Abstract
Context
Increasing rates of opioid abuse, particularly fentanyl, may lead to more presentations of unusual effects of opioid toxicity. Diffuse alveolar hemorrhage is a rare complication of fentanyl overdose.
Case Details
A 45-year-old male presented in hypoxic respiratory failure secondary to diffuse alveolar hemorrhage requiring intubation. Comprehensive drug screening detected fentanyl without exposure to cocaine. Further history upon the patient’s recovery revealed exposure to snorted fentanyl powder immediately prior to presentation.
Discussion
Diffuse alveolar hemorrhage is a potential, though rare, presentation of opioid intoxication.
Conclusions
Recognition of less common complications of opioid abuse such as diffuse alveolar hemorrhage is important in proper management of overdoses.
Keywords: Fentanyl, Diffuse alveolar hemorrhage, Opioid toxicity, Pulmonary hemorrhage
Introduction
Fentanyl is a synthetic opioid of the phenylpiperidine class [1]. The analgesic effect of fentanyl occurs primarily via the mu1-receptor, while fentanyl-induced respiratory depression is mediated by the mu2-receptor [1]. The most common prescription form, the transdermal patch, is easily manipulated to extract active drug so that it can be consumed intravenously, nasally, orally, or by smoking [2, 3]. Toxicity from fentanyl includes centrally mediated respiratory depression, complications related to the method of ingestion, and infections [4]. Pulmonary hemorrhage associated with use of fentanyl and synthetic fentanyl analogues has been reported rarely in the literature, and the mechanism and risk factors for this presentation are not known [5, 6]. The rate of fentanyl abuse rapidly escalated in the early 2000s, and therefore, this manifestation of fentanyl toxicity may become more common [5, 6]. We present a novel case of diffuse alveolar hemorrhage secondary to nasal insufflation of fentanyl powder.
Case
A 45-year-old male with coronary artery disease, gambling addiction, and polysubstance abuse with prior use of cocaine, anabolic steroids, and alcohol, had a witnessed that he collapsed while playing video lottery terminals at a casino. Initial vital signs taken by emergency medical services (EMS) 6 min after loss of consciousness were pulse rate 142 bpm, pulse oximetry 60 %, respiratory rate 6/min, temperature 36.9 °C, and blood pressure 40/0 mmHg. Glasgow Coma Score was 3/15. EMS attempted bag mask ventilation at a respiratory rate of 15/min with an improvement of oxygen saturation to 72 % and blood pressure to 218/149 mmHg. Frank blood was visualized in both nares and the oropharynx. His pupils were symmetric, 2 mm, and non-reactive. He was cool, diaphoretic, and mottled in his extremities. At 33 min, on-scene intubation was performed for hypoxic respiratory failure using ketamine and succinylcholine. Post-intubation, pulse oximetry increased to 85 on 100 % FiO2, pulse rate was 102 bpm, and blood pressure was 192/107 mmHg. A bottle containing green powder was found in the patient’s personal belongings.
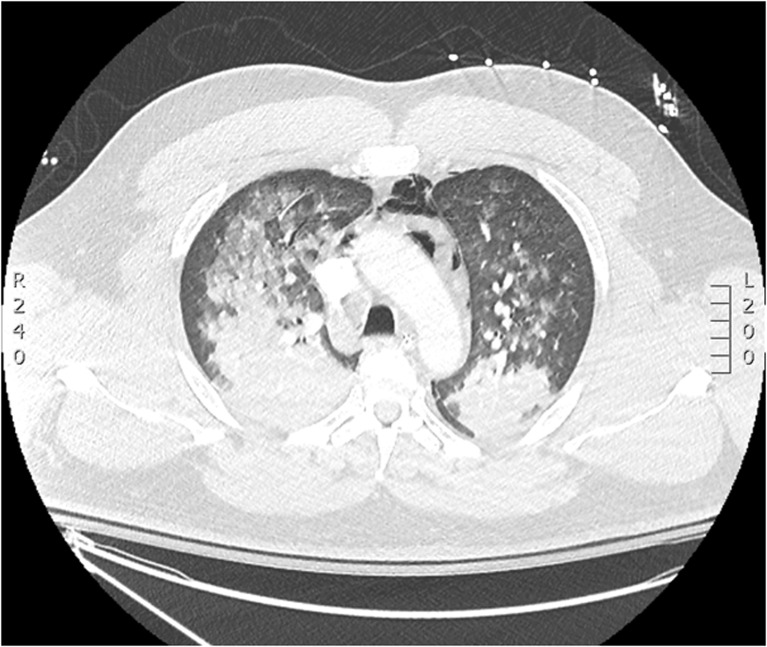
Vitals signs upon arrival to the emergency department at 1 h were pulse rate 100 bpm, pulse oximetry 87 %, blood pressure 154/90 mmHg, and GCS 3 T. Arterial blood gas obtained in the emergency department while intubated on 100 % FiO2 showed pH 7.01, PaCO2 77 mmHg, PaO2 75 mmHg, and bicarbonate 19 mmol/L. An electrocardiogram demonstrated long-standing, unchanged anteroseptal ST segment elevation. Complete blood count, electrolytes, and creatinine were within normal range. The initial high-sensitivity troponin was 14 ng/L, a repeat level drawn at 3 h peaked at 183 ng/L and decreased to 83 ng/L at 6 h (normal range 0–14 ng/L). Acetaminophen and salicylates were undetectable, and ethanol level was 10 mg/dL. Portable chest X-ray (CXR) demonstrated bilateral infiltrates. Chest computed tomography (CT) demonstrated diffuse ground glass opacities involving all lobes with peripheral sparing and mediastinal emphysema (Fig. 1).
Fig. 1.
Computed tomography of the chest obtained on day 1 of the patient’s admission demonstrates diffuse, bilateral ground glass opacities and pneumomediastinum
The patient received piperacillin-tazobactam and vancomycin as empiric therapy for pneumosepsis and acetylsalicylic acid and heparin for a potential acute coronary syndrome. The patient did not receive naloxone. He was admitted to the intensive care unit. A transthoracic echocardiogram obtained on day 2 of admission demonstrated normal left ventricular function and a mildly dilated right ventricle with borderline reduction in right ventricular systolic function. Ongoing suction of blood via the endotracheal tube continued past 12 h post initial presentation. Bronchoscopy was performed on day 2 of admission. Frank blood was seen throughout the airway, and gross blood was returned on bronchoalveolar lavage from the right upper lobe. No focal source or upper airway trauma was identified on direct visualization. Autoimmune panel including anti-glomerular basement membrane antibody, anti-neutrophil cytoplasmic antibody, and anti-nuclear antibody was negative. Urinalysis did not demonstrate casts or dysmorphic red blood cells. Gram stain and culture of blood and bronchoalveolar lavage were negative, and antibiotics were discontinued (Table 1).
Table 1.
Results of serologic testing from the case patient
| Variable | Reference range, adults | Result |
|---|---|---|
| Acetaminophen (μg/mL) | 10–30 | 0 |
| Ethanol (mg/dL) | 0 | 10 |
| Salicylates (mgdL) | 15–30 | 0 |
| Anti-nuclear antibody | Negative | Negative |
| Anti-neutrophil cytoplasmic antibody | Negative | Negative |
| Anti-myeloperoxidase antibody (AI) | <0.1 | <0.1 |
| Anti-PR3 antibody (AI) | <0.1 | <0.1 |
| Anti-glomerular antibody | Negative | Negative |
| HIV serology | Negative | Negative |
| Blood culture | No growth | No growth |
Comprehensive urine drug screen (UDS) via gas chromatography-mass spectrometry (GC-MS) was performed on urine obtained on presentation to the emergency department. Results are summarized in Table 2. Of note is that fentanyl was not administered for therapeutic use at any point in his hospitalization. GC-MS analysis of the green powder from the patient’s belongings identified fentanyl, quetiapine, and caffeine. In light of these results, the patient’s urine GC-MS results were reviewed again; neither quetiapine nor caffeine was detected.
Table 2.
Gas chromatography-mass spectrometry analysis of the patient’s urine and the green powder found in the patient’s personal belongings
| Compound | Detected | Administered in hospital |
|---|---|---|
| Urine | ||
| Caffeine | No | No |
| Cannabinoids | Yes | No |
| Cocaine | No | No |
| Fentanyl | Yes | No |
| Ketamine | Yes | Yes |
| Lidocaine | Yes | Yes |
| Midazolam | Yes | Yes |
| Norvenlafaxine | Yes | Home medication |
| Propofol | Yes | Yes |
| Quetiapine | No | No |
| Ranitidine | Yes | Yes |
| Green powder | ||
| Caffeine | Yes | |
| Cocaine | No | |
| Fentanyl | Yes | |
| Quetiapine | Yes | |
By day 4 of admission, suction of blood via the patient’s endotracheal tube had subsided, and his oxygenation had improved. He was successfully extubated. At that time, further history was obtained. His home medications included only venlafaxine and naproxen. He denied cocaine use in 8 months prior to admission. He admitted to snorting fentanyl powder weekly for the previous 6 months. On the day of his presentation, he had snorted crushed green fentanyl tablets 2 to 3 h prior to his collapse. He was gradually titrated off supplemental oxygen and was on room air prior to his discharge on day 6. Follow-up CXR and pulmonary function tests obtained 6 weeks post discharge were normal.
Discussion
The case describes a patient with hypoxic respiratory failure secondary to diffuse alveolar hemorrhage in the context of intranasal fentanyl use. Diffuse alveolar hemorrhage is a subgroup of pulmonary hemorrhage that originates from alveolar capillaries and is diagnosed by bronchoscopy [7]. The differential diagnosis for diffuse alveolar hemorrhage is broad and includes small vessel vasculitis, connective tissue disease, infection, and crack cocaine abuse [7].
In our patient, diffuse alveolar hemorrhage was confirmed on bronchoscopy. Analysis of the green powder found with the patient was positive for fentanyl, and the patient admitted to intranasal administration of this powder 1 to 2 h prior to his collapse. The temporal association of the onset of symptoms after insufflation of fentanyl, and the exclusion of other etiologies of diffuse alveolar hemorrhage, supports fentanyl as the underlying cause of the patient’s pulmonary hemorrhage.
Pulmonary hemorrhage secondary to fentanyl overdose has been reported sparingly in the literature. Pulmonary hemorrhage was reported with insufflation of butyrfentanyl, a fentanyl analogue with no clinical applications, in a 16-year-old male who presented with hypoxic respiratory failure and required intubation [8]. Autopsy of a 36-year-old male who died from acute respiratory distress syndrome after an overdose of intravenous fentanyl demonstrated tracheobronchial congestion, non-cardiogenic pulmonary edema, and diffuse pulmonary hemorrhage [9]. A Swedish case series of nine autopsies performed in patients who had died in the context of recent fentanyl abuse demonstrated pulmonary congestion in five patients and hemosiderin-laden macrophages from the lung in three patients [10]. The relationship between fentanyl consumption and alveolar hemorrhage in this series is complicated by the high frequency of co-ingestants such as amphetamines [10]. Similarly, a Russian autopsy series of patients who overdosed on 3-methylfentanyl, a synthetic fentanyl analogue, found that nearly 60 % of patients had evidence of pulmonary edema and 18 % had alveolar hemorrhage [11]. Diffuse alveolar hemorrhage appears to be a class effect of opioids as it has also been reported in morphine, codeine, and heroin poisonings [12].
The mechanism of pulmonary hemorrhage secondary to opioid use is not known. It is independent of the route of opioid ingestion and so is not likely to be a direct toxic effect [13]. There may be a link between the development of noncardiogenic pulmonary edema (NCPE) and diffuse alveolar hemorrhage, as autopsies have found evidence of subclinical pulmonary hemorrhage in the lungs of patients who died from NCPE due to opioid toxicity without overt hemoptysis [9, 14]. It is possible that diffuse alveolar hemorrhage is a manifestation of severe endothelial dysfunction that is more commonly associated with NCPE in opioid toxicity.
Pulmonary hemorrhage is commonly reported with cocaine and crack cocaine abuse, with up to 5.7 % of abusers reporting hemoptysis associated with use [15]. Evidence of acute and chronic diffuse alveolar hemorrhage was found in over half of patients who died of cocaine overdose in one autopsy series, including cases where pulmonary hemorrhage was not clinically apparent [14, 15]. Diffuse alveolar hemorrhage was found in the absence of pulmonary infarction, suggesting that infarction-reperfusion injury is not the primary mechanism [14, 15]. Studies have implicated anoxic endothelial dysfunction and alveolar-capillary membrane injury as potential mechanisms [16]. In our patient, the result of the urine comprehensive drug analysis by GC-MS was negative for cocaine, implying a lack of exposure to cocaine for several days prior to his loss of consciousness.
Factors which limit our ability to definitively identify fentanyl as the causative agent of the case patient’s alveolar hemorrhage include the detection of caffeine and quetiapine in the powder that the patient snorted prior to presentation. It is possible that the patient’s presentation could be explained by one of these substances. However, neither substance has been previously linked to pulmonary hemorrhage in the literature and was identified within the patient’s urine GC-MS, suggesting that they were not substantially absorbed. These substances may have been present in the patient’s urine in amounts below the detection limit of our institution’s GC-MS method. In addition, it is possible that an additional adulterant may have been present in the green tablets that was not identified by the GC-MS analysis and may have been responsible for the patient’s diffuse alveolar hemorrhage. The GC-MS method used at our institution cannot make quantitative determination about the amount of substance within a particular sample.
Rates of opioid abuse increased rapidly worldwide from the early 1990s, followed by a recent plateau beginning in 2013 coinciding with a reduction in opioid prescription availability [5, 6]. More than three quarters of pharmaceutical overdoses in 2010 in the USA were attributed to opioids [5]. Opioid-related mortality in Ontario, Canada, doubled from 1991 to 2007 with a concurrent fivefold increase in fentanyl prescription rates [5]. The most commonly available formulation of fentanyl is transdermal patches intended for patients with chronic pain [3]. Extraction of fentanyl from transdermal patches for intravenous, oral, inhaled, or insufflated use is commonly reported by abusers [2, 3]. Non-pharmacologic grade fentanyl powder has been illegally synthesized and distributed in eastern Europe during a recent epidemic of fentanyl abuse [2].
A 20-fold increase in deaths and overdoses related to the use of illegally synthesized fentanyl tablets from an unknown source has been reported in Alberta, Canada, where the patient lives and presented, in the recent year [17, 18]. The Canadian Center on Substance Abuse (CSA) and the Calgary Police Service believe that fentanyl powder is being produced illegally overseas, most likely in China, and smuggled into western Canada. A portion of the fentanyl powder is incorporated into other street drugs such as heroin, while some is dyed green and pressed into tablets by organized-crime groups in Canada and sold as OxyContin. These pills are known as “greenies” or “green beans” [18]. This description of non-pharmacologically produced fentanyl tablets is compatible with the substance found in the patient’s belongings. Physicians must be familiar with the potential manifestations and complications of opioid toxicity as abuse rates are at epidemic proportions and methods of use have expanded.
Conclusion
Opioid toxicity leading to respiratory failure is commonly associated with central nervous system mediated respiratory depression and classically with non-cardiogenic pulmonary edema. Diffuse pulmonary hemorrhage has rarely been reported with opioid overdose and to the best of our knowledge, only once before with fentanyl. Recognition of this complication is important in light of the ongoing fentanyl abuse epidemic.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Footnotes
This work has not previously been reported or presented.
References
- 1.Cheng J, Smith ER, Cahill M, Cohen R, Fishman JB. The opioid receptor binding of dezocine, morphine, fentanyl, butorphanol and nalbuphine. Life Sci. 1992;52:389–396. doi: 10.1016/0024-3205(93)90152-S. [DOI] [PubMed] [Google Scholar]
- 2.Mounteney J, Giraudon I, Denissov G, Griffiths P. Fentanyls: are we missing the signs? Highly potent and on the rise in Europe.Int J of Drug Policy. 2015. [DOI] [PubMed]
- 3.Katz N, Dart RC, Bailey E, Trudeau J, Osgood E, Paillard F. Tampering with prescription opioids: nature and extent of the problem, health consequences, and solutions. Am J Drug Alcohol Abuse. 2011;37:205–215. doi: 10.3109/00952990.2011.569623. [DOI] [PubMed] [Google Scholar]
- 4.Yamanaka T, Sadikot RT. Opioid effect on lungs. Off J Asian Pac Soc Resp. 2013;18:255–262. doi: 10.1111/j.1440-1843.2012.02307.x. [DOI] [PubMed] [Google Scholar]
- 5.King NB, Fraser V, Boikos C, Richardson R, Harper S. Determinants of increased opioid-related mortality in the United States and Canada, 1990–2013: a systematic review. Am J Public Health. 2014;104(8):e32–e42. doi: 10.2105/AJPH.2014.301966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 7.Lara AR, Schwarz MI. Diffuse alveolar hemorrhage. Chest. 2010;137(5):1164–1171. doi: 10.1378/chest.08-2084. [DOI] [PubMed] [Google Scholar]
- 8.Cole JB, Dunbar JF, McIntire SA, Regelmann WE, Slusher TM. Butyrfentanyl overdose resulting in diffuse alveolar hemorrhage. Pediatrics. 2015;135(3):e740–e742. doi: 10.1542/peds.2014-2878. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi AK, Baird JR, Rao NGS. A death due to self-administered fentanyl. J Anal Toxicol. 1990;14:385–387. doi: 10.1093/jat/14.6.385. [DOI] [PubMed] [Google Scholar]
- 10.Kronstrand R, Druid H, Holmgren P, Rajs J. A cluster of fentanyl-related deaths among drug addicts in Sweden. Forensic Sci Int. 1997;88:185–195. doi: 10.1016/S0379-0738(97)00068-6. [DOI] [PubMed] [Google Scholar]
- 11.Safrai AE. Peculiarities of morphologic changes in fatal 3-methylfentanyl poisoning. Abstracts/Forensic Sci Int. 2007;169S:S10–S11. [Google Scholar]
- 12.Porter R, O’Reilly H. Pulmonary hemorrhage: a rare complication of opioid overdose. Pediatr Emerg Care. 2011;27(8):742–744. doi: 10.1097/PEC.0b013e318226df00. [DOI] [PubMed] [Google Scholar]
- 13.Megarbane B, Chevillard L. The large spectrum of pulmonary complications following illicit drug use: features and mechanisms. Chem Biol Interact. 2013;206:444–451. doi: 10.1016/j.cbi.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Tseng W, Sutter ME, Albertson TW. Stimulants and the lung. Clin Rev Allerg Immunol. 2014;46:82–100. doi: 10.1007/s12016-013-8376-9. [DOI] [PubMed] [Google Scholar]
- 15.Albertson TE, Walby WF, Derlet RW. Stimulant-induced pulmonary toxicity. Chest. 1995;108:1140–1149. doi: 10.1378/chest.108.4.1140. [DOI] [PubMed] [Google Scholar]
- 16.Tomashefski JF, Felo JA. The pulmonary pathology of illicit drug and substance abuse. Curr Diagn Pathol. 2004;10:413–426. doi: 10.1016/j.cdip.2004.04.009. [DOI] [Google Scholar]
- 17.Southwick, R. Deadly painkiller linked to rising number of Alberta deaths. In: The Calgary Herald. 2015. Retrieved from www.thecalgaryherald.com. Accessed 26 June 2015.
- 18.Canadian Centre on Substance Abuse. Deaths involving fentanyl in Canada, 2009–2014. Canadian Community Epidemiology Network on Drug Use Bulletin. 2015.