Abstract
Cyanide toxicity is common after significant smoke inhalation. Two cases are presented that provide framework for the discussion of epidemiology, pathogenesis, presenting signs and symptoms, and treatment options of inhalational cyanide poisoning. An evidence-based algorithm is proposed that utilizes point-of-care testing to help physicians identify patients who benefit most from antidotal therapy.
Keywords: Cyanide, Hydroxocobalamin, Smoke inhalation, Fire, Sodium thiosulfate
Case Series
As the medical toxicologist for a tertiary care academic center, you are notified by EMS that your emergency department will be receiving several patients from a house fire with varying levels of injury/acuity. The most critically ill patient is an elderly woman found unresponsive in bed. She was intubated in the field and received a cyanide antidote by paramedics.
How Common Is Cyanide Toxicity After Smoke Inhalation?
Fire departments in the USA respond to approximately 1,389,500 fires annually [1]. While only 29.1 % of fires involve private homes, they represent 75.7 and 79.1 % of all fire-related fatalities and injuries, respectively [1]. Frequently, both building occupants and firefighters experience inhalation exposure to smoke. On average, there are 2580 deaths and 13,280 injuries per year associated with fires in the USA [1], most of them due to the inhalation of smoke [2]. While the composition of smoke is highly variable and depends on fuel available for pyrolysis, temperature, and oxygen availability [3], two common inhalational toxicants found in smoke are carbon monoxide and hydrogen cyanide. Both gases are cellular asphyxiants, respectively competing for oxygen binding sites on hemoglobin or inhibiting cytochrome c oxidase in mitochondrial oxidative phosphorylation. Experts suspect that the amount of hydrogen cyanide in fire-related smoke has increased over the past decades due to use of novel, synthetic building and furnishing materials [4]. Cyanide is detectable in the blood of almost 60 % of fire-related fatalities and 50 % of survivors of enclosed-space fires [3].
What Is the Pathophysiology of Cyanide Toxicity?
Hydrogen cyanide gas is a cellular asphyxiant which quickly dissociates into both hydrogen and cyanide ions when dissolved. The cyanide ion has a high affinity for metalloproteins and can affect up to 40 different enzyme systems [5]. When bound to the ferric iron (Fe3+) in cytochrome a3, a heme group within cytochrome c oxidase, non-competitive inhibition results in arrest of oxidative phosphorylation. Affected cells are subsequently forced into anaerobic metabolism. Organs highly susceptible to ATP depletion (brain, heart) are primarily affected [5]. From rodent studies, it is further believed that cyanide also alters neurotransmitter concentrations in the brain, mainly increasing glutamate and dopamine while decreasing γ-aminobutyric acid (GABA) [6].
Another asphyxiant produced in house fires is carbon monoxide. This toxicant tightly binds to hemoglobin, thereby inhibiting oxygen delivery to tissues. Like cyanide, it also binds to cytochrome a3 of cytochrome c oxidase and inhibits its function [7]. Not surprisingly, carbon monoxide and cyanide act synergistically to inhibit cellular respiration. Despite sublethal levels of either toxicant, fatalities have been reported in humans and demonstrated in animal trials [8, 9].
Case Series Continued
The critical patient is the first to arrive in the ED. Paramedics report that prior to intubation, she had a GCS of 6 (withdrawal to pain). Fluid resuscitation has been initiated in the field, and hydroxocobalamin has been infused en route to the hospital, but the patient has not improved.
What Are the Published Indications for Antidotal Therapy in Cyanide Toxicity from Smoke Inhalation?
The approach to resuscitation for critically ill patients is similar for the treatment of victims of smoke inhalation. After circulation, airway, and breathing have been assessed and stabilization has been initiated as appropriate, the pre-hospital provider must decide whether to administer an antidote for cyanide toxicity. Ideally, the treatment for cyanide toxicity is initiated in the field due to the time-sensitive nature of this condition.
In 2011, O'Brien et al. published an excellent review of cyanide toxicity, with a proposed algorithm on the empiric management of cyanide toxicity in the pre-hospital setting [10]. In their work, they divided patients into four categories: mild, moderate, and severe smoke inhalation, and cardiac arrest. As the focus of this algorithm is directed to pre-hospital providers, the decision to administer antidote is based on vital signs and mental status. Patients who show evidence of smoke inhalation (e.g., soot around mouth or nose), confusion, or abnormal vital signs are advocated to receive cyanide antidote. There is no inclusion of point-of-care testing parameters. While appropriate for EMS, physicians may have to apply stricter triage criteria in mass casualty events with limited supply of medication or decide which patients require additional antidotal therapy. In these instances, point-of-care evaluation of laboratory markers can guide clinicians in their decision-making.
We could not find a published consensus defining significant toxicity. For example, the National Health Service (NHS) in Great Britain recommends antidote administration only if the patient is unconscious, convulsing, or demonstrates a rapidly deteriorating clinical status [11], although this guideline was devised for victims of cyanide ingestion or cyanide gas inhalation. Given the complexity of toxic substances in smoke and their potential synergism [12], we recommend immediate antidotal treatment of patients who display evidence of cardiotoxicity (bradycardia or acute myocardial infarction) or significant respiratory distress (hyperpnea, tachypnea, or bradypnea) in addition to the indications put forth by NHS guidelines. Patients presenting in cardiac arrest should also be treated empirically [13, 14]. If none of the above signs and symptoms are present, antidotal treatment can likely be withheld for several minutes to gather additional data and provide optimal care (discussed below).
What Are the Available Cyanide Antidotes, and How Are They Administered?
Current antidotal approaches include the use of hydroxocobalamin (Cyanokit®, Merck Santé s.a.s., Semoy, France), sodium thiosulfate, or a combination of sodium nitrite and sodium thiosulfate (see Table 1). While recent animal data indicate efficacy of sodium thiosulfate alone in cyanide poisoning [15], human efficacy data are lacking, even in the form of case reports. After an extensive literature review and evaluation of more than 400 published and unpublished clinical cases, a Task Force of the European Centre for Ecotoxicology and Toxicology of Chemicals concluded that hydroxocobalamin is the preferred antidote in severe cyanide toxicity due to smoke inhalation, with sodium thiosulfate serving as a second-line antidote [16]. Other authors have supported this recommendation [17, 18], although it remains controversial [19]. Hydroxocobalamin is the most widely used antidote in the USA and Europe, and the use of other cyanide antidotes is decreasing [20].
Table 1.
Summary of available cyanide antidotes in the USA with mechanism of action and adverse effects
| Antidote | Mechanism | Adverse events/complications |
|---|---|---|
| Hydroxocobalamin | Binds cyanide to form cyanocobalamin (vit. B12), which is excreted renally | Hypertension [49], chromaturia [50–53], and acneiform rash. Interference with photometry-based laboratory tests (carboxyhemoglobin, methemoglobin, oxyhemoglobin) [54–56], aspartate aminotransferase, total bilirubin, creatinine, magnesium, and iron [57]. Blood leak alarm during dialysis [44, 58, 59]. Falsely elevated cyanide levels [60]. Allergic reactions [61–63] |
| Sodium nitrite | Induces methemoglobinemia. Cyanide binds ferric iron, forms cyanomethemoglobin [64] | Methemoglobinemia impairs tissue oxygenation [64–66, 22]. Hypotension, syncope, arrhythmias, seizures, acidosis, methemoglobin formation, and coma/death [67] |
| Sodium thiosulfate | Acts as a sulfur donor to cyanide to form thiocyanate, relatively non-toxic, renally excreted [68] | Hypotension, prolonged bleeding time, and persistent vomiting [67] |
Amyl nitrite and sodium nitrite are cyanide antidotes with high oxidative potential. These agents induce methemoglobinemia, which drives the conversion of cyanide to cyanomethemoglobin; additionally, these nitrites cause vasodilation and conversion to nitric oxide [21–23]. While an appropriate antidote for cyanide ingestion, nitrites can precipitate clinical deterioration in patients with suspected cyanide exposure from smoke inhalation. Inevitably, significant smoke inhalation produces varying degrees of carboxyhemoglobinemia. The creation of methemoglobinemia from nitrite administration further reduces oxygen carrying capacity in the smoke inhalation victim. Additionally, the nitrites can worsen hypotension typically seen in the setting of cyanide toxicity [22]. For these reasons, either sodium thiosulfate or hydroxocobalamin administration is preferred in smoke inhalation. We recommend avoiding amyl nitrite and sodium nitrite for the treatment of suspected cyanide toxicity after smoke inhalation.
Case Series Continued
After initial ED stabilization, the endotracheal tube is exchanged for a larger caliber tube to allow for anticipated bronchoscopy. Carbonaceous sputum is noted by the intubating resident. After intubation, she has good bilateral breath sounds and oxygen saturation of 95 %. The nurse reports the initial vital signs as BP 80/palp with a heart rate of 140 bpm. Additional IV access is obtained and fluid resuscitation continued.
The secondary survey reveals 2-mm pupils and soot in the nasal passages, but no apparent significant trauma or burns. Beside ultrasonography does not show any evidence of pneumothorax or free intraperitoneal fluid. A bedside glucose is 128 mg/dL. Current GCS is 3T. The patient remains hypotensive and tachycardic.
What Are Typical Clinical Findings in Cyanide Toxicity After Smoke Inhalation?
Cyanide toxicity is commonly associated with physiologic signs, symptoms, and abnormal biomarkers. Most data is from cyanide ingestion rather than inhalation, but likely the symptoms are similar. Physical exam findings can be subdivided into external, neurological, respiratory, cardiovascular, and gastrointestinal signs and symptoms.
External: Patients may have soot on their face, neck, oral cavity, or sputum [24].
Neurological: Patients initially experience headache, dizziness, and nausea that may rapidly progress to vomiting, confusion, and gradual decrease in the level of consciousness, which ultimately result in coma [4, 14, 24–27]. In a case series of 11 laboratory-confirmed cyanide toxicity cases (10 by ingestion, 1 inhalation), 18 % of patients had transient seizures [28]. In a retrospective review of 21 cases of cyanide poisoning (most by ingestion, most confirmed by serum cyanide levels), 71 % of patients with severe cyanide toxicity were unconscious at the time of presentation [25]. The mean Glasgow Coma Scale (GCS) score of patients with detectable serum cyanide levels after enclosed-space fire was 12.7 as compared to 14.7 in patients with no detectable cyanide [24].
Respiratory: Due to the cyanide-induced metabolic acidosis, patients present with dyspnea [4, 24, 25] and tachypnea [4, 24] in the early stages of toxicity. This will progress to bradypnea and apnea. In a retrospective analysis of 21 patients with confirmed cyanide toxicity, all patients who had initial respiratory symptoms (dyspnea, rapidly decreasing respiratory rate, pulmonary edema, or apnea) were severely toxic. Of the patients with only mild toxicity, none had respiratory complaints [25].
Cardiovascular: The initial tachycardia, likely the result of a catecholamine surge, progresses to bradycardia with subsequent hypotension [25–28]. EKG may show ST-segment changes, as well as a variety of arrhythmias [29]. Ultimately, respiratory and cardiac arrest ensues [4, 14, 24–27]. Yen et al. noted that cardiovascular symptoms indicated severe toxicity [25].
Gastrointestinal: Patients may develop gastrointestinal distress, nausea, and vomiting after inhalational cyanide exposure. GI symptoms were found in both mild and severe cyanide toxicity [25].
How Is the Diagnosis of Cyanide Toxicity Made?
While carbon monoxide poisoning is readily diagnosed by bedside CO-oximetry, the diagnosis of hydrogen cyanide poisoning must be made clinically, as results of serum or whole blood cyanide levels may not be available to the treating clinician for hours to days. Clinical markers must be used to determine the need for emergent antidotal therapy.
Patients with early cyanide toxicity display non-specific signs such as a Glasgow Coma Score of less than 15 or dilated or constricted pupils [24, 30]. A GCS of less than 10 is highly suggestive of cyanide toxicity fire victims without evidence of trauma [24]. However, the presence of ethanol or sedative/hypnotics may also contribute to depressed mental status in this population.
Patients who present with objective signs of significant smoke inhalation (soot around mouth/nose, elevated carboxyhemoglobin levels) are at risk of symptomatic cyanide toxicity, but may initially look stable due to hemodynamic compensation or benign appearance of early cyanide toxicity. In these instances, point-of-care evaluation of laboratory markers can guide the clinician on whether a patient requires an antidote.
Arteriolization of venous blood occurs when cells no longer extract oxygen. Central venous oxygen saturation ranges from 76.8 to 87.2 % in healthy individuals. Johnson et al. described a patient with cyanide toxicity with an initial peripheral venous oxygen saturation of 95.4 % which declined to 59.7 % after the administration of antidote [31]. Several others have reported similar findings in case studies, utilizing central venous oxygen saturation [4, 32, 33]. Johnson reported that a ScVO2 of >90 % should be considered evidence of significant cyanide toxicity in the right clinical setting. However, Yeh et al. report a case of significant ingested cyanide toxicity with normal ScVO2 [34]. The R.I.S.K. study was unable to detect a difference in venous oxygen pressures between patients with undetectable and elevated serum cyanide levels. A ScVO2 above 90 % after smoke inhalation is likely specific for significant cyanide toxicity, but animal studies do not support this test and its sensitivity is unknown [15].
Acidosis with hyperlactatemia may result from hypoxia before extrication, as well as from trauma, under-resuscitation, or carbon monoxide poisoning. However, when due to carbon monoxide, it is usually mild (median 2.3 mmol/L) [35, 36]. Baud et al. found in a retrospective case series of 11 patients with cyanide ingestion (smoke inhalation was excluded) that a serum lactate level >8 mmol/L was 94 % sensitive and 70 % specific for significant cyanide toxicity (>100 μg/dL). The specificity increases to 84 % in patients who have not received catecholamines. Several other authors have described lactate as a useful indicator for cyanide toxicity [4, 13, 37, 38]. While specificity is greatly increased when the diagnostic lactate threshold is raised to 10 mmol/L, we recommend a treatment threshold of 8 mmol/L for this treatment algorithm given the associated improvement in sensitivity.
Victims of smoke inhalation should be assessed for carbon monoxide poisoning. An observational study of over 100 patients with smoke inhalation found that patients with multiple areas affected by soot, especially sputum, had higher blood cyanide levels [24]. Many of the symptoms of early or mild cyanide toxicity are non-specific, and there is significant overlap with symptoms of carbon monoxide toxicity [13]. The presence of carboxyhemoglobinemia greater than 10 % is evidence of significant smoke inhalation, placing the patient at higher risk for having inhaled other toxins, including cyanide. Carbon monoxide levels and cyanide levels are correlated according to several case series of patients with smoke inhalation, and a retrospective analysis of 285 fire-related fatalities [3, 13, 24, 27, 39]. Both toxins are cellular asphyxiants, and several authors proposed that carbon monoxide toxicity is synergistic to that of cyanide; death may result despite sublethal levels of either toxin [8, 9, 12].
Case Series Continued
An arterial blood gas shows a pH of 6.9 with a lactate of 11.6 mmol/L. Given the patient’s history of smoke exposure, severely depressed mental status, and acidosis with hyperlactatemia, the decision is made to administer a second dose of hydroxocobalamin.
How Is Hydroxocobalamin Administered?
Each hydroxocobalamin kit (Cyanokit) contains two bottles of 2.5 g of lyophilized hydroxocobalamin, which must be diluted with 100 mL of normal saline. The recommended dose of 70 mg/kg leads to a typical, initial adult dose of 5 g administered over 15 min, followed by an additional 5 g over 15 min to 2 h if indicated by the patient’s clinical condition [40]. However, in cardiac arrest or severe hypotension, animal studies support a faster infusion rate over only 1–3 min without apparent adverse effects [41].
What Are the Adverse Effects Associated with Hydroxocobalamin Administration?
After hydroxocobalamin administration, common adverse events include hypertension, chromaturia, pink skin discoloration, and interference with common laboratory assays (including hemoglobin, methemoglobin, lactate, and carboxyhemoglobin) [42]. Hydroxocobalamin potentially interferes with dialysis. A falsely detected “blood leak” from the staining of the filtration membrane by hydroxocobalamin triggers an alarm in some dialysis machines that cannot be overridden. This interference has been shown to occur with Fresenius 2008K™ dialysis machine, but not with the Gambro Phoenix X36™. This poses a problem for patients who require hemodialysis for pH correction or are dialysis dependent at baseline. Therefore, it may be feasible to choose thiosulfate alone or with sodium nitrite if the dialysis machines at the institution treating the patient are prone to hydroxocobalamin-induced error [13].
Case Series Continued
After stabilization and transfer to the ICU of this critically ill patient, the doctor turns his attention to another family member who had been in the house at the time of the fire. This middle-aged man had been able to escape the house, but prior to arrival of the fire fighters, he attempted to extract family members from the burning building. However, the flames and heavy smoke forced him to retreat. He now presents for medical evaluation due headache and feeling dizzy. He suffered no significant burns or injuries. His bedside CO-oximetry reads 7 % carboxyhemoglobin.
In Which Patients with Evidence of Cyanide Toxicity Can Antidotal Therapy be Withheld?
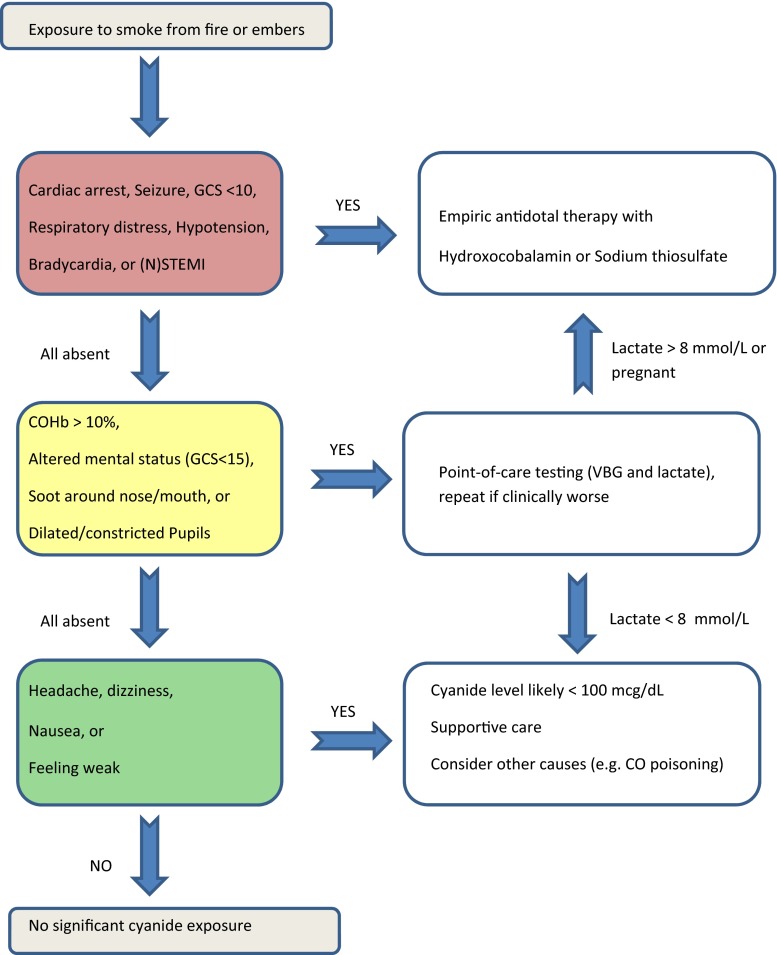
An overly permissive scheme for empiric cyanide antidote treatment after smoke inhalation can outstrip the available antidote supply in mass casualty scenarios and lead to unnecessary treatment and cost in low-risk exposures. An overly restrictive approach will delay the resuscitation of cyanide-poisoned patients. In our review, we found that cyanide toxicity produces a variety of clinical signs and symptoms as well as laboratory abnormalities that are rapidly detectable at the bedside. We developed an algorithm based on these findings (see Fig. 1), but the algorithm has not been validated by prospective clinical trials. The purpose of the algorithm is to guide focused diagnostic testing and antidotal therapy for smoke-related cyanide toxicity based on published clinical and preclinical reports. In our proposed algorithm, we assume that bedside providers have initiated standard resuscitative therapies including airway management, supplemental oxygen, and hemodynamic support (intravenous fluids and vasopressors) when indicated. We established a high-risk category based on the emergent need for (repeat) antidotal therapy, an intermediate category requiring further diagnostics to guide antidotal therapy, and a low-risk group where antidotal therapy is not required. Based on our review of the current literature, we subdivided presenting signs, symptoms, and vital signs into these same categories. Furthermore, our analysis found several laboratory tests that can be obtained at point-of-care, providing data to the treating physician within minutes.
Fig. 1.
Proposed diagnostic and treatment algorithm for inhalational cyanide toxicity after smoke exposure
Low-risk patients without indications for antidotal treatment represent the population that is most poorly described in published reports, perhaps due to underreporting of these cases. However, patients from this category are most likely to overwhelm available resources. For example, in March of 2006, 27 firefighters from Providence, RI, presented to the ED with concern for cyanide toxicity after one of their colleagues had suffered a ventricular fibrillation cardiac arrest and was found to have a whole blood cyanide level of 66 μg/dL. None of them had alarming symptoms or vital sign abnormalities. A consensus on required treatment is important for these low-risk patients.
Mild cyanide toxicity may lead to non-specific symptoms such as headache, dizziness, and nausea. However, smoke inhalation victims are also at risk for carbon monoxide poisoning, dehydration, stress, and other processes that lead to a similar clinical picture. Symptomatic treatment is important, but there is no current indication for extensive testing or antidotal treatment, and the clinician should maintain a high index of suspicion for other causes.
Literature regarding long-term outcome after cyanide toxicity is by its very nature self-selective as the toxicity had to be recognized. As such, undiagnosed patients with cyanide toxicity who did not receive antidotal treatment cannot not be assessed for long-term outcomes. While chronic low-dose cyanide exposure is known to lead to konzo (a neurologic disease associated with ingestion of cyanogens in cassava), no data exists on the outcome of acute low-dose untreated cyanide exposure from smoke.
Are There Any Special Populations That Require Consideration?
In pregnant patients, the fetus is highly susceptible to cyanide toxicity. Fetal carboxyhemoglobin levels are approximately 10–15 percentage points above maternal levels [43] and may potentiate the effects of cyanide. In addition, the fetus is already in a relatively hypoxic environment at baseline, with a left-ventricular oxygen saturation of only 65 % under normal physiologic conditions [44]. Hydroxocobalamin is a pregnancy category C drug; animal studies have shown soft tissue abnormalities at therapeutic doses when given during organogenesis and increased fetal death at supratherapeutic doses [40]. As such, we recommend that the treatment threshold for pregnant women outside of the organogenic period be lower. Curry et al. showed in an animal study that sodium thiosulfate provides fetal protection of cyanide toxicity during maternal sodium nitroprusside infusion [45]. Sodium thiosulfate does not cross the placenta and likely creates a cyanide gradient toward the maternal circulation [46]. Therefore, we recommend thiosulfate as an antidote during early pregnancy instead of hydroxocobalamin. However, if thiosulfate is not immediately available, the benefit of treating a pregnant cyanide-poisoned patient with hydroxocobalamin outweighs its risks.
Conclusion
Cyanide toxicity is a common but often underappreciated effect of smoke inhalation. No real-time confirmatory tests are available, but an array of clinical markers allows clinicians to gauge the likelihood and severity of cyanide toxicity. Patients with severe toxicity and significant laboratory abnormalities require emergent administration of antidote. A select group of patients with evidence of mild cyanide toxicity likely does not require antidote if demand outstrips supply.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Sources of Funding
There was no funding for this project.
References
- 1.Residential and nonresidential building fire estimates. FEMA, Emmitsburg, MD 21727. 2014. http://www.usfa.fema.gov/statistics/estimates/index.shtm. Accessed 9/18 2013.
- 2.Einhorn IN. Physiological and toxicological aspects of smoke produced during the combustion of polymeric materials. Environ Health Perspect. 1975;11:163–89. doi: 10.1289/ehp.7511163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabowska T, Skowronek R, Nowicka J, Sybirska H. Prevalence of hydrogen cyanide and carboxyhaemoglobin in victims of smoke inhalation during enclosed-space fires: a combined toxicological risk. Clin Toxicol (Phila) 2012;50(8):759–63. doi: 10.3109/15563650.2012.714470. [DOI] [PubMed] [Google Scholar]
- 4.Borron SW. Recognition and treatment of acute cyanide poisoning. J Emerg Nurs. 2006;32(4 Suppl):S12–8. doi: 10.1016/j.jen.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Anseeuw K, Delvau N, Burillo-Putze G, De Iaco F, Geldner G, Holmstrom P, et al. Cyanide poisoning by fire smoke inhalation: a European expert consensus. Eur J Emerg Med. 2013;20(1):2–9. doi: 10.1097/MEJ.0b013e328357170b. [DOI] [PubMed] [Google Scholar]
- 6.Persson SA, Cassel G, Sellstrom A. Acute cyanide intoxication and central transmitter systems. Fundam Appl Toxicol. 1985;5(6 Pt 2):S150–9. doi: 10.1016/0272-0590(85)90124-1. [DOI] [PubMed] [Google Scholar]
- 7.Nelson L. Goldfrank’s toxicologic emergencies. 9. New York: McGraw Hill; 2010. [Google Scholar]
- 8.Moore SJ, Ho IK, Hume AS. Severe hypoxia produced by concomitant intoxication with sublethal doses of carbon monoxide and cyanide. Toxicol Appl Pharmacol. 1991;109(3):412–20. doi: 10.1016/0041-008X(91)90004-X. [DOI] [PubMed] [Google Scholar]
- 9.Norris JC, Moore SJ, Hume AS. Synergistic lethality induced by the combination of carbon monoxide and cyanide. Toxicology. 1986;40(2):121–9. doi: 10.1016/0300-483X(86)90073-9. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien DJ, Walsh DW, Terriff CM, Hall AH. Empiric management of cyanide toxicity associated with smoke inhalation. Prehosp Disaster Med. 2011;26(5):374–82. doi: 10.1017/S1049023X11006625. [DOI] [PubMed] [Google Scholar]
- 11.Department of Health. Use of antidotes in cyanide poisoning. 2009. http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/browsable/DH_4875689 Accessed 9/23 2013.
- 12.Eckstein M, Maniscalco PM. Focus on smoke inhalation—the most common cause of acute cyanide poisoning. Prehosp Disaster Med. 2006;21(2):s49–55. doi: 10.1017/s1049023x00015909. [DOI] [PubMed] [Google Scholar]
- 13.Baud FJ, Barriot P, Toffis V, Riou B, Vicaut E, Lecarpentier Y, et al. Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med. 1991;325(25):1761–6. doi: 10.1056/NEJM199112193252502. [DOI] [PubMed] [Google Scholar]
- 14.Borron SW, Baud FJ, Barriot P, Imbert M, Bismuth C. Prospective study of hydroxocobalamin for acute cyanide poisoning in smoke inhalation. Ann Emerg Med. 2007;49(6):794–801. doi: 10.1016/j.annemergmed.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 15.Renard C, Borron SW, Renaudeau C, Baud FJ. Sodium thiosulfate for acute cyanide poisoning: study in a rat model. Ann Pharm Fr. 2005;63(2):154–61. doi: 10.1016/S0003-4509(05)82266-7. [DOI] [PubMed] [Google Scholar]
- 16.Baud F. Efficacy and safety of antidotes for acute poisoning by cyanides. Brussels, Belgium2013. Report No.: Technical Report No. 121.
- 17.Fortin JL, Ruttiman M, Domanski L, Kowalski JJ. Hydroxocobalamin: treatment for smoke inhalation-associated cyanide poisoning. Meeting the needs of fire victims. Jems. 2004;29(suppl):18–21. [PubMed] [Google Scholar]
- 18.Hall AH, Dart R, Bogdan G. Sodium thiosulfate or hydroxocobalamin for the empiric treatment of cyanide poisoning? Ann Emerg Med. 2007;49(6):806–13. doi: 10.1016/j.annemergmed.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Kerns W, 2nd, Beuhler M, Tomaszewski C. Hydroxocobalamin versus thiosulfate for cyanide poisoning. Ann Emerg Med. 2008;51(3):338–9. doi: 10.1016/j.annemergmed.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 20.Streitz MJ, Bebarta VS, Borys DJ, Morgan DL. Patterns of cyanide antidote use since regulatory approval of hydroxocobalamin in the United States. Am J Ther. 2014;21(4):244–9. doi: 10.1097/MJT.0b013e31824ea656. [DOI] [PubMed] [Google Scholar]
- 21.Kirk MA, Gerace R, Kulig KW. Cyanide and methemoglobin kinetics in smoke inhalation victims treated with the cyanide antidote kit. Ann Emerg Med. 1993;22(9):1413–8. doi: 10.1016/S0196-0644(05)81988-2. [DOI] [PubMed] [Google Scholar]
- 22.Hall AH, Kulig KW, Rumack BH. Suspected cyanide poisoning in smoke inhalation: complications of sodium nitrite therapy. J Toxicol Clin Exp. 1989;9(1):3–9. [PubMed] [Google Scholar]
- 23.Modin A, Bjorne H, Herulf M, Alving K, Weitzberg E, Lundberg JO. Nitrite-derived nitric oxide: a possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol Scand. 2001;171(1):9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 24.Geldner G, Koch EM, Gottwald-Hostalek U, Baud F, Burillo G, Fauville JP, et al. Report on a study of fires with smoke gas development: determination of blood cyanide levels, clinical signs and laboratory values in victims. Anaesthesist. 2013;62(8):609–16. doi: 10.1007/s00101-013-2209-3. [DOI] [PubMed] [Google Scholar]
- 25.Yen D, Tsai J, Wang LM, Kao WF, Hu SC, Lee CH, et al. The clinical experience of acute cyanide poisoning. Am J Emerg Med. 1995;13(5):524–8. doi: 10.1016/0735-6757(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 26.Baud FJ. Cyanide: critical issues in diagnosis and treatment. Hum Exp Toxicol. 2007;26(3):191–201. doi: 10.1177/0960327107070566. [DOI] [PubMed] [Google Scholar]
- 27.Fortin JL, Giocanti JP, Ruttimann M, Kowalski JJ. Prehospital administration of hydroxocobalamin for smoke inhalation-associated cyanide poisoning: 8 years of experience in the Paris Fire Brigade. Clin Toxicol (Phila) 2006;44(Suppl 1):37–44. doi: 10.1080/15563650600811870. [DOI] [PubMed] [Google Scholar]
- 28.Baud FJ, Borron SW, Megarbane B, Trout H, Lapostolle F, Vicaut E, et al. Value of lactic acidosis in the assessment of the severity of acute cyanide poisoning. Crit Care Med. 2002;30(9):2044–50. doi: 10.1097/00003246-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Fortin JL, Desmettre T, Manzon C, Judic-Peureux V, Peugeot-Mortier C, Giocanti JP, et al. Cyanide poisoning and cardiac disorders: 161 cases. J Emerg Med. 2010;38(4):467–76. doi: 10.1016/j.jemermed.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien B, Quigg C, Leong T. Severe cyanide toxicity from ‘vitamin supplements’. Eur J Emerg Med. 2005;12(5):257–8. doi: 10.1097/00063110-200510000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Johnson RP, Mellors JW. Arteriolization of venous blood gases: a clue to the diagnosis of cyanide poisoning. J Emerg Med. 1988;6(5):401–4. doi: 10.1016/0736-4679(88)90014-5. [DOI] [PubMed] [Google Scholar]
- 32.Peddy SB, Rigby MR, Shaffner DH. Acute cyanide poisoning. Pediatr Crit Care Med. 2006;7(1):79–82. doi: 10.1097/01.PCC.0000192508.92993.D1. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Bermudez R, Maestre-Romero A, Goni-Belzunegui MV, Bautista-Lorite A, Arenas-Cabrera C. Venous blood arteriolization and multiple organ failure after cyanide poisoning. Intensive Care Med. 1997;23(12):1286. doi: 10.1007/s001340050502. [DOI] [PubMed] [Google Scholar]
- 34.Yeh MM, Becker CE, Arieff AI. Is measurement of venous oxygen saturation useful in the diagnosis of cyanide poisoning? Am J Med. 1992;93(5):582–3. doi: 10.1016/0002-9343(92)90590-8. [DOI] [PubMed] [Google Scholar]
- 35.Baud FJ. Acute poisoning with carbon monoxide (CO) and cyanide (CN) Ther Umsch. 2009;66(5):387–97. doi: 10.1024/0040-5930.66.5.387. [DOI] [PubMed] [Google Scholar]
- 36.Benaissa ML, Megarbane B, Borron SW, Baud FJ. Is elevated plasma lactate a useful marker in the evaluation of pure carbon monoxide poisoning? Intensive Care Med. 2003;29(8):1372–5. doi: 10.1007/s00134-003-1866-0. [DOI] [PubMed] [Google Scholar]
- 37.Megarbane B, Delahaye A, Goldgran-Toledano D, Baud FJ. Antidotal treatment of cyanide poisoning. J Chin Med Assoc. 2003;66(4):193–203. [PubMed] [Google Scholar]
- 38.Baud FJ, Borron SW, Bavoux E, Astier A, Hoffman JR. Relation between plasma lactate and blood cyanide concentrations in acute cyanide poisoning. Bmj. 1996;312(7022):26–7. doi: 10.1136/bmj.312.7022.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeoh MJ, Braitberg G. Carbon monoxide and cyanide poisoning in fire related deaths in Victoria. Australia. J Toxicol Clin Toxicol. 2004;42(6):855–63. doi: 10.1081/CLT-200035211. [DOI] [PubMed] [Google Scholar]
- 40.Pfizer. Cyanokit product insert. http://www.cyanokit.com/pdf/CYANOKIT_Perscribing_Information_5-06-2011.pdf. Accessed 9/28 2013.
- 41.Bebarta VS, Tanen DA, Lairet J, Dixon PS, Valtier S, Bush A. Hydroxocobalamin and sodium thiosulfate versus sodium nitrite and sodium thiosulfate in the treatment of acute cyanide toxicity in a swine (Sus scrofa) model. Ann Emerg Med. 2010;55(4):345–51. doi: 10.1016/j.annemergmed.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 42.Avila J, Prasad D, Weisberg LS, Kasama R. Pseudo-blood leak? A hemodialysis mystery. Clin Nephrol. 2013;79(4):323–5. doi: 10.5414/CN107447. [DOI] [PubMed] [Google Scholar]
- 43.Longo LD. The biological effects of carbon monoxide on the pregnant woman, fetus, and newborn infant. Am J Obstet Gynecol. 1977;129(1):69–103. doi: 10.1016/0002-9378(77)90824-9. [DOI] [PubMed] [Google Scholar]
- 44.Murphy PJ. The fetal circulation. Contin Educ Anaesth Crit Care Pain. 2005;5(4):107–12. doi: 10.1093/bjaceaccp/mki030. [DOI] [Google Scholar]
- 45.Curry SC, Carlton MW, Raschke RA. Prevention of fetal and maternal cyanide toxicity from nitroprusside with coinfusion of sodium thiosulfate in gravid ewes. Anesth Analg. 1997;84(5):1121–6. doi: 10.1097/00000539-199705000-00031. [DOI] [PubMed] [Google Scholar]
- 46.Graeme KA, Curry SC, Bikin DS, Lo Vecchio FA, Brandon TA. The lack of transplacental movement of the cyanide antidote thiosulfate in gravid ewes. Anesth Analg. 1999;89(6):1448–52. doi: 10.1097/00000539-199912000-00023. [DOI] [PubMed] [Google Scholar]