Abstract
Background
Decreased sensitivity to pleasant stimuli is associated with a higher vulnerability to nicotine dependence in youths and with difficulty quitting in adult smokers. Recently, we showed that smokers showing lower brain reactivity to non-cigarette-related pleasant images than to cigarette-related ones have lower chances of achieving longer-term abstinence during a quit attempt.
Methods
We tested whether individual differences in brain responses to cigarette-related and pleasant stimuli require a long history of smoking to develop by measuring the late positive potential (LPP) to cigarette cues, emotional, and neutral stimuli in 45 young, light smokers (ages 18-25). k-means cluster analysis was used to partition smokers into two groups based on the magnitude of their LPPs.
Results
Group 1 was characterized by larger LPPs to pleasant pictures than cigarette-related pictures whereas Group 2 showed the opposite pattern.
Conclusions
Our results suggest that individual differences in brain responses to cigarette-related and pleasant cues do not require a long smoking history to develop.
Keywords: Addiction, smoking, emotion, young adults
1. INTRODUCTION
Chronic drug use is hypothesized to result in the attribution of excessive motivational value to drugs at the expense of natural rewards (Volkow et al., 2016, 2010). Recently, we found neurophysiological evidence to support this hypothesis in smokers using the late positive potential (LPP), an event-related potential (ERP) measure of emotional arousal (Cuthbert et al., 2000). Using k-means cluster analysis, which is a multivariate statistical technique designed to partition individual cases (participants) into k-groups such that variance is minimized within groups and maximized between groups (Hair and Black, 2000; Johnson and Wichern, 2002), we identified two distinct groups of smokers based on their LPPs to cigarette-related, pleasant, unpleasant, and neutral cues. One group (Group 1) was characterized by larger LPPs to pleasant stimuli than to cigarette-related cues. The other group (Group 2) was characterized by larger LPPs to cigarette-related cues than to pleasant stimuli. Importantly, smokers in Group 2, i.e., those with larger LPPs to cigarette cues than to pleasant stimuli, had a reduced likelihood of achieving long-term smoking abstinence over the course of a six-month smoking-cessation clinical trial (Versace et al., 2012). In another study (Versace et al., 2014), we used functional magnetic resonance imaging (fMRI) to identify specific brain regions where individual smokers differ in their brain responses to cigarette-related and pleasant stimuli. Again, we used cluster analysis to divide smokers into two groups and found that smokers in Group 1 showed larger brain responses to pleasant stimuli than to cigarette cues, and those in Group 2 showed the opposite pattern of brain responses. As was the case in the LPP study, the smokers in Group 2 in the fMRI study were also less likely to achieve long-term abstinence over the course of a six-month quit attempt. Importantly, the differences in brain activation in response to pleasant stimuli and cigarette cues were observed not only in the visual areas (the neural generators of the LPP; Keil et al., 2002; Liu et al., 2012; Sabatinelli et al., 2007), but also in the striatum, anterior cingulate cortex, and medial prefrontal cortex, all of which have been implicated in reward processing (Jasinska et al., 2014). These results support the hypothesis that, in some smokers (i.e., those in Group 2), brain reward circuits are biased toward cigarette cues at the expense of other forms of reinforcement (Volkow et al., 2010).
It is unknown whether blunted brain responses to pleasant stimuli are a consequence of nicotine use (Volkow et al., 2010), or if they precede smoking initiation and increase the risk of nicotine dependence (Audrain-McGovern et al., 2012). Our previous studies were conducted in regular smokers who, on average, smoked 20 cigarettes per day for 25 years (Versace et al., 2014, 2012). Thus, the different brain reactivity profiles that we observed could have been pre-existing, or they could have emerged at some point after smoking initiation (e.g., at the transition from casual smoking to nicotine dependence). It is important to identify when smokers begin to show differences in brain responses to cigarette cues and pleasant stimuli because, in addition to predicting the likelihood of successfully quitting, it might also be possible to use this neural biomarker to predict other outcomes, such as smoking initiation or the transition from relatively early stages of cigarette use to nicotine dependence.
Ultimately, longitudinal studies will be necessary to determine whether differential brain responses to cigarette cues and pleasant stimuli predict smoking initiation or escalation. Prior to undertaking longitudinal research, it is prudent to determine whether a similar pattern of brain responses to those seen in heavy smokers attempting to quit can also be observed in younger, lighter smokers. Hence, to achieve this goal, we decided to apply the same cluster analytic method that we used in our studies of heavy smokers to a previously-unpublished LPP dataset collected as part of a larger study about emotional reactivity in smokers (Engelmann et al., 2011). Most of the smokers in this study were 18-25 years old, which is when smoking prevalence peaks (Substance Abuse and Health Services Administration, 2012) and patterns of cigarette use start to solidify (Hu et al., 2012). We partitioned smokers into k=2 groups based on the amplitude of their LPPs to cigarette-related, pleasant, unpleasant, and neutral stimuli. We decided to use k=2 groups because, based on our previous research, we expected to find individual differences in relative reactivity to cigarette-related and pleasant cues, i.e., one group with larger brain responses to cigarette cues than to pleasant stimuli, and another group with significantly larger brain responses to pleasant stimuli than to cigarette cues.
2. MATERIALS AND METHODS
2.1. Participants
Of 81 participants enrolled in the parent study, 45 daily smokers aged 18-25 with LPP data available were included in the cluster analysis. Participants not included in the cluster analysis consisted of 2 individuals who withdrew from the study, 5 for whom equipment failure resulted in a loss of data, 3 with excessive artifact in their EEG data, 20 non-smokers, and 6 smokers over the age of 25. Non-smokers were not included in the cluster analysis because the goal of this analysis was to determine whether young smokers show a pattern of individual differences in brain responses to pleasant and cigarette-related cues that was similar to what we previously observed in heavy smokers interested in quitting. Thus, we had no specific a priori hypotheses about how non-smokers would respond to cigarette cues, or about how their responses to cigarette cues would differ from pleasant stimuli. However, we did include the non-smokers (n=19) in an exploratory analysis for which they were used as a reference group against which to compare the two groups of smokers (data from 1 non-smoker over the age of 25 were excluded).
Participants were recruited via advertisements seeking smokers not currently interested in quitting. Smokers were included if they reported smoking at least 1 cigarette per day for at least the past 30 days. Non-smokers were included if they reported not smoking a single cigarette over the past 6 months, and smoking no more than 100 cigarettes in their lifetime. Participants were excluded if they reported current uncontrolled psychiatric or medical illness, or the use of medications that might influence the ERP recording. All participants provided informed consent and all procedures were approved by the University of Minnesota’s institutional review board. Participants received $50 or course credit for completing the study.
2.2. Procedure
The full procedure is described elsewhere (Engelmann et al., 2011). Briefly, participants attended three study visits: baseline, psychophysiological recording, and follow-up. During the baseline visit, smokers were randomly assigned to an abstinent or non-abstinent condition. At the time of psychophysiological recording, the abstinent smokers (n=23) were 24 h into a 48 h abstinence period, whereas the non-abstinent smokers (n=22) were instructed to smoke normally during the same period, and to smoke one additional cigarette at the start of the session (approximately 20 minutes elapsed between when this cigarette was smoked and the start of data collection).
During the baseline visit, nicotine dependence was assessed using the Fagerström Test of Nicotine Dependence (FTND; Heatherton et al., 1991) and Heaviness of Smoking Index (HSI; Heatherton et al., 1989). At the start of all visits, the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes and Hatsukami, 1998) and Factor 1 of the Questionnaire of Smoking Urges (QSU; Tiffany and Drobes, 1991) were used to assess nicotine withdrawal symptoms and cigarette craving. Due to a recording error, FTND, HSI, MNWS, and QSU data were lost from 1 participant assigned to the non-abstinent condition.
Participants viewed a series of 60 pictures, 15 from each of four categories: cigarette, pleasant, unpleasant, and neutral. Pictures were presented for 6 s each in a random order, separated by an intertrial interval lasting 18-24 s (Cuthbert et al., 2000). Pictures were selected from the International Affective Picture System (IAPS; Lang et al., 2005) and from picture sets developed by the authors (Carter et al., 2006; Engelmann et al., 2011). The electroencephalogram (EEG) was recorded from electrodes placed at the Fz, Cz, and Pz sites of the International 10-20 system (Jasper, 1958), referenced to linked mastoids. Vertical electrooculogram (vEOG) was measured for the purpose of correcting eye-movement artifacts in the EEG. Using established procedures for measuring the LPP (Cuthbert et al., 2000; Sabatinelli et al., 2007; Schupp et al., 2000; Versace et al., 2011), the EEG and vEOG were bandpass filtered (0.1-40 Hz), amplified, and continuously sampled at a rate of 125 Hz using a PC running VPM software (Cook, 2003).
2.3. Statistical Analysis
We analyzed data from the Cz electrode site, which is where the LPP is most reliably observed (Cuthbert et al., 2000; Keil et al., 2002; Schupp et al., 2000) and where the LPP was localized in our previous study of individual differences in LPP magnitude in smokers (Versace et al., 2012). EEG data were processed using established procedures, which included digital filtering (0.1-30 Hz), epoch extraction (120 ms before through 1000 ms after picture onset), artifact detection and rejection, averaging across trials within each participant and picture category to compute the ERP, eye-movement correction (Gratton et al., 1983), and baseline correction. In cases where there were fewer than 10 artifact-free trials in any stimulus category for a particular participant, the data for that participant were excluded from further analysis (n=3). Averaging across subjects, the mean (SD) number of trials that remained in the analysis after artifact rejection was 14.6 (0.7), 14.7 (0.7), 14.6 (0.9), and 14.7 (0.6) for cigarette-related, pleasant, neutral, and unpleasant stimuli, respectively.
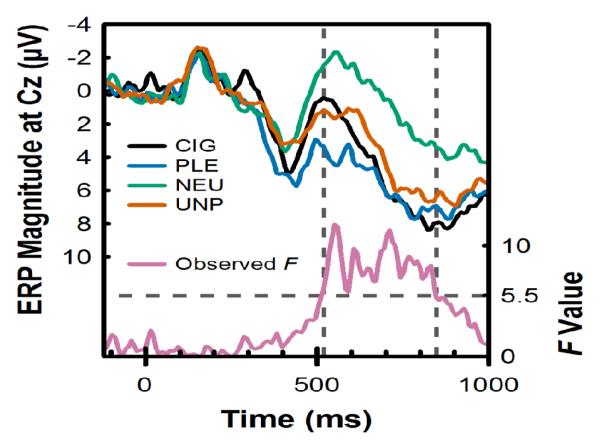
The time window for the LPP was determined empirically using a permutation-based statistical testing approach similar to that used previously in our laboratory (Robinson et al., 2015; Versace et al., 2011) and elsewhere (Maris, 2004). First, we built a permutation distribution associated with the hypothesis of interest (i.e., the effect of picture category on the LPP). The permutation distribution was obtained by repeating the following two steps 10,000 times: a) the data labels (i.e., picture category) for the values (i.e., the ERP voltage value at the Cz electrode site) recorded at each time point were randomly shuffled; b) a within-subjects ANOVA for picture category was conducted at each time point and the highest F-value was added to the distribution. Second, the F-values of the actual data from the picture category ANOVA at each time point were compared to the 95th percentile of the F-values in the permutation distribution. If the F-value for any given time point of the actual data exceeded the permutation F, it was considered to be significant at the 0.05 level. Contiguous significant time points were considered part of the LPP time window of interest and the voltage from these time points were averaged together. This procedure resulted in the mean ERP voltage between 520-848 ms after picture onset being defined as the LPP (Figure 1).
Figure 1.
The time window for computing the LPP was identified via permutation testing. Upper traces, left vertical axis: Grand-average event-related potential (ERP) at Cz in response to cigarette-related (CIG), pleasant (PLE), neutral (NEU), and unpleasant (UNP) pictures. Time is plotted on the horizontal axis, where stimulus onset occurred at 0 ms. Lower trace (Observed F), right vertical axis: F-statistic for the effect of picture category observed at each time point. The dashed horizontal line indicates the threshold for identifying time points with significant effects of picture category. This value (F > 5.5) was obtained using a permutation-based statistical testing approach (for details, see Statistical Analysis). The dashed vertical lines illustrate the time window (520-848 ms) used to compute the LPP magnitude.
To divide smokers into two groups, the LPPs for cigarette, pleasant, unpleasant, and neutral pictures were standardized within-subjects and entered into a k-means cluster analysis, implemented in Statistica software (version 10, StatSoft Inc., Tulsa, OK, USA). k-means cluster analysis is a multivariate technique that partitions observations into k groups through an iterative process. First, participants are randomly separated into k-groups. Next, the algorithm shuffles the group assignments until variance is minimized within-groups and maximized between-groups (Hair and Black, 2000; Johnson and Wichern, 2002). Based on our previous studies (Versace et al., 2014, 2012), we specified that the algorithm partition the sample into k=2 groups of smokers.
To compare LPPs between the two groups, we used general linear models, also implemented in Statistica. Cluster membership (Group 1, Group 2) was entered into the model as a between-subjects factor, and picture category (cigarette, pleasant, unpleasant, and neutral) as a within-subjects factor. To control for possible effects of abstinence on LPP magnitude, carbon-monoxide (CO)-confirmed abstinence on the day of the psychophysiological recording session was also included in the model as a between-subjects factor. To be considered abstinent, a participant had to report not smoking over the past 24 h and produce a CO level < 6 parts per million [ppm] or half of his/her baseline level (whichever was lower) (Engelmann et al., 2011; Marrone et al., 2010). Per this criterion, 1 participant who was assigned to the abstinent condition was unable to remain abstinent for 24 h prior to the psychophysiological recording session, and was thus classified as non-abstinent in the analysis.
The cluster analysis divided the smokers into two groups with different patterns of brain responses. Thus, we expected to find a significant cluster membership × picture category interaction. It is important to note that, although we specified the number of clusters to extract based on the results of our previous studies, the k-means algorithm is unsupervised; the specific LPP response profile that each cluster might show is not pre-determined and could take different forms. To determine the specific pattern of differences within and between the groups that contributed to the interaction, we used pairwise comparisons of means, controlling for the Type I error rate (α = .05) using Bonferroni correction.
To determine whether different proportions of individuals were assigned to each cluster group on the basis of abstinence status, gender, race, or ethnicity, we used chi-square tests. Next, we examined whether the two groups differed on any self-report measures using general linear models. For questionnaires that were administered only at baseline, cluster membership (Group 1, Group 2) was the only predictor in the model. For questionnaires that were administered at baseline and during the psychophysiological recording visit, cluster membership and abstinence status were included as between-subjects factors, and the score on the same questionnaire at baseline was included as a covariate.
Finally, we conducted an exploratory analysis in which we compared the LPP between the two groups of smokers and a reference group of non-smokers with the same age range (18-25) as the smokers. For this analysis, we used a GLM with group (smoker group 1, smoker group 2, and non-smokers) as a between-subjects factor and picture category (cigarette, pleasant, unpleasant, and neutral) as a within-subjects factor. Significant main effects and interactions were followed-up with pairwise comparisons of means, controlling the Type I error rate (α = .05) using Bonferroni correction. We also compared the LPP between nonsmokers, non-abstinent smokers, and abstinent smokers independent of cluster group assignment using a GLM with group (non-smoker, non-abstinent smoker, abstinent smoker) as a between-subjects factor and picture category (cigarette, pleasant, unpleasant, and neutral) as a within-subjects factor.
3. RESULTS
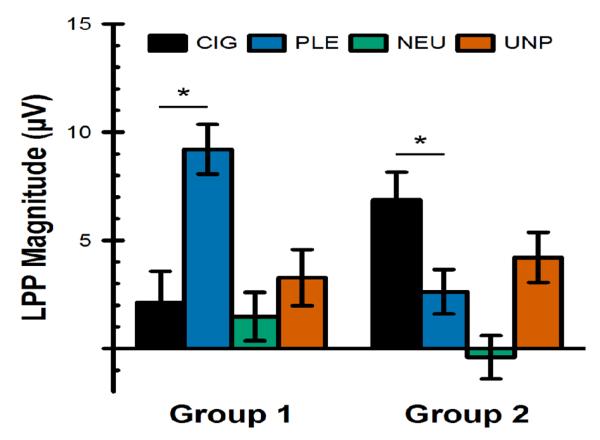
On average, our smokers were 21 years old and smoked 7.4 cigarettes per day (range = 1-20; see Table 1). The k-means cluster analysis algorithm assigned 20 smokers to Group 1 and 25 smokers to Group 2. The general linear model for LPP magnitude (Figure 2) found a significant cluster membership × picture category interaction: F(3,123) = 15.82, p < .0001. As hypothesized, this interaction was driven by larger brain responses to pleasant pictures than cigarette cues in Group 1 (within-group comparison: F[1,123] = 31.25, p < .0001, Bonferroni-corrected p < .0001), and the opposite pattern in Group 2 (within-group comparison: F[1,123] = 14.59, p = .0002, Bonferroni-corrected p = .006). Between-groups comparisons found a significant difference between the two groups in LPP magnitude to pleasant pictures (F[1,123] = 17.47, p < .0001, Bonferroni-corrected p = .0015). The difference between the two groups in LPP magnitude to cigarette-related pictures was not statistically significant after correction for multiple comparisons (F[1,123] = 5.57, p = .02, Bonferroni-corrected p = .55). There was no evidence of a significant difference between the groups in LPP magnitude to unpleasant (F[1,123] = 0.05, p = .83) or neutral (F[1,123] = 1.23, p = .27) pictures. The main effect of abstinence (F[1,41] = 1.39, p = .25), and the abstinence × cluster membership (F[1,41] = 1.03, p = .32), abstinence × picture category (F[3,123] = 0.55, p = .65), and abstinence × cluster membership × picture category (F[3,123] = 1.23, p = .30) interactions were not statistically significant.
Table 1.
Demographics, smoking history, and questionnaire scores
| Group 1 (n = 20) |
Group 2 (n = 25) |
Total (n = 45) |
||
|---|---|---|---|---|
|
| ||||
| % (n) | % (n) | % (n) | ||
|
|
||||
| Gender | ||||
| Female | 45.0 (9) | 48.0 (12) | 46.7 (21) | |
| Race | ||||
| White | 70.0 (14) | 80.0 (20) | 75.6 (34) | |
| Other | 30.0 (6) | 20.0 (5) | 24.4 (11) | |
| Ethnicity | ||||
| Non-Hispanic | 90.0 (18) | 100.0 (25) | 95.6 (43) | |
| Hispanic | 10.0 (2) | 0.0 (0) | 4.4 (2) | |
| Visit | Mean (SD) | Mean (SD) | Mean (SD) | |
|
|
||||
| Age (years) | 1 | 20.6 (1.6) | 21.3 (1.5) | 21.0 (1.6) |
| Years smoking | 1 | 4.5 (2.0) | 5.2 (3.2) | 4.9 (2.7) |
| Current smoking rate (cigarettes/day) | 1 | 6.9 (4.7) | 7.9 (5.8) | 7.4 (5.3) |
| Expired CO (ppm) | 1 | 8.0 (4.9) | 9.4 (5.4) | 8.8 (5.2) |
| Nicotine dependence (FTND) | 1 | 2.2 (1.8) | 2.5 (1.7) | 2.3 (1.7) |
| Heaviness of smoking index | 1 | 1.2 (1.0) | 1.5 (1.4) | 1.4 (1.3) |
| Withdrawal Symptoms (MNWS) | 1 | 1.3 (1.0) | 1.3 (0.8) | 1.3 (0.9) |
| 2 | 1.3 (0.8) | 1.6 (0.7) | 1.4 (0.8) | |
| Cigarette Craving (QSU Factor 1) | 1 | 4.7 (1.5) | 4.8 (1.2) | 4.8 (1.3) |
| 2 | 4.8 (1.5) | 4.8 (1.5) | 4.8 (1.4) | |
Note: Visit 1 = baseline, Visit 2 = ERP recording. CO = carbon monoxide, FTND = Fagerström Test of Nicotine Dependence, MNWS = Minnesota Nicotine Withdrawal Scale, QSU = Questionnaire of Smoking Urges, SD = Standard Deviation.
Figure 2.
Magnitude of the late positive potential (LPP) at Cz in response to cigarette-related (CIG), pleasant (PLE), neutral (NEU), and unpleasant (UNP) pictures in young smokers who were classified into two groups using k-means cluster analysis. For Group 1, n = 20. For Group 2, n = 25. * = p < .05 for the within-group difference between cigarette-related and pleasant pictures (Bonferroni-corrected for all pairwise comparisons).
In cluster 1, there were 13 non-abstinent smokers and 7 abstinent smokers. In cluster 2, there were 10 non-abstinent smokers and 15 abstinent smokers. The proportion of non-abstinent vs. abstinent smokers in each cluster group did not significantly differ (χ2[1] = 2.78, p = .10). Demographic and self-report data, broken down by cluster membership, are shown in Table 1. The proportion of males vs. females (χ2[1] = 0.04, p = .84), whites vs. non-whites (χ2[1] = 0.60, p = .44), and Hispanics vs. non-Hispanics (χ2[1] = 2.62, p = .11) did not significantly differ between the two groups. The two cluster groups did not significantly differ on age (F[1,43] = 2.02, p = .16), the number of years that they had been smoking cigarettes (F[1,43] = 0.74, p = .40), current smoking rate (F[1,43] = .45, p = .51), FTND scores (F[1,42] = 0.44, p = .51), HSI scores (F[1,42] = 1.06, p = .31), baseline MNWS scores (F[1,42] = 0.08, p = .78), or baseline QSU scores (F[1,44] = 0.09, p = .78). As previously published (Engelmann et al., 2011), withdrawal symptom and craving scores significantly increased during abstinence, as indicated by significant main effects of abstinence (Fs[1,39] > 2.8, ps < .01, data not shown). However, there was no significant abstinence × cluster membership interaction for either measure (Fs[1,39] < 1, ps > .1).
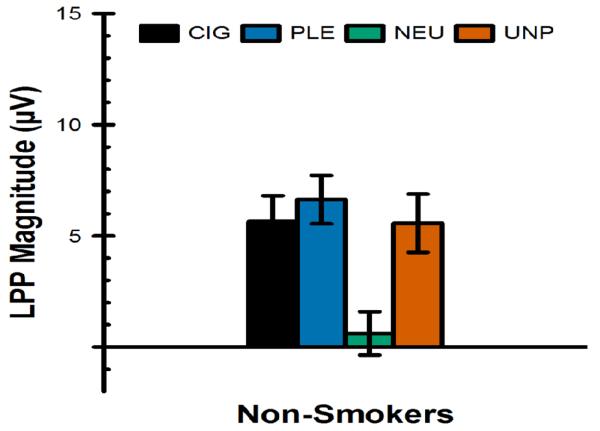
LPP magnitude measured in the reference group of non-smokers (n=19, 13 female, mean age = 20.5, SD = 1.6) is presented in Figure 3. The GLM with cluster group (smoker group 1, smoker group 2, and non-smokers) as a between-subjects factor and picture category (cigarette, pleasant, unpleasant, and neutral) as a within-subjects factor found a significant group × picture category interaction. No between-group comparisons involving non-smokers were statistically significant after Bonferroni correction (ps > .1). Rather, the interaction was driven by differences between the two smoker groups, as detailed above in the results of the GLM that only included smokers. Within the non-smokers group, the LPP to cigarette-related (F[1,183] = 14.98, p = .0002, Bonferroni-corrected p = .01), pleasant (F[1,183] = 21.44, p < .0001, Bonferroni-corrected p = .0005), and unpleasant (F[1,183] = 14.59, p = .0002, Bonferroni-corrected p = .0122) pictures was significantly larger than it was to neutral pictures. The LPP to cigarette-related pictures did not significantly differ from the LPP to pleasant (F[1,183] = 0.58, p = .45) and unpleasant (F[1,183] = 0.002, p = .96) pictures. Finally, the non-smokers did not differ from non-abstinent or abstinent smokers as a whole (i.e., independent of cluster group assignment): The group (non-smoker, non-abstinent smoker, and abstinent smoker) × picture category (cigarette, pleasant, unpleasant, and neutral) interaction did not approach statistical significance (F[6,183] = 0.64, p = .70).
Figure 3.
Magnitude of the late positive potential (LPP) at Cz in response to cigarette-related (CIG), pleasant (PLE), neutral (NEU), and unpleasant (UNP) pictures in a reference group of non-smokers, plotted on the same scale as that used for smokers (see Figure 2).
4. DISCUSSION
Previously, we used cluster analysis to partition smokers into two groups on the basis of their brain responses to cigarette-related, pleasant, neutral, and unpleasant stimuli. In those studies, we found that smokers with larger brain responses to cigarette cues than to pleasant stimuli are less likely to achieve long-term abstinence over the course of a 6-month smoking-cessation attempt (Versace et al., 2014, 2012). The goal of the current analysis was to determine whether a similar pattern of brain responses to those seen in heavy smokers attempting to quit can also be observed in younger, lighter smokers.
Compared to typical samples of adult smokers interested in quitting (e.g., Cinciripini et al., 2013), the young smokers studied here smoked fewer cigarettes per day, had lower FTND and HSI scores, and had lower baseline CO levels. We used cluster analysis to partition the smokers into k=2 groups based on their brain responses to cigarette-related, pleasant, neutral, and unpleasant stimuli. Based on our previous research, we predicted that one group would have larger LPPs to pleasant stimuli than to cigarette cues, and that the other group would have larger LPPs to cigarette cues than to pleasant stimuli. In fact, we observed this pattern of brain activity. The 20 smokers assigned to Group 1 had significantly larger LPPs to pleasant stimuli than to cigarette cues, whereas the 25 smokers assigned to Group 2 had significantly larger LPPs to cigarette cues than to pleasant stimuli.
The LPP is considered a measure of emotional arousal (Cuthbert et al., 2000). Our result suggests that smokers in Group 2, but not in Group 1, find pleasant stimuli less arousing than cigarette cues. Observing differences only in response to pleasant stimuli and cigarette cues — but not unpleasant or neutral stimuli — suggests that this effect could be attributed to individual differences in the motivational significance of cigarette-related versus pleasant stimuli. Reduced sensitivity to natural rewards is associated with higher vulnerability to nicotine dependence in youths (Audrain-McGovern et al., 2012). However, the role of sensitivity to drug-related stimuli and natural rewards in substance abuse has been studied almost exclusively using self-report measures, which might have limited validity because of cultural and social desirability biases (e.g., Leventhal et al., 2006). Thus, a biological measure indexing individual reactivity to different types of emotional and drug-related stimuli might be useful. Our results suggest that the LPP might represent this biological measure. In fact, previous fMRI research in our laboratory found that this pattern of individual differences in brain responses to pleasant stimuli and cigarette cues is not limited to visual areas, but also occurs in the brain’s appetitive motivational system (e.g., dorsal striatum, anterior cingulate, medial prefrontal cortex; Versace et al., 2014). Other fMRI studies have found that adolescent smokers not only show larger responses to cigarette cues than to neutral cues (Rubinstein et al., 2011b), but also show smaller brain responses to pleasant cues than do nonsmokers (Peters et al., 2011; Rubinstein et al., 2011a). These results are consistent with our finding here that a long history of nicotine dependence typically seen in adult smokers trying to quit is not necessary for the development of this effect.
The two cluster groups did not significantly differ on self-report measures of nicotine dependence, withdrawal symptoms, or cigarette craving. This finding is consistent with the results of our previous findings from the study of adult smokers interested in quitting: members of the two cluster groups did not significantly differ on self-report measures of nicotine dependence, affect, craving, or withdrawal symptoms that were measured at baseline and during the ERP or fMRI recording sessions (Versace et al., 2014, 2012). However, cluster membership was predictive of long-term smoking abstinence. This is consistent with recent findings from other areas of neuroscience, in which neural metrics of other constructs (e.g., working memory capacity) may be accurate predictors of abstinence (Loughead et al., 2015).
The LPPs of the two cluster groups studied here did not significantly differ as a function of 24-h nicotine deprivation, nor did the LPP differ between non-abstinent and abstinent smokers as a whole. This could be due to insufficient power to detect such an effect (i.e., only 7 abstinent smokers were assigned to Group 1 whereas 15 were assigned to Group 2). Indeed, previous research has shown that nicotine deprivation modulates brain responses during anticipation of reward (Fedota et al., 2015) and under conditions that require sustained attention (Beaver et al., 2011). Alternatively, our passive picture-viewing paradigm may not be as sensitive to abstinence manipulations as are the active tasks used in other studies (Beaver et al., 2011; Fedota et al., 2015). Furthermore, 24 h of nicotine deprivation may be insufficient to produce reliable abstinence effects using this task, whereas data from our laboratory show that extended abstinence may have resulted in changes in the LPP (Robinson et al., 2015).
In addition to finding no differences between abstinent and non-abstinent smokers, we also found no evidence that the pattern of responses in non-smokers significantly differed from smokers in either cluster group. Instead, we found that non-smokers showed significantly larger LPPs to cigarette-related cues than to neutral cues. This is not necessarily surprising, as this pattern of results has been previously observed in our laboratory (Deweese et al., 2016; Robinson et al., 2015) and elsewhere (Littel et al., 2012). The LPP is considered a measure of emotional arousal (Cuthbert et al., 2000). Thus, one possible explanation for the elevated LPP to cigarette-related cues in non-smokers is that, whereas the smokers found the cigarette-related cues to be pleasant, the non-smokers found these cues to be unpleasant (Deweese et al., 2016; Geier et al., 2000; Robinson et al., 2015). This is consistent with self-reported valence and arousal ratings of the pictures obtained from the current sample that have been previously published (Engelmann et al., 2011): while both smokers and non-smokers rated the cigarette-related pictures as mildly arousing, the smokers rated them as pleasant whereas the non-smokers rated them as unpleasant.
Cluster analytic techniques such as the k-means methodology used in the current study are advantageous because they provide a data-driven means of identifying individual differences. There are, however, two disadvantages to this approach that must be acknowledged. First, the number of groups (k) is decided by the researcher, not by the algorithm. For this study, we decided to partition smokers into two groups based on our previous research in which we found two distinct groups of smokers based on their relative reactivity to cigarette-related and pleasant stimuli. However, our selection of two groups should not be interpreted as implying that there are only two types of smokers. Like most traits, LPP amplitude exists along a continuum, but we decided to dichotomize this trait to better isolate it and study its consequences. Second, because cluster analysis is used to maximize differences between groups, it is not surprising that we found a significant cluster group × picture category interaction. However, cluster analysis does not place any constraints on how the groups differ. Thus, the pairwise comparisons that followed the significant interaction provided information about the nature of the differences between the groups. In this case, the two groups of smokers differed in their brain responses to cigarette-related and pleasant stimuli, but not to unpleasant or neutral stimuli. Because this was a preliminary study aimed at determining whether similar clusters would emerge in a sample of smokers younger than those we previously studied, we used the same analytic strategy that we used in our previous research. We are currently developing an algorithm that will allow us to classify each individual before the entire sample is collected.
We acknowledge that the current study has additional limitations. First, this study was not designed to include an outcome measure, such as transition to heavy smoking or difficulty quitting smoking in adulthood. Based on our previous research of smokers interested in quitting, a compelling hypothesis is that young smokers in Group 2 (i.e., those with larger brain responses to cigarette cues than to pleasant stimuli) will be characterized by poorer outcomes later in time (i.e., more likely to become heavy smokers, less likely to successfully quit smoking). However, prospective studies are needed to assess whether individual differences in brain responses to cigarette cues and natural rewards in young smokers are stable over time and predict these outcomes. Second, although the smokers included in this study were considerably younger than those in most ERP and fMRI studies of smokers, including our previous studies that first identified the two cluster groups, the smokers studied here had been smoking for about 5 years, and all were at least 18 years old. This may indeed be sufficient time for these neuroadaptations to take place, but what we have established is that it may not require a protracted history of smoking to emerge. Nevertheless, it is necessary to study smokers under the age of 18 who are still experimenting with cigarettes. It is also necessary to conduct a longitudinal study of whether variation in brain responses to natural rewards in non-smoking adolescents is predictive of smoking initiation, which is important for evaluating whether these individual differences are a contributing factor to the emergence of regular smoking (Audrain-McGovern et al., 2012), a consequence of smoking (Volkow et al., 2010), or both. Rubinstein and colleagues (2011a) found that adolescent smokers 13-17 years old who smoke 1-5 cigarettes per day show reduced brain responses to pleasurable food stimuli. This is similar to our finding that smokers in Group 2 had reduced LPPs to pleasant stimuli. We suggest that blunted brain responses to pleasant stimuli also occur in a subgroup of adolescents who are experimenting with cigarettes, and that these individuals might be at greater risk of becoming regular smokers. Third, the two groups of smokers did not differ on any self-report of behavioral measures. Thus, our neurobiological measure still needs to be validated against other objective measures of reactivity to rewarding stimuli and/or behavioral measures of reward experience (Der-Avakian et al., 2016; Pizzagalli et al., 2008). Fourth, many of the smokers in this study were college students, which limits the generalizability of our findings to the larger population of young smokers. We are currently conducting research that will address these limitations.
Although limited in scope, this study further demonstrates the promise of ERPs as a neural metric of the relative reactivity to cigarette-related and pleasant stimuli in smokers, including young smokers with low levels of nicotine dependence. We observed the effect by measuring the LPP with three electrodes, which is a cost- and time-effective method of assessing brain responses to cigarette-related cues and pleasant stimuli. Relative differences in the LPP to pleasant and cigarette cues that are predictive of smoking abstinence in long-term smokers trying to quit are present in young, light smokers with a shorter smoking history. Future research is necessary to determine whether this phenotype predicts risk for smoking escalation. If so, identifying those who are at risk as early as possible might lead to improved strategies for preventing the transition from casual to regular smoking.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jeffrey M. Engelmann, Department of Behavioral Science, The University of Texas MD Anderson Cancer Center
Francesco Versace, Oklahoma Tobacco Research Center, Stephenson Cancer Center, and Department of Family & Preventive Medicine, University of Oklahoma Health Sciences Center.
Jonathan C. Gewirtz, Department of Psychology, University of Minnesota
Paul M. Cinciripini, Department of Behavioral Science, The University of Texas MD Anderson Cancer Center
References
- Audrain-McGovern J, Rodriguez D, Leventhal AM, Cuevas J, Rodgers K, Sass J. Where is the pleasure in that? Low hedonic capacity predicts smoking onset and escalation. Nicotine Tob. Res. 2012;14:1187–96. doi: 10.1093/ntr/nts017. doi:10.1093/ntr/nts017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Long CJ, Cole DM, Durcan MJ, Bannon LC, Mishra RG, Matthews PM. The effects of nicotine replacement on cognitive brain activity during smoking withdrawal studied with simultaneous fMRI/EEG. Neuropsychopharmacology. 2011;36:1792–800. doi: 10.1038/npp.2011.53. doi:10.1038/npp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Robinson JD, Lam CY, Wetter DW, Tsan JY, Day SX, Cinciripini PM. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine Tob. Res. 2006;8:361–9. doi: 10.1080/14622200600670215. doi:10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam C, Versace F, Brown VL, Engelmann JM, Wetter DW. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry. 2013;70:522–533. doi: 10.1001/jamapsychiatry.2013.678. doi:10.1001/jamapsychiatry.2013.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EW. VPM Reference Manual. Author: Birmingham, AL: 2003. [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol. Psychol. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Barnes SA, Markou A, Pizzagalli DA. Translational assessment of reward and motivational deficits in psychiatric disorders. Curr. Top. Behav. Neurosci. 2016 doi: 10.1007/7854_2015_5004. doi:10.1007/7854_2015_5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deweese MM, Robinson JD, Cinciripini PM, Versace F. Conditioned cortical reactivity to cues predicting cigarette-related or pleasant images. Int. J. Psychophysiol. 2016;101:59–68. doi: 10.1016/j.ijpsycho.2016.01.007. doi:10.1016/j.ijpsycho.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Gewirtz JC, Cuthbert BN. Emotional reactivity to emotional and smoking cues during smoking abstinence: potentiated startle and P300 suppression. Psychophysiology. 2011;48:1656–1668. doi: 10.1111/j.1469-8986.2011.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedota JR, Sutherland MT, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Reward anticipation is differentially modulated by varenicline and nicotine in smokers. Neuropsychopharmacology. 2015;40:2038–46. doi: 10.1038/npp.2015.54. doi:10.1038/npp.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology (Berl.) 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hair JF, Black WC. Cluster analysis. In: Grimm LG, Yarnold PR, editors. Reading and Understanding More Multivariate Statistics. American Psychological Association; Washington, D.C.: 2000. pp. 147–205. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br. J. Addict. 1989;84:791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Hu M-C, Griesler PC, Schaffran C, Wall MM, Kandel DB. Trajectories of criteria of nicotine dependence from adolescence to early adulthood. Drug Alcohol Depend. 2012;125:283–289. doi: 10.1016/j.drugalcdep.2012.03.001. doi:10.1016/j.drugalcdep.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J, Hatsukami DK. Errors in using tobacco withdrawal scale. Tob. Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci. Biobehav. Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. doi:10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958;10:157–165. [PubMed] [Google Scholar]
- Johnson RA, Wichern DW. Applied Multivariate Statistical Analysis. 4th Prentice Hall; Upper Saddle River, NJ: 2002. [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. doi:10.1017.S0048577202394162. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report no. A-6. University of Florida; Gainesville, FL: 2005. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. J. Clin. Psychol. 2006;62:1545–1558. doi: 10.1002/jclp.20327. doi:10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- Littel M, Euser AS, Munafò MR, Franken IHA. Electrophysiological indices of biased cognitive processing of substance-related cues: a meta-analysis. Neurosci. Biobehav. Rev. 2012;36:1803–1816. doi: 10.1016/j.neubiorev.2012.05.001. doi:10.1016/j.neubiorev.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang H, McGinnis-Deweese M, Keil A, Ding M. Neural substrate of the late positive potential in emotional processing. J. Neurosci. 2012;32:14563–14572. doi: 10.1523/JNEUROSCI.3109-12.2012. doi:10.1523/JNEUROSCI.3109-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Ruparel K, Falcone M, Hopson R, Gur R, Lerman C. Working memory-related neural activity predicts future smoking relapse. Neuropsychopharmacology. 2015;40:1311–1320. doi: 10.1038/npp.2014.318. doi:10.1038/npp.2014.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E. Randomization tests for ERP topographies and whole spatiotemporal data matrices. Psychophysiology. 2004;41:142–151. doi: 10.1111/j.1469-8986.2003.00139.x. doi:10.1111/j.1469-8986.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- Marrone GF, Paulpillai M, Evans RJ, Singleton EG, Heishman SJ. Breath carbon monoxide and semiquantitative saliva cotinine as biomarkers for smoking. Hum. Psychopharmacol. 2010;25:80–83. doi: 10.1002/hup.1078. doi:10.1002/hup.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, Conrod PJ, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Martinot J-L, Paus T, Poline J-B, Robbins TW, Rietschel M, Smolka M, Ströhle A, Struve M, Loth E, Schumann G, Büchel C, IMAGEN Consortium Lower ventral striatal activation during reward anticipation in adolescent smokers. Am. J. Psychiatry. 2011;168:540–549. doi: 10.1176/appi.ajp.2010.10071024. doi:10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J. Psychiatr. Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. doi:10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JD, Versace F, Engelmann JM, Cui Y, Slapin A, Oum R, Cinciripini PM. The motivational salience of cigarette-related stimuli among former, never, and current smokers. Exp. Clin. Psychopharmacol. 2015;23:37–48. doi: 10.1037/a0038467. doi:10.1037/a0038467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein ML, Luks TL, Dryden WY, Rait MA, Simpson GV. Adolescent smokers show decreased brain responses to pleasurable food images compared with nonsmokers. Nicotine Tob. Res. 2011a;13:751–755. doi: 10.1093/ntr/ntr046. doi:10.1093/ntr/ntr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein ML, Luks TL, Moscicki A-B, Dryden W, Rait MA, Simpson GV. Smoking-related cue-induced brain activation in adolescent light smokers. J. Adolesc. Health. 2011b;48:7–12. doi: 10.1016/j.jadohealth.2010.09.016. doi:10.1016/j.jadohealth.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Keil A, Bradley MM. Emotional perception: correlation of functional MRI and event-related potentials. Cereb. Cortex. 2007;17:1085–1091. doi: 10.1093/cercor/bhl017. doi:10.1093/cercor/bhl017. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Substance Abuse and Health Services Administration Results from the 2012 National Survey on Drug Use and Health: National findings [WWW Document] 2012 URL http://archive.samhsa.gov/data/NSDUH/2012SummNatFindDetTables/DetTabs/NSDUH-DetTabsSect6peTabs1to54-2012.htm#Tab6.5B.
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br. J. Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Versace F, Engelmann JM, Robinson JD, Jackson EF, Green CE, Lam CY, Minnix JA, Karam-Hage MA, Brown VL, Wetter DW, Cinciripini PM. Prequit fMRI responses to pleasant cues and cigarette-related cues predict smoking cessation outcome. Nicotine Tob. Res. 2014;16:697–708. doi: 10.1093/ntr/ntt214. doi:10.1093/ntr/ntt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Lam CY, Engelmann JM, Robinson JD, Minnix JA, Brown VL, Cinciripini PM. Beyond cue reactivity: blunted brain responses to pleasant stimuli predict long-term smoking abstinence. Addict. Biol. 2012;17:991–1000. doi: 10.1111/j.1369-1600.2011.00372.x. doi:10.1111/j.1369-1600.2011.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Minnix JA, Robinson JD, Lam CY, Brown VL, Cinciripini PM. Brain reactivity to emotional, neutral and cigarette-related stimuli in smokers. Addict. Biol. 2011;16:296–307. doi: 10.1111/j.1369-1600.2010.00273.x. doi:10.1111/j.1369-1600.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. Neurobiologic advances from the brain disease model of addiction. N. Engl. J. Med. 2016;374:363–371. doi: 10.1056/NEJMra1511480. doi:10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. doi:10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]