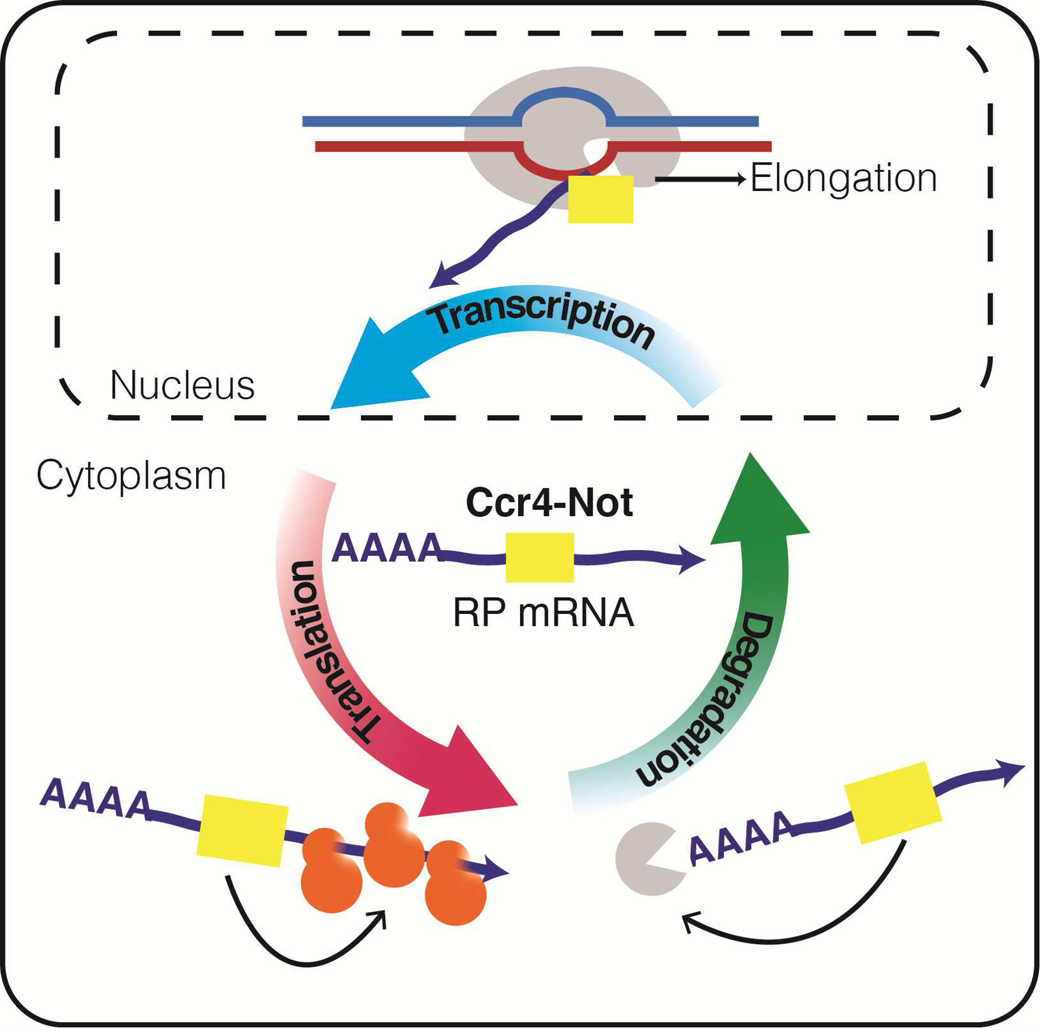
Summary
Current understanding of gene expression considers transcription and translation to be independent processes. Challenging this notion, we found that translation efficiency is determined during transcription elongation through imprinting of mRNAs with Not1, the central scaffold of the Ccr4-Not complex. We determined that another subunit of the complex, Not5, defines Not1 binding to specific mRNAs, particularly those produced from ribosomal protein genes. This imprinting mechanism specifically regulates ribosomal protein gene expression, which in turn determines the translational capacity of cells. We validate our model by SILAC and polysome profiling experiments. As a proof of concept, we demonstrate that enhanced translation compensates for transcriptional elongation stress. Taken together our data indicates that in addition to defining mRNA stability, components of the Ccr4-Not imprinting complex regulate RNA translatability, thus ensuring global gene expression homeostasis.
Keywords: Ccr4-Not, Not5, mRNA fate, translation, transcription, yeast, genomewide, circuitry of gene expression, mRNA stability, protein stability, control of global translation, production of ribosomal proteins
eTOC blurb
Transcriptional determination of translation rates enables cells to buffer genetic and environmental perturbations early on during gene expression and thus confers fitness advantage by making gene expression robust to changes. Gupta et al. show that Not5-dependent Not1 imprinting of mRNAs occurs during transcription and regulates translation. Moreover since ribosomal mRNAs are a major target of Not5, these findings establish a direct role for Not5 in regulating the abundance of the translation machinery and hence defining global translation levels in the cell.
Introduction
There is growing evidence that different levels of gene expression are interconnected to form a network. Constant feedback in all directions is given by components of the different cellular machineries acting to finally produce functional proteins. This serves to ensure homeostasis in gene expression, for example by inducing compensatory changes in production and degradation of mRNAs to maintain a steady state level. The circuitry buffering mRNA abundance was revealed first by the finding that different yeast species with different mRNA decay rates had nevertheless similar mRNA levels (Dori-Bachash et al., 2011). These findings were supported and extended by evidence that mutations affecting machineries involved in mRNA synthesis and decay have coevolved (Sun et al., 2012). It was also demonstrated that mutations in promoter elements induced coupled changes in synthesis and decay rates, suggesting that transcription factors binding to promoters might ensure that the two processes are linked (Dori-Bachash et al., 2012; Trcek et al., 2011).
The first evidence that a factor involved in transcription can have functions in decay emerged from the discovery of the roles of the RNA polymerase II (RNAPII) subunit Rpb4 in the decay of a specific class of mRNAs (Lotan et al., 2005). Our recent finding that Not5 is necessary for cytoplasmic functions of Rpb4 suggested that the multisubunit Ccr4-Not complex might also play a key role in regulating the gene expression circuitry (Villanyi et al., 2014). Ccr4-Not is a conserved multifunctional eukaryotic regulator composed of nine subunits in the yeast S.cerevisiae. It has been proposed to be responsible for the integration of environmental signals that coordinate multiple nuclear and cytoplasmic steps in gene expression (reviewed in (Chapat and Corbo, 2014; Collart and Panasenko, 2012; Collart et al., 2013; Collart and Timmers, 2004)). The Ccr4-Not complex plays roles in both regulation of transcription in the nucleus and mRNA degradation in the cytoplasm. Hence it is tempting to hypothesize that components of the Ccr4-Not complex are also loaded onto mRNAs to play the role of a global orchestrator of gene expression that defines mRNA fate later in the cytoplasm (Haimovich et al., 2013).
Current models of a gene expression circuitry ignore the possible cross-talk between the processes of transcription and translation. Several evidences point towards the Ccr4-Not complex mediating this cross-talk. Recently, we have shown that Not5 plays a role in translatability and assembly of the RNAPII complex (Villanyi et al., 2014). In addition, components of the Ccr4-Not complex, particularly Not4 and Not5, are important for transcription elongation (Kruk et al., 2011) and protein quality control (Dimitrova et al., 2009; Halter et al., 2014; Preissler et al., 2015). Hence these components of the Ccr4-Not complex are prime candidates to coordinate transcription with translation.
Studies investigating the role played by the Ccr4-Not complex in regulating the fate of mRNA genome-wide have followed two lines of experimentation in yeast. First, analysis of deletion mutants of the subunits of the Ccr4-Not complex revealed that the complex controls expression of most of the genome (Azzouz et al., 2009b; Cui et al., 2008), with particular impacts on snoRNAs (Azzouz et al., 2009a; Halter et al., 2014) and SAGA regulated genes (Cui et al., 2008). Second, genome-wide ChIP experiments revealed the presence of the Ccr4-Not complex on SAGA regulated genes (Venters et al., 2011). The former studies indicated targets of the Ccr4-Not complex, both direct and indirect, without differentiating between transcriptional or post-transcriptional regulation of mRNA levels. The latter study shed some light on regulation by Ccr4-Not at the transcriptional level. To date no genome-wide study has addressed which mRNAs are bound by the components of the Ccr4-Not complex, what determines the binding specificity of the complex subunits and finally what is the extent to which the complex subunits might regulate the post-transcriptional fate of mRNAs genome-wide.
In this work, to find the mRNAs whose cytoplasmic fate might be directly affected by the Ccr4-Not complex, we first determined the core set of mRNAs that are bound by the Not1 scaffold of the Ccr4-Not complex using native RNA immunoprecipitation (RIP). Next, we determined what defines the binding of mRNAs by Not1. We extended previous single gene studies genome-wide, to show that the Not5 subunit of the complex regulates Not1 mRNA binding, specifically on ribosomal and nuclear encoded mitochondrial protein mRNAs. Using polysome profiling and stable isotope labeling by amino acids in cell culture (SILAC) experiments, we found that Not5-dependent Not1-bound mRNAs are actively translated and that Not5 affects the translation of ribosomal genes. We determined that inhibition of transcriptional elongation enhanced Not5-dependent Not1 binding of mRNAs and also their translatability. Finally, we show that tethering Not5 to the cytoplasm affects polysomal presence of specific mRNAs as predicted by our model. These findings indicate that Not5-dependent Not1 binding of mRNAs occurs during transcription and regulates translation. We therefore refer to this binding as mRNA imprinting as proposed before for proteins that associate with mRNA co-transcriptionally to regulate the cytoplasmic fate of the imprinted mRNA (Choder, 2011). Moreover since ribosomal mRNAs are a major target of Not5, these findings establish a direct role for Not5 in regulating the abundance of the translation machinery and hence defining global translation levels in the cell.
Results
Not1 is enriched over 1/5th of the yeast transcriptome
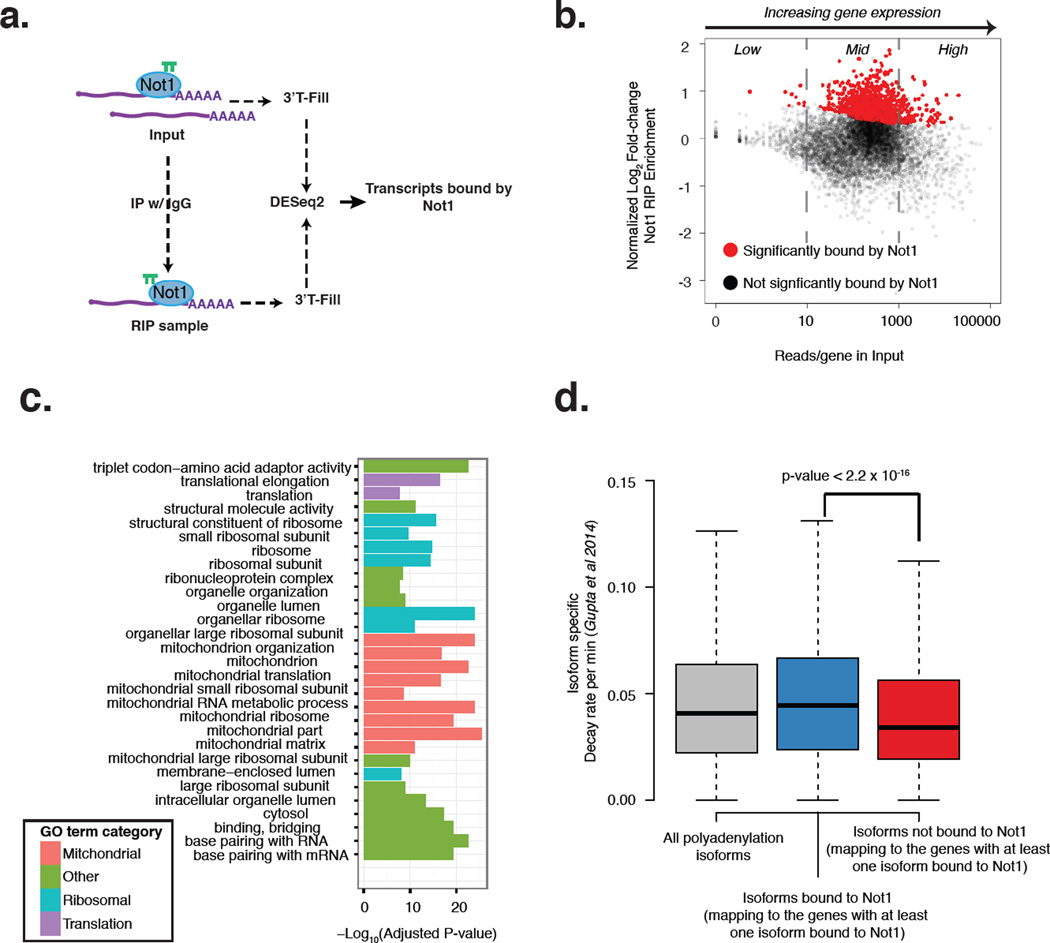
We performed RIP experiments with a yeast strain expressing tagged Not1 (Fig. 1a). RNA from total extracts and Not1 immunoprecipitated samples were sequenced using base-pair resolution mapping of polyadenylation isoforms to a read-depth of more than 4 million (Fig. S1a). Biological duplicates showed high reproducibility at the level of gene expression (Fig. S1b). We called differential enrichment of mRNAs in the RIP sample over the total extract at a false discovery rate (FDR) < 10%, removing the bias of gene expression on the signal for RIP enrichment (Fig. 1b) as described previously (Gupta et al., 2014). In order to account for non-specific binding of mRNAs we performed a negative control RIP using a strain without tagged Not1, and the non-specific binders were removed from further analysis. We found that 1030 (out of 5400) of the protein coding mRNAs were significantly enriched in Not1 RIPs (Table S1), with enrichment for gene ontology (GO) terms belonging to the mitochondrion and ribosome categories (Fig. 1c). Only 25 of the annotated non-coding RNAs (ncRNAs) such as CUTs and SUTs were significantly enriched in Not1 RIPs (Fig. S1c).
Figure 1. Native RIP reveals that Not1 binds 1/5 of the transcriptome.
The native RIP was performed in biological duplicates. a: Schematic view of the workflow used to identify Not1 bound mRNAs. b: Plot of the applied cut-off to identify significantly bound or unbound mRNAs by Not1. c: Chart of gene ontology categories of Not1-enriched mRNAs. d: Boxplots show decay rates of polyadenylation isoforms in wild-type yeast obtained in a previous study (Gupta et al., 2014) for all genes (gray), for polyadenylation isoforms that are enriched in Not1 RIP (blue) and for polyadenylation isoforms that are not enriched in Not1 RIP but belong to genes which have at least one polyadenylation isoform enriched in Not1 RIPs (red). See also Figure S1 and Table S1.
Next, we compared the binding of Not1 to specific polyadenylation isoforms of a gene. In line with the known role played by the Ccr4-Not complex in mRNA degradation, we found that all the polyadenylation isoforms of genes enriched in Not1 RIPs had higher degradation rates as defined in a previous study (Gupta et al., 2014) when compared to other polyadenylation isoforms from the same genes that were not bound by Not1 (Fig. 1d). Further we probed the intervening sequence between two differentially bound polyadenylated isoforms of the same gene for existing sequence elements like RNA binding protein (RBP) motifs (Riordan et al., 2011) (Fig. S1d). Confirming previous evidence of a functional interaction between RBPs and the Ccr4-Not complex, we found that the presence of an RBP motif in the 3’ UTR of a gene, such as Puf3 (Chatenay-Lapointe and Shadel, 2011), Khd1 (Ito et al., 2011), Vts1 (Rendl et al., 2008) and Pab1 (Hogan et al., 2008) was associated with increased Not1 binding. We also observed increased Not1 binding to mRNA isoforms that carried motifs recognized by RBPs such as Pub1 or Nrd1, suggesting functional connections also between these RBPs and the Ccr4-Not complex (Fig. S1d).
Not5 regulates Not1 binding to determine RNA abundance
Previous studies have shown that Not5 is required for association of Not1 to specific mRNAs (Villanyi et al., 2014) and that in the absence of Not5 the expression of nuclear encoded mitochondrial protein mRNAs was upregulated (Azzouz et al., 2009b; Cui et al., 2008). In not5Δ Not1 interaction with most other Ccr4-Not subunits is reduced (Fig. S2a). Many of these subunits have affinity for RNA. Therefore, we hypothesized that Not5 might globally affect the way Not1 is associated with mRNAs. To test this, we performed RIP with tagged Not1 in the not5Δ background.
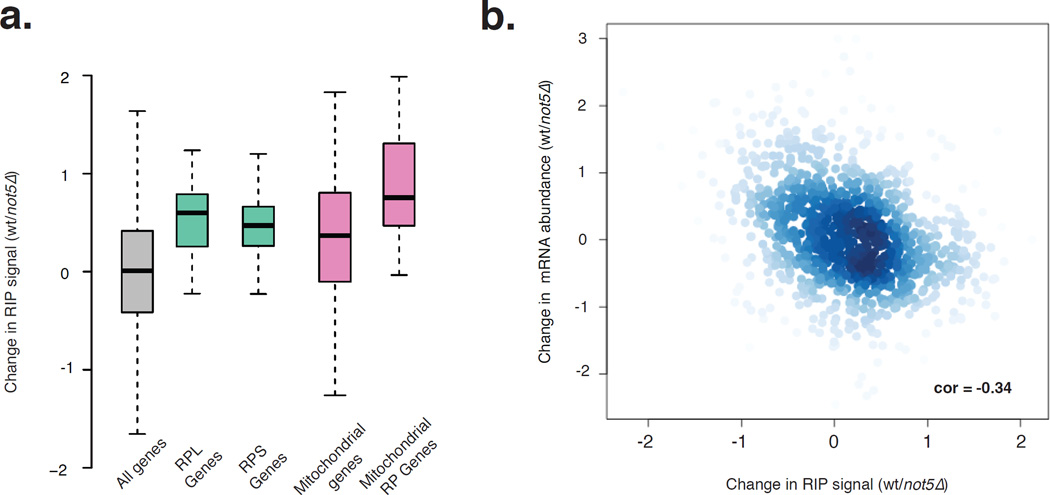
Mitochondrial and ribosomal protein (RP) mRNAs were 2-fold less enriched in the Not1 RIP from not5Δ when compared to the RIP from the wild type, indicating that Not5 plays an important role in Not1 binding to these transcripts (Fig. 2a, Fig. S2b). We observed that the change in Not1 binding of mRNAs genome-wide was negatively correlated with the change in gene expression between wild type and not5Δ (Fig. 2b). The enhanced expression of a gene upon loss of Not1 binding could be due to enhanced in vivo stability of transcripts that lose Not1 binding. To verify this we tested the decay rate of 2 such Not1 target mRNAs (NHP2 and RPS8) and one non-target (SED1), by a 1,10-phenanthroline pulse-chase (Fig. S2c). NHP2 and RPS8 mRNAs were indeed more stable in the mutant but in contrast the SED1 decay curve was not different between not5Δ and wild type cells. Anti-correlation between the levels of the mRNAs and their detection by RIP of Not1 from extracts indicates that they are in vivo targets of Not5-dependent Not1 binding.
Figure 2. Not5 regulates Not1 binding to determine RNA abundance.
a: Boxplot representing the distribution of loss in Log2 fold of Not1 enrichment in not5Δ (calculated as the difference between the RIP signal between wild type (wt) and not5Δ) for all genes (gray), ribosomal large subunit (RPL) genes (green), ribosomal small subunit (RPS) genes (orange), mitochondrial genes (violet) and mitochondrial ribosomal genes (pink). All these specific categories of genes lost Not1 binding significantly in not5Δ as denoted by the p-values from a student’s t-test comparing the distribution of all genes in gray with the distribution of each category of genes. b: Scatter plot between change in per gene RNA abundance and change in Log2 fold Not1 enrichment from wt to not5Δ shows a negative Spearman correlation. See also Figure S2 and Table S1.
It is important to mention at this point that while most slow growth phenotypes related to a deficient growth medium or to stress conditions correlate with a global reduction in the RNA abundance of RP genes (Gasch et al., 2000) in the case of not5Δ cells the expression of a majority of RP genes is upregulated or unchanged (Fig. S2d). Moreover, the global change in gene expression in not5Δ is not correlated with the slow growth gene expression signature reported by Holstege and colleagues (Fig. S2e) (O'Duibhir et al., 2014).
mRNAs bound by Not1 in a Not5 dependent manner are translated
Previous work has indicated that Not5 is important for the presence of certain mRNAs in polysomes (Villanyi et al., 2014). Moreover Not5 is needed for association of a newly produced protein with its chaperone (Villanyi et al., 2014). These findings have revealed that Not5 is needed for translation of specific mRNAs.
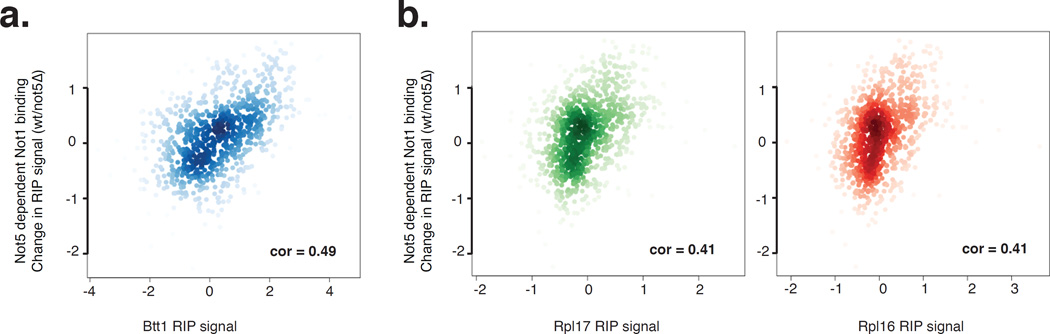
To determine whether Not5 may have a global function in translation, we compared the Not5 dependent Not1-bound RIP signal on mRNAs (measured as the difference between Not1 binding in wild type and not5Δ) with the published RIP enrichment signal for the Btt1 protein (Fig. 3a). Btt1 is a ribosome-associated chaperone that binds to nascent peptides (for review see (Rospert et al., 2002)). It has been shown to interact with the Ccr4-Not complex (Liu et al., 2001) and to be associated with mRNAs being translated, in particular with mRNAs encoding mitochondrial proteins and RPs (del Alamo et al., 2011), the same category of mRNAs bound by Not1 in a Not5-dependent manner. We found that the Not5-dependent Not1 RIP signal correlated (Spearman cor: 0.49) with the Btt1 RIP signal for genes that were significantly enriched in the wild type Not1 RIP. In contrast, much less of a correlation was obtained if we looked at genes enriched in Not1 RIPs in not5Δ but not in the wild type (Spearman cor: 0.3; Fig. S3).
Figure 3. Not5 dependent Not1 mRNA binding correlates with Rpl16, Rpl17 and Btt1 mRNA binding.
Scatter plot between the Log2 fold change in Not1 RIP signal strength (wt/not5Δ) with previously published Btt1 (a), Rpl17 and Rpl16 (b) RIP signal (del Alamo et al., 2011) for genes significantly enriched in Not1 RIP in the wild type background only, shows a positive Spearman correlation (inset). See also Figure S3.
Btt1 was reported to have a pattern of RIP enrichment over specific mRNAs different from the pool of mRNAs globally being translated, as reflected by the RIP signal with the 2 ribosomal proteins Rpl16 and Rpl17 (del Alamo et al., 2011). Nevertheless, we also found that the Not5-dependent Not1 RIP signal correlated (Spearman cor: 0.41) with the RIP enrichment of ribosomal protein subunits Rpl16 and Rpl17 RIP (Fig. 3b). Again, much less of a correlation was obtained if we looked at genes enriched in Not1 RIPs in not5Δ only (Fig. S3). Taken together these results suggest that Not5-dependent Not1-bound mRNAs are being translated.
Not5 affects the translation of RP genes
In order to study the role of Not5 in regulating translation genome-wide, we profiled mRNA from the polysome fraction in both wild type and not5Δ (Table S2). The abundance of mRNAs in the polysomes should directly reflect the translatability of a particular mRNA.
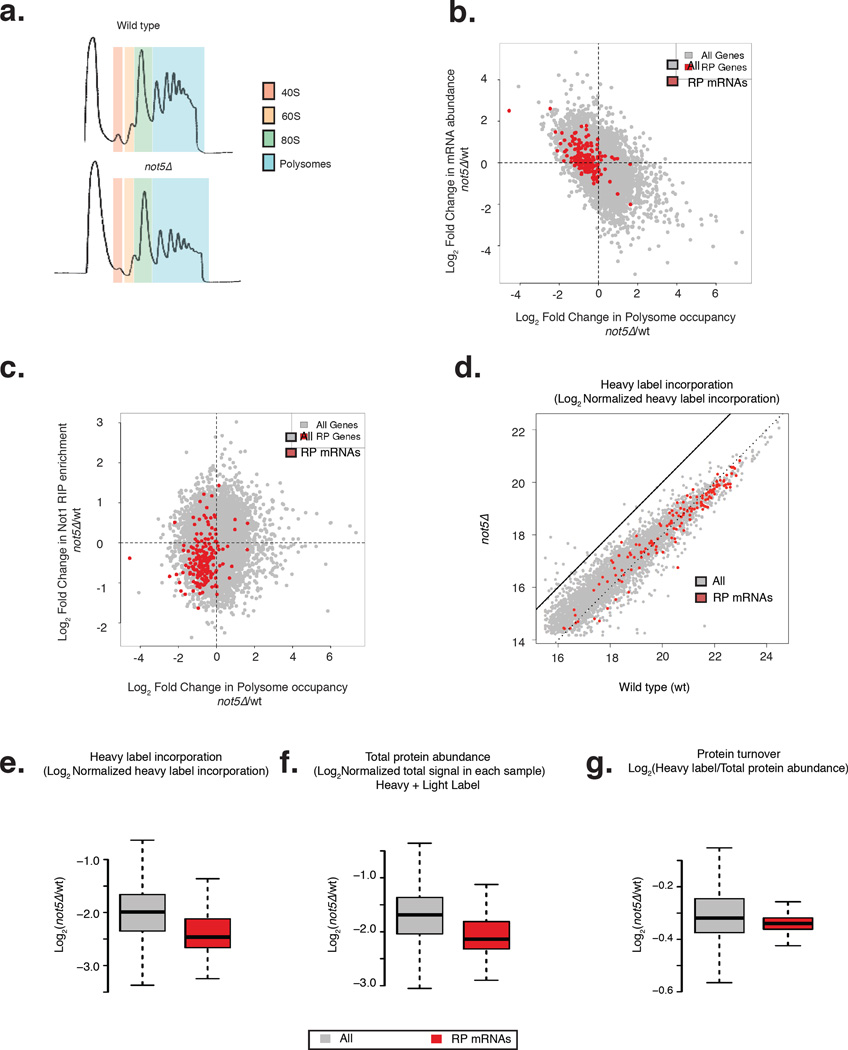
We saw drastically reduced polysomes in not5Δ (Fig. 4a) as previously observed (Panasenko and Collart, 2012). 273 mRNAs had greater than 40% loss in polysome occupancy (calculated as enrichment in polysomes over total RNA abundance) in not5Δ as compared to wild type cells (Fig. 4b). Of these, 125 mRNAs had similar or higher mRNA abundance in total extracts of not5Δ, clearly demonstrating that reduction from not5Δ polysomes was due to reduced translatability and not due to reduced RNA abundance. Amongst the 273 mRNAs we found almost all of RP mRNAs and most of the other mRNAs encode ribosome biogenesis factors (Table S2). Unlike most stress responses where the mRNA abundance of RP genes globally decreases, (Weiner et al., 2012) in not5Δ we find that the level of a majority of the RP mRNAs is either unchanged or upregulated, yet the majority of these mRNAs are less abundant in polysomes (98/139) (Fig. 4b). It is important to note that although the number of polysomes in the cell is diminished in not5Δ, only the class of RP mRNAs is significantly depleted from the polysomes. Therefore, RP genes lost both Not1 binding and polysome occupancy in not5Δ (Fig. 4c).
Figure 4. Not5 affects the translation of ribosomal protein genes.
a: Polysome traces in wt cells (top) and not5Δ cells (bottom) with different ribosomal fractions highlighted. b: Scatter plot between Log2 fold change in mRNA abundance from not5Δ to wt against the Log2 fold change in polysomal occupancy from not5Δ to wt. c: Scatter plot between Log2 fold change of Not1 RIP enrichment from not5Δ to wt is plotted against polysomal occupancy change from not5Δ to wt. d: Scatter plot between Log2 transformed heavy label (lysine) incorporation in wt and not5Δ strains from SILAC experiment. e: Box plots for the Log2 fold change in heavy label incorporation from not5Δ to wt. f: Box plots for the Log2 fold change in total protein abundance (sum of both heavy and light lysine) from not5Δ to wild type. g: Box plots for the Log2 fold change in protein turnover (measured by the Log2 ratio of heavy label incorporation over the total protein abundance) from not5Δ to wt. b–g, all mRNAs are in gray and RP mRNAs are in red. See also Tables S2 and S3.
To get a global picture of the translation phenotype in not5Δ we conducted SILAC for 3 hours by substituting growth medium with a heavier amino acid (Experimental Procedures). We were able to quantify protein turnover of 4350 proteins (Table S3). We found that the amount of heavy label incorporation and subsequently the total protein abundance was lower in the mutant as compared to the wild type globally (Fig. 4d) consistent with reduced polysomes in not5Δ cells. We found that heavy label incorporation for RPs was significantly lower (p-value < 2.2 × 10−16) than for the bulk of proteins in not5Δ (Fig. 4e). This was also true for the total abundance of RPs (p-value < 2.2 × 10−16). Consistently, the protein turnover rates, measured as the ratio of heavy amino acid incorporation to total amino acid incorporation, remained the same for RPs in not5Δ compared to the wild type (Fig. 4g). Thus, the deletion of Not5 led to loss of Not1 binding for RP mRNAs, and to their reduced translation.
Not1 binding is a co-transcriptional event and depends on Not5
Not5-dependent Not1 binding to RP mRNAs correlates with reduced expression of these mRNAs indicative of a role of Not1 in decay of these mRNAs, but it also correlates with presence of these mRNAs in polysomes and production of RPs. Therefore we questioned where Not5 might be important for Not1 binding to mRNAs, in the nucleus or in the cytoplasm?
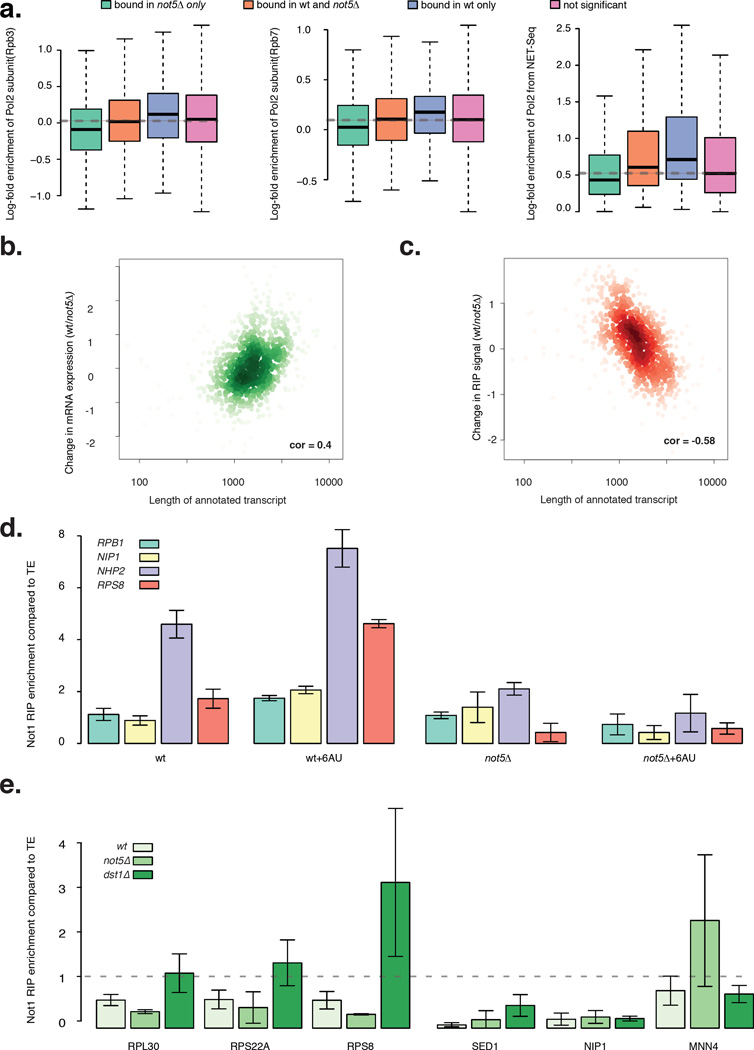
GLAM assays (gene-length dependent accumulation of mRNA) have previously shown that Ccr4-Not contributes to transcription elongation (Kruk et al., 2011) and a more recent study has indicated that Not1 is associated with elongating polymerase and contributes to TFIIS’s function in transcription elongation (Dutta et al., 2015). Hence it could be that Not1 mRNA binding is coupled to transcription elongation. In support of this hypothesis, we found that mRNAs that are bound by Not1 in a Not5-dependent manner (enriched in Not1 RIPs in wild type cells with at least 40% reduction in binding in not5Δ cells) have significantly higher occupancy of RNAPII subunits such as Rpb3 (Mayer et al., 2010) (Fig. 5a, left) and Rpb7 (Jasiak et al., 2008) (Fig. 5a, center) on the encoding genes. We found a similar pattern of significant accumulation of NET-Seq (native elongating transcript sequencing) signal (Churchman and Weissman, 2011) (Fig. 5a, right).
Figure 5. Not5–dependency for Not1 mRNA binding correlates with elongating polymerase.
a: Box-plots show how four different categories of genes categorized by their Not1 binding properties (bound in not5Δ only, bound in wt and not5Δ, bound in wt only and not significantly bound) correlate with polymerase occupancy on genes: Rpb3 (Mayer et al., 2010), Rpb7 (Jasiak et al., 2008) and with Net seq signal identifying nascent mRNAs (Churchman and Weissman, 2011). b: Scatter plot between change in RNA abundance from wt to not5Δ (wt/not5Δ) and transcript length is positively correlated. c: Scatter plot between change in Not1 RIP signal strength (wt/not5Δ) and transcript length is negatively correlated. d: Barplot for Log2 fold change in Not1 enrichment over 4 mRNAs (RPS8, NHP2, RPB1 and NIP1) in wt and not5Δ after transcriptional inhibition upon 6AU treatment. e: Barplot for fold enrichment in Not1 RIP compared to total extract, for several mRNAs in wt, not5Δ and dst1Δ. d–e, error bars representing SD. See also Figures S4, S5 and Table S4.
These findings suggested that Not5 could be defining Not1 binding to mRNAs during transcription. To determine whether Not1 was binding mRNAs before their export to the cytoplasm, we determined whether intronic sequences were present in the Not1 immunoprecipitates. Few genes in yeast have introns, but fortunately for this study, they are mostly RP genes, a major target of Not5-dependent Not1 binding. Introns are removed from newly produced RNAs prior to their export to the cytoplasm. Therefore, we analyzed total RNA, polysomal RNA and Not1 RIPed RNA for the levels of exonic and intronic sequences belonging to RPL30, RPS7A and ACT1. We detected intronic sequences in Not1 RIPs. The relative levels of intron to exon sequences were a magnitude lower in polysomal RNA than in total extracts and in Not1-RIPs (Fig. S4). These results support that Not1 is binding mRNAs prior to their association with ribosomes.
We observed that expression of longer genes was reduced in not5Δ (Fig. 5b) and that the increase in Not1 binding in not5Δ was significantly correlated with gene length (Fig. 5c). These findings confirmed that binding of Not1 to mRNAs correlated with reduced expression, and indicated that in the absence of Not5, association of Not1 with mRNAs is mainly defined by length. Binding of Not1 in presence of Not5 instead seems to favor RP mRNAs, some of which are very short, so this led us to question what might define RP mRNAs as a target for Not5-dependent Not1 binding. Transcription of RP genes has been well characterized, and depends upon 2 prevalent RPG promoter types, with respect to the localized binding of 4 transcription factors (TFs) (Rap1, Fhl1/Ifh1 and Hmo1). 9 RP genes do not seem bound by any of these 4 TFs (Knight et al., 2014). Not1 RIP and polysome presence of RP mRNAs in wild type versus Not5 was not correlated with any specific promoter type, suggesting that promoter elements are unlikely to determine the specificity of Not1 targeting to RP mRNAs. Transcription of RP genes has been reported to occur with intense backtracking of RNAPII (Gomez-Herreros et al., 2012). The Ccr4-Not complex can associate with the elongating polymerase and promote elongation from backtracked polymerase (Kruk et al., 2011) so we considered the possibility that Not5 might promote Not1 association to newly produced mRNAs under these conditions. If this model is correct, then by impairing transcription elongation we might promote association of Not1 with mRNAs.
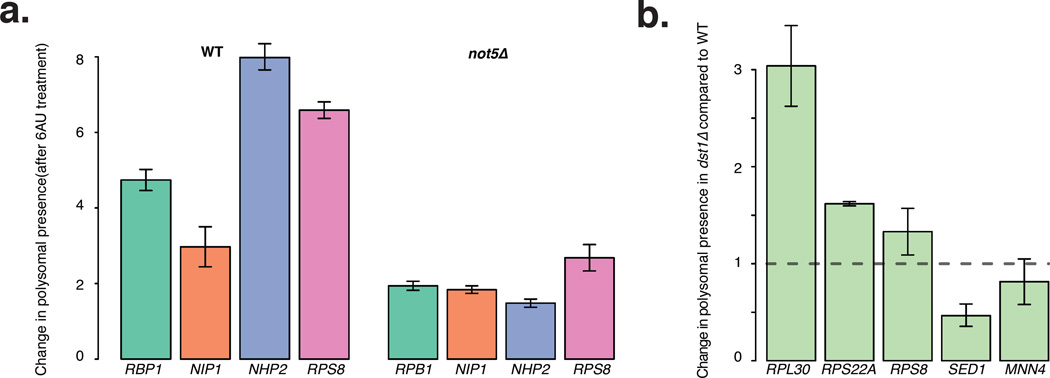
To test this model we treated wild type and not5Δ cells with 6-azauracil (6AU), which impairs transcription elongation by limiting available GTPs, and then performed the Not1 RIP. In cells treated for 90 minutes with 6AU, Not1 association with mRNAs was globally improved in wild-type cells (Fig. 5d). This was not the case in absence of Not5 (Fig. 5d).
To orthogonally test whether Not1 was associating co-transcriptionally with mRNAs we analyzed cells lacking TFIIS (dst1Δ). Indeed efficient transcription elongation of RP genes is highly dependent upon TFIIS (Gomez-Herreros et al., 2012). We performed Not1 RIP from wild type and dst1Δ, and compared the presence of 6 mRNAs in the RIPs, 3 RP-encoding mRNAs, namely RPS8, RPS22A and RPL30 and 3 mRNAs as controls, namely NIP1, MNN4 and SED1. The RP mRNAs were associated with Not1 to a greater extent in dst1Δ compared to the wild type (Fig. 5e) whereas none of the control mRNAs had significant enrichment in the Not1 RIP from dst1Δ. This effect was not due to changes in Not1 protein abundance as Not1 was similarly immunoprecipitated from wild type, not5Δ and dst1Δ strains (Fig. S5).
Taken together, these results indicate that binding of Not1 to mRNAs is coupled to transcription elongation, and that transcription elongation stress permits improved Not1 binding, but that this requires Not5.
Transcriptional stress leads to a Not5-dependent increase in translation
Not1 binding to RP mRNAs under transcription elongation conditions stress requires Not5, which is also needed for the optimal presence of RP mRNAs in polysomes in normal conditions. To determine whether better Not1 binding under elongation stress is connected to better polysome presence of the bound mRNA, we tested for the presence of mRNAs in polysomes in cells treated with 6AU. Inhibition of transcription elongation led to increased presence of all tested mRNAs in polysomes for wild type cells, but this was much less the case in the absence of Not5 (Fig. 6a and 6b). We then tested the presence of mRNAs in wild type and dst1Δ polysomes, and similarly observed that increased binding of Not1 to RPS8, RPS22A and RPL30 shown above correlated with increased presence in polysomes. In contrast, in the case of the SED1 and MNN4 controls, neither a significant change in Not1 binding nor any increased polysomal presence was observed in dst1Δ (Fig. 5e and 6b).
Figure 6. Inhibition of transcription elongation is compensated by upregulation of translatability.
a: Barplots of mRNA abundance in polysomes upon treatment or not with 6AU for 4 mRNAs tested previously for RIP enrichment (Fig. 5d) in wt and not5Δ. b: Barplots showing the abundance of various mRNAs normalized to NIP1 mRNA levels, in not5Δ and dst1Δ polysomes relative to wt polysomes. a–b, error bars representing SD. See also Table S4.
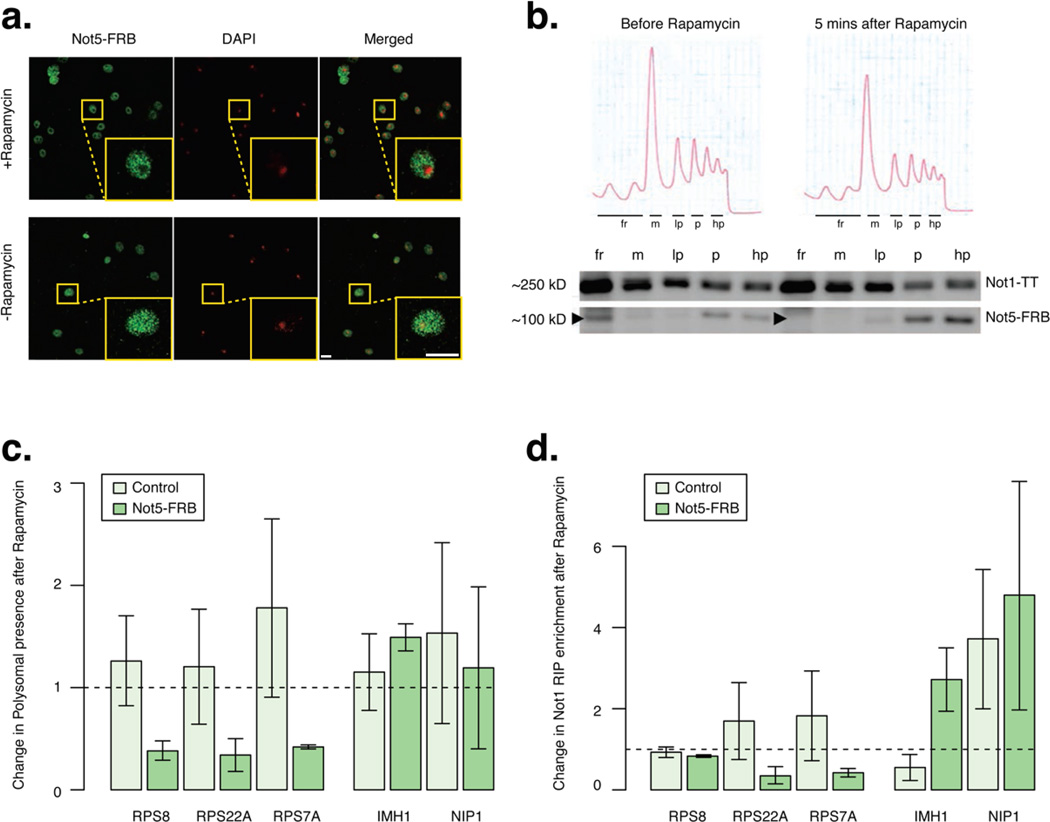
Since transcription elongation stress leads to a Not5-dependent increase in Not1 association with mRNAs that enhances their abundance in polysomes, particularly for RP mRNAs, we wanted to confirm that nuclear Not5 has an impact on RP mRNA translatability. We used the tether away system where fusing a protein of interest to the FRB domain of human mTOR in a rapamycin resistant strain in which the human FKBP12 is fused to Rpl13A leads to tethering of the protein of interest to the cytoplasm upon treatment of cells with rapamycin (Haruki et al., 2008). We fused Not5 to FRB in the parental strain. Cells expressing the fusion protein did not display any detectable growth phenotype, and when treated with rapamycin started growing slower than the parental strain only after 2 hours. We first looked at localization of fused Not5 before and after treatment with rapamycin for 5 minutes (Fig. 7a). Not5 was localized both in the cytoplasm and nucleus before treatment, but it lost nuclear localization after treatment. Consistently the distribution of fused Not5 across a sucrose gradient was changed 5 minutes after rapamycin treatment: the Not5 that was initially detected in the free fraction had been removed and accumulated in polysomes (Fig. 7b). This was not the case in the control strain that did not express tagged Not5 (Fig. S6a). It is intriguing to note that the tethering of Not5 to ribosomes did not change the distribution of Not1 across the sucrose gradient (Fig. 7b) or Not1’s nuclear staining (Fig. S6b).
Figure 7. Translatability of specific mRNAs is regulated by nuclear Not5.
Tethering of Not5 to the cytoplasm leads to reduced presence of RP encoding-mRNAs and increased presence of IMH1 mRNA in polysomes. a: Localization of FRB-fused Not5 in cells before and after treatment for 5 minutes with rapamycin. Nucleus is shown in red (DAPI), Not5-FRB is shown in green (Alexa-fluor). In response to rapamycin nuclear localization of Not5-FRB was lost and the nuclei are detected as dark holes, which co-localize with DAPI staining. Scale bars represent 5 µm b: Presence of Not5-FRB and Not1 before and after 5 minutes rapamycin treatment in free fractions (fr), monsomes (m), and in 3 polysome fractions, light (lp), medium (p) and heavy (hp). Arrows indicate Not5-FRB. Polysome traces before and after rapamycin treatment for 5 minutes are displayed above the corresponding blots.
c: Barplots of the change in abundance of several mRNAs in polysomes after treatment for 5 minutes with rapamycin in control cells, or cells expressing Not5-FRB.
d: Barplot for fold change in Not1 RIP enrichment over total extract after rapamycin compared to before for several mRNAs, in control cells and in cells expressing Not5-FRB. c–d, error bars representing SD. See also Figure S6 and Table S4.
We then measured the presence of different mRNAs in polysomes before and after treatment. In parallel we determined the association of these mRNAs with Not1 by RIP. RPS8A, RPS22A and RPS7A mRNAs were decreased in polysomes already 5 minutes after rapamycin addition (Fig. 7c). This correlated with a reduced association with Not1 after treatment (Fig. 7d). Inversely, the polysomal presence of IMH1, an mRNA that gains Not1 binding in not5Δ, and that gained binding after rapamycin addition (Fig. 7d), was enhanced after tethering of Not5 to the cytoplasm (Fig. 7c). These correlated changes in polysome presence and Not1 RIP were not observed in the control strain. Moreover NIP1 mRNA presence in polysomes or Not1 RIP did not change after rapamycin treatment.
Taken together these results confirm that Not1 mRNA targets are imprinted in the nucleus, that nuclear Not5 defines the mRNA targets for Not1 imprinting and that Not1 imprinting is facilitating translation.
Discussion
Specific mRNAs are imprinted by Not1 co-transcriptionally
In this work, we have investigated at a genome-wide scale using RIP experiments, which mRNAs can be detected in association with Not1, the core subunit of the Ccr4-Not complex. We found that Not1-bound mRNAs define 1/5th of the coding transcriptome, with gene ontology enrichment for mitochondrial and RP genes. Association of Not1 with these specific targets in particular, was very much dependent on the Not5 subunit of the Ccr4-Not complex.
A caveat with RIP experiments is that some level of binding could be occurring in vitro. We observed that Not1 was less bound to mRNAs in not5Δ even when they were more abundant in the extracts, but Not1 also lost association with several subunits of the Ccr4-Not complex that have RNA binding affinity in not5Δ. However, we found a genome-wide correlation between the loss of Not1 binding to RP mRNAs and reduced translation of these mRNAs, measured by polysome profiling and SILAC (Fig. 4), suggesting that the RIP values reported in our study describe an in vivo phenomenon. Our single gene experiments also reinforced that Not1 binding measured by RIP correlates with translatability of mRNAs (Fig 5d, 6a).
Our findings strongly indicate that besides the RNA binding protein- or miRNA associated-tethering of Not1 to mRNAs in the cytoplasm (for review see (Collart and Panasenko, 2012)), Not1 also binds to mRNA co-transcriptionally. We provide experimental evidences for the existence of this nuclear Not1 mRNA imprinting. First, Not1 was associated with intronic RNA sequences that are hardly detectable in polysomes (Fig. S4). Second, generating transcription elongation stress by treating cells with 6AU leads to better Not1 binding to mRNAs (Fig. 5d). Third, impairing transcription elongation by deletion of TFIIS also leads to better Not1 mRNA imprinting (Fig 5e). Fourth, Not1 binding, as measured by the amount of mRNA that can be co-immunoprecipitated with Not1, correlates with polymerase occupancy on genes globally. This correlation is the strongest for mRNAs that are bound by Not1 in a Not5 dependent manner (Fig 5a). Finally, the tethering of Not5 away from the nucleus leads within 5 minutes to a correlated change in the presence of mRNAs in polysomes and binding of these mRNAs to Not1 that additionally correlate with how the binding of these mRNAs by Not1 changes in not5Δ (Fig. 7). These results confirm that Not5 is needed in the nucleus to promote Not1 binding, which in turn promotes mRNA translatability.
Our study suggests that Not1 associates better with mRNAs under transcription elongation stress. The Ccr4-Not complex binds to the elongating polymerase and promotes elongation, but it does not affect elongation of un-arrested polymerase (Babbarwal et al., 2014; Dutta et al., 2015). The window of opportunity for Not1 to associate with the nascent transcripts is likely to be greater when the elongation process is slowed by backtracking or limiting nucleotides. Under normal conditions, this concerns specifically RP mRNAs (Dutta et al., 2015; Gomez-Herreros et al., 2012; Pelechano et al., 2009). Under global transcription elongation stress such as 6AU treatment, it will concern all genes.
How might Not5 be important for the association of Not1 with specific mRNAs during transcription? Association of Ccr4-Not with the elongating polymerase complex was shown to depend upon transcript length, suggesting that Ccr4-Not is interacting with the nascent transcript (Babbarwal et al., 2014). Not5 might connect Not1 to the elongating polymerase or to the mRNAs, as a direct tether, or because it is important for other Ccr4-Not subunits to associate with Not1. Not5 has RNA binding activity (Bhaskar et al., 2013), as do other subunits of the Ccr4-Not complex such as the deadenylase subunits or Not4 that has an RRM motif (Albert et al., 2000). As mentioned above, association of Not1 with different isoforms of thesame gene was more likely for the isoforms with binding sites for RBPs. This could indicate that RBPs facilitate the retention of Not1 on mRNAs after Not1 is imprinted during the transcription elongation phase. Finally, the poly(A) binding protein itself might contribute to anchor Not1 to the mRNA.
Co-transcriptional imprinting by Not1 regulates translation and mRNA degradation
Polyadenylation isoforms of a gene that showed better association with Not1 were those previously determined to have higher degradation rates (Gupta et al., 2014) (Fig. 1d). This is in line with the role of the Ccr4-Not complex as the major yeast deadenylase. Moreover, RP mRNAs that are a major target of Not5-mediated Not1 binding have been reported to be a preferred target of Ccr4 (Grigull et al., 2004). This raised the question whether Not1 was co-transcriptionally associated with mRNAs solely to define their subsequent decay rate. We were able to globally correlate the mRNAs dependent upon Not5 for Not1 binding with translated mRNAs, defined as those associated with 2 different ribosomal proteins, Rpl16 or Rpl17, or those associated with a ribosome-associated chaperone, Btt1 (Fig. 3). Indeed, previous work has suggested that mRNA decay and translation are coupled processes (Hu et al., 2009; Pelechano et al., 2015). We observed that less RPs were produced in not5Δ. So we reflected on how an impact on mRNA degradation could indirectly affect translation. The Ccr4-Not complex is important for mRNA deadenylation, and interaction of Not1 with the Ccr4 deadenylase is reduced in not5Δ. However in this study we measure polyadenylated mRNAs, and find that polyadenylated RP mRNAs are reduced in polysomes in not5Δ. Inefficient deadenylation of RP mRNAs in the mutant cannot explain this observation.
Not1 imprinting determines translational capacity
Our findings reveal that Not1 imprinting plays an important role in coupling different steps of gene expression, facilitating both translation of the imprinted mRNA and its subsequent decay. Since Not1 imprinting of RP mRNA happens co-transcriptionally, it also links the decay and translation machinery to the status of global transcription. An exciting finding in this work is that we were able to correlate the necessity of Not5 for Not1 imprinting during transcription with translation of RP mRNAs and the level of total polysomes. Indeed, without Not5, RP mRNAs are less imprinted by Not1 (Fig. 4c), ribosomal proteins are less produced and polysome levels are reduced (Fig 4a and e). During a transcriptional elongation stress that renders transcription generally less efficient (Gomez-Herreros et al., 2012), Not1 associates with mRNAs better via Not5 (Fig. 5d), and consequently these mRNAs are better translated (Fig. 6a). Since RP mRNAs are particularly affected by this mechanism, this allows the cell to produce more ribosomes and increase global translation levels to overcome transcriptional stress even for mRNAs not imprinted by Not1. Our data indicates that loss of Not5-mediated Not1 imprinting is visible within 5 minutes of removing Not5 out of the nucleus and it also affects presence of RP mRNAs in polysomes within 5 minutes. It is true that there is still substantial binding of Not1 to RPS8, and that newly produced RPS8 mRNAs are unlikely to constitute an essential part of the steady state RPS8 mRNA but nevertheless these observations suggest that newly exported and imprinted RP mRNAs contribute importantly to the pool of RP mRNAs being translated.
We observed that tethering of Not5 to ribosomes did not lead to the co-tethering of Not1, suggesting that either the tethering artificially pulled Not5 away from the Not1 scaffold, or alternatively that in vivo the Ccr4-Not complex is not a single entity at all times. This is an exciting observation because the question of whether the Ccr4-Not complex exists only in one form in cells to perform its’ multiple and sometimes conflicting functions is an open and important one.
It is interesting to note here that we recently showed that Not5 controls assembly of RNAPII during translation. While we have not demonstrated that the level of transcription is directly affected by the co-translational assembly of the polymerase, these results are highly suggestive that Not5 could in turn determine transcription levels during translation.
According to our knowledge this is the first demonstration that inhibition of global transcription can be compensated via a direct physical link to an increase in translation globally. These studies demonstrate that the different gene expression levels, transcription, translation and mRNA degradation, are linked in a gene expression circuitry by the Ccr4-Not complex, a complex that clearly plays a major role in gene expression that extends way beyond its function as the major deadenylase of eukaryotic cells.
Experimental procedures
Strains and growth media
Strains used in the present study are summarized in Table S4. Growth media were standard. In some experiments 6AU was added at 25 µg/ml for 90 minutes. For others to obtain complete transcription inhibition to follow mRNA decay rates we used 1,10 phenanthroline as described previously (Grigull et al., 2004).
Native RIP
Exponentially growing cells were harvested at OD 0.6–0.9 and broken with glass beads. An aliquot of lysate was taken as Input control, the remaining was immunoprecipitated (IP) with Dyna M280 sheep anti rabbit IgG for 2h at 4°C and analyzed as described previously (Gupta et al., 2014). To follow single mRNAs with RIP we performed quantitative real time PCR (q-RT-PCR) on input and IP samples utilizing specific primers as described (Villanyi et al., 2014). The input and immunoprecipitated RNA samples obtained were subjected to polyadenylation profiling using sequencing as previously described (Gupta et al., 2014) and the reads were aligned to the R64 S.cerevisiae genome.
qPCR analysis
1 µg of total RNA obtained from polysomes or from total extracts was reversed transcribed with oligo(dT) primers in a total volume of 25 µL. After synthesis, cDNAs were diluted to a final volume of 250 µL and 5 µL were utilized for qPCR using gene specific primers as described in (Villanyi et al., 2014). Gene specific primers are summarized in Table S4.
Polysome profiling
The polysome profiling was done as described previously (Panasenko and Collart, 2012). The input and polysomal RNA samples obtained were subjected to polyadenylation profiling using sequencing as previously described (Wilkening et al., 2013) and the reads were aligned to the R64 S.cerevisiae genome.
SILAC experiment
Wild type and not5Δ cells were grown until saturation overnight in SC-complete medium (2% glucose) containing light lysine (12C6, 14N2). Next day the samples were diluted to an OD of 0.1–0.2 and grown for 3 h in SC-complete medium with light lysine. The cells were then shifted to medium containing heavy lysine (13C6, 15N2) and were grown for another 3 h, harvested and sent for LC/MS as described previously (Hughes et al., 2014).
Not5 tether away and immunolocalization of Not5-FRB
Yeast cells expressing NOT5-FRB and RPL13A-FKBP12 alleles were cultured in YPD medium at 30°C. Exponentially growing cultures were treated with 1 µg/ml rapamycin (Enzo Laboratories) and 100 ml of cells were collected for polysome profiling. 5 ml of cells were collected for immunofluorescence microscopy. For microscopy cells were fixed with 1 ml of 37% formaldehyde at RT for 2 h. Cell pellets were washed with PBS containing 0.1% tween 20, and resuspended in 1 ml of spheroblasting buffer (1.2 M sorbitol, 20 mM potassium phosphate, pH 7.4). 0.1 ml of cell suspension was treated with 3.2 µl of 1.42 M b-mercaptoethanol and 5 µl of 5 mg/ml zymolyase 100T for 30 minutes at 30°C. 20 µl of spheroblasts were immobilized on polylysine coated microscope slides. Staining was done using rabbit polyclonal anti-Not5 antibody followed Alexa Fluor 488 anti Rabbit IgG (Life technologies) and DAPI. Images were taken before and 15 minutes after addition of rapamycin using an Olympus DeltaVision microscope equipped with GFP/mRFP filter set (Chroma, Bellow falls USA) and a CoolSNAP HQ camera. Images were obtained by optical sectioning (taking several z-stacks) with a step size of 0.2 µm and further processed with ImageJ-win64.
Statistical Methods
In order to calculate fold change enrichments between input and Not1 RIPs, we used DESeq2 R Bioconductor package as described previously (Gupta et al., 2014) and these values are given in Table S1. The polysomal occupancy was calculated as the fold change enrichment in the polysomal fraction over the total input RNA also using DESeq2 (Love et al., 2014) and these values are provided in Table S2. The raw values from the mass spectrometry run were normalized and log-transformed by variance stabilizing using ‘vsn’ package in R Bioconductor and are provided in the Table S3. For calculating changes in light or heavy label incorporation we variance-stabilized either only light labeled or heavy labeled wild type and mutant samples together. To calculate changes in total protein levels the heavy and light label raw values were added and then variance-stabilized for wild type and mutant samples together. To calculate the protein turnover for each sample the raw value heavy label by total protein as calculated previously were variance-stabilized for wild type and mutant samples together.
Supplementary Material
Highlights.
Inhibition of transcription elongation is balanced by enhanced translation.
Co-transcriptional Not1 mRNA imprinting regulates mRNA stability and translatability.
Not5 determines global ribosomal protein gene mRNA imprinting
Not5 determines the translational capacity of the cells
Acknowledgments
This work was supported by grant 31003a_135794 from the Swiss National Science awarded to MAC, by grant (P01 HG000205) from the US National Institutes of Health awarded to LMS and by grant STE 1422/3-1 from Deutsche Forschungsgemeinschaft awarded to LMS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Access
Datasets generated in this study have been submitted to the EBI ArrayExpress under the accession E-MTAB-3195 (https://www.ebi.ac.uk/arrayexpress/). The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD003871.
Author Contributions
Conceptualization, IG ZV and MAC.; Investigation, Methodology IG ZV SK CH and OOP.; Software, IG.; Formal Analysis, IG ZV LMS and MAC.; Resources, IG ZV SK CH OOP LMS and MAC.; Writing - Original Draft, IG ZV LMS and MAC.; Writing – Review & Editing IG ZV OOP LMS and MAC.; Visualization, IG and ZV.; Supervision, LMS and MAC.; Funding Acqusition, LMS and MAC.
Contributor Information
Ishaan Gupta, Email: ishaanpbs@gmail.com.
Zoltan Villanyi, Email: zoltan.villanyi@unige.ch.
Sari Kassem, Email: sari.kassem@unige.ch.
Christopher Hughes, Email: chughes@bcgsc.ca.
Olesya O. Panasenko, Email: olesya.panasenko@unige.ch.
Lars M. Steinmetz, Email: larsms@embl.de.
References
- Albert TK, Lemaire M, van Berkum NL, Gentz R, Collart MA, Timmers HT. Isolation and characterization of human orthologs of yeast CCR4-NOT complex subunits. Nucleic Acids Res. 2000;28:809–817. doi: 10.1093/nar/28.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzouz N, Panasenko OO, Colau G, Collart MA. The CCR4-NOT complex physically and functionally interacts with TRAMP and the nuclear exosome. PLoS One. 2009a;4:e6760. doi: 10.1371/journal.pone.0006760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzouz N, Panasenko OO, Deluen C, Hsieh J, Theiler G, Collart MA. Specific roles for the Ccr4-Not complex subunits in expression of the genome. Rna. 2009b;15:377–383. doi: 10.1261/rna.1348209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbarwal V, Fu J, Reese JC. The Rpb4/7 module of RNA polymerase II is required for Carbon Catabolite Repressor Protein 4-Negative on TATA (Ccr4-Not) complex to promote elongation. J Biol Chem. 2014;289:33125–33130. doi: 10.1074/jbc.C114.601088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar V, Roudko V, Basquin J, Sharma K, Urlaub H, Seraphin B, Conti E. Structure and RNA-binding properties of the Not1-Not2-Not5 module of the yeast Ccr4-Not complex. Nat Struct Mol Biol. 2013;20:1281–1288. doi: 10.1038/nsmb.2686. [DOI] [PubMed] [Google Scholar]
- Chapat C, Corbo L. Novel roles of the CCR4-NOT complex. Wiley Interdiscip Rev RNA. 2014;5:883–901. doi: 10.1002/wrna.1254. [DOI] [PubMed] [Google Scholar]
- Chatenay-Lapointe M, Shadel GS. Repression of mitochondrial translation, respiration and a metabolic cycle-regulated gene, SLF1, by the yeast Pumilio-family protein Puf3p. PLoS One. 2011;6:e20441. doi: 10.1371/journal.pone.0020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choder M. mRNA imprinting: Additional level in the regulation of gene expression. Cell Logist. 2011;1:37–40. doi: 10.4161/cl.1.1.14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchman LS, Weissman JS. Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature. 2011;469:368–373. doi: 10.1038/nature09652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart MA, Panasenko OO. The Ccr4--not complex. Gene. 2012;492:42–53. doi: 10.1016/j.gene.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Collart MA, Panasenko OO, Nikolaev SI. The Not3/5 subunit of the Ccr4-Not complex: a central regulator of gene expression that integrates signals between the cytoplasm and the nucleus in eukaryotic cells. Cell Signal. 2013;25:743–751. doi: 10.1016/j.cellsig.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Collart MA, Timmers HT. The eukaryotic Ccr4-not complex: a regulatory platform integrating mRNA metabolism with cellular signaling pathways? Prog Nucleic Acid Res Mol Biol. 2004;77:289–322. doi: 10.1016/S0079-6603(04)77008-7. [DOI] [PubMed] [Google Scholar]
- Cui Y, Ramnarain DB, Chiang YC, Ding LH, McMahon JS, Denis CL. Genome wide expression analysis of the CCR4-NOT complex indicates that it consists of three modules with the NOT module controlling SAGA-responsive genes. Mol Genet Genomics. 2008;279:323–337. doi: 10.1007/s00438-007-0314-1. [DOI] [PubMed] [Google Scholar]
- del Alamo M, Hogan DJ, Pechmann S, Albanese V, Brown PO, Frydman J. Defining the specificity of cotranslationally acting chaperones by systematic analysis of mRNAs associated with ribosome-nascent chain complexes. PLoS Biol. 2011;9:e1001100. doi: 10.1371/journal.pbio.1001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova LN, Kuroha K, Tatematsu T, Inada T. Nascent peptide-dependent translation arrest leads to Not4p-mediated protein degradation by the proteasome. J Biol Chem. 2009;284:10343–10352. doi: 10.1074/jbc.M808840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dori-Bachash M, Shalem O, Manor YS, Pilpel Y, Tirosh I. Widespread promoter-mediated coordination of transcription and mRNA degradation. Genome Biol. 2012;13:R114. doi: 10.1186/gb-2012-13-12-r114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dori-Bachash M, Shema E, Tirosh I. Coupled evolution of transcription and mRNA degradation. PLoS Biol. 2011;9:e1001106. doi: 10.1371/journal.pbio.1001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A, Babbarwal V, Fu J, Brunke-Reese D, Libert DM, Willis J, Reese JC. Ccr4-Not and TFIIS function cooperatively to rescue arrested RNA polymerase II. Mol Cell Biol. 2015 doi: 10.1128/MCB.00044-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Herreros F, de Miguel-Jimenez L, Morillo-Huesca M, Delgado-Ramos L, Munoz-Centeno MC, Chavez S. TFIIS is required for the balanced expression of the genes encoding ribosomal components under transcriptional stress. Nucleic Acids Res. 2012;40:6508–6519. doi: 10.1093/nar/gks340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigull J, Mnaimneh S, Pootoolal J, Robinson MD, Hughes TR. Genome-wide analysis of mRNA stability using transcription inhibitors and microarrays reveals posttranscriptional control of ribosome biogenesis factors. Mol Cell Biol. 2004;24:5534–5547. doi: 10.1128/MCB.24.12.5534-5547.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta I, Clauder-Munster S, Klaus B, Jarvelin AI, Aiyar RS, Benes V, Wilkening S, Huber W, Pelechano V, Steinmetz LM. Alternative polyadenylation diversifies post-transcriptional regulation by selective RNA-protein interactions. Molecular systems biology. 2014;10:719. doi: 10.1002/msb.135068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich G, Medina DA, Causse SZ, Garber M, Millan-Zambrano G, Barkai O, Chavez S, Perez-Ortin JE, Darzacq X, Choder M. Gene expression is circular: factors for mRNA degradation also foster mRNA synthesis. Cell. 2013;153:1000–1011. doi: 10.1016/j.cell.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Halter D, Collart MA, Panasenko OO. The Not4 E3 ligase and CCR4 deadenylase play distinct roles in protein quality control. PLoS One. 2014;9:e86218. doi: 10.1371/journal.pone.0086218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruki H, Nishikawa J, Laemmli UK. The anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol Cell. 2008;31:925–932. doi: 10.1016/j.molcel.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Hogan DJ, Riordan DP, Gerber AP, Herschlag D, Brown PO. Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system. PLoS Biol. 2008;6:e255. doi: 10.1371/journal.pbio.0060255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Sweet TJ, Chamnongpol S, Baker KE, Coller J. Co-translational mRNA decay in Saccharomyces cerevisiae. Nature. 2009;461:225–229. doi: 10.1038/nature08265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CS, Foehr S, Garfield DA, Furlong EE, Steinmetz LM, Krijgsveld J. Ultrasensitive proteome analysis using paramagnetic bead technology. Mol Syst Biol. 2014;10:757. doi: 10.15252/msb.20145625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito W, Li X, Irie K, Mizuno T, Irie K. RNA-binding protein Khd1 and Ccr4 deadenylase play overlapping roles in the cell wall integrity pathway in Saccharomyces cerevisiae. Eukaryot Cell. 2011;10:1340–1347. doi: 10.1128/EC.05181-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasiak AJ, Hartmann H, Karakasili E, Kalocsay M, Flatley A, Kremmer E, Strasser K, Martin DE, Soding J, Cramer P. Genome-associated RNA polymerase II includes the dissociable Rpb4/7 subcomplex. J Biol Chem. 2008;283:26423–26427. doi: 10.1074/jbc.M803237200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight B, Kubik S, Ghosh B, Bruzzone MJ, Geertz M, Martin V, Denervaud N, Jacquet P, Ozkan B, Rougemont J, et al. Two distinct promoter architectures centered on dynamic nucleosomes control ribosomal protein gene transcription. Genes Dev. 2014;28:1695–1709. doi: 10.1101/gad.244434.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk JA, Dutta A, Fu J, Gilmour DS, Reese JC. The multifunctional Ccr4-Not complex directly promotes transcription elongation. Genes Dev. 2011;25:581–593. doi: 10.1101/gad.2020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Chiang YC, Pan J, Chen J, Salvadore C, Audino DC, Badarinarayana V, Palaniswamy V, Anderson B, Denis CL. Characterization of CAF4 and CAF16 reveals a functional connection between the CCR4-NOT complex and a subset of SRB proteins of the RNA polymerase II holoenzyme. J Biol Chem. 2001;276:7541–7548. doi: 10.1074/jbc.M009112200. [DOI] [PubMed] [Google Scholar]
- Lotan R, Bar-On VG, Harel-Sharvit L, Duek L, Melamed D, Choder M. The RNA polymerase II subunit Rpb4p mediates decay of a specific class of mRNAs. Genes Dev. 2005;19:3004–3016. doi: 10.1101/gad.353205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Lidschreiber M, Siebert M, Leike K, Soding J, Cramer P. Uniform transitions of the general RNA polymerase II transcription complex. Nat Struct Mol Biol. 2010;17:1272–1278. doi: 10.1038/nsmb.1903. [DOI] [PubMed] [Google Scholar]
- O'Duibhir E, Lijnzaad P, Benschop JJ, Lenstra TL, van Leenen D, Groot Koerkamp MJ, Margaritis T, Brok MO, Kemmeren P, Holstege FC. Cell cycle population effects in perturbation studies. Mol Syst Biol. 2014;10:732. doi: 10.15252/msb.20145172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasenko OO, Collart MA. Presence of Not5 and ubiquitinated Rps7A in polysome fractions depends upon the Not4 E3 ligase. Mol Microbiol. 2012;83:640–653. doi: 10.1111/j.1365-2958.2011.07957.x. [DOI] [PubMed] [Google Scholar]
- Pelechano V, Jimeno-Gonzalez S, Rodriguez-Gil A, Garcia-Martinez J, Perez-Ortin JE, Chavez S. Regulon-specific control of transcription elongation across the yeast genome. PLoS Genet. 2009;5:e1000614. doi: 10.1371/journal.pgen.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelechano V, Wei W, Steinmetz LM. Widespread Co-translational RNA Decay Reveals Ribosome Dynamics. Cell. 2015;161:1400–1412. doi: 10.1016/j.cell.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preissler S, Reuther J, Koch M, Scior A, Bruderek M, Frickey T, Deuerling E. Not4-dependent translational repression is important for cellular protein homeostasis in yeast. EMBO J. 2015;34:1905–1924. doi: 10.15252/embj.201490194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendl LM, Bieman MA, Smibert CA. S. cerevisiae Vts1p induces deadenylation-dependent transcript degradation and interacts with the Ccr4p-Pop2p-Not deadenylase complex. RNA. 2008;14:1328–1336. doi: 10.1261/rna.955508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan DP, Herschlag D, Brown PO. Identification of RNA recognition elements in the Saccharomyces cerevisiae transcriptome. Nucleic Acids Res. 2011;39:1501–1509. doi: 10.1093/nar/gkq920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rospert S, Dubaquie Y, Gautschi M. Nascent-polypeptide-associated complex. Cell Mol Life Sci. 2002;59:1632–1639. doi: 10.1007/PL00012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Schwalb B, Schulz D, Pirkl N, Etzold S, Lariviere L, Maier KC, Seizl M, Tresch A, Cramer P. Comparative dynamic transcriptome analysis (cDTA) reveals mutual feedback between mRNA synthesis and degradation. Genome Res. 2012;22:1350–1359. doi: 10.1101/gr.130161.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trcek T, Larson DR, Moldon A, Query CC, Singer RH. Single-molecule mRNA decay measurements reveal promoter- regulated mRNA stability in yeast. Cell. 2011;147:1484–1497. doi: 10.1016/j.cell.2011.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venters BJ, Wachi S, Mavrich TN, Andersen BE, Jena P, Sinnamon AJ, Jain P, Rolleri NS, Jiang C, Hemeryck-Walsh C, et al. A comprehensive genomic binding map of gene and chromatin regulatory proteins in Saccharomyces. Mol Cell. 2011;41:480–492. doi: 10.1016/j.molcel.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanyi Z, Ribaud V, Kassem S, Panasenko OO, Pahi Z, Gupta I, Steinmetz L, Boros I, Collart MA. The not5 subunit of the ccr4-not complex connects transcription and translation. PLoS Genet. 2014;10:e1004569. doi: 10.1371/journal.pgen.1004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A, Chen HV, Liu CL, Rahat A, Klien A, Soares L, Gudipati M, Pfeffner J, Regev A, Buratowski S, et al. Systematic dissection of roles for chromatin regulators in a yeast stress response. PLoS Biol. 2012;10:e1001369. doi: 10.1371/journal.pbio.1001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkening S, Pelechano V, Jarvelin AI, Tekkedil MM, Anders S, Benes V, Steinmetz LM. An efficient method for genome-wide polyadenylation site mapping and RNA quantification. Nucleic Acids Res. 2013;41:e65. doi: 10.1093/nar/gks1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.