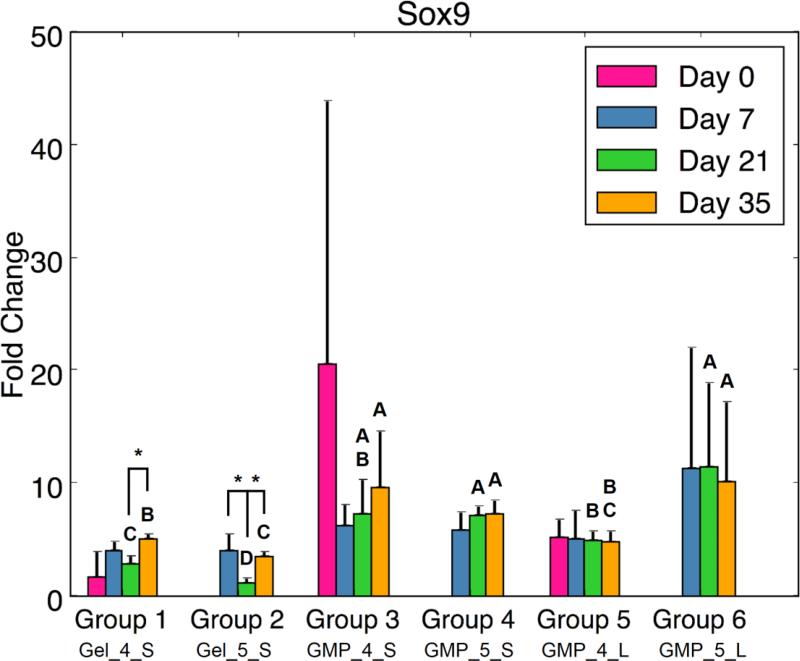
In addition to evaluating the synthetic activity of the cell-laden hydrogel composites, real-time RT-PCR analysis was also performed to assess chondrogenic and/or osteogenic differentiation potential. In examining markers for chondrogenic differentiation, certain trends were consistently seen for COL2, ACAN, and SOX9 gene expression. Of note, all MSC-GMP groups exhibited greater chondrogenic gene expression at D21 than did the MSC + GMP control groups (Fig 5). Differences between MSC-GMP (Groups 3-6) and MSC + GMP (Groups 1-2) hydrogel composites were also observed at other time points: MSCs seeded on 5mM GMPs demonstrated higher ACAN and SOX9 fold change at D35 compared to the MSC + 5mM GMP control (Fig 5b,c). Similarly, Groups 3 and 5 (4mM MSC-GMPs) had a greater COL2 fold change at D35 compared to the MSC + 4mM GMP control (Fig 5a). At D7, MSCs seeded on 5mM GMPs exhibited greater ACAN gene expression than the MSC + 5mM GMP control (Fig 5b). Significant differences were also seen between 4mM GMP (Groups 1, 3, and 5) and 5mM GMP (Groups 2, 4, and 6) hydrogel composites. Group 1 had higher COL2, ACAN, and SOX9 expression at D21 and D35 compared to Group 2. Additionally, the larger sized, 5mM MSC-GMP group (Group 6) had higher ACAN and SOX9 gene expression than its 4mM counterpart (Group 5) at D21.
Figure 5.
(A) Type II Collagen expression, (B) Aggrecan expression, and (C) Sox9 expression for each experimental group at various time points. At each time point, groups connected with different letters are significantly different (p < 0.05). Within each group, time points connected by lines and noted with (*) are significantly different. The x-axis labels are denoted by Gel or GMP, representing MSCs co-encapsulated with GMPs vs seeded on GMPs; 4 or 5, representing the crosslinking extent of GMPs at either 4mM vs 5mM; and S or L, representing smaller 50-100μm GMPs vs larger 100-150μm GMPs. Error bars correspond to standard deviation for n=4 samples.
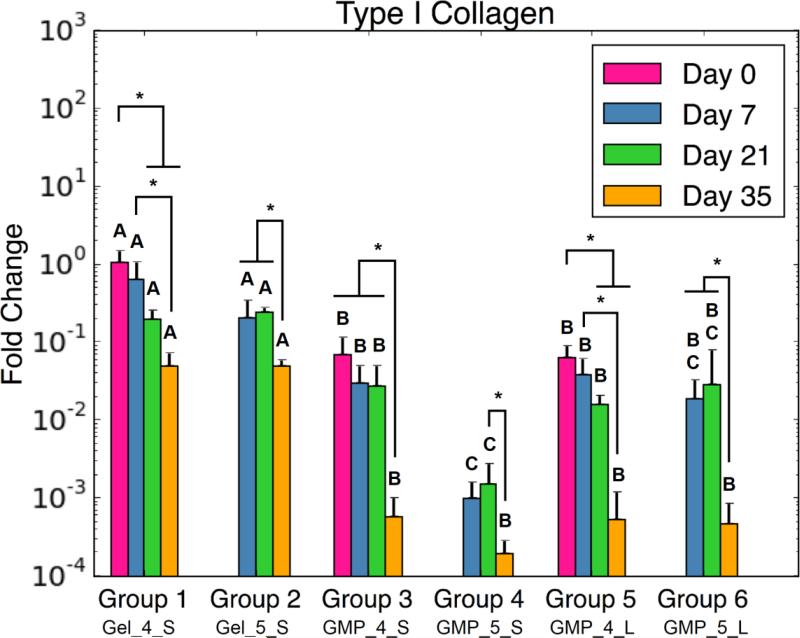
In assessing markers of osteogenic differentiation, COL1 gene expression was consistently down regulated over time for all groups (Fig 6a). Moreover, MSC-GMP groups had decreased COL1 fold change compared to the MSC + GMP controls at all time points. RUNX2 expression also revealed similar down regulation for MSC-GMP groups compared to MSC + GMP groups at both D21 and D35 (Fig 6b). Interestingly, minimal differences in osteogenic gene expression were observed between smaller and larger GMP size groups. Regarding GMP crosslinking, Group 4 had reduced COL1 and RUNX2 expression at D21 compared to Group 3.
Figure 6.
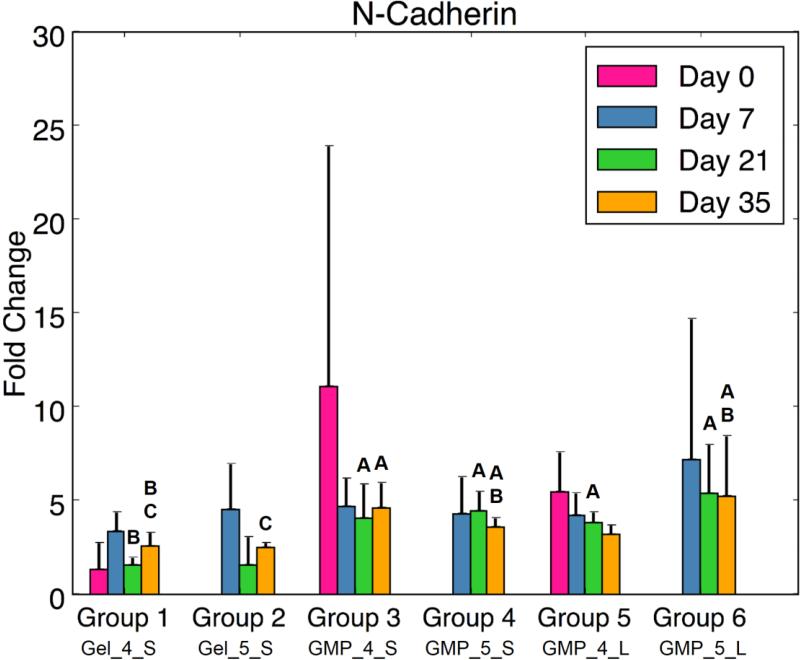
(A) Type I Collagen, (B) Runx2, and (C) N-Cadherin expression for each experimental group at various time points. At each time point, groups connected with different letters are significantly different (p < 0.05). Within each group, time points connected by lines and noted with (*) are significantly different. The x-axis labels are denoted by Gel or GMP, representing MSCs co-encapsulated with GMPs vs seeded on GMPs; 4 or 5, representing the crosslinking extent of GMPs at either 4mM vs 5mM; and S or L, representing smaller 50-100μm GMPs vs larger 100-150μm GMPs. Error bars correspond to standard deviation for n=4 samples.
Lastly, the presence and formation of cell-cell contacts through CDH2 expression was investigated (Fig 6c). While there was no significant difference between groups with MSCs seeded on GMPs and MSCs homogenously encapsulated in the hydrogel at early time points, D21 and D35 revealed the impact of the GMPs as a cell substrate: MSC-GMP groups showed greater CDH2 fold change compared to Group 1 at D21. At D35, MSC-5mM GMP groups showed higher N-cadherin expression than the MSC + 5mM GMP control. Similarly, Group 3 exhibited more CDH2 gene expression than Group 1 at D35.
Discussion
The primary objective of this study was to utilize enzymatically-degradable GMPs as a temporary adherent-substrate for MSCs within a hydrogel matrix. MSCs were seeded onto GMPs of different sizes and crosslinking densities and assessed for chondrogenesis/osteogenesis as well as their condensation behavior over 35 days. Specifically, we investigated 1) whether the GMPs could act as a temporary adherent-substrate for MSCs, allowing for MSC aggregation following GMP degradation, and 2) if the initial seeding of MSCs on the GMPs could improve their differentiation potential.
In order to evaluate GMPs as a temporary adherent-substrate, GMPs of varying crosslinking densities were fabricated and subjected to MSC seeding tests as well as collagenase-containing medium for GMP degradation modeling. Following GMP synthesis, 2 and 3mM GMPs were discovered to be unsuitable for MSC seeding and disappeared before the end of the seeding period. Since 2 and 3mM GMPs did not completely dissolve in the absence of collagenase within 24 h (Fig 1a,b), these loosely crosslinked GMPs were likely degraded via enzymes secreted by the MSCs during the seeding period. Consequently, 4 and 5mM GMPs were chosen as the temporary adherent-substrate for initial MSC seeding. However, this initial enzymatic activity on the GMPs may not have lasted throughout the culture period since microparticles still remained within the hydrogel scaffolds at D35. Indeed, while MSCs are known secretors of MMPs, tissue inhibitors of metalloproteinases (TIMPs) are often produced alongside MMPs, and most TIMPs are highly specific for MMPs, binding in a 1:1 stoichiometric ratio.30 In a study by Lozito and Tuan, despite secretion of MMP-1 and MMP-2 (a gelatinase) by MSCs cultured in a serum-free, growth factor-free medium, actual MMP activity was significantly lower due to endogenous production of TIMP-1 and TIMP-2.15 However, certain chemokines have been found to modulate the expression of MMPs and TIMPs in MSCs: expression of MMP-2 in a serum-free, growth factor-free medium was up-regulated with the exogenous addition of transforming growth factor-β1 while TIMP expression was not affected.24 As a result, to fully realize the potential of GMPs as a temporary adherent-substrate for cell delivery, the inclusion of serum or growth factors may be necessary.
Due to incomplete GMP degradation, macropores could not form within the hydrogel scaffold, and the space necessary for MSC aggregation was not achieved. Certainly, other methods have been developed to induce cell aggregation within a hydrogel: MSCs seeded within macroprous OPF hydrogels,14 chondrocytes encapsulated within uncrosslinked GMPs and released into a porous hydrogel bulk upon GMP dissolution,12 and MMPs used to degrade crosslinked GMPs as hepatocyte cell carriers within an alginate hydrogel.11 Nevertheless, an objective of the current study entailed the use of GMPs as temporary adherent-substrates for the anchorage-dependent MSCs, and it is unclear what the effects of a large dose of MMPs to degrade the GMPs would be on stem cells, as opposed to a differentiated cell phenotype (e.g., hepatocytes).
Despite the lack of MSC aggregation, biochemical and real-time RT-PCR analysis revealed that employing GMPs as an adherent-substrate within OPF hydrogels resulted in greater MSC chondrogenic potential as compared to MSCs co-encapsulated with GMPs. At D35, synthetic GAG activity and type II collagen expression was higher for MSC-4mM GMP groups than the MSC + 4mM GMP control. Furthermore, aggrecan and SOX9 fold changes were larger for the MSC-5mM GMP groups than for the controls at D35. Overall chondrogenic gene expression was higher at D21 for all MSC-GMP groups compared to MSC + GMP groups. While expression of these genes did not necessarily increase over time, the significant differences are corroborated by enhanced N-cadherin expression for MSC-GMP groups at both D21 and D35. Indeed, cell-cell contacts provide additional communication that has been demonstrated to improve adipogenic, osteogenic, and/or chondrogenic differentiation through cadherin signaling,26 and the disruption of such cell-cell adhesion has been shown to reduce MSC chondrogenic potential.5
While MSC-GMPs exhibited marked chondrogenic differentiation potential, markers of osteogenesis decreased over time for all groups. Interestingly, type I collagen and RUNX2 expression were significantly lower at D35 for MSCs seeded on GMPs compared to MSCs co-encapsulated with GMPs in hydrogels. However, substrate stiffness and the physical environment also play a large role in determining cellular response. Osteoblastic markers such as RUNX2 are often only expressed when the matrix stiffness is close to that of native bone tissue.23 Although mechanical properties of the hydrogel composites were not tested, the soft adherent substrate presented by the loosely crosslinked GMPs may have precluded significant osteogenic potential.
Lastly, the extent of GMP crosslinking and GMP size were also investigated as factors for influencing MSC activity in the hydrogel composites. While the variable of GMP size had minimal effects on differentiation and condensation, differences between 4mM and 5mM GMPs were found. Total HYP amount was consistently greater for 5mM GMP groups than 4mM GMP groups at D21 and D35, likely due to the increased crosslinking extent of 5mM GMPs. Additionally, differences between Group 1 and Group 2 were seen: greater normalized ALP expression was seen at D7 for MSCs co-encapsulated with 5mM GMPs compared to 4mM GMPs. This was followed by higher COL2, ACAN, and SOX9 gene expression for Group 1 compared to Group 2 at D21 and D35. Indeed, the microenvironment plays a large role in stimulating the activity of encapsulated MSCs and the relatively softer substrate of 4mM GMPs in Group 1 may have improved the chondrogenic potential of co-encapsulated MSCs. However, a similar trend was not consistently seen in groups with MSCs seeded on GMPs. 4mM MSC-GMPs groups displayed greater initial ALP activity compared to 5mM MSC-GMPs groups, yet smaller sized 4mM MSC-GMPs also had greater synthetic GAG activity at D35 compared to 5mM MSC-GMPs. In contrast, larger sized 5mM MSC-GMPs had higher ACAN and SOX9 expression than their 4mM counterpart at D21. Future investigation with a broader range of GMP crosslinking densities would be necessary to clarify the effect of GMP crosslinking. Additionally, the variables of size and crosslinking are anticipated to play a larger role in influencing MSC aggregation following complete degradation of the GMPs. Further studies incorporating serum or growth factors in the culture medium would be warranted to elucidate the effect of these variables on the differentiation and condensation potential of seeded MSCs.
Conclusion
In summary, MSCs were seeded onto GMPs of varying crosslinking densities and sizes and subsequently encapsulated in OPF hydrogels. MSCs co-encapsulated with the GMPs were included as controls, and the cell-laden hydrogel composites were cultured in a serum-free, growth factor-free OM for 35 days. Results indicate that the encapsulated GMPs did not completely degrade during the culture period, and crosslinking density and GMP size were not found to influence MSC differentiation potential. Nonetheless, MSCs seeded on GMPs exhibited greater N-cadherin expression than their MSC and GMP co-encapsulated counterparts. Additionally, MSCs seeded on GMPs showed greater chondrogenic differentiation potential, while osteogenic markers were found to down-regulate over time. Overall, these results demonstrate the potential of GMPs as an adherent-substrate within hydrogel scaffolds for facilitating cell-cell contacts and enhancing MSC differentiation.
Acknowledgements
This work was supported by the National Institutes of Health (R01-AR048756 and R01-AR068073). The authors acknowledge the assistance of Brandon T. Smith with the rabbit bone marrow harvest, Marco Santoro and Tiffany N. Vo with MSC culture, and Kelsea M. Hubka with immunocytochemistry.
Abbreviations
- ACAN
aggrecan
- ALP
alkaline phosphatase
- APS
ammonium persulfate
- CC-PBS
collagenase-containing PBS
- CDH2
N-cadherin
- COL1
type I collagen
- COL2
type II collagen
- ddH2O
distilled, deionized water
- FBS
fetal bovine serum
- GAG
glycosaminoglycan
- GMP
gelatin microparticle
- HYP
hydroxyproline
- LG-DMEM
Low Glucose-Dulbecco's modified Eagle's medium
- MMP
matrix metalloproteinases
- MSC
mesenchymal stem cell
- OM
osteochondral medium
- OPF
oligo(poly(ethylene glycol) fumarate
- PBS
phosphate-buffered saline
- PEG
poly(ethylene glycol)
- RT-PCR
reverse transcription polymerase chain reaction
- RUNX2
runt-related transcription factor 2
- SOX9
(sex determining region Y)-box 9
- TEMED
N,N,N’,N’-tetramethylethylenediamine
References
- 1.Bates RC, Edwards NS, Yates JD. Spheroids and cell survival. Crit Rev Oncol Hematol. 2000;36:61–74. doi: 10.1016/s1040-8428(00)00077-9. [DOI] [PubMed] [Google Scholar]
- 2.Bhumiratana S, Eton RE, Oungoulian SR, Wan LQ, Ateshian GA, Vunjak-Novakovic G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc Natl Acad Sci U S A. 2014;111:6940–6945. doi: 10.1073/pnas.1324050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bian L, Guvendiren M, Mauck RL, Burdick JA. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci U S A. 2013;110:10117–10122. doi: 10.1073/pnas.1214100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao L, Yang F, Liu G, Yu D, Li H, Fan Q, Gan Y, Tang T, Dai K. The promotion of cartilage defect repair using adenovirus mediated Sox9 gene transfer of rabbit bone marrow mesenchymal stem cells. Biomaterials. 2011;32:3910–3920. doi: 10.1016/j.biomaterials.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Chen AX, Hoffman MD, Chen CS, Shubin AD, Reynolds DS, Benoit DS. Disruption of cell-cell contact-mediated notch signaling via hydrogel encapsulation reduces mesenchymal stem cell chondrogenic potential: winner of the Society for Biomaterials Student Award in the Undergraduate Category, Charlotte, NC, April 15 to 18, 2015. J Biomed Mater Res A. 2015;103:1291–1302. doi: 10.1002/jbm.a.35383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo X, Liao J, Park H, Saraf A, Raphael RM, Tabata Y, Kasper FK, Mikos AG. Effects of TGF-beta 3 and preculture period of osteogenic cells on the chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in a bilayered hydrogel composite. Acta Biomaterialia. 2010;6:2920–2931. doi: 10.1016/j.actbio.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi K, Tabata Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater. 2011;7:2797–2803. doi: 10.1016/j.actbio.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Ho IAW, Chan KYW, Ng WH, Guo CM, Hui KM, Cheang P, Lam PYP. Matrix Metalloproteinase 1 Is Necessary for the Migration of Human Bone Marrow-Derived Mesenchymal Stem Cells Toward Human Glioma. Stem Cells. 2009;27:1366–1375. doi: 10.1002/stem.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinard LA, Kasper FK, Mikos AG. Synthesis of oligo(poly(ethylene glycol) fumarate). Nature Protocols. 2012;7:1219–1227. doi: 10.1038/nprot.2012.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam J, Lu S, Meretoja VV, Tabata Y, Mikos AG, Kasper FK. Generation of osteochondral tissue constructs with chondrogenically and osteogenically predifferentiated mesenchymal stem cells encapsulated in bilayered hydrogels. Acta Biomaterialia. 2014;10:1112–1123. doi: 10.1016/j.actbio.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau TT, Lee LQ, Leong W, Wang DA. Formation of model hepatocellular aggregates in a hydrogel scaffold using degradable genipin crosslinked gelatin microspheres as cell carriers. Biomed Mater. 2012;7:065003. doi: 10.1088/1748-6041/7/6/065003. [DOI] [PubMed] [Google Scholar]
- 12.Leong W, Lau TT, Wang DA. A temperature-cured dissolvable gelatin microsphere-based cell carrier for chondrocyte delivery in a hydrogel scaffolding system. Acta Biomater. 2013;9:6459–6467. doi: 10.1016/j.actbio.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Rodrigues J, Tomas H. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem Soc Rev. 2012;41:2193–2221. doi: 10.1039/c1cs15203c. [DOI] [PubMed] [Google Scholar]
- 14.Lim CT, Ren X, Afizah MH, Tarigan-Panjaitan S, Yang Z, Wu Y, Chian KS, Mikos AG, Hui JH. Repair of osteochondral defects with rehydrated freeze-dried oligo[poly(ethylene glycol) fumarate] hydrogels seeded with bone marrow mesenchymal stem cells in a porcine model. Tissue Eng Part A. 2013;19:1852–1861. doi: 10.1089/ten.TEA.2012.0621. [DOI] [PubMed] [Google Scholar]
- 15.Lozito TP, Tuan RS. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J Cell Physiol. 2011;226:385–396. doi: 10.1002/jcp.22344. [DOI] [PubMed] [Google Scholar]
- 16.Lu S, Lam J, Trachtenberg JE, Lee EJ, Seyednejad H, van den Beucken JJJP, Tabata Y, Wong ME, Jansen JA, Mikos AG, Kasper FK. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials. 2014;35:8829–8839. doi: 10.1016/j.biomaterials.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mannello F, Tonti GA, Bagnara GP, Papa S. Role and function of matrix metalloproteinases in the differentiation and biological characterization of mesenchymal stem cells. Stem Cells. 2006;24:475–481. doi: 10.1634/stemcells.2005-0333. [DOI] [PubMed] [Google Scholar]
- 18.Meretoja VV, Dahlin RL, Wright S, Kasper FK, Mikos AG. The effect of hypoxia on the chondrogenic differentiation of co-cultured articular chondrocytes and mesenchymal stem cells in scaffolds. Biomaterials. 2013;34:4266–4273. doi: 10.1016/j.biomaterials.2013.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakajima K, Fujita J, Matsui M, Tohyama S, Tamura N, Kanazawa H, Seki T, Kishino Y, Hirano A, Okada M, Tabei R, Sano M, Goto S, Tabata Y, Fukuda K. Gelatin Hydrogel Enhances the Engraftment of Transplanted Cardiomyocytes and Angiogenesis to Ameliorate Cardiac Function after Myocardial Infarction. PLoS One. 2015;10:e0133308. doi: 10.1371/journal.pone.0133308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen AH, Wang Y, White DE, Platt MO, McDevitt TC. MMP-mediated mesenchymal morphogenesis of pluripotent stem cell aggregates stimulated by gelatin methacrylate microparticle incorporation. Biomaterials. 2016;76:66–75. doi: 10.1016/j.biomaterials.2015.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:149–165. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28:3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reilly GC, Engler AJ. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech. 2010;43:55–62. doi: 10.1016/j.jbiomech.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055–4063. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- 25.Santoro M, Tatara AM, Mikos AG. Gelatin carriers for drug and cell delivery in tissue engineering. J Control Release. 2014;190:210–218. doi: 10.1016/j.jconrel.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sart S, Tsai AC, Li Y, Ma T. Three-dimensional aggregates of mesenchymal stem cells: cellular mechanisms, biological properties, and applications. Tissue Eng Part B Rev. 2014;20:365–380. doi: 10.1089/ten.teb.2013.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 28.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabata Y, Hijikata S, Muniruzzaman M, Ikada Y. Neovascularization effect of biodegradable gelatin microspheres incorporating basic fibroblast growth factor. Journal of Biomaterials Science-Polymer Edition. 1999;10:79–94. doi: 10.1163/156856299x00298. [DOI] [PubMed] [Google Scholar]
- 30.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Lu S, Lam J, Kasper FK, Mikos AG. Fabrication of cell-laden macroporous biodegradable hydrogels with tunable porosities and pore sizes. Tissue Eng Part C Methods. 2015;21:263–273. doi: 10.1089/ten.tec.2014.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]