Abstract
Intravenous lipid emulsion (ILE), a component of parenteral nutrition, consists of a fat emulsion of soy bean oil, egg phospholipids, and glycerin. Case reports suggest that ILE may reverse hypotension caused by acute poisoning with lipophilic drugs such as verapamil, but the mechanism remains unclear. The methods used are the following: (1) measurement of ILE concentration in serum samples from a patient with verapamil poisoning treated with ILE, (2) measurement of free verapamil concentrations in human serum mixed in vitro with increasing concentrations of ILE, and (3) measurement of murine ventricular cardiomyocyte l-type Ca2+ currents, intracellular Ca2+, and contractility in response to verapamil and/or ILE. Maximum patient serum ILE concentration after infusion of 1 L ILE over 1 h was approximately 1.6 vol%. In vitro GC/MS verapamil assays showed that addition of ILE (0.03–5.0 vol%) dose-dependently decreased the free verapamil concentration in human serum. In voltage-clamped myocytes, adding ILE to Tyrode’s solution containing 5 μM verapamil recovered l-type Ca2+ currents (ICa). Recovery was concentration dependent, with significant ICa recovery at ILE concentrations as low as 0.03 vol%. ILE had no effect on ICa in the absence of verapamil. In field-stimulated intact ventricular myocytes exposed to verapamil, adding ILE (0.5 %) resulted in a rapid and nearly complete recovery of myocyte contractility and intracellular Ca2+. Our in vitro studies indicate that ILE acts as a lipid sink that rapidly reverses impaired cardiomyocyte contractility in the continued presence of verapamil.
Electronic supplementary material
The online version of this article (doi:10.1007/s13181-015-0511-y) contains supplementary material, which is available to authorized users.
Keywords: Lipid emulsion, Verapamil, Poisoning, Antidote, Lipid sink
Introduction
According to the 2013 report of the American Association of Poison Control Center National Poison Data System (NPDS), there were 995 exposures to cardiovascular (CV) agents that caused moderate clinical outcome, major outcome, or death [1]. CV agents were the third most common cause of deaths attributed to pharmaceutical agents following analgesics and drug abuse. Of the CV agents, calcium channel blockers (CCB) were responsible for approximately 50 % patients with significant morbidity or mortality. Among the fatalities caused by CCBs, verapamil was the CCB most commonly listed, followed by amlodipine and diltiazem [1]. However, it is difficult to determine the actual number of deaths as reporting is voluntary, but it is likely that the exposures are underreported. Hence, it is important to find treatments that will improve the outcome of CCB ingestion, in particular those due to verapamil exposure.
Although there are no therapies that consistently reverse cardiovascular collapse caused by CCB poisoning, both high-dose insulin/euglycemia (HIE) therapy [2–4] and intravenous lipid emulsion (ILE) [5–7] have been shown to improve hemodynamics and survival following intravenous administration of verapamil in animals. On the other hand, while several case reports indicate amelioration of CCB-induced cardiotoxicity by the administration of ILE [8–11], others report no benefit [12]. Hence, there are many open questions remaining such as the mechanism of ILE action, the optimal time and dose of ILE administration [9, 11, 13], and the interaction of ILE with other therapy being administrated. A working group charged with developing evidence-based recommendations on the use of ILE therapy in poisoning has recently been formed [14].
The objective of our experiments was to conduct in vitro studies aimed at determining the mechanism by which ILE may reverse verapamil-induced cardiotoxicity. The study was motivated by a case of verapamil poisoning where ILE infusion completely reversed hemodynamic collapse despite unchanged serum verapamil concentrations [15]. We performed studies mixing human serum with verapamil, and studies of ILE in freshly isolated cardiomyocytes exposed to verapamil to test the hypothesis that ILE acts as a lipid sink in verapamil cardiotoxicity.
Methods
Determination of Patient ILE Serum Concentration
The residual serum samples from a patient with CCB poisoning treated with ILE (Supplemental Fig. 1) were used to estimate the ILE serum concentration reached after ILE infusion. Our clinical laboratory has IRB approval for the use of de-identified residual fluid specimens for instrument and method validation with reference range verification. We utilized the clinical assay for triglycerides (TG, Beckman-Coulter DxC800, Brea, CA), which is calibrated against a physiological mix of fatty acids. The instrument was re-calibrated using purchased drug-free serum (UTAK Laboratories, Valencia, CA) given known amounts of ILE. The patient’s physiological TG at 7 h post-ingestion (and prior to ILE administration) was 132 mg/dL and was assumed to remain at this value throughout. Thus, all measured TG above 132 mg/dL was assumed to be ILE derived and re-calculated accordingly (measured TG = 1.787 × ILE TG + 1.4 mg/dL).
In Vitro Mixing Study
Certified drug free human serum (UTAK) was purchased and spiked with verapamil to a final concentration of 20 μM and equilibrated at room temperature (21 °C) for 4 h. Aliquots were then mixed with varying amounts of ILE, in a matched pairs design adding ILE and phosphate buffered saline such that the final volume, and the concentrations of serum proteins, verapamil, and TG were identical in all samples (final serum albumin was 2.85 g/dL; TG was 74 mg/dL; and individual protein fractions were not assayed). After gentle rocking at 20 cycles/min for 20 min, duplicate 3.5 mL portions were placed in 30-kDa cut-off ultrafiltration devices in a fixed angle rotor centrifuge for 25 min at 3000 rpm (21 °C). This produced approximately 450 μL clear filtrate, in which the concentration of verapamil (free concentration) was determined by GC-MS. In a preliminary experiment, measurement of the drug concentration in both the filtrate and retentate showed there was no significant loss of drug due to binding to the filtration device.
Verapamil concentrations were measured by GC-MS after a standard basic extraction into ethyl acetate. Briefly, 800 μL serum or filtrate was vortexed for 60 s with 50-μL internal standard (20 mg/L aqueous), 50 μL 4 M ammonium hydroxide, and 300 μL ethylacetate. After centrifugation, the organic phase was recovered and evaporated to dryness under air at 56 °C. The residue was reconstituted in 30 μL ethyl acetate and injected onto the gas chromatograph. Selected ion monitoring of the column effluent (m/z 303 for verapamil, m/z 289 for norverapamil, and m/z 120 for the internal standard O-desmethylvenlafaxine) allowed quantitation against a standard curve prepared in drug-free human serum and extracted simultaneously. All chemicals were obtained from Sigma (St. Louis, MO).
Myocyte Isolation and Ca2+ Indicator Loading
The University Committee on Use and Care of Animals at Vanderbilt University Medical Center approved our animal protocols. Ventricular myocytes from 12- to 16-week-old mice (C57BL/6 strain) were isolated using modified collagenase/protease method, as previously described [16]. All chemicals, unless otherwise specified, were obtained from Sigma (St. Louis, MO). Experiments on isolated myocytes were conducted in Tyrode’s solution containing (in mM): NaCl = 134, KCl = 5.4, MgCl2 = 1, glucose = 10, HEPES = 10, adjusted to pH 7.4 with NaOH. For the measurements of cytosolic [Ca2+] and cell shortening, cells were loaded with membrane-permeable fluo-4 acetoxymethyl ester (fluo-4 AM). Myocytes were incubated in Tyrode’s solution, containing 1 mM Ca2+, 6.6 μM fluo-4 AM, and 0.16 % Pluronic F127 for 20 min at room temperature to load indicator in the cytosol. Then supernatant was removed and cells were washed in Tyrode’s solution, containing 1.2 mM Ca2+, twice for 25 min. A minimum of 30 min were allowed for de-esterification of the indicator before using the cells.
Voltage-Clamp Experiments
Freshly isolated murine ventricular myocytes were whole-cell patched in Tyrode’s solution. For l-type Ca2+ current (ICa) measurements, solution was changed to K, Na-free containing (in mM): N-Methyl-d-glucamine (NMDG) = 138; CsCl = 5; MgCl2 = 1; CaCl2 = 2; BaCl2 = 0.15; glucose = 10; Hepes = 10; and pH = 7.4. The pipette solution contained (in mM): CsCl = 110; MgCl2 = 1; CaCl2 = 3; MgATP = 5; cAMP = 0.2; EGTA = 14; Hepes = 10; and pH = 7.25. Currents were elicited by 200-ms depolarizing pulses to 0 mV from the holding potential −60 mV. For monitoring current–voltage relationship, currents were elicited with 200-ms depolarizing steps from −40 to 20 mV with 10-mV increment from the holding potential of −60 mV. For every experiment, the number of myocytes used is listed in corresponding figure legend. For example, for experiments testing the dose–response effect of ILE on ICa in the presence of verapamil (Fig. 2), a total of 19 myocytes (1 myocyte = 1 experiment) from 15 animals were used. For I–V curves (Fig. 3), five myocytes from three different animals were used. All measurements were carried out at room temperature.
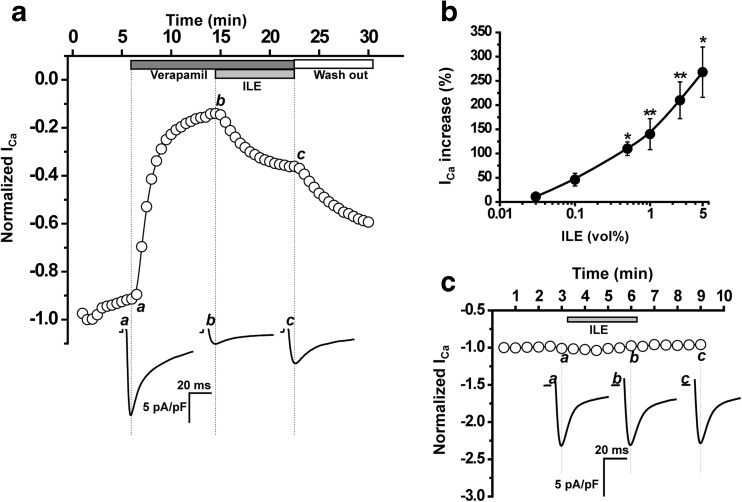
Fig. 2.
ILE recovers ICa in the presence of verapamil. a Time course of changes in peak ICa amplitude elicited by 200-ms depolarizing step from −70 to 0 mV in voltage-clamped ventricular myocyte. Application of 5 μM verapamil to the external solution dramatically inhibited current amplitude (to 10 % of its initial level), and subsequent addition of ILE (2.5 vol%) significantly recovers the current. Traces below are representative ICa records. b Relative increase in ICa amplitude in response to different ILE concentrations (0.03, 0.1, 0.5, 1, 2.5, and 5.0 vol%) in the presence of 5 μM verapamil. n = 5–7 myocytes per concentration (19 total), *P < 0.05, **P < 0.005 compared to 0 % ILE by the paired Student’s t test. c Time course in the absence of verapamil. ILE (2.5 vol%) had no effect on ICa amplitude
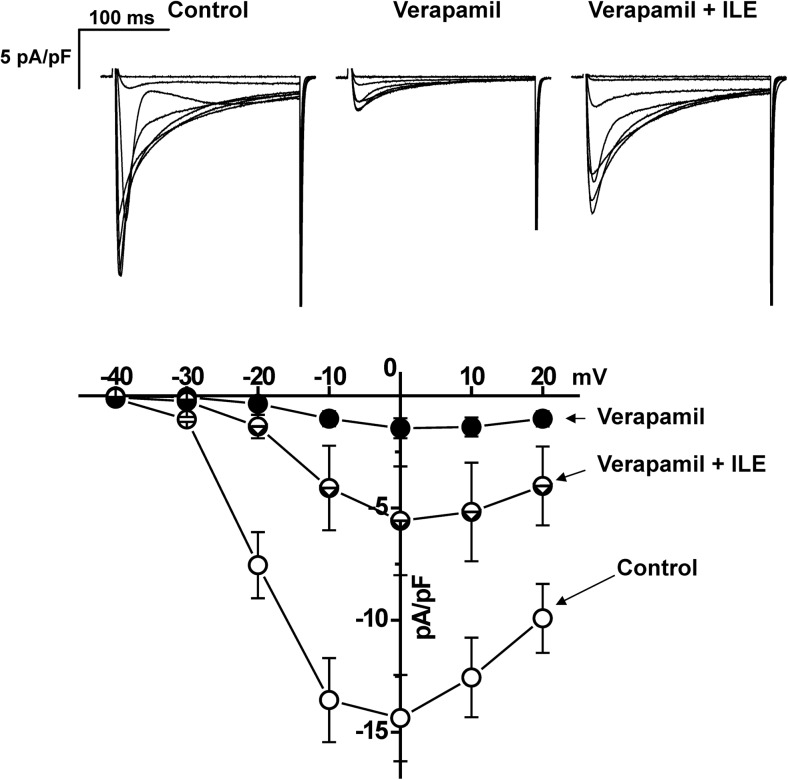
Fig. 3.
ILE has no effect on the voltage dependence of ICa activation. Examples of superimposed current traces (top) and current–voltage relationship of ICa (bottom) in control, in the presence of 5 μM verapamil and after adding ILE (2.5 vol%). Note that ILE recovers the peak current amplitude at all voltages but has no effect on the voltage dependence of ICa activation. Currents are elicited by 200-ms-long 10-mV depolarizing steps from −40 to 20 mV applied from holding potential −60 mV. n = 5
Ca2+ Fluorescence and Myocyte Shortening Measurements
Fluo-4-loaded healthy rod-shaped isolated ventricular myocytes were loaded in the experimental chamber, superfused with 2 mM Ca2+ Tyrode’s solution and field stimulated. Intracellular Ca2+ transients and cell shortening were simultaneously measured using fluorescence photometry setup (IonOptix Corp.). Ca2+ fluorescence and cell shortening were first measured after 3–5 min of steady-state pacing at 2 Hz. After that, Tyrode’s solution containing 10 μM verapamil was applied using rapid concentration clamp system, followed by addition of ILE once the maximum effect of verapamil on Ca2+ transients and shortening was reached. Cells were illuminated at 488 nm, and emitted fluorescence was measured at >515 nm. All experiments were conducted at room temperature (≈23 °C). Ca2+ and cell shortening transients were analyzed using commercial software (IonWizard; IonOptix Corp.). A total of 17 myocytes from seven different animals were used.
Statistical Analysis
Dependent datasets (Figs. 2, 4, and 5) were compared using the paired Student’s t test. Results were considered statistically significant if the P value was <0.05. Unless otherwise indicated, results are expressed as arithmetic means ± SE.
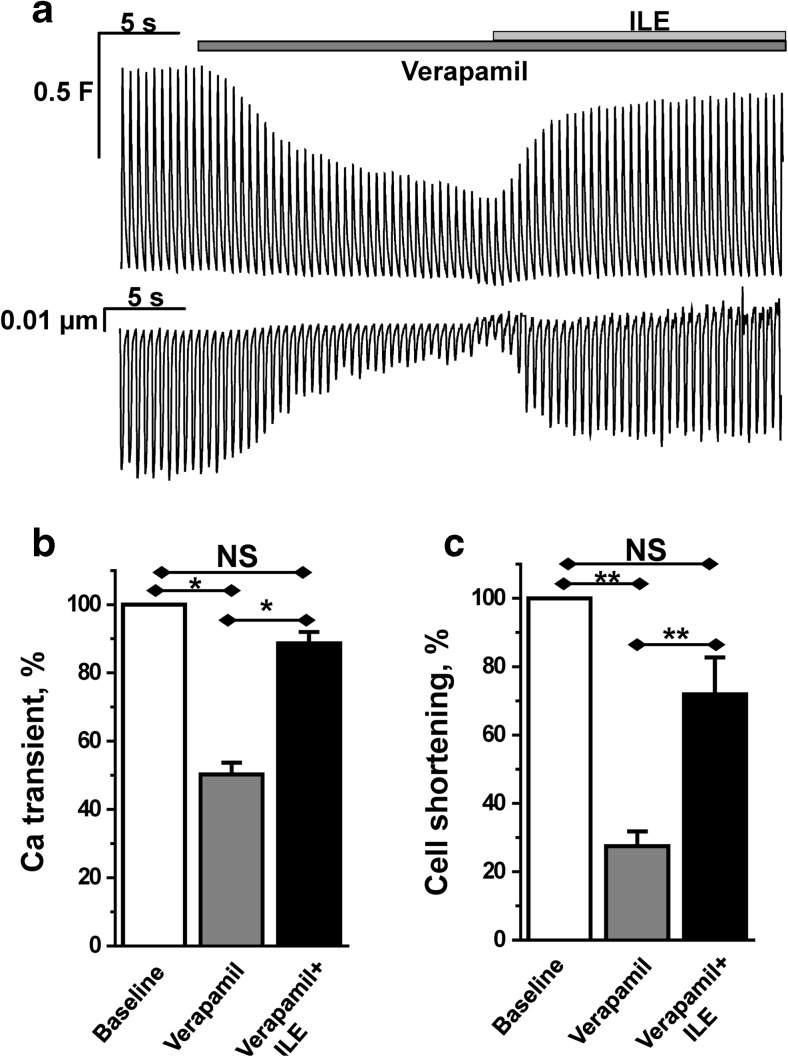
Fig. 4.
ILE rescues cardiomyocyte Ca2+ transients and contractility in the presence of verapamil. a Representative example of intracellular Ca2+ transients (top trace) and cell shortening (bottom trace) recorded from Fluo-4 AM-loaded ventricular myocytes field-stimulated at 2-Hz frequency. Application of verapamil (10 μM) causes substantial inhibition of both cell shortening and Ca2+ transient amplitude. Addition of ILE (0.5 vol%) results in rapid and almost complete recovery of both parameters. b, c Bar graphs report average data of Ca2+ transient amplitude and cell shortening, respectively, before and after application of verapamil and verapamil + ILE. All values were normalized to baseline values (control) before drug or ILE application. For Ca2+ transients, n = 17, for cell shortening, n = 10, 3–6 myocytes per animal. *P < 0.05, **P < 0.005 by paired two-tailed Student’s t test
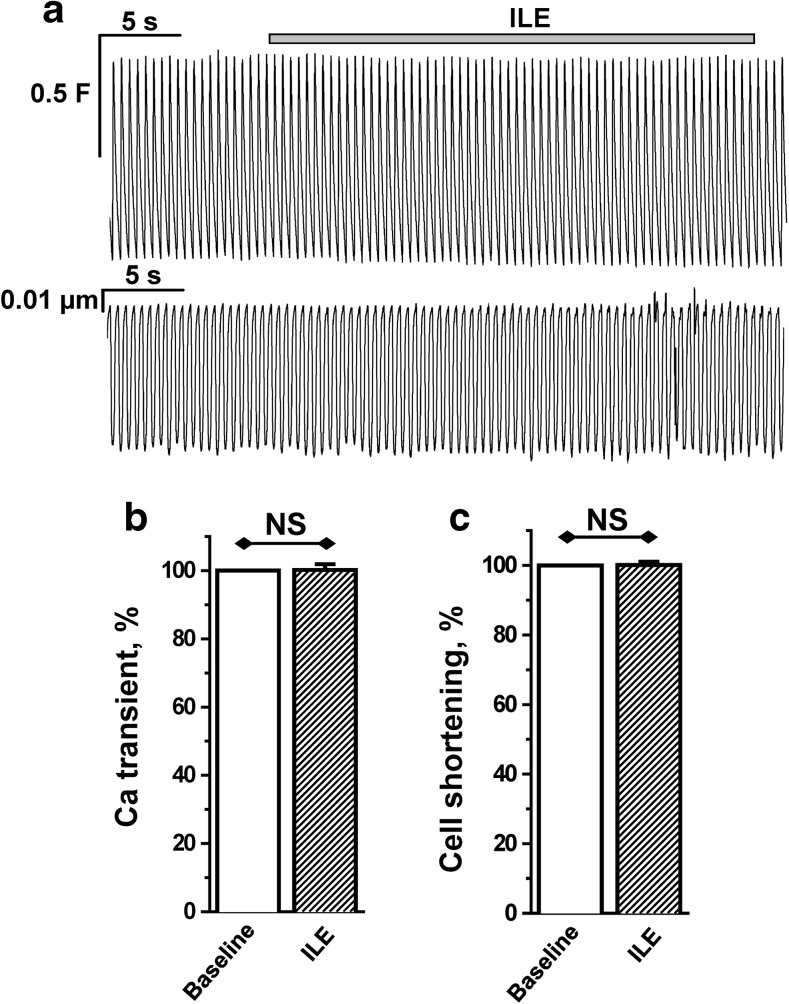
Fig. 5.
ILE alone has no effect on cardiomyocyte Ca2+ transient amplitude or contractility. a Representative example of intracellular Ca2+ transients (top trace) and cell shortening (bottom trace) recorded from Fluo-4 AM-loaded ventricular myocytes field-stimulated at 2-Hz frequency. Addition of ILE has no effect on Ca2+ transient amplitude or cell shortening. b, c Bar graphs report average data of Ca2+ transient amplitude and cell shortening, respectively, before and after application of ILE. All values were normalized to baseline values (control) before ILE application. n = 10 cells from two animals. Paired two-tailed Student’s t test was used to compare the two groups
Results
Estimating the Serum ILE Concentration Associated With Clinical Benefit in a Verapamil Poisoning Case
In order to know what ILE concentration to use for our in vitro testing, we first calculated the serum ILE concentrations reached in a patient after infusing 1 L ILE over 1 h (Supplemental Fig. 1). Serum TG prior to ILE administration were 132 mg/dL. After assay re-calibration against ILE-derived TG, TG were calculated at 670 and 296 mg/dL at 3 and 8 h post-ILE, corresponding to a serum ILE concentration of 0.8 and 0.25 vol%, respectively. Based on ILE concentrations of 0.8 and 0.25 vol% at 3 and 8 h after ILE infusion, the ILE half-life in the patient was approximately 3 h. Because ILE elimination from serum follows first-order kinetics [17], the maximum ILE concentration reached at the end of the ILE infusion can be calculated as 1.6 vol%.
Mixing Studies of Verapamil-Spiked Human Serum with ILE
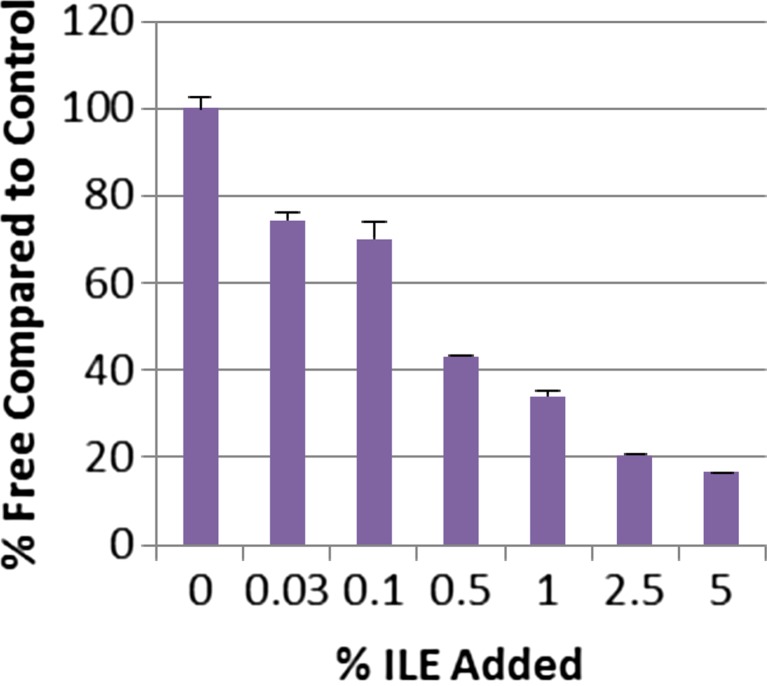
To begin investigating the mechanism of ILE action, we next mixed verapamil-spiked human serum with increasing concentrations of ILE in vitro (Fig. 1) that bracket the 1.6 % ILE serum concentration found in our index case. ILE lowered free verapamil in a concentration-dependent manner, with 1 % ILE reducing the free verapamil concentration by approximately 70 % (Fig. 1). These results indicate that ILE effectively lowers the free verapamil fraction in human serum over a wide range of ILE concentrations.
Fig. 1.
ILE increases free verapamil concentration in human plasma. Concentration–response relationship of ILE on the free verapamil concentration in human serum containing 20-μM total verapamil. Free verapamil was determined by ultrafiltration and gas chromatography after adding increasing concentrations of ILE (0.03, 0.1, 0.5, 1, 2.5, and 5 vol%). Data are means and SD of eight measurements for each concentration
l-Type Ca2+ Current Measurements
Because clinically ILE is also used for delivering lipophilic drugs (i.e., propofol), it is possible that drug (i.e., verapamil) delivery to its target could actually be enhanced by ILE. Furthermore, in the setting of verapamil poisoning, verapamil is already bound to its pharmacological target in the heart and vasculature, the l-type Ca2+ channel, prior to administering ILE. Hence, we next tested whether lowering the free verapamil concentration with ILE will remove verapamil from its binding site on the l-type Ca2+ channel. To test this hypothesis, ICa was recorded in murine cardiomyocytes. Addition of 5 μM verapamil to the bath solution blocked approximately 90 % of ICa (Fig. 2a). Subsequent addition of ILE (in the continued presence of verapamil) caused a rapid and substantial recovery of ICa (Fig. 2a). The effect of ILE was concentration dependent, with significant ICa recovery occurring at ILE concentrations as low as 0.5 % (Fig. 2b). Importantly, even high (2.5 vol%) concentrations of ILE had no effect on ICa in the absence of verapamil (Fig. 2c). The blocking effect of verapamil on ICa is voltage dependent. Thus, we next measured the ICa current–voltage (I–V) relationship in the presence of verapamil and verapamil + ILE (Fig. 3) to exclude the possibility that application of ILE shifts the voltage dependence of verapamil block. Recovery of ICa was observed at all voltages tested (Fig. 3).
Cardiomyocyte Shortening and Ca2+ Transient Measurements
In heart muscle, Ca2+ influx via l-type Ca2+ channel is required to release Ca2+ from intracellular stores, such as those in the sarcoplasmic reticulum [18]. The ensuing rise in intracellular Ca2+ activates myocyte contraction. Hence, we hypothesized that ILE-mediated recovery of ICa (Figs. 2 and 3) rescues the impaired contractility caused by verapamil. To test this hypothesis, we measured intracellular Ca2+ and myocyte contractility in intact murine ventricular myocytes. Myocytes were paced by field-stimulation and intracellular Ca2+ and cell shortening recorded (Fig. 4). Verapamil block of l-type Ca2+ channels suppressed intracellular Ca2+ and drastically decreased myocyte contractility (Fig. 4a). On average, verapamil reduced intracellular Ca2+ transient height by 50 % (Fig. 4b) and contractility by over 75 % (Fig. 4c). Addition of ILE in the continued presence of verapamil rapidly restored intracellular Ca2+ and contractility (Fig. 4a–c). Importantly, ILE in the absence of verapamil had no effect on intracellular Ca2+ and contractility (Fig. 5).
Discussion
CCB poisoning is associated with cardiovascular collapse that remains difficult to treat [19]. One possible reason for the high mortality is the loss of cardiac contractility due to impairment of the cellular excitation–contraction coupling in the heart, which requires Ca2+ influx via l-type Ca2+ channels [18]. Strategies for restoring contractility caused by CCB poisoning would include maximizing the residual Ca2+ current, increasing intracellular Ca2+ stores, or enhancing the contractile response to intracellular Ca2+. Common therapeutic approaches such as administration of glucagon, catecholamines, insulin and glucose, or Ca2+ infusion utilize these strategies for the treatment of verapamil toxicity. However, given the absolute requirement of functional l-type Ca2+ channels for cardiac excitation contraction coupling, these strategies are of limited benefit when l-type Ca2+ channels are almost completely blocked by CCBs such as verapamil. A potentially more effective strategy would be to remove verapamil from its binding site at the l-type Ca2+ channel and restore ICa in the heart. Results of our in vitro studies suggest that ILE does just that.
ILE is a mixture of soybean oil, glycerol, and egg phospholipids used for parenteral nutritional support. ILE given rapidly and at much higher doses has been used to reverse the cardiovascular complications caused by local anesthetics and other lipid-soluble medications such as verapamil [20]. While the mechanism of ILE action is debatable, case reports support ILE efficacy in acute verapamil poisoning [19]. A number of mechanisms have been proposed: simple serum volume expansion, ILE particles sequestering the lipophilic drug akin to a “lipid sink”, enhanced drug distribution, increased hepatic drug metabolism, inotropic action due to increased cardiac fat metabolism, and increase of l-type Ca2+ current by direct action of free fatty acids [21–23]. Our in vitro studies are most consistent with ILE acting as a lipid sink: 0.5 % ILE rapidly reversed verapamil-induced Ca2+ channel block and restored myocyte contractility despite the continued presence of verapamil in the experimental solutions (Fig. 4). Importantly, our studies speak against a direct enhancement of Ca2+ currents by ILE, since ILE in the absence of verapamil had no effect on ICa (Fig. 2). A possible explanation could be that the fatty acids reported to enhance Ca2+ current in cardiomyocytes [24] are not in their free form when applied as ILE (i.e., their carboxyl group is not available for binding). Also, for most fatty acids, it should take at least several minutes to see substantial effect on ICa [24], whereas in our study, in the presence of verapamil, we see an effect on ICa, as well as on cell shortening and intracellular Ca2+ transients, almost immediately (within seconds, Figs. 2 and 4). Furthermore, 1 vol% ILE was sufficient to reduce free verapamil in human serum by greater than 70 % (Fig. 1). Our serum TG measurements after infusion of 1 L ILE in a clinical case suggest that ILE serum concentration of 1 vol% is readily achievable in patients. Published reports of poisoning with verapamil as well as other drugs, such as bupropion and lamotrigine, demonstrate increased total serum drug concentrations following ILE administration [25]. This finding is also consistent with ILE acting as lipid sink that works by lowering free rather than total verapamil concentration in the blood. On the other hand, a recent study in a rat model of bupivacaine toxicity suggests ILE causes a cardiotonic response that complements sequestration [21]. The authors postulate that these results (i.e., existence of several complementing mechanisms) explain how ILE provides beneficial effect in the treatment of less-lipophilic drugs that also provoke cardiovascular toxicity [8, 25]. While we did not observe any inotropic effect (Fig. 4), it is possible that this effect may occur at higher ILE concentrations [21].
Limitations
Our in vitro study has a number of limitations that need to be considered. We only tested verapamil but did not examine the effect of ILE on other CCB such as dihydropyridines (e.g., amlodipine) that are increasingly used as antihypertensive agents in the clinic. However, verapamil remains the drug most commonly implicated in fatalities due to cardiovascular agents in the 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System [1]. While not tested here, lowering the free drug concentration by ILE in principle should be effective in poisoning with lipophilic dihydropyridines. However, this remains to be tested, because ILE reduced the free serum concentration of some but not all lipophilic local anesthetics [26]. Another concern is that ILE action as a lipid sink may enhance gastrointestinal absorption and therefore increase verapamil serum levels. While not tested for verapamil, a recent rodent study reported that ILE infusion increased serum drug concentrations and cardiotoxicity of the lipophilic agent amitriptyline when administered concomitantly with oral amitriptyline [27]. Another limitation is that we only used serum from a single patient to calculate serum concentrations reached after ILE infusion. But even much lower ILE concentrations than what was achieved in our index case were effective in reducing free verapamil in our in vitro mixing studies (Fig. 1). Finally, given that we only tested ILE acutely in vitro, our experiments do not exclude that ILE has additional benefits in CCB poisoning such as volume expansion and/or increasing mitochondrial metabolism [20].
Conclusions
Our in vitro studies indicate that ILE acts as a lipid sink that lowers free verapamil concentration in human serum and rapidly reverses impaired cardiomyocyte contractility in the continued presence of verapamil. While not directly tested here, our results are consistent with case reports of rapid intravenous administration of ILE being an effective antidote for hypotension caused by acute poisoning with verapamil and possibly other lipophilic CCB. Prospective observational studies followed ideally by randomized placebo-controlled clinical trials are needed to provide definitive answers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 43 kb)
Acknowledgments
The work was supported in part by American Heart Association grants (post-doctoral fellowship grant to D.O.K. and established investigator grant to B.C.K.)
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Mowry JB, Spyker DA, Cantilena LR, Jr, McMillan N, Ford M. 2013 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st annual report. Clin Toxicol. 2014;52(10):1032–283. doi: 10.3109/15563650.2014.987397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engebretsen KM, Morgan MW, Stellpflug SJ, Cole JB, Anderson CP, Holger JS. Addition of phenylephrine to high-dose insulin in dihydropyridine overdose does not improve outcome. Clin Toxicol. 2010;48(8):806–12. doi: 10.3109/15563650.2010.521753. [DOI] [PubMed] [Google Scholar]
- 3.Kline JA, Tomaszewski CA, Schroeder JD, Raymond RM. Insulin is a superior antidote for cardiovascular toxicity induced by verapamil in the anesthetized canine. J Pharmacol Exp Ther. 1993;267(2):744–50. [PubMed] [Google Scholar]
- 4.Engebretsen KM, Kaczmarek KM, Morgan J, Holger JS. High-dose insulin therapy in beta-blocker and calcium channel-blocker poisoning. Clin Toxicol. 2011;49(4):277–83. doi: 10.3109/15563650.2011.582471. [DOI] [PubMed] [Google Scholar]
- 5.Perez E, Bania TC, Medlej K, Chu J. Determining the optimal dose of intravenous fat emulsion for the treatment of severe verapamil toxicity in a rodent model. Acad Emerg Med. 2008;15(12):1284–9. doi: 10.1111/j.1553-2712.2008.00259.x. [DOI] [PubMed] [Google Scholar]
- 6.Bania TC, Chu J, Perez E, Su M, Hahn IH. Hemodynamic effects of intravenous fat emulsion in an animal model of severe verapamil toxicity resuscitated with atropine, calcium, and saline. Acad Emerg Med. 2007;14(2):105–11. doi: 10.1111/j.1553-2712.2007.tb01752.x. [DOI] [PubMed] [Google Scholar]
- 7.Tebbutt S, Harvey M, Nicholson T, Cave G. Intralipid prolongs survival in a rat model of verapamil toxicity. Acad Emerg Med. 2006;13(2):134–9. doi: 10.1111/j.1553-2712.2006.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 8.Bates N, Chatterton J, Robbins C, Wells K, Hughes J, Stone M, et al. Lipid infusion in the management of poisoning: a report of 6 canine cases. Vet Rec. 2013;172(13):339. doi: 10.1136/vr.101036. [DOI] [PubMed] [Google Scholar]
- 9.French D, Armenian P, Ruan W, Wong A, Drasner K, Olson KR, et al. Serum verapamil concentrations before and after Intralipid(Registered Trademark) therapy during treatment of an overdose. Clin Toxicol. 2011;49(4):340–4. doi: 10.3109/15563650.2011.572556. [DOI] [PubMed] [Google Scholar]
- 10.Young AC, Velez LI, Kleinschmidt KC. Intravenous fat emulsion therapy for intentional sustained-release verapamil overdose. Resuscitation. 2009;80(5):591–3. doi: 10.1016/j.resuscitation.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Doepker B, Healy W, Cortez E, Adkins EJ. High-dose insulin and intravenous lipid emulsion therapy for cardiogenic shock induced by intentional calcium-channel blocker and beta-blocker overdose: a case series. J Emerg Med. 2014;46(4):486–90. doi: 10.1016/j.jemermed.2013.08.135. [DOI] [PubMed] [Google Scholar]
- 12.Cole JB, Stellpflug SJ, Engebretsen KM. Asystole immediately following intravenous fat emulsion for overdose. J Med Toxicol. 2014;10(3):307–10. doi: 10.1007/s13181-014-0382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine M, Curry SC, Padilla-Jones A, Ruha AM. Critical care management of verapamil and diltiazem overdose with a focus on vasopressors: a 25-year experience at a single center. Ann Emerg Med. 2013;62(3):252–8. doi: 10.1016/j.annemergmed.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Gosselin S, Morris M, Miller-Nesbitt A, Hoffman RS, Hayes BD, Turgeon AF, et al. Methodology for AACT evidence-based recommendations on the use of intravenous lipid emulsion therapy in poisoning. Clin Toxicol. 2015;53(6):557–64. doi: 10.3109/15563650.2015.1052498. [DOI] [PubMed] [Google Scholar]
- 15.Kryshtal D, Dawling S, Seger D, Knollmann BC. Intralipid as Novel Antidote for Verapamil Poisoning. Circulation. 2010;122(21).
- 16.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116(9):2510–20. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlotzer E, Kanning U. Elimination and tolerance of a new parenteral lipid emulsion (SMOF)—a double-blind cross-over study in healthy male volunteers. Ann Nutr Metab. 2004;48(4):263–8. doi: 10.1159/000080461. [DOI] [PubMed] [Google Scholar]
- 18.Nabauer M, Callewaert G, Cleemann L, Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989;244(4906):800–3. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- 19.St-Onge M, Dube PA, Gosselin S, Guimont C, Godwin J, Archambault PM, et al. Treatment for calcium channel blocker poisoning: a systematic review. Clin Toxicol. 2014;52(9):926–44. doi: 10.3109/15563650.2014.965827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinberg GL. Lipid emulsion infusion: resuscitation for local anesthetic and other drug overdose. Anesthesiology. 2012;117(1):180–7. doi: 10.1097/ALN.0b013e31825ad8de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fettiplace MR, Akpa BS, Ripper R, Zider B, Lang J, Rubinstein I, et al. Resuscitation with lipid emulsion: dose-dependent recovery from cardiac pharmacotoxicity requires a cardiotonic effect. Anesthesiology. 2014;120(4):915–25. doi: 10.1097/ALN.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo I, Akpa BS. Validity of the lipid sink as a mechanism for the reversal of local anesthetic systemic toxicity: a physiologically based pharmacokinetic model study. Anesthesiology. 2013;118(6):1350–61. doi: 10.1097/ALN.0b013e31828ce74d. [DOI] [PubMed] [Google Scholar]
- 23.Partownavid P, Umar S, Li J, Rahman S, Eghbali M. Fatty-acid oxidation and calcium homeostasis are involved in the rescue of bupivacaine-induced cardiotoxicity by lipid emulsion in rats. Crit Care Med. 2012;40(8):2431–7. doi: 10.1097/CCM.0b013e3182544f48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang JM, Xian H, Bacaner M. Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc Natl Acad Sci U S A. 1992;89(14):6452–6. doi: 10.1073/pnas.89.14.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sirianni AJ, Osterhoudt KC, Calello DP, Muller AA, Waterhouse MR, Goodkin MB, et al. Use of lipid emulsion in the resuscitation of a patient with prolonged cardiovascular collapse after overdose of bupropion and lamotrigine. Ann Emerg Med. 2008;51(4):412–5. doi: 10.1016/j.annemergmed.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Clark LA, Beyer J, Graudins A. An in vitro analysis of the effects of intravenous lipid emulsion on free and total local anaesthetic concentrations in human blood and plasma. Crit Care Res Pract. 2014;2014:236520. doi: 10.1155/2014/236520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perichon D, Turfus S, Gerostamoulos D, Graudins A. An assessment of the in vivo effects of intravenous lipid emulsion on blood drug concentration and haemodynamics following oro-gastric amitriptyline overdose. Clin Toxicol. 2013;51(4):208–15. doi: 10.3109/15563650.2013.778994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 43 kb)