Abstract
Background
Bariatric surgery prevents and induces remission of type 2 diabetes in many patients. The effect of preoperative glucose status on long-term healthcare costs is unknown.
Methods
The Swedish Obese Subjects (SOS) study is a prospective, matched, controlled intervention study conducted in the Swedish healthcare system including 2010 adults who underwent bariatric surgery and 2037 contemporaneously matched controls recruited between 1987 and 2001. Prescription drug costs were retrieved via questionnaires and the nationwide Swedish Prescribed Drug Register. Data on hospital admissions and outpatient visits were retrieved from the Swedish National Patient Register. The sample linked to register data (n=4030; 2836 euglycemic; 591 prediabetes; 603 diabetes) was followed over up to 15 years. Mean differences were adjusted for baseline characteristics.
Findings
Drug costs did not differ between the surgery and control group in the euglycemic subgroup (adjusted mean difference −$225; 95%CI −2080 to 1631), but were lower in surgery patients in the prediabetes (−$3329; 95%CI −5722 to −937) and diabetes subgroups (−$5487; 95%CI −7925 to −3049). Greater hospital costs were observed in the surgery group for the euglycemic ($22,931; 95%CI 19,001–26,861), prediabetes ($27,152; 95%CI 18,736–35,568) and diabetes subgroups ($18,697; 95%CI 9992–27,402). No differences in outpatient costs were observed. Total healthcare costs were higher in surgery patients in the euglycemic ($22,390; 95%CI 17,358–27,423) and prediabetes subgroups ($26,292; 95%CI 16,738–35,845), while no difference was detected between treatment groups in patients with diabetes ($9081; 95%CI −1419 to 19,581).
Interpretation
Long-term healthcare cost results support prioritizing obese patients with diabetes for bariatric surgery.
INTRODUCTION
The severely obese population (body mass index [BMI] ≥35kg/m2) has grown faster than less severe obesity sub-classes(1, 2), and the risk of type 2 diabetes increases dramatically with BMI(3, 4). Severely obese patients have difficulty sustaining weight loss through lifestyle interventions.(5) In contrast, bariatric surgery facilitates sustained weight loss(6), and for patients with type 2 diabetes, remission or improvements for most patients(7–9). The Swedish Obese Subjects (SOS) study has previously demonstrated superior type 2 diabetes remission(10) and prevention(11) in patients receiving bariatric surgery relative to conventional treatment. These improvements were not associated with baseline BMI.
It is foreseeable that diabetes prevention and remission induced by bariatric surgery will prevent future diabetes-related healthcare and drug use over the long-term, and therefore offset at least some of the costs associated with the bariatric procedure. Therefore prioritizing patients with prediabetes or diabetes may lead to greater returns on investment.
We previously reported that over 20 years, compared with obese controls, surgically treated patients used more inpatient and nonprimary outpatient care during the first 6-year period after undergoing bariatric surgery but not thereafter, while drug costs from years 7 through 20 were lower for surgery than control patients.(12) Whether these overall findings are consistent across preoperative glucose status has not been investigated.
In the current study, we assessed healthcare costs over 15 years by obese patients treated conventionally or with bariatric surgery in subgroups with euglycemia, prediabetes and type 2 diabetes prior to intervention. Linkage with Swedish healthcare registers was undertaken and prescription drug costs, inpatient and nonprimary outpatient care costs were assessed.
METHODS
The SOS study has previously been described(13) and details are provided in the eMethods. Briefly, recruitment campaigns were undertaken in the mass media, 25 Swedish surgical departments and 480 primary healthcare centers. 2010 individuals chose to undergo surgery, and a contemporaneously matched obese control group of 2037 participants was created using 18 variables from a matching examination and a group matching procedure. The surgery and control groups had identical inclusion (age 37–60 years; BMI of ≥34/≥38 in men/women) and exclusion criteria. Mortality was the primary outcome, while healthcare use and economic outcomes were predefined secondary objectives.
In the surgery group, 13% underwent gastric bypass; 19% gastric banding; and 68% vertical-banded gastroplasty. Eighty-nine percent of the patients underwent open surgery. Control patients received the customary nonsurgical obesity treatment at their registration center. No attempt was made to standardize their treatment, which ranged from sophisticated lifestyle intervention and behavior modification to no treatment. Treatment protocols for diabetes were also not prescribed. The SOS trial has been registered in the ClinicalTrials.gov registry (NCT01479452). The study was approved by seven ethics committees in Sweden, and informed consent was obtained from all participants.
Healthcare use and costs
Healthcare use outcomes were retrieved from nationwide registers managed by the Swedish National Board of Health and Welfare (except drug data for certain years which were collected by self-report) and linked to the study database via the unique personal identity number assigned to each Swedish resident.
Drug costs for follow-up years 7–15 were retrieved from the Prescribed Drug Register, which captures date, drug, therapeutic categories, and cost of all dispensed prescription drugs in Sweden from July 2005, as described previously for the full SOS study population.(12) Drug data for follow-up years 1–6 were from questionnaires completed by participants at baseline, years 1, 2, 3, 4 and 6 (data for years 8, 10 and 15 were also used in the imputed datasets). Information on prescription drug use, including dosage and strength were collected, and unit costs were applied as detailed previously.(14) Costs were inflation-adjusted to 2013 Swedish kronor (SEK) using the Swedish drug price index(15), and converted to US$ (exchange rate, 7SEK:$1).
Data on inpatient and nonprimary care outpatient visits were retrieved from the National Patient Register. Nonprimary outpatient care includes consultations with hospital-based medical specialists and excludes consultations with community-based general practitioners. Inpatient care has been recorded since 1964, and attained national coverage in 1987. The outpatient care component commenced nationwide in 2001. Registered diagnostic-related group (DRG) costs were used to calculate inpatient (1998–2013) and outpatient costs (2001–2013). For visits years 1986–1997, and in instances where DRG data were missing thereafter, costs were estimated by applying the average cost per day, or outpatient visit, from the years when DRG costs were available. Costs are reported in 2013 US$.
Preoperative glucose status
The SOS study started in 1987, before guidelines recommended repeated glucose measurements or HbA1c measurements for the diagnosis of diabetes. We have therefore used single fasting glucose measurements and/or self-reported diabetes medication to define diabetes. Three categories of preoperative glucose status were defined: type 2 diabetes (≥110mg/dL fasting blood glucose [corresponding to ≥126mg/dL fasting plasma glucose(16, 17)], or diabetes medication), prediabetes (90–110mg/dL fasting blood glucose [corresponding to 100–126mg/dL fasting plasma glucose(18,19)]), and euglycemic patients. Diabetes medication was not used to define prediabetes.
Follow-up
The index date was the day of surgery for patients in the surgery group and their matched controls. Hence, follow-up year 1 was the first 365 days after (and including) the index date. Patients were followed from the index date (or date of first data capture in the registers) for 15 years, until death, emigration, or December 31, 2013, whichever came first. Recruitment occurred between 1987 and 2001, therefore patients contributed healthcare register data in different follow-up years as the register for outpatient care commenced in 2001 (the final year of study recruitment; data presented for followup years 2–15), and the Prescribed Drug Register in 2005.
Statistical analysis
Analyses were by intention to treat. Adjusted mean differences for 15-year aggregated drug, outpatient, inpatient and total healthcare costs were estimated using generalised linear models employing gamma distribution with identity link.(20) Robust standard errors were estimated to derive confidence intervals. Adjustment was made for age, sex, BMI, drug costs the year before the index date (for drug and total costs), inpatient costs the year before the index date (for inpatient and total costs), smoking and inclusion period (recruitment </≥ 1995). Mean annual differences, adjusted for the same variables, were estimated using linear regression with 95% confidence intervals derived via bias-corrected accelerated bootstrapping. Due to large number of annual zero costs, a sensitivity analysis of the annual differences was conducted using a two-part model (eResults, eFigure 3).
Complete register data were available for all years for inpatient care, but not for drug costs and outpatient care. If data from the Prescribed Drug Register were not available, self-reported drug data were used. To handle missing outpatient and drug cost data, multiple imputation was used (eMethods, eFigure 1, eFigure 2).
Subgroup analysis was performed by diabetes duration (above/below the median). In a sensitivity analysis, the observed length of stay for the index surgery (mean 7.1 days) was replaced with that observed in Sweden in 2012 (1.9 days(21)) to make the results more generalisable to modern-day procedures. Inpatient costs over 15 years were also estimated excluding the index year.
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc) and Stata version 11. Reported P values are 2-sided, and P values <·05 were considered statistically significant.
Role of the funding source
The funders were not involved in the study design or conduct; collection, management, analysis, and interpretation of the data; preparation, review, or manuscript approval; and decision to submit for publication. LSMC, MN and JE had access to the raw data. The corresponding author (MN) had full access to all data and the final responsibility to submit for publication.
RESULTS
Exclusions included 2 patients who requested to be deleted from the database, 4 with type 1 diabetes, and 11 with missing baseline glucose. Therefore the analytical sample was 4030. During follow-up, 1 patient obtained an unlisted personal identity number and 29 patients emigrated, making linkage thereafter impossible. These patients were included the available follow-up years. The mean observation times were 14·5/14·4 [surgery/control], 14·2/14·1, and 14·0/13·7 years for euglycemic, prediabetes, and diabetes patients, respectively.
At baseline, patients in the surgery group were on average 6·3kg heavier, 1·3 years younger, more frequently smokers, and less likely to have a university degree than control patients. Similar trends were observed across all glucose categories (Table 1). At 15 years, the relative total weight loss in the surgery group/control group was 16%/0%, 18%/0% and 18%/0% in the euglycemic, prediabetes and diabetes groups, respectively.
Table 1.
Patient Characteristics at Baseline (mean (standard deviation))
| Euglycemic | Prediabetes | Diabetes | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Surgery (n=1355) |
Controls (n=1481) |
P | Surgery (n=301) |
Controls (n=290) |
P | Surgery (n=343) |
Controls (n=260) |
P | |
| Women, n (%) | 998 (74%) | 1083 (73%) | ·75 | 213 (71%) | 203 (70%) | ·84 | 202 (59%) |
156 (60%) |
·78 |
| Age, years | 47 (6) | 48 (6) | <·001 | 48 (6) | 50 (6) | <·001 | 49 (6) | 50 (6) | ·001 |
| Procedure type, n (%) | |||||||||
| VBGa | 938 (69%) | – | 200 (66%) | – | 227 (66%) | – | |||
| Gastric banding | 259 (19%) | – | 52 (17%) | – | 61 (18%) | – | |||
| Gastric bypass | 158 (12%) | – | 49 (16%) | – | 55 (16%) | – | |||
| Height, cm | |||||||||
| Women | 165 (6) | 165 (6) | ·85 | 164 (6) | 165 (6) | ·75 | 164 (6) | 166 (6) | ·10 |
| Men | 179 (6) | 180 (7) | ·01 | 179 (7) | 179 (7) | ·66 | 180 (7) | 178 (7) | ·03 |
| Weight, kg | |||||||||
| Women | 116 (13) | 110 (14) | <·001 | 119 (15) | 114 (16) | ·001 | 115 (14) | 112 (14) | ·09 |
| Men | 132 (16) | 125 (16) | <·001 | 132 (18) | 126 (15) | ·03 | 135 (18) | 123 (18) | <·001 |
| Body mass index, kg/m2 | |||||||||
| Women | 42·7 (4·2) | 40·5 (4·5) | <·001 | 44·0 (4·5) | 41·9 (4·8) | <·001 | 42·4 (4·4) | 40·9 (4·5) | ·002 |
| Men | 41·2 (4·7) | 38·4 (4·7) | <·001 | 40·9 (4·8) | 39·5 (4·9) | ·054 | 41·8 (5·0) | 38·7 (4·6) | <·001 |
| Waist circumference, cm | |||||||||
| Women | 123 (10) | 118 (11) | <·001 | 126 (10) | 121 (11) | <·001 | 126 (11) | 122 (9) | <·001 |
| Men | 130 (10) | 123 (11) | <·001 | 130 (11) | 126 (10) | ·04 | 133 (12) | 125 (10) | <·001 |
| Smoking, n (%) | 360 (27%) | 301 (20%) | <·001 | 70 (23%) | 66 (23%) | ·90 | 85 (25%) | 53 (20%) | ·24 |
| University education, n (%) | 177 (13%) | 330 (22%) | <·001 | 40 (13%) | 55 (19%) | ·06 | 39 (11%) | 45 (17%) | ·04 |
Vertical-banded gastroplasty
Mean imputed 15-year aggregated drug costs did not differ between the surgery and control group in euglycemic patients ($10,511 vs $10,680, adjusted difference −$225; 95%CI −2080 to 1631; P=·812; Figure 1). The very wide confidence intervals during years 10 to 13 were driven by certain expensive drugs (Figure 2). In patients with prediabetes, lower 15-year drug costs were observed in the surgery than the control group ($10,194 vs $13,186, adjusted difference −$3329; 95%CI −5722 to −937; P=·007), with diabetes drug cost savings from year 4 (Figure 3). 15-year drug costs were also lower for the surgery group in patients with diabetes ($14,346 vs $19,511, adjusted difference −$5487; 95%CI −7925 to −3049, P<·0001), a difference driven by diabetes drugs (Figure 3).
Figure 1. Aggregated 15-year healthcare costs.
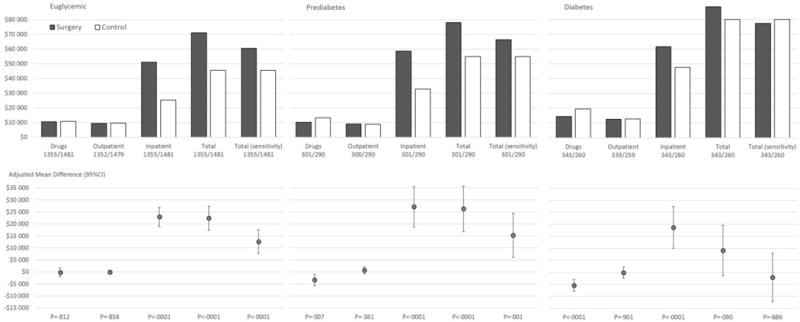
Mean differences adjusted for baseline age, sex, smoking, body mass index, inclusion period (</≥ 1995), drug costs and hospital costs the year prior to the index date. Hospital days and costs were retrieved from the National Patient Register between 1987 and 2013, and data were not imputed (apart from missing cost data which were calculated from the observed number of hospital days). Outpatient care data were retrieved from the National Patient Register between 2001 and 2013 for observation years 2 to 15, with multiple imputation used for missing data. Drug cost data were retrieved from the Prescribed Drug Register between 2005 and 2013, and from self-reported drug use in SOS (years 1, 2, 3, 4, 6, 8, 10, 15), with multiple imputation used for missing data. *Total (sensitivity): Sensitivity analysis where the length of stay for the index bariatric surgery reflected practice in Sweden in 2012. Costs are reported in 2013 US$.
Figure 2. Mean Annual Prescription Drug Costs from Year 0 to 15 (Years 0–6: Questionnaire data; Years 7–15: Register data from the Prescribed Drug Register, data capture 2005–2013).
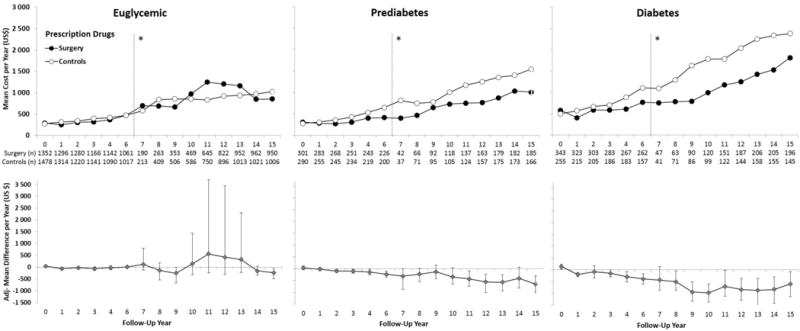
Adjusted for baseline age, sex, smoking, body mass index and inclusion period (</≥ 1995), and drug costs the year prior to the index date (year 0). Error bars indicate 95%CIs derived by nonparametric bootstrapping. Data were not collected in year 5 and therefore no data are presented. Results are based on observed data. Imputed vs observed data are shown in eFigure 2. *Self-reported drug data collected for follow-up years 0–6 (left) and Swedish Prescribed Drug Register data for follow-up years 7–15 (2005–2013) (right). Costs are reported in 2013 US$.
Figure 3. Mean Annual Diabetes Drug Costs from Year 0 to 15 (Years 0–6: Questionnaire data; Years 7–15: Register data from the Prescribed Drug Register, data capture 2005–2013).
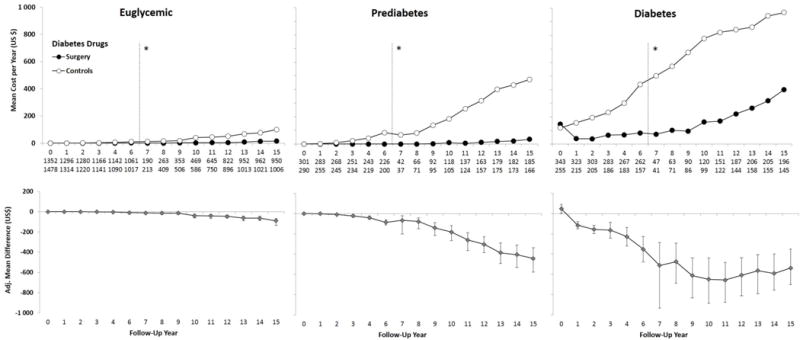
Adjusted for baseline age, sex, smoking, body mass index and inclusion period (</> 1995), and diabetes drug costs the year prior to the index date (year 0). Error bars indicate 95%CIs derived by nonparametric bootstrapping. Data were not collected in year 5 and therefore no data are presented. Results are based on observed data. Imputed vs observed data are shown in eFigure 2. Drugs presented are those included in ATC group “A10 – Drugs used in Diabetes”. *Self-reported drug data collected for follow-up years 0–6 (left) and Swedish Prescribed Drug Register data for follow-up years 7–15 (2005–2013; right). Costs are reported in 2013 US$.
Greater 15-year inpatient costs were observed in the surgery group for all glucose subgroups. In euglycemic patients, mean 15-year inpatient costs were $51,225 in the surgery group and $25,313 in the control group (adjusted difference, $22,931; 95%CI 19,001–26,861; P<·0001; Figure 1 & Figure 4). Corresponding costs in patients with prediabetes were $58,699 and $32,861 (adjusted difference, ($27,152; 95%CI 18,736–35,568; P<·0001) and in patients with diabetes $61,569 and $47,569 (adjusted difference, $18,697; 95%CI 9992–27,402; P<·0001). If excluding the first year altogether, euglycemic and prediabetes surgery patients still incurred higher inpatient costs during follow-up years 2–15, while no difference was detected for patients with diabetes (eTable 1).
Figure 4. Mean Annual Hospital Costs from Years 1–15 (From the National Patient Register, data capture 1987–2013).
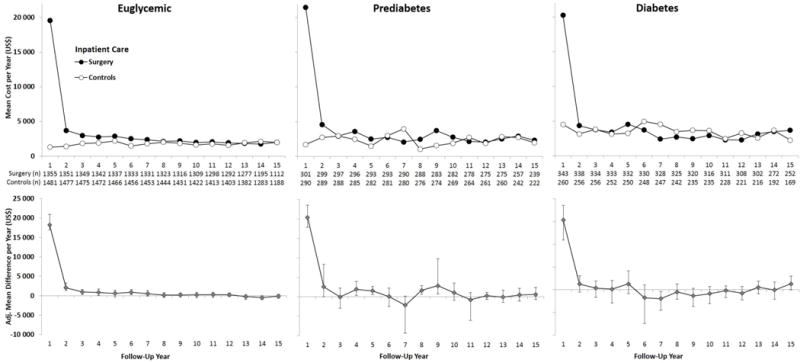
Adjusted for baseline age, sex, smoking, body mass index and inclusion period (</≥ 1995) and hospital days in the year prior to the index date. Error bars indicate 95%CIs derived by nonparametric bootstrapping. Results are based on observed data.
During years 2–15, there was no difference between treatment groups in mean imputed outpatient costs for any glucose subgroup (Figure 1 & Figure 5). In the diabetes subgroup, the very wide confidence intervals during years 10 to 14 were driven by visits for end-stage renal disease (Figure 5).
Figure 5. Mean Annual Outpatient Care Costs from Years 2 to 15 (From the National Patient Register, data capture 2001–2013).
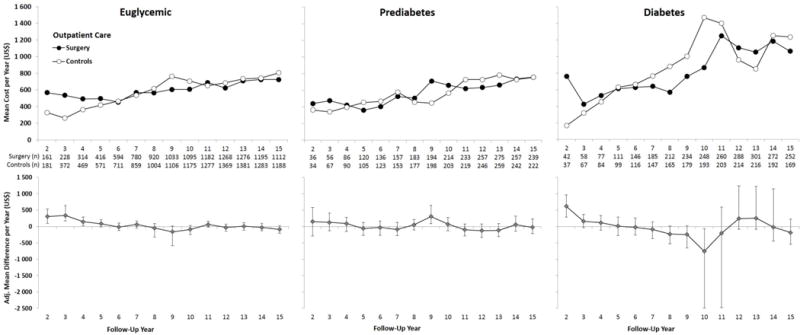
Adjusted for baseline age, sex, smoking, body mass index and inclusion period (</≥ 1995). Error bars indicate 95%CIs derived by nonparametric bootstrapping. Results are based on observed data. Imputed vs observed data are shown in eFigure 1.
Total healthcare costs were higher in surgery patients in the euglycemic ($22,390; 95%CI 17,358–27,423; P<·0001) and prediabetes subgroups ($26,292; 95%CI 16,738–35,845; P<·0001), while no difference was detected between treatment groups in patients with diabetes ($9081; 95%CI −1419 to 19,581; P=·090; Figure 1). In the sensitivity analysis where the length of stay for the index bariatric surgery reflected practice in Sweden in 2012, total costs remained higher for surgically treated patients in the euglycemic and prediabetes group and no difference was detected for patients with diabetes. In patients with diabetes, total costs were no different from controls in patients with diabetes duration below the median ($4390; 95%CI −7672 to 16,451; P=·476) while those above the median had statistically significantly higher total costs ($21,780; 95%CI 4907–38,653; P=·011; eTable 2).
DISCUSSION
Recent bariatric surgery reviews have highlighted “examining long term cost data”, and “which patient level factors predict success” as priorities for research(22–24). Our study partly fills these evidence gaps by analysing total prescription drug, inpatient, and outpatient costs for obese patients treated conventionally or with bariatric surgery over up to 15 years according to their baseline glucose status. We observed that 15-year drug costs did not differ between treatment groups in euglycemic patients, but were lower in surgery patients with prediabetes or diabetes. Further, 15-year inpatient costs were higher in the surgery group in all glucose subgroups, while no differences were observed for outpatient costs. Finally, the total healthcare costs (drug, inpatient and outpatient costs combined) were higher for euglycemic and prediabetes surgery patients, but no difference was detected versus the control group for patients with diabetes. Diabetes duration was also found to matter, with no difference in total costs for patients with a duration of 1 year or less at baseline, but statistically significantly higher costs in surgery versus control patients with longer disease duration.
Mean 15-year drug cost savings of $5500 were accrued in surgery patients with diabetes, while a $3300 cost saving was accrued in prediabetes patients compared to controls. In prediabetes patients, the annual drug cost difference increased over time and is therefore likely to grow larger with longer follow-up. The savings were primarily driven by reduced costs for diabetes drugs. For prediabetes patients, the mean annual cost of diabetes drugs was $0–$39 during years 1–15 for surgery patients, similar to the $0–$16 range observed in euglycemic surgery patients. This is consistent with diabetes prevention results from SOS; progression to diabetes was prevented in 87% of glucose-impaired surgery patients over 15 years.(11)
A large part of the inpatient costs in the surgery group was due to the index bariatric surgery, and to a lesser extent, revisional bariatric surgery, plastic surgery, gallstones and anemia.(14) Since patients in our study received bariatric surgery between 1987 and 2001, surgical procedures have changed. The rate of laparoscopic surgery in our study was 11% compared to 97% in 2012 in Sweden(21), and vertical-banded gastroplasty, the dominant bariatric procedure in SOS, is no longer used. Also many SOS surgery patients receiving gastric banding and vertical-banded gastroplasty were later converted to gastric bypass. Therefore results may be more favourable to surgery patients in contemporary settings who likely have lower frequency of re-operations. The total healthcare costs remained higher (euglycemic and prediabetes patients) or similar (diabetes patients) in a sensitivity analysis where the observed index bariatric surgery length of hospital stay was replaced with the much shorter length of stay observed in Sweden in 2012.
We did not observe any differences between treatment groups for outpatient care costs. However, we did not have nationwide register data for primary care, which plays a significant role in the management of type 2 diabetes. Exclusion of this component may result in underestimation of differences between surgery and control groups. On the other hand, many adverse effects of bariatric surgery are also handled in primary care, which could affect cost estimates in the opposite direction.
The use of bariatric surgery globally is largely governed by a US National Institutes of Health consensus statement defining eligible patients as those with a BMI≥40 or BMI≥35 combined with a serious obesity-related morbidity.(25) Our previously reported clinical endpoints including remission(10) and prevention of type 2 diabetes(11) and the prevention of cardiovascular events(26) were more favourable in patients with prediabetes and diabetes at baseline, relative to euglycemic patients. Consequently, we have recommended that a measure of glucose impairment (rather than BMI), be used to identify obese patients who can achieve the greatest health benefits from surgery. This recommendation was also advocated by Cummings and Cohen(27), and is partially reflected in recommendations in the new National Institute for Health and Care Excellence obesity guidelines for the United Kingdom. These guidelines recommend expedited assessment for bariatric surgery in patients with a BMI≥35 who have recent-onset type 2 diabetes, and they recommend considering an assessment for people with BMI 30–34.9 who have recent-onset type 2 diabetes(28). In the current study, we report for the first time, that long-term economic outcomes favour the diabetes subgroup (relative to the euglycemic and prediabetes subgroups), adding further evidence to support prioritizing obese patients with type 2 diabetes for bariatric surgery. We also found that duration of diabetes was of importance, with shorter duration being associated with improved cost outcomes.
To date, the SOS study is the largest and longest prospective, controlled study of bariatric surgery. To our knowledge, this is the first prospectively controlled study to assess long-term healthcare costs in bariatric surgery patients according to their preoperative glucose status versus matched controls. Furthermore, the analysis of healthcare register data enabled near complete follow-up for the years when the registers were available (all years for inpatient care, since 2001 for outpatient care, and since 2005 for prescription drugs). Also, universal healthcare access in Sweden ensured that healthcare use is likely a reasonable reflection of overall morbidity, rather than of insurance status.
However, our study was limited by the ethical review boards not permitting randomization. We aimed to reduce any residual confounding by multivariable adjustments. Our analysis adopted intention to treat methods, although approximately 10% of controls subsequently received bariatric surgery during the 15 years of follow-up, effectively increasing hospital costs due to surgery and decreasing diabetes drug costs in controls. The sample size for the prediabetes and diabetes subgroups were smaller than for euglycemic patients. It is possible that some differences did not reach statistical significance due to this reason.
We were also limited by the fact that we did not have register data on outpatient visits and drugs for the whole period because no nationwide registers for these services existed when recruitment started in 1987. Therefore, fewer patients were available for these register outcomes during the earlier follow-up years. We utilized self-reported drug use data and multiple imputation to handle missing register data. Apart from missing data, certain healthcare cost categories were not assessed, for example non-prescription drugs, nursing home costs, medical equipment for the home, and allied health services (dietician, physiotherapy, psychologists, and more).
Published models indicate that bariatric surgery is cost-effective for treating severe obesity in the currently eligible population(29), but no previous long-term cost studies and few cost-effectiveness analyses have examined heterogeneity in costs across patient subgroups(24). Based on our previous clinical(11, 13), and current healthcare use outcomes favouring the diabetes group, economic evaluations of the total eligible population likely overestimate the benefits for the euglycemic subgroup and underestimate them for the diabetes subgroup. A cost-effectiveness analysis based on long-term real-world data and stratified by glucose status is needed.
In conclusion, we demonstrate that for obese patients with type 2 diabetes, the upfront costs of bariatric surgery appear to be largely offset by the prevention of future healthcare and drug use. This finding of cost-neutrality is seldom found for healthcare interventions, nor is it a requirement of funding in most settings. Most commonly, buying health benefits at an acceptable cost (for example, £20,000 per quality-adjusted life-year in the United Kingdom) is the economic benchmark adopted by payors when assessing new interventions. Bariatric surgery should be held to the same economic standards as other medical interventions.
Supplementary Material
Research in context.
Evidence before this study
Long-term clinical endpoints after bariatric surgery including remission and prevention of type 2 diabetes and the prevention of cardiovascular events favour patients with prediabetes or diabetes at the time of surgery, relative to euglycemic patients. Consequently, several groups have recommended that a measure of glucose impairment (rather than BMI alone), be used to prioritize obese patients to receive bariatric surgery. So far, the long-term effect of bariatric surgery (relative to conventional therapy) on healthcare costs in obese patients according to preoperative glucose status has not been assessed using real-world data.
Added value of this study
We report that long-term healthcare cost outcomes favour the diabetes subgroup, relative to the euglycemic and prediabetes subgroups. Over 15 years after surgery, in the prediabetes and diabetes subgroups, a drug cost saving was observed for bariatric surgery patients (relative to controls). Higher total healthcare costs were seen in surgery patients in both the euglycemic and prediabetes subgroups, while no difference was detected for patients with diabetes. In patients with diabetes, recent disease onset was associated with more favourable cost outcomes.
Implications of all the available evidence
The combined long-term clinical and economic evidence supports prioritizing obese patients with diabetes for bariatric surgery, especially patients with recent disease onset.
Acknowledgments
Declaration of Interests
This analysis based on a registry linkage of the SOS study data to the National Patient Register and the Prescribed Drug Register was supported by a grant from Arbetsmarknadens Försäkringsoch Aktiebolag (AFA) to KN and MN. MN was also supported by the Swedish Scientific Research Council (K2014-99X-22495-01-3). CK was supported by a partnership grant from Deakin University (Australia). KS was supported by a grant from the Swedish Agency for Innovation Systems. The SOS study was supported by grants from the Swedish Research Council (K2012-55X-22082-01, K2013-54X-11285-19, K2013-99X-22279-01), the Swedish federal government under the LUA/ALF agreement concerning research and education of doctors, and Diabetesfonden. The SOS study has previously been supported by grants to authors from Hoffmann–La Roche, AstraZeneca, Cederroth, Sanofi-Aventis, and Johnson & Johnson.
CK previously led an independent research grant from Allergan Australia. MN has received lecture and consulting fees from Abbott, Sanofi-Aventis, Itrim International, Pfizer and Strategic Health Resources; research grants from Cambridge Weight Plan, Novo Nordisk and Astra Zeneca; and royalty payments from Studentlitteratur for co-authoring chapters in a Swedish textbook on obesity. KS owns stock in Pfizer. KN has received royalty payments for co-authoring a chapter in a Swedish textbook on obesity. JKE reports participating in research projects fully or partly funded by Novo Nordisk and Combine Sweden, and serving as an external consultant to AbbVie. LMSC reports receiving consulting fees from Astra Zeneca, lecture fees from Johnson & Johnson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov registry: NCT01479452
Contributors
LMSC, LS, CK, MN and KN conceived the study. KS prepared the SOS drug data. MN, KN, LMSC and KS organised register linkage with the Swedish authorities. CK, MN and JKE conducted the statistical analysis with support from MP. CK wrote the paper with contributions from all authors. All authors have approved the final draft of the manuscript. LMSC, MN and JKE had full access to all of the data (including statistical reports and tables) and can take responsibility for the integrity of the data and the accuracy of the data analysis. LMSC and LS contributed equally and are both guarantors.
Additional Contributions
We thank the staff members at 25 surgical departments and 480 primary healthcare centers in Sweden. No one received compensation for their contribution besides salary.
Contributor Information
Catherine Keating, Deakin Health Economics, Deakin University, Melbourne, Australia; Obesity and Population Health, Baker IDI Heart and Diabetes Institute, Melbourne, Australia.
Martin Neovius, Clinical Epidemiology Unit, Department of Medicine, Karolinska Institutet, Stockholm, Sweden.
Kajsa Sjöholm, Institute of Medicine, The Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Markku Peltonen, Department of Chronic Disease Prevention, National Institute for Health and Welfare, Helsinki, Finland.
Kristina Narbro, Regional Health Department, Region Vastra Gotaland, Gothenburg, Sweden (KN).
Jonas K Eriksson, Clinical Epidemiology Unit, Department of Medicine, Karolinska Institutet, Stockholm, Sweden.
Lars Sjöström, Institute of Medicine, The Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Lena MS Carlsson, Institute of Medicine, The Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
References
- 1.Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public health. 2007;121(7):492–6. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neovius M, Teixeira-Pinto A, Rasmussen F. Shift in the composition of obesity in young adult men in Sweden over a third of a century. Int J Obes (Lond) 2008;32(5):832–6. doi: 10.1038/sj.ijo.0803784. [DOI] [PubMed] [Google Scholar]
- 3.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122(7):481–6. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17(9):961–9. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 5.Norris SL, Zhang X, Avenell A, et al. Long-term effectiveness of lifestyle and behavioral weight loss interventions in adults with type 2 diabetes: a meta-analysis. Am J Med. 2004;117(10):762–74. doi: 10.1016/j.amjmed.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. Journal of internal medicine. 2013;273(3):219–34. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 7.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–56. doi: 10.1016/j.amjmed.2008.09.041. e5. [DOI] [PubMed] [Google Scholar]
- 8.Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon JB, le Roux CW, Rubino F, Zimmet P. Bariatric surgery for type 2 diabetes. Lancet. 2012;379(9833):2300–11. doi: 10.1016/S0140-6736(12)60401-2. [DOI] [PubMed] [Google Scholar]
- 10.Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–304. doi: 10.1001/jama.2014.5988. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367(8):695–704. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- 12.Neovius M, Narbro K, Keating C, et al. Health care use during 20 years following bariatric surgery. JAMA. 2012;308(11):1132–41. doi: 10.1001/2012.jama.11792. [DOI] [PubMed] [Google Scholar]
- 13.Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. The New England journal of medicine. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 14.Narbro K, Ågren G, Jonsson E, Näslund I, Sjöström L, Peltonen M. Pharmaceutical costs in obese individuals: comparison with a randomly selected population sample and long-term changes after conventional and surgical treatment: the SOS intervention study. Arch Intern Med. 2002;162(18):2061–9. doi: 10.1001/archinte.162.18.2061. [DOI] [PubMed] [Google Scholar]
- 15.ApotekensService. Drug Price Index (Lä kemedelsprisindex) 2012 [August 8, 2012]. Available from: http://www.apotekensservice.se/Global/Externa%20webben/statistik/Lakemedelsprisindex_med_arsmedeltal_2012.pdf.
- 16.Schreier E, Hohne M, Kunkel U, Berg T, Hopf U. Hepatitis GBV-C sequences in patients infected with HCV contaminated anti-D immunoglobulin and among i.v. drug users in Germany. Journal of hepatology. 1996;25(3):385–9. doi: 10.1016/s0168-8278(96)80126-7. [DOI] [PubMed] [Google Scholar]
- 17.Pilgrim H, Lloyd-Jones M, Rees A. Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation. Health technology assessment. 2009;13(10):iii, ix–xi, 1–103. doi: 10.3310/hta13100. [DOI] [PubMed] [Google Scholar]
- 18.Executive summary: standards of medical care in diabetes–2011. Diabetes Care. 2011;34(Suppl 1):S4–10. doi: 10.2337/dc11-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–9. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 20.Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. Journal of health services research & policy. 2004;9(4):197–204. doi: 10.1258/1355819042250249. [DOI] [PubMed] [Google Scholar]
- 21.Annual Report SOReg 2012 (Scandinavian Obesity Surgery Register) 2013 [cited Nov 21, 2013]. Available from: http://www.ucr.uu.se/soreg/
- 22.Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ. 2014;349:g3961. doi: 10.1136/bmj.g3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Courcoulas AP, Yanovski SZ, Bonds D, et al. Long-term Outcomes of Bariatric Surgery: A National Institutes of Health Symposium. JAMA surgery. 2014 doi: 10.1001/jamasurg.2014.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maciejewski ML, Arterburn DE. Cost-effectiveness of bariatric surgery. JAMA. 2013;310(7):742–3. doi: 10.1001/jama.2013.276131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115(12):956–61. [PubMed] [Google Scholar]
- 26.Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 27.Cummings DE, Cohen R. Beyond BMI: the need for new guidelines governing the use of bariatrc and metabolic surgery. Lancet Diabetes Endocrinol. 2014;2:175–81. doi: 10.1016/S2213-8587(13)70198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institute for Health and Care Excellence (NICE UK. Obesity: identification, assessment and management of overweight and obesity in children, young people and adults. 2014 [PubMed] [Google Scholar]
- 29.Padwal R, Klarenbach S, Wiebe N, et al. Bariatric surgery: a systematic review of the clinical and economic evidence. Journal of general internal medicine. 2011;26(10):1183–94. doi: 10.1007/s11606-011-1721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


