Summary
Hematopoietic stem cell (HSC) transplantation is the most prevalent stem cell therapy, but it remains a risky procedure. To improve this treatment, it is important to understand how transplanted stem cells rebuild the blood and immune systems and how this process is impacted by transplantation variables such as the HSC dose. Here we find that in the long term following transplantation, 70–80% of donor-HSC-derived clones do not produce all measured blood cell types. High HSC doses lead to more clones that exhibit balanced lymphocyte production while low doses produce more T-cell specialized clones. High HSC doses also produce significantly higher proportions of early-differentiating clones compared to low doses. These complex differentiation behaviors uncover the clonal-level regeneration dynamics of hematopoietic regeneration, and suggest that transplantation dose can be exploited to improve stem cell therapy.
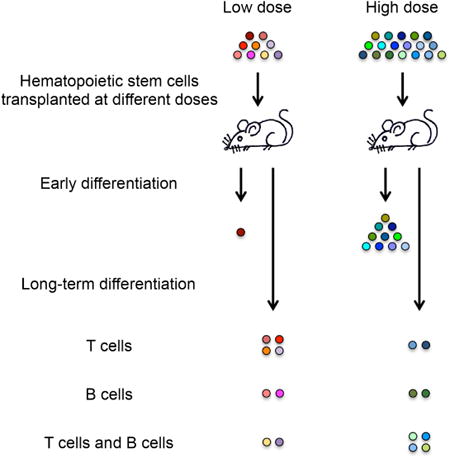
Introduction
Hematopoietic stem cells (HSCs) replenish the blood and immune systems. Residing in the bone marrow, each HSC is capable of generating every blood and immune cell type (Barker et al., 2010; Bryder et al., 2006). Since the mid-20th century, scientists have recognized HSCs as a potential cure for patients suffering from hematologic diseases or injuries (Copelan, 2006). HSC transplantation, also known as bone marrow transplantation, is currently used to treat a variety of blood diseases, to reset the immune system during organ transplantation, and to regenerate blood systems destroyed by radiation and chemotherapy during cancer treatment (Kondo et al., 2003). It remains the only cure option for many diseases. While millions of patients could potentially benefit from HSC transplantation, only a small fraction of these patients undergo the procedure due to high treatment-related mortality (Copelan, 2006). Most adverse incidents arise from infection or from graft-versus-host complications following the procedure. In addition, patients with hematological malignancies such as leukemia often suffer relapse following disease remission. A better understanding of how HSCs rebuild the blood and immune system post transplantation will help develop a safer and more effective therapy.
While much has been learned about HSC transplantation in recent years, most of our knowledge comes from population-level analyses. In these studies, a population of HSCs is isolated using cell-surface markers, and their progeny analyzed at the population level. Limiting dilution assays of HSC transplantation suggest that the number of donor HSCs quantitatively determines the fraction of blood cells that they produce (Eaves et al., 1997; Purton and Scadden, 2007). These experiments support a simple model for HSC coordination in which individual HSCs play equal roles and uniformly alter their blood production in response to changes in hematopoiesis. This simple, homogeneous model was challenged by recent work from our group and others indicating the heterogeneity of HSC differentiation at the single-cell level (Beerman et al., 2010; Benz et al., 2012; Dykstra et al., 2007; Ergen et al., 2012; Lu et al., 2011; McKenzie et al., 2006; Sieburg et al., 2006; Yamamoto et al., 2013). For instance, individual HSC clones supply differential amounts of blood cells in mice and in human patients (McKenzie et al., 2006) (Weksberg et al., 2008)(Fehse and Roeder, 2008)(Roeder et al., 2005)(Nienhuis, 2008) (Yamamoto et al., 2013). They also exhibit distinct differentiation preferences for myeloid or lymphoid lineages post transplantation (Beerman et al., 2010; Cho et al., 2008; Dykstra et al., 2007; Lu et al., 2011; Sieburg et al., 2006). In addition, recent studies of native hematopoiesis suggest that different blood cell types have distinct clonal origins as well (Pietras et al., 2015; Sun et al., 2014). These findings raise the question of how the diverse differentiation programs of individual HSCs are coordinated following transplantation. Manipulating this coordination may provide alternative approaches to controlling HSC differentiation and to improving stem cell therapy.
Previous studies showed that the regeneration of the blood supply post transplantation occurs in two phases (Camargo et al., 2006; Eaves, 2015; Morrison and Weissman, 1994). Immediately after transplantation, HSCs and short-term hematopoietic progenitors collectively supply blood cells. Four months later, HSCs are thought to be the only cells to supply every blood cell type as short-term progenitor cells lack the capacity for long-term self-renewal. This two-phase mode of blood supply suggests that the coordination of HSC blood production changes during the blood reconstitution process. Immediately after transplantation, HSC clones must respond to the presence of short-term progenitors and to the urgent need for blood cells, while four months later, HSCs only have to contend with themselves. A full understanding of HSC differentiation requires knowledge of HSC clonal behavior during both phases of blood production.
Understanding how individual HSC clones heterogeneously differentiate over time after transplantation has significant clinical implications. For example, identifying and promoting HSCs that differentiate immediately following the transplantation procedure may reduce the risk for infection, one of the most serious side effects of HSC transplantation. In addition, after decades of practice, no gold standard exists for determining the optimal HSC transplantation dose. It is even unknown what proportion of transplanted HSCs actually engrafts and produces blood. Thus, in practice, patients receive donor HSCs over a wide range of doses. Studies have reported mixed results on the effects of transplantation dose in various groups of patients (Díez-Campelo et al., 2005; Dominietto et al., 2002; Gorin et al., 2006; Mehta et al., 2009; Panse et al., 2005; Perez-Simon et al., 2003). These differences may have arisen from HSC clonal behaviors that were not readily apparent at the population level.
To better understand how individual HSCs supply blood cells post transplantation and how they are affected by the transplantation dose, we used a genetic barcoding technology (Lu et al., 2011; Wu et al., 2014) to track the blood production of individual mouse HSCs transplanted over a wide range of doses. We found that the majority of transplanted HSCs do not exhibit the multipotent behavior manifested at the population level, but rather only produces one or two measured cell types in a “specialized” manner. More importantly, these “specialized” differentiation programs and their temporal patterns change with the transplantation dose.
Results
More Donor HSCs Engraft and Produce Every Cell Type at High Transplantation Doses
Purified mouse HSCs were labeled with genetic barcodes as described (Lu et al., 2011; Wu et al., 2014) and transplanted at four doses into lethally irradiated mice. The highest dose used is equal to approximately a quarter to a half of a mouse's total endogenous HSC population. The doses used in most human bone marrow transplantations range between the middle two doses that we used (Copelan, 2006). Half million non-barcoded, unpurified whole bone marrow cells were co-transplanted alongside the purified barcoded HSCs as helper cells. The purified HSCs (lineage (CD3, CD4, CD8, B220, Gr1, Mac1, Ter119)-/ckit+/Sca1+/Flk2-/CD34-/CD150+) have been shown to produce all blood cell types at the population level and in single cell transplantation experiments (Seita and Weissman, 2010). We assessed HSC differentiation after transplantation using the four most abundant white blood cell types: granulocytes, B cells, CD4 T cells and CD8 T cells. Together, these four cell types constitute 70–80% of all white blood cells in the peripheral blood (Figure S1).
Before transplantation, individual HSC clones were labeled with unique genetic barcodes that are inherited by progeny cells (Lu et al., 2011; Wu et al., 2014). These genetic barcodes were recovered from genomic DNA and precisely quantified by high-throughput sequencing. Barcode data and flow cytometry data were combined together to quantify the contribution of individual HSC clones to each blood cell type. Clones that contributed less than 0.01% of an examined blood cell population were considered to be absent and removed from analysis. Our clonal tracking system offers high reproducibility (Figure S2) and high fidelity (Figure S3), two qualities that are essential for distinguishing between various HSC differentiation programs.
We found that the donor chimerisms of all measured blood cell types were proportional to the transplantation dose, consistent with previous limiting dilution experiments (Figure 1A and S4) (Eaves et al., 1997; Purton and Scadden, 2007). One month after transplantation, host cells rapidly cleared from the granulocyte and B-cell populations, but still sustained nearly 80% of the T-cell population. T-cell production by host cells dropped sharply after one month, and was unaffected by the donor HSC dose. Non-barcoded helper cells contain HSCs that assisted in blood production. Identical quantities of non-barcoded whole bone marrow cells supplied differential amounts of blood cells when combined with different doses of barcoded HSCs, suggesting that blood production was coordinated between the two populations of transplanted cells. Despite differences in donor chimerisms across different HSC transplantation doses, the relative sizes of the granulocyte, B-cell, CD4 T-cell and CD8 T-cell populations were the same for all HSC transplantation doses (Figure S1).
Figure 1. More Donor HSCs Engraft and Produce Every Cell Type at High Transplantation Doses.
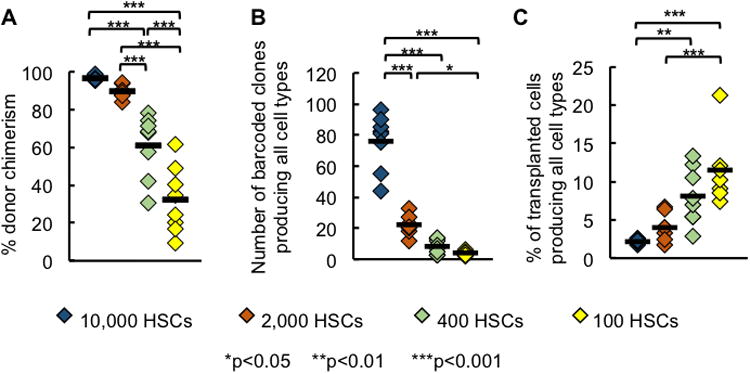
(A) Granulocyte donor chimerism at each transplantation dose.
(B) Number of barcoded clones that produced every measured cell type (granulocytes, B cells, CD4 T and CD8 T cells) at each HSC transplantation dose.
(C) Percentage of transplanted clones that produced every measured cell type at each HSC transplantation dose. Data in Figure 1B was divided by the number of transplanted HSCs that carry barcodes as shown by GFP expression.
Each diamond represents one mouse; horizontal black lines represent group means. All data were collected six months post transplantation. 7-8 mice were used per transplantation dose.
HSCs are defined as cells that supply every hematopoietic cell type long term after transplantation. We quantified the number of HSC clones that produced every measured blood cell types (granulocytes, B cells, CD4 T cells, and CD8 T cells) six months after transplantation. By this time, hematopoiesis has reached a steady state and a distinct group of HSC clones consistently supply all blood cells over time (Jordan and Lemischka, 1990; Prchal, 1996; Wu et al., 2014). We found that the number of HSC clones that produced every measured cell type is correlated with the HSC transplantation dose (Figure 1B). However, the fraction of donor-derived HSCs that supplied every cell type is inversely correlated with the transplantation dose (Figure 1C), indicating a limit to engraftment. This limit may arise from limited niche availability (Czechowicz et al., 2007; Zhang et al., 2003). Overall, the number of long-term clones that produced every cell type was only a small fraction of the entire transplanted HSC population, ranging from 1.5% at the highest transplantation dose to 10% at the lowest transplantation dose. Therefore, it is important to assess the activities of donor-derived clones that differentiate early (Figure 2), as well as clones that do not contribute to every blood cell type six months after transplantation (Figure 3).
Figure 2. High HSC Doses Increase the Number of Early Differentiating Clones.
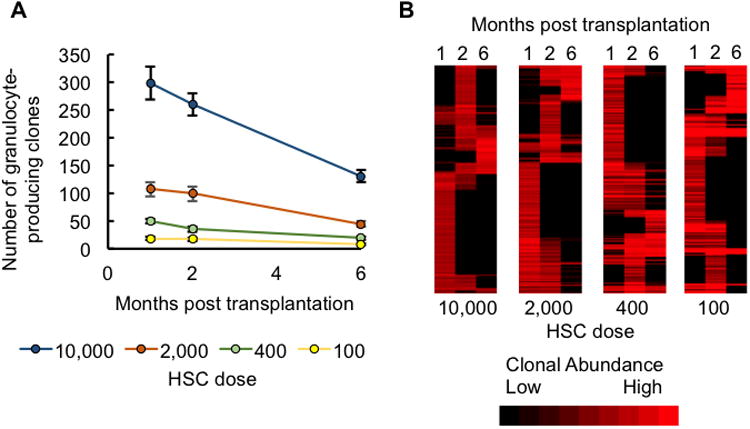
(A) The number of clones that produced granulocytes at one, two, and six months post transplantation. Data points represent group means. Error bars represent standard error of the mean. See Table S1 for significance values.
(B) A heatmap depicting granulocyte production by individual HSC clones at one, two, and six months post transplantation. Clones from all examined mice at each transplantation dose are shown. The presence of a red band in a column represents granulocyte production by an HSC clone at that time point. A red band stretching across multiple columns represents an HSC clone producing granulocytes at multiple time points. Bright red bands represent high levels of granulocyte production. Black represents the lack of granulocyte production by an HSC clone at that time point. Clones are clustered based on the similarity of their granulocyte production over time. There were 7-8 mice per dose group.
Figure 3. Most Long-Term Clones Do Not Produce Every Blood Cell Type.
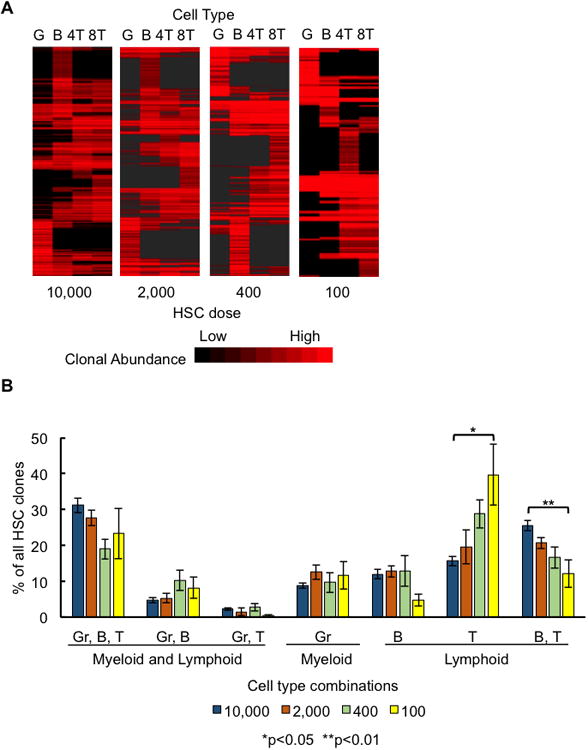
(A) A heatmap depicting the production of granulocytes, B cells, CD4T, and CD8T cells by individual HSC clones. Clones from all examined mice at each transplantation dose are shown. A red band in a column represents an HSC clone producing that specific cell type. A red band stretching across multiple columns represents an HSC clone producing multiple cell types. Bright red bands indicate high levels of blood cell production. Black represents the lack of production of that cell type by an HSC clone. Clones are clustered based on the similarity of their white blood cell production six months after transplantation.
(B) Clones were categorized by the combination of cell types they produced six months post transplantation. All possible combinations of measured cell types are displayed in the graph. A clone was considered to produce T cells if CD4T and/or CD8T cells were detected among its progeny. Bar graphs display the percentage of clones belonging to that category. Percentages were calculated separately for each mouse and then averaged across each HSC dose group. Error bars represent standard error of the mean. 7-8 mice were used per transplantation dose.
High HSC Doses Significantly Increase the Number of Early Differentiating Clones
We examined early differentiating clones using granulocytes harvested one and two months after transplantation (Figure 2), because granulocyte production has been shown to best represent HSC differentiation in the bone marrow (Czechowicz et al., 2007). At month one, significantly more granulocyte-producing clones were found in mice transplanted with more HSCs (Figure 2A and Table S1). At month two, the difference in the number of granulocyte-producing clones between the two lowest HSC transplantation doses had greatly narrowed. By month six, the three lowest HSC transplantation doses showed little difference in the number of granulocyte-producing clones. Mice transplanted with the highest dose showed a steady drop in the number of blood-supplying clones over time, such that the difference between the highest and lowest doses was substantially reduced. Moreover, most clones did not persist in producing granulocytes overtime (Figure 2B). These results suggest that the HSC transplantation dose strongly affects the number of early differentiating clones, and that its impact diminishes over time. The increase in the number of early-differentiating clones at high transplantation doses can account for the rapid regeneration of blood immediately after transplantation especially in the lymphoid lineages, as evidenced by the donor chimerisms of purified HSCs (Figure S4).
Most Long-Term Clones Do Not Produce Every Blood Cell Type
Previous population-level studies had suggested that the hematopoietic progenitors that produce restricted subsets of blood cell types are short-lived cells that only supply blood cells during the first four months after transplantation. After that initial period, all blood cell types were believed to be supplied by multipotent HSCs (Camargo et al., 2006; Eaves, 2015; Morrison and Weissman, 1994). Surprisingly, we found that most long-term clones did not produce every measured blood cell type six months after transplantation (Figure 3A). The fraction of long-term clones that produced every measured cell type only ranged between 20–30%, and increased with the transplantation dose (Figure 3B). The other 70–80% of clones specialized in producing only a single or a subset of blood cell types. These specialized HSCs produced a significant portion of the measured blood cells (Figure 5).
Figure 5. All-Cell-Type Clones Produce Disproportionately Large Numbers of Blood Cells.
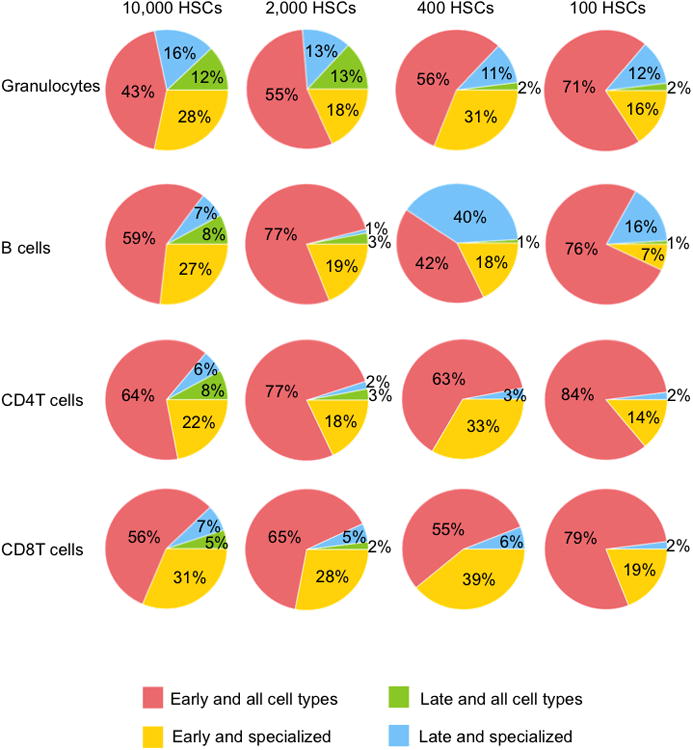
Clones were categorized as “early and all cell types” (produced granulocytes one and/or two months post transplantation, and produced every measured cell type six months after transplantation), “late and all cell types” (produced every measured cell type six months after transplantation, but did not produce granulocytes one or two months post transplantation), early and specialized (produced granulocytes one and/or two months post transplantation, but did not produce every cell type six months post transplantation) or late and specialized (did not produce granulocytes one or two months post transplantation, and did not produce every cell type six months post transplantation). Percentages represent the average amount of blood cell production by a category of clones six months post transplantation. If the average amount of blood production by a category of clones was less than 0.5%, it was not shown. See also Tables S4-S5. 7-8 mice were used per transplantation dose.
A significant number of long-term clones specialized to produce just a single cell type (Figure 3A). Approximately 10% of all HSC clones produced only granulocytes at every HSC dose (Figure 3B). A similar percentage of clones specialized in producing B cells. The fraction of long-term clones exclusively producing T cells is inversely correlated with the transplantation dose, reaching 40% at the lowest transplantation dose. The increase in T cell–only clones suggests that T cell generation is prioritized when donor HSCs are limited. In contrast, clones producing both B and T cells showed the opposite dose-dependent effects and are less abundant at lower HSC doses. These dose-dependent changes suggest that the HSC differentiation program is not predetermined, but is rather significantly influenced by the transplantation dose.
Individual HSC Clones Exhibit Distinct Differentiation and Temporal Patterns
Individual HSC clones exhibit distinct patterns of differentiation over time (Figure 2-3). To classify these patterns, we separated long-term HSC clones into two categories: (1) “all-cell-type” clones that produced every measured cell type, and (2) “specialized” clones that only produced a subset of cell types. We also classified “early-differentiating” clones based on granulocyte data from months one and two post transplantation. The number of clones in each category and the overlaps between different categories vary significantly with the transplantation dose (Figure 4 and Tables S2-S3).
Figure 4. HSC Clones Exhibit Various Differentiation and Temporal Patterns.
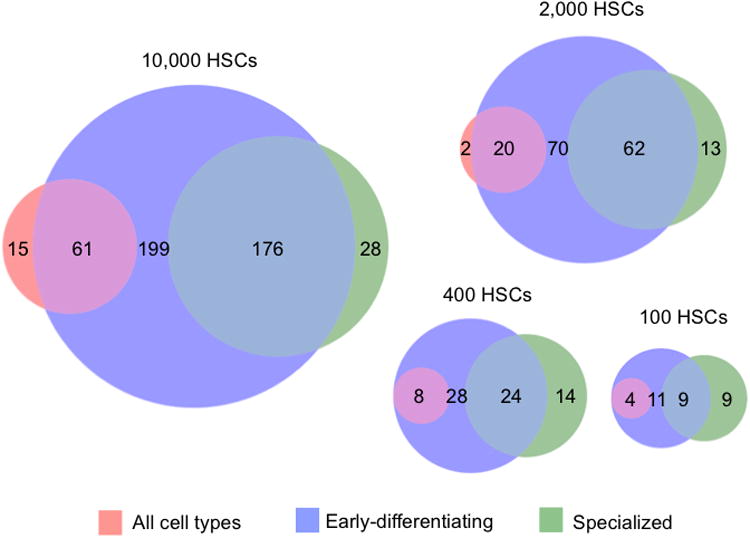
Clones were categorized as “early-differentiating” (produced granulocytes one and/or two months post transplantation), “all cell types” (produced every measured cell type six months post transplantation), and/or “specialized” (did not produce every measured cell type six months post transplantation). The Venn diagrams show the size and overlap of these three categories of clones at each HSC transplantation dose. Numbers represent the mean clone counts for each category by HSC dose. See also Tables S2-S3 for significance values. 7-8 mice were used per transplantation dose.
At high transplantation doses, a large number of transplanted HSCs differentiated early after transplantation (Figure 4 and Table S2). A small portion of transplanted clones produced every measured blood cell type. These clones often differentiated early at all transplantation doses. At the two lowest HSC transplantation doses, all HSC clones that produced every cell type had differentiated early. Thus, early differentiation does not prevent HSC clones from self-renewing and maintaining their HSC identity. Conversely, specialized clones constitute a large proportion of all clones across all HSC transplantation doses. The number of specialized clones that differentiated early is proportional to the transplantation dose.
To determine the probability for an HSC to commit to a particular differentiation program, we calculated the fraction of donor-derived clones in each differentiation category (Table S3). We found that significantly larger fractions of clones differentiated at early time points when transplanted at the highest two doses compared to the lowest dose. Also, a larger proportion of clones appeared late and produced every cell type at the highest dose than at the three lower doses. Conversely, a larger proportion of clones appeared late and produced specialized cell types at the lowest transplantation dose than at the two highest doses. Therefore, at a high transplantation dose, donor HSC clones are more likely to differentiate early. Furthermore, late differentiating clones are more likely to produce all cell types when transplanted at a high dose, and are more likely to exhibit specialized blood production when transplanted at a low dose. These dose-dependent changes again demonstrate how the HSC differentiation program changes with the transplantation dose.
All-Cell-Type Clones Produce Disproportionately Large Numbers of Blood Cells
HSC clones that exhibit different differentiation patterns also differ in the quantity of their blood production. Here, we quantified how much each category of clones contributed to the production of granulocytes, B cells, CD4 T cells, and CD8 T cells six months after transplantation (Figure 5 and Table S4). At the highest HSC transplantation dose, all categories of clones contributed to every measured blood cell type. At lower transplantation doses, late/all-cell-type clones contributed little-to-no blood production, while early/all-cell-type clones produced the majority of blood cells. Early-differentiating and specialized clones consistently contributed a considerable proportion of blood cells across all transplantation doses, and this tended to decrease with the transplantation dose (Figures 5 and Table S4). Late-differentiating and specialized clones contributed significantly less to blood production than the other categories of clones, especially at low doses (Figures 5 and Table S4). An exception is B cells where late-differentiating and specialized clones significantly contributed at low transplantation doses (Figures 5 and Table S5). The data suggest that low HSC transplantation doses compel early-differentiating clones to self-renew and to continue producing every blood cell type at later time points. At high transplantation doses, early-differentiating clones are replaced by other clones at later time points and are not as crucial to long-term hematopoiesis. Across the range of HSC transplantation doses assessed in this experiment, the number of all-cell-type clones was low, but their contribution to blood production was disproportionately high (Figures 5 and Tables S4-S5). This was especially true at low HSC transplantation doses.
To characterize the donor-derived clones that supply blood immediate after transplantation, we quantified granulocyte production from months one and two after transplantation (Figure S5). These early-differentiating clones were categorized based on their later differentiation patterns: all cell type, specialized cell types, or no cell types (“early-only”). The HSC transplantation dose most strongly affected early-only clones. They produced a significantly larger proportion of early granulocytes at high transplantation doses (Table S6). At low HSC transplantation doses, a large proportion of early granulocytes were produced by clones that would later produce “all cell types” (Table S7). This is consistent with the previous analysis at month six post transplantation (Figure 5) where early-differentiating clones were more likely to persist and produce all cell types at low HSC doses.
Maximum Blood Production by an Individual HSC Clone Is Not Affected by the Transplantation Dose
Even though fewer clones supplied blood at low transplantation doses (Figure 4), we found surprisingly that the most abundant clones produced similar amounts of blood cells across different transplantation doses six months after transplantation (Figure 6A). However, dose-dependence was observed one and two months after transplantation, when granulocyte production by the most abundant clones was significantly less at high HSC doses than at the low HSC doses (Figure 6B). At the highest transplantation dose, granulocyte production by the most abundant clone significantly increased over time after transplantation. Other transplantation doses also exhibited similar temporal trends. A few outliers supplied a large portion of blood cells particularly in granulocyte production and at low transplantation doses (Figures 6A and 6B). These outliers were not present at the highest transplantation dose (Figures 6A and 6B). Overall, the maximum blood production of single clones is largely independent of the transplantation dose, indicating a physiological limit for HSC clonal expansion in supplying blood.
Figure 6. Maximum Blood Production by Individual HSC Clones Is Not Affected by the Transplantation Dose.
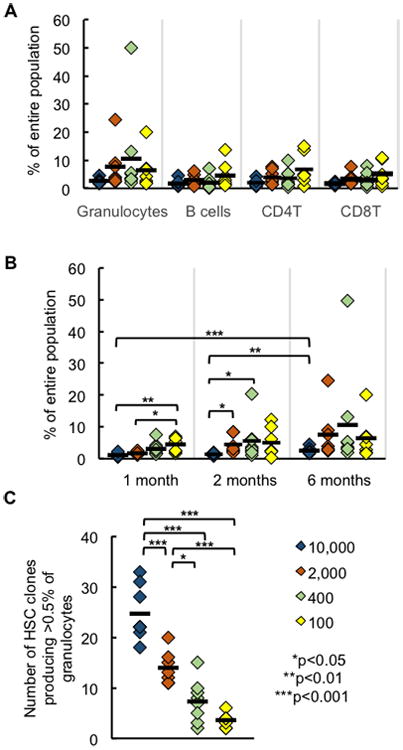
(A) Percentage of blood cells produced by the most abundant clone six months after transplantation.
(B) Percentage of granulocytes produced by the most abundant clone at different time points.
(C) Number of clones that produced at least 0.5% of all granulocytes in each mouse.
Each diamond represents one mouse; horizontal black lines represent group means. All data were collected six months post transplantation. 7-8 mice were used per transplantation dose.
While HSC transplantation dose had little effect on the magnitude of clonal expansion, it altered the number of expanded clones (Figure 6C and S6). We quantified the number of clones that produced at least 0.5% of all granulocytes at each transplantation dose. Our calculations revealed that high HSC transplantation doses resulted in the expansion of significantly more HSC clones (Figure 6C). We obtained similar results by quantifying the number of clones producing at least 0.1% of all granulocytes (Figure S6). Thus, the number of HSC clones that expanded is correlated with donor HSC dose, although their maximum expansion levels were not influenced by transplantation dose.
Increased Lineage Balance is Associated with High HSC Doses
Recent studies suggest that HSC differentiation can be biased towards a specific hematologic lineage (Beerman et al., 2010; Cho et al., 2008; Dykstra et al., 2007; Lu et al., 2011; Sieburg et al., 2006). To determine how this bias is impacted by the transplantation dose, we measured the lineage bias of individual HSCs at different transplantation doses. In particular, we compared the clonal production between granulocytes and B cells, and between B cells and T cells six months after transplantation (Figure 7 and S7). A clone was categorized as “specialized” to a cell type if it only produced that cell type, “biased” to a cell type if it favored producing that cell type while also contributing to the other cell type, and “balanced” if it produced similar amounts of both cell types.
Figure 7. Balanced Lymphoid Production Is Associated with High HSC Doses.
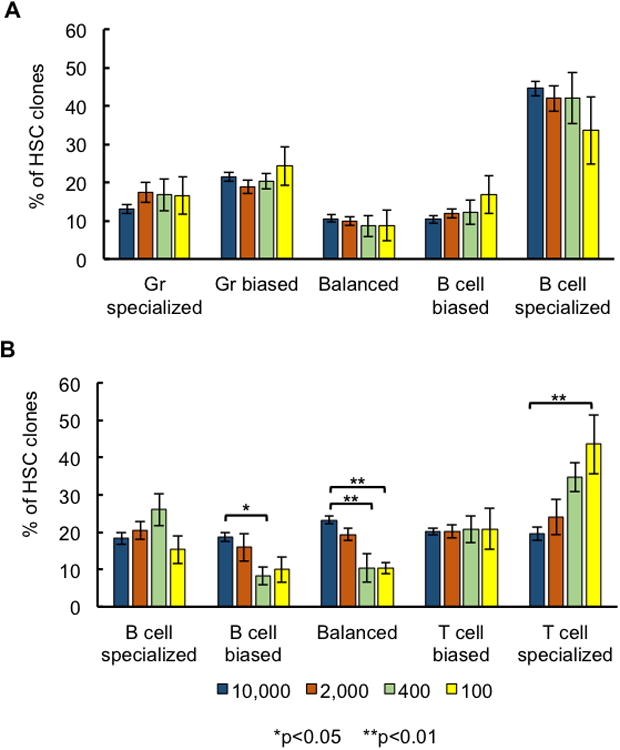
A clone was considered to be “specialized” to a cell type if it only produced that cell type, “biased” to a cell type if it favored the production of that cell type, and “balanced” if its relative production of the two cell types was similar. The mean percent is displayed for each HSC dose. Error bars represent the standard error of the mean. (A) Granulocyte production was compared to B-cell production for each HSC clone. (B) B-cell production was compared to T-cell production for each HSC clone. Error bars represent standard error of the mean. 7-8 mice were used per transplantation dose.
We found that the number of specialized, biased, and balanced clones producing granulocytes over B cells and over T cells is consistent across all transplantation doses (Figure 7A and S7). However, in the production of B cells over T cells, higher HSC doses yielded significantly more donor HSC clones with balanced lymphocyte production (Figure 7B). In addition, high HSC doses were associated with an increase in B-cell biased clones and a decrease in T-cell specialized clones (Figure 7B). These results demonstrate that HSC dose alters the clonal production of lymphocytes, with high HSC doses generating a greater proportion of balanced lymphocyte-producing clones, and low HSC doses generating a greater proportion of biased lymphocyte-producing clones.
Discussion
In this study, we demonstrated that the differentiation programs of individual stem cells are specialized and are coordinated differently at different transplantation doses. While our data is consistent with previous studies at the population level (Figures S1 and S4) (Eaves et al., 1997; Purton and Scadden, 2007; Spangrude et al., 1988), our discoveries at the clonal level reveal unexpected stem cell differentiation characteristics that differ from their collective population-level behaviors.
We discovered that HSCs are specialized in their differentiation to an unexpected degree. Previous population-level studies suggest that HSCs are the only cells that supply blood long term after transplantation, and that they simultaneously supply every blood lineage. In contrast, we have discovered at the clonal level that 70–80% of all donor-HSC-derived clones do not supply every measured blood cell type six months after transplantation (Figure 3). This unexpected discovery is counter to results from previous single-cell transplantation experiments where a single HSC is forced to supply every blood cell type to ensure the survival of the organism (Dykstra et al., 2007; Osawa et al., 1996; Yamamoto et al., 2013). In these single-cell transplantation experiments, long-term specialized differentiation was also observed, although not extensively discussed. Recent studies suggest that a similar specialized differentiation program takes place under homeostasis (Sun et al., 2014) and in human HSCs (Notta et al., 2015). Here, our data show that HSCs specialize and coordinate their differentiation programs when transplanted as a population. The specialized differentiation program may be induced by the local environment as the cytokine milieu post transplantation may not be uniformly distributed to all HSCs throughout the body (Morrison and Scadden, 2014).
HSCs adapt their differentiation programs to different transplantation doses. Particularly, low transplantation doses produced significantly more T-cell-specialized HSCs, while high transplantation doses produced a greater proportion of balanced lymphocyte-producing clones (Figures 3B and 7B). Decades of studies to identify and isolate stem cells have relied on the assumption, or functional definition, that stem cells will reliably differentiate in a predetermined manner. Here, we found that stem cells changed their differentiation programs when transplanted at different doses. These changes may be modulated by HSC niche or by co-transplanted helper bone marrow cells. Future studies on how HSC transplantation dose influences niche cells and short-term progenitor cells can provide further insights. We have shown here that altering the HSC transplantation dose results in long-term changes to HSC behavior. While the specializations of individual HSCs necessitate their coordination to ensure overall balanced blood production, the dose-dependency suggest this coordination is altered by adding or removing HSCs.
In this study, granulocytes, B cells and T cells were examined as differentiation end products. Other cell types, such as natural killer cells, may derive from a different repertoire of HSC clones (Wu et al., 2014). With our genetic barcoding technique, we could not directly measure red blood cells and platelets due to their lack of genomic DNAs. Additionally, our analyses of early differentiating clones relied on measurements at the one and two-month time points post transplantation, and may have missed clones that differentiated at other times. However, additional data will not change the conclusions from this study that a substantial fraction of HSC clones do not produce all blood cell types and that the transplantation dose changes HSC differentiation programs at the clonal level.
Our results have important medical implications. For example, to help patients survive the critical immune-deficient period immediately following transplantation, it is useful to know that high transplantation dose promotes a greater number of transplanted HSCs to immediately supply blood (Figure 2). This may help recipients better survive the immune-deficient period which is a significant risk to patients post transplantation (Dominietto et al., 2002). While immediate differentiation after transplantation is generally thought to be stressful to cells, our data suggest that this does not prevent early differentiating clones from self-renewing and supplying all blood cell types later on. In fact, most clones that supply all blood cell types over the long term had supplied blood shortly after transplantation, especially at low transplantation doses (Figure 4).
While the “all-cell-type” clones were few in number, the actual blood production from these clones was significantly higher than other clones (Figure 5). This illustrates the importance of the all-cell-type clones in reconstituting the hematopoietic system after transplantation, despite their relative scarcity. Promoting the all-cell-type phenotype could substantially improve the efficacy of bone marrow transplantation. This could be achieved in future studies by isolating HSC clones pre-disposed towards the all-cell-type phenotype or by exploiting the regulatory mechanism underlying stem cell coordination to promote the all-cell-type phenotype.
Our data also reveals a physiological limit for donor stem cell expansion during differentiation (Figure 6). Previously, a few dominant clones were reported to supply the majority of blood production after mouse and human HSC transplantation (McKenzie et al., 2006) (Weksberg et al., 2008)(Fehse and Roeder, 2008)(Roeder et al., 2005)(Nienhuis, 2008) (Yamamoto et al., 2013). We had expected lower transplantation doses to produce higher levels of clonal dominance. But surprisingly, we found that the magnitude of clonal dominance does not change over the range of transplantation doses that we examined (Figure 6). Thus, normal HSC differentiation seems to have a limit for expansion. This has profound implications for cell or gene therapies that rely on donor cell expansion, as it may be more challenging to repopulate the patient with altered genetic material than previously expected.
In summary, our data provide a clonal-level perspective on HSC differentiation post transplantation and on its alteration by HSC transplantation dose. These findings offer insights into the complex effects that transplantation dose has on patient survival during bone marrow transplantation (Díez-Campelo et al., 2005; Dominietto et al., 2002; Gorin et al., 2006; Mehta et al., 2009; Panse et al., 2005; Perez-Simon et al., 2003). Patients with different pathogeneses might benefit from distinct differentiation programs associated with particular transplantation doses.
Experimental Procedures
Mice
The donor mice used in the experiments were 8–12-week-old C57BL6/Ka (CD45.1+) mice. The recipient mice were 8–12-week-old C57BL6/Ka (CD45.2+) mice. At each transplantation dose, 7–8 mice were examined. Helper cells that were co-transplanted with HSCs consisted of F1 whole bone marrow cells (CD45.2+/ CD45.1+). 950 cGy irradiation was performed on all mice immediately before transplantation. All animal procedures were approved by the International Animal Care and Use Committee.
Cell Isolation and Transplantation
HSCs (lineage (CD3, CD4, CD8, B220, Gr1, Mac1, Ter119)-/ckit+/Sca1+/Flk2-/CD34-/CD150+) were obtained from the crushed bones of donor mice and isolated using double fluorescence-activated cell sorting (FACS) with the FACS-Aria II (BD Biosciences, San Jose, CA) after enrichment using CD117 microbeads (AutoMACS, Miltenyi Biotec, Auburn, CA). HSCs were infected with lentivirus carrying barcodes and GFP before transplantation for 10 hours. HSC clonal labeling was as described in Lu et al., 2011. Helper cells (i.e., whole bone marrow cells) were flushed from the femurs of F1 mice and added to HSCs right before transplantation. Half million helper cells were transplanted per mouse. Transplantation was performed via retro-orbital injection.
Blood Sample Collection and FACS Analysis
Blood samples were collected into PBS containing 10 mM EDTA via a small transverse cut in the tail vein. To separate red blood cells, 2% dextran was added, and the remaining blood cells were treated with ammonium-chloride-potassium lysis buffer on ice for 5 minutes to remove residual red blood cells. After a 30-minute antibody incubation at 4 °C, samples were resuspended in propidium iodide solution (1:5000 in PBS with 2% FBS). Cells were sorted using a FACS-Aria II cell sorter and separated into granulocytes, B cells, CD4T cells and CD8T cells. Antibodies were obtained from eBioscience and BioLegend as described in Lu et al., 2011. Donor cells were sorted based on the CD45 marker. The following cell-surface markers were used to sort the different hematopoietic populations:
Granulocytes: CD4-/CD8-/B220-/CD19-/Mac1+/Gr1+/side scatterhigh
B cells: CD4-/CD8-/Gr1-/Mac1-/B220+/CD19+
CD4 T cells: B220-/CD19-/Mac1-/Gr1-/TCRαβ+/CD4+/CD8-
CD8 T cells: B220-/CD19-/Mac1-/Gr1-/TCRαβ+/CD4-/CD8+
Flow cytometry data were analyzed using FlowJo software version 9.6.1 (Tree Start, Ashland, OR).
DNA Barcode Extraction and Sequencing
Genomic DNA (gDNA) was extracted from hematopoietic cells and amplified using Phusion PCR mastermix (Thermo Scientific, Waltham, MA). The PCR reactions were halted once they had progressed halfway through the exponential phase. PCR product was purified and analyzed using high-throughput sequencing at the USC Epigenome Center. Sequencing data were analyzed as described (Lu et al., 2011; Wu et al., 2014). The lenti viral vector that delivers the genetic barcodes also conveys GFP expression that marks the barcoded cells. We combined the sequencing data with the FACS data to generate clonal abundance for each HSC clone:
Statistical Analysis
All group comparisons were evaluated by 1-way analysis of variance (ANOVA). Post-hoc analysis was done by Tukey test. For nonparametric data, group comparisons were evaluated via Kruskal-Wallis test with Dunn post-hoc test. Significance was defined as p<0.05. Error bars in all figures represent the standard error of the mean.
Supplementary Material
Acknowledgments
We thank A. Nogalska, Q. Liu, C. Lytal, G. Howard, and Z. Huang for helpful discussions. We also thank A. Nogalska for laboratory management; thank L. Barsky for FACS core management; thank C. Nicolet for helping with the high-throughput sequencing. This work is supported by NIH-R00-HL113104 and NCI P30CA014089. C.B. is supported by CIRM-TG2-01161.
Footnotes
Author Contributions: C.B. and R.L. designed and performed the experiments. E.C. wrote custom Python codes for data analysis. M.C. assisted with experiments. All authors analyzed the data. C.B. and R.L. wrote the manuscript. All authors edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker N, Bartfeld S, Clevers H. Tissue-Resident Adult Stem Cell Populations of Rapidly Self-Renewing Organs. Cell Stem Cell. 2010;7:656–670. doi: 10.1016/j.stem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, Rossi DJ. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci USA. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz C, Copley MR, Kent DG, Wohrer S, Cortes A, Aghaeepour N, Ma E, Mader H, Rowe K, Day C, et al. Hematopoietic Stem Cell Subtypes Expand Differentially during Development and Display Distinct Lymphopoietic Programs. Cell Stem Cell. 2012;10:273–283. doi: 10.1016/j.stem.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo FD, Chambers SM, Drew E, McNagny KM, Goodell MA. Hematopoietic stem cells do not engraft with absolute efficiencies. Blood. 2006;107:501–507. doi: 10.1182/blood-2005-02-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copelan EA. Hematopoietic Stem-Cell Transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez-Campelo M, Pérez-Simón JA, Ocio EM, Castilla C, González-Porras JR, Sánchez-Guijo FM, Vázquez L, Caballero MD, Cañizo MC, San Miguel JF. CD34 + cell dose and outcome of patients undergoing reduced-intensity-conditioning allogeneic peripheral blood stem cell transplantation. Leuk Lymphoma. 2005;46:177–183. doi: 10.1080/10428190400014900. [DOI] [PubMed] [Google Scholar]
- Dominietto A, Lamparelli T, Raiola AM, Van Lint MT, Gualandi F, Berisso G, Bregante S, Di Grazia C, Soracco M, Pitto A, et al. Transplant-related mortality and long-term graft function are significantly influenced by cell dose in patients undergoing allogeneic marrow transplantation. Blood. 2002;100:3930–3934. doi: 10.1182/blood-2002-01-0339. [DOI] [PubMed] [Google Scholar]
- Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee SJ, Brinkman R, Eaves C. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1:218–229. doi: 10.1016/j.stem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Eaves CJ. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood. 2015;125:2605–2613. doi: 10.1182/blood-2014-12-570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves C, Miller C, Cashman J, Conneally E, Petzer A, Zandstra P, Eaves A. Hematopoietic stem cells: inferences from in vivo assays. Stem Cells Dayt Ohio. 1997;15 Suppl 1:1–5. doi: 10.1002/stem.5530150802. [DOI] [PubMed] [Google Scholar]
- Ergen AV, Boles NC, Goodell MA. Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood. 2012;119:2500–2509. doi: 10.1182/blood-2011-11-391730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehse B, Roeder I. Insertional mutagenesis and clonal dominance: biological and statistical considerations. Gene Ther. 2008;15:143–153. doi: 10.1038/sj.gt.3303052. [DOI] [PubMed] [Google Scholar]
- Gorin NC, Labopin M, Boiron JM, Theorin N, Littlewood T, Slavin S, Greinix H, Cahn JY, Alessandrino EP, Rambaldi A, et al. Results of genoidentical hemopoietic stem cell transplantation with reduced intensity conditioning for acute myelocytic leukemia: higher doses of stem cells infused benefit patients receiving transplants in second remission or beyond--the Acute Leukemia Working Party of the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24:3959–3966. doi: 10.1200/JCO.2006.05.5855. [DOI] [PubMed] [Google Scholar]
- Jordan CT, Lemischka IR. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990;4:220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- Lu R, Neff NF, Quake SR, Weissman IL. Tracking single hematopoietic stem cells in vivo using high-throughput sequencing in conjunction with viral genetic barcoding. Nat Biotechnol. 2011;29:928–933. doi: 10.1038/nbt.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie JL, Gan OI, Doedens M, Wang JCY, Dick JE. Individual stem cells with highly variable proliferation and self-renewal properties comprise the human hematopoietic stem cell compartment. Nat Immunol. 2006;7:1225–1233. doi: 10.1038/ni1393. [DOI] [PubMed] [Google Scholar]
- Mehta J, Mehta J, Frankfurt O, Altman J, Evens A, Tallman M, Gordon L, Williams S, Winter J, Krishnamurthy J, et al. Optimizing the CD34 + cell dose for reduced-intensity allogeneic hematopoietic stem cell transplantation. Leuk Lymphoma. 2009;50:1434–1441. doi: 10.1080/10428190903085944. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Nienhuis AW. Development of gene therapy for blood disorders. Blood. 2008;111:4431–4444. doi: 10.1182/blood-2007-11-078121. [DOI] [PubMed] [Google Scholar]
- Notta F, Zandi S, Takayama N, Dobson S, Gan OI, Wilson G, Kaufmann KB, McLeod J, Laurenti E, Dunant CF, et al. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science. 2015 doi: 10.1126/science.aab2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Hanada Ki, Hamada H, Nakauchi H. Long-Term Lymphohematopoietic Reconstitution by a Single CD34-Low/Negative Hematopoietic Stem Cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Panse JP, Heimfeld S, Guthrie KA, Maris MB, Maloney DG, Baril BB, Little MT, Chauncey TR, Storer BE, Storb R, et al. Allogeneic peripheral blood stem cell graft composition affects early T-cell chimaerism and later clinical outcomes after non-myeloablative conditioning. Br J Haematol. 2005;128:659–667. doi: 10.1111/j.1365-2141.2005.05363.x. [DOI] [PubMed] [Google Scholar]
- Perez-Simon JA, Diez-Campelo M, Martino R, Sureda A, Caballero D, Canizo C, Brunet S, Altes A, Vazquez L, Sierra J, et al. Impact of CD34+ cell dose on the outcome of patients undergoing reduced-intensity-conditioning allogeneic peripheral blood stem cell transplantation. Blood. 2003;102:1108–1113. doi: 10.1182/blood-2002-11-3503. [DOI] [PubMed] [Google Scholar]
- Pietras EM, Reynaud D, Kang YA, Carlin D, Calero-Nieto FJ, Leavitt AD, Stuart JM, Göttgens B, Passegué E. Functionally Distinct Subsets of Lineage-Biased Multipotent Progenitors Control Blood Production in Normal and Regenerative Conditions. Cell Stem Cell. 2015;17:35–46. doi: 10.1016/j.stem.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prchal JT. Clonal stability of blood cell lineages indicated by X-chromosomal transcriptional polymorphism. J Exp Med. 1996;183:561–567. doi: 10.1084/jem.183.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell. 2007;1:263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Roeder I, Kamminga LM, Braesel K, Dontje B, de Haan G, Loeffler M. Competitive clonal hematopoiesis in mouse chimeras explained by a stochastic model of stem cell organization. Blood. 2005;105:609–616. doi: 10.1182/blood-2004-01-0282. [DOI] [PubMed] [Google Scholar]
- Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2:640–653. doi: 10.1002/wsbm.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburg HB, Cho RH, Dykstra B, Uchida N, Eaves CJ, Muller-Sieburg CE. The hematopoietic stem compartment consists of a limited number of discrete stem cell subsets. Blood. 2006;107:2311–2316. doi: 10.1182/blood-2005-07-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Sun J, Ramos A, Chapman B, Johnnidis JB, Le L, Ho YJ, Klein A, Hofmann O, Camargo FD. Clonal dynamics of native haematopoiesis. Nature. 2014;514:322–327. doi: 10.1038/nature13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksberg DC, Chambers SM, Boles NC, Goodell MA. CD150- side population cells represent a functionally distinct population of long-term hematopoietic stem cells. Blood. 2008;111:2444–2451. doi: 10.1182/blood-2007-09-115006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Li B, Lu R, Koelle SJ, Yang Y, Jares A, Krouse AE, Metzger M, Liang F, Loré K, et al. Clonal tracking of rhesus macaque hematopoiesis highlights a distinct lineage origin for natural killer cells. Cell Stem Cell. 2014;14:486–499. doi: 10.1016/j.stem.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Morita Y, Ooehara J, Hamanaka S, Onodera M, Rudolph KL, Ema H, Nakauchi H. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154:1112–1126. doi: 10.1016/j.cell.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


