Abstract
Objectives:
To investigated serum cortisol and serum dehydroepiandrosterone-sulphate (DHEA-S) levels between fibromyalgia (FMS) patients and a control group, and the effect of balneotherapy (BT) on these hormones.
Methods:
Seventy-two patients with FMS and 39 healthy volunteers were included in the study. This prospective and cross-sectional study was carried out in the Medical Faculty, Physical Medicine and Rehabilitation Clinic, Cumhuriyet University, Cumhuriyet, Turkey between June 2012 and June 2013. Patients were divided into 2 groups. There were 40 patients in the first group, consisting of BT and physical therapy (PT) administered patients. There were 32 FMS patients in the second group who were only administered PT. Thirty-nine healthy volunteers were enrolled as a control group.
Result:
Cortisol was observed to be lower in FMS patients compared with the controls (10.10±4.08 μg/dL and 11.78±3.6 μg/dL; p=0.033). Serum DHEA-S level was observed to be lower in FMS patients compared with the controls (89.93±53.96 μg/dL and 143.15±107.92 μg/dL; p=0.015). Average serum cortisol levels of patients receiving BT were determined to be 9.95±3.20 μg/dL before treatment and 9.06±3.77μg/dL after treatment; while average serum DHEA-S levels were 77.60±48.05 μg/dL before treatment, and 76.84±48.71 μg/dL after treatment. No significant changes were determined in serum cortisol and DHEA-S levels when measured again after BT and PT.
Conclusion:
Low levels of serum cortisol and DHEA-S were suggested to be associated with the physiopathology of FMS.
Fibromyalgia syndrome (FMS) is a musculoskeletal system disorder accompanied with conditions such as diffuse body pain, increased fatigue, sensitivity in specific anatomic areas, and sleeping disorder.1,2 Physical therapy (PT) modalities used in FMS treatment are superficial heat, ultrasound, electrotherapy, hydrotherapy, and biofeedback. Many studies regarding the efficiency of hydrotherapy and balneotherapy (BT) were performed in recent years.3 Physical activities of patients with FMS are restricted; thus, their aerobic performance capacities decreased. Exercise programs have positive effects on FMS through the alleviation of FMS symptoms. Serum beta endorphin level and immunoreactivity increase after exercise programs, revealing positive psychological effects (decreased dysphoria), better sleep quality, and decreased pain sensitivity.4 Etiopathogenesis of FMS has been investigated for a long time; however, no single factor has been determined to be the reason neither in fibromyalgia, or other disorders causing chronic pain. In many stress-related cases such as fibromyalgia, characterized with decreased response in various levels of hypothalamic pituitary adrenal (HPA) axis were identified.1,3,5 By affecting the electrical activity of neurons, cortisol regulates stimulability, behaviors and mood of individuals. Dehydroepiandesterone-sulphate (DHEA-S) is a neuroactive steroid interacting with N-methyl-D-aspartate (NMDA) and gamma-aminobutyric acid (GABA) receptors. However, DHEA-S binds to sigma receptors and these receptors regulate neuronal excitability and plasticity since they possess ion channel characteristics.6 The ways in which serum cortisol and serum DHEA-S levels change in FMS have not been fully clarified. In studies performed for this purpose, different results were obtained, and the literature contains no information regarding changes in serum DHEA-S levels during treatment processes.7,8 If the pattern changes, these hormones in disease and treatment processes could be elucidated, they could possibly be used as biological markers to both clarify the pathophysiology and estimate disease progression, which are substantial in clinical terms. The purpose of this study is to investigate cortisol and serum DHEA-S levels in FMS patients and determine whether BT and PT have an effect on these hormones.
Methods
Ethical committee approval was obtained from the Ethics Committee of the Medical Faculty of Cumhuriyet University, and the study was performed in accordance with the Declaration of Helsinki. Participants were informed regarding the subject before enrolment into the study, and necessary consent documents were obtained.
Seventy-two patients diagnosed with primary FMS criteria according to American College of Rheumatology (ACR) (1990)9 and 39 healthy volunteers were included in the study. This prospective and cross-sectional study, was carried out in the Medical Faculty, Physical Medicine and Rehabilitation Clinic, Cumhuriyet University, Cumhuriyet, Turkey, between June 2012 and June 2013.
The inclusion criteria were patients aged between 18-65 years, having obtained a primary FMS diagnosis according to the ACR (1990) diagnosis criteria, agreeing to participate in the study, receiving a stable drug dose, or no drug treatment for the last 2 weeks, or prior to the study, and no known psychiatric, or metabolic disorders. The control group consisted of volunteers who had applied to the general internal medicine outpatient clinic and were determined to be healthy. The control group’s criteria included 18-65 years old, agreeing to participate in the study, and no known psychiatric, or metabolic disorders. Both the patient and control groups were evaluated by a psychiatrist before inclusion. Patients with major psychiatric disorders were not included in the study.
Routinely, complete blood count, erythrocyte sedimentation rate, C-reactive protein, liver and renal function tests, blood sugar, electrolytes, thyroid function tests, and complete urine urinalysis were performed in all cases. If any of these tests had anomalies, those cases were excluded from the study. In addition, new or past history of psychiatric disorders that may affect serum cortisol and serum DHEA-S levels (major depression, alcohol addiction, substance abuse, schizophrenia or paranoid disorders, personality disorder, somatoform disorder, immunological problem, endocrin, neurological, inflammatory or clinically significant chronic disorders such as diabetes mellitus, rheumatoid arthritis, inflammatory bowel disease and organic brain diseases), and pregnant cases were excluded from the study. It was observed that all cases that were enrolled in the study did not experience infection, inflammation, or allergic reactions, and did not use preparations known to affect immune and endocrine system for at least 2 weeks before.
Seventy-two patients diagnosed with FMS and 39 healthy volunteers were enrolled in the study. Patients were divided into 2 groups according to treatment methods they received. There were 40 patients in the first group, consisting of BT, PT, and EP. There were 32 FMS patients in the second group who were administered PT and EP. Both groups were treated with 20-minute hot pack, 5 days a week for 3 weeks for a total of 15 sessions, transcutaneous electrical nerve stimulation (TENS), ultrasound (US), and EP; while only the first group was treated with BT. We performed TENS therapy using a Fizyotens 4000 (Fizyomed Medical Devices Ltd. Sti., Ankara, Turkey). A total of 4 carbon-silicon composite electrodes (5x5 cm in size) were placed over the region of the pain. The current frequency was set at 50-100 Hz, the current time was set at 60 microseconds, and the amplitude was calculated to avoid discomfort, and to remain under the motor threshold. The TENS therapy was performed in both groups for 20 minutes with the conventional method. The exercise program consisted of flexibility (trunk, hips, ankle, shoulders, and wrist) movement, stretching, and strengthening exercises were performed in 10 minute period (deltoid, latissimus dorsi, pectoralis major, abdominalis, gluteus and biceps muscle groups). The stretching exercises included the following muscle groups (1x10 repetitions for each of the neck, shoulder, dorsal, lumbar, gluteal, thigh, and cruris muscle groups). We performed therapeutic US using a ULS 1000 (ZMI Electronics Ltd., Kaohsiung, Taiwan). Ultrasound gel was applied during the examination. Both groups were administered a dose of 1.5 Watt/cm2 at a frequency of 1 MHz for 6 minutes in a mode of continuous and circular motion. Balneotherapy was delivered in the form of daily 20-min in warm water on 15 consecutive days. During sessions, patients reclined and relaxed in a therapeutic pool, no specific treatment was delivered. Thermal water, that is 40°C and rich in terms of Calcium (Ca) and bicarbonate (HCO3), was used for BT. Blood samples were taken from the patients before and after treatment, and scales were applied. The content of the mineral substances of the thermal water used for BT were sodium: 337 mg/L, chloride: 257 mg/L, Ca: 655 mg/L, sulphate: 65 mg/L, magnesium: 104 mg/L, fluoride: 2.24 mg/L, HCO3: 2003 mg/L, and silicate: 32 mg/L.
Materials used in the study. Fibromyalgia Impact Questionnaire (FIQ)
In order to assess the functional status of patients, progression, and results of the disease, an FIQ consisting of 20 questions was used to evaluate the state of physical function, job status, depression, anxiety, sleep, pain, stiffness, fatigue, and wellness.10
Biochemical analyses
In order to detect cortisol and DHEA-S levels, blood samples were taken from patient and control groups compliant with the study criteria, between the hours of 08:00-09:00 in the morning. Two blood samples were taken from the patients before and after treatment. Blood samples were centrifuged and serum was stored at -70o after being separated. Serum cortisol and DHEA-S levels were measured in an Axsym-Abbott® (Abbott Laboratories, Abbott Park, IL, USA) device with Microparticle Enzyme Immunoassay method in the Laboratory Department of Biochemistry, Faculty of Medicine, Cumhuriyet University.
Statistical methods
Data was loaded into the Statistical Package for Social Sciences version 14.0 program (SPSS Inc., Chicago, IL, USA). Data obtained from groups was presented as mean±standard deviation. Chi-square test was used for assessing sociodemographic differences in groups, significance test for 2 proportions (difference in proportions test) was used for assessing inter-group differences for parametric variables, and Man Whitney-U test was used for non-parametric variables. Also, Pearson and Spearman correlation test was used for parameter-assessment for correlation analysis. Receiver-operating characteristic analysis was performed for evaluating sensitivity of prediction of serum cortisol and serum DHEA-S levels in fibromyalgia diagnosis, and for measuring specificity. Our data is presented in tables as arithmetic average ± standard deviation, number and percentage of subjects, and level of significance was considered as 0.05.
Results
Sociodemographic characteristics of samples
A total of 72 patients and 39 controls participated in the study. The average age in the patient group was 45.16±11.5 years, while it was 43.07±7.43 years in the control group, and intergroup difference was not found to be statistically significant with regard to age (t=1.02; p=0.309). Sixty-eight subjects in the patient group (94.4%) were female, 4 were (5.6%) were male, while 35 subjects in the control group (89.7%) were female and 4 (10.3%) were male. Sociodemographic characteristics of patient and control groups are presented in Table 1.
Table 1.
Sociodemographic characteristics of FMS patient and control groups.
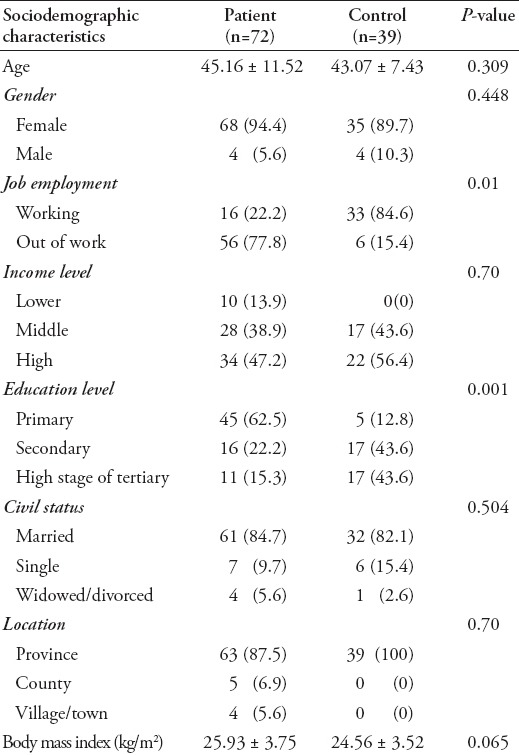
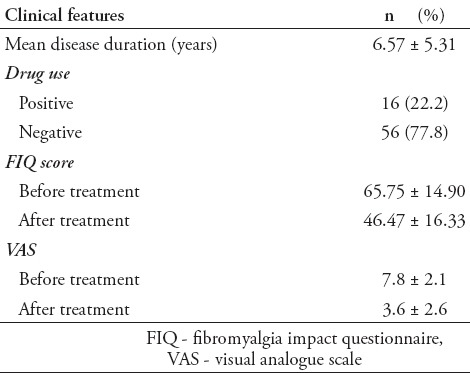
Approximately 22.2% of subjects in the patient group were receiving drugs. While there was no psychiatric disease family history in the control group, 8.3% from the patient group had a family history of psychiatric disease. The average disease period was determined as 6.57±5.31 years. When measurements before and after treatment were compared for the FIQ values in the patient group, the difference was determined to be statistically significant (p=0.001). Clinical characteristics of patients are presented in Table 2.
Table 2.
Clinical features of 72 patients with fibromyalgia syndrome in the Medical Faculty, Physical Medicine and Rehabilitation Clinic, Cumhuriyet University, Cumhuriyet, Turkey.
Comparison of serum cortisol measurements in patient and control groups
Average serum cortisol levels were 10.10±4.08 µg/dL for the patient group before treatment, while serum cortisol levels were determined to be 11.78±3.6 µg/dL for the control group. Serum cortisol levels of the patient group measured before treatment were determined to be lower than the control group at a statistically significantly level (p=0.033). When serum cortisol levels fell <9.82 in receiver-operating characteristic (ROC) curve analysis, it was determined that cortisol may be a predictor for fibromyalgia diagnosis with 51.4% sensitivity and 74.4% specificity (p=0.017) (area under curve: 0.637, 95% confidence interval (CI): 0.541-0.726) (Figure 1). When the effect of treatment on serum cortisol levels are evaluated; serum cortisol levels of all patients measured before treatment were 10.10±4.08 µg/dL, and serum cortisol levels after treatment were 9.16±4.09 µg/dL. The difference between serum cortisol levels measured before and after treatment was not statistically significant (p=0.683). Serum cortisol levels of groups according to the treatment method administered to patients (PT group, BT+PT group) are presented in Table 3.
Figure 1.
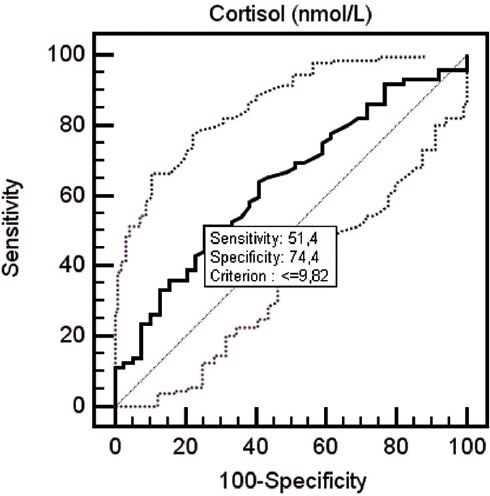
Receiver operating characteristic curve showing the performance of cortisol in patients with fibromyalgia.
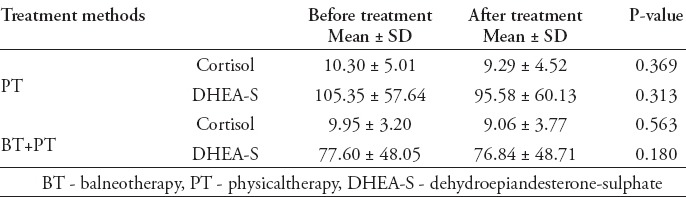
Table 3.
Serum cortisol and serum DHEA-S levels of groups according to treatment method administered to patients.
The serum DHEA-S levels of the patient and control groups
Serum DHEA-S level of the patient group was determined to be 89.93±53.96 µg/dL, and of the control group was 143.15±107.92 µg/dL. The serum DHEA-S level of the patient group was determined to be lower than the control group at a statistically significantly level (p=0.015). When serum DHEA-S level decreased <123 in ROC curve analysis, it was determined that DHEA-S may be a predictor for fibromyalgia diagnosis with 79.2% sensitivity and 56.4% specificity (p=0.014) (area under curve: 0.641, 95% CI: 0.545-0.730) (Figure 2). When the effects of treatment on serum DHEA-S levels were evaluated; the serum DHEA-S levels of all patients measured before treatment was 89.93±53.96 µg/dL, and serum DHEA-S levels after treatment was 85.61±54.58 µg/dL. The difference between serum DHEA-S levels measured before and after treatment was not statistically significant (p=0.769). The serum DHEA-S levels of groups according to treatment method administered to patients (PT group, BT+PT group) are presented in Table 3.
Figure 2.
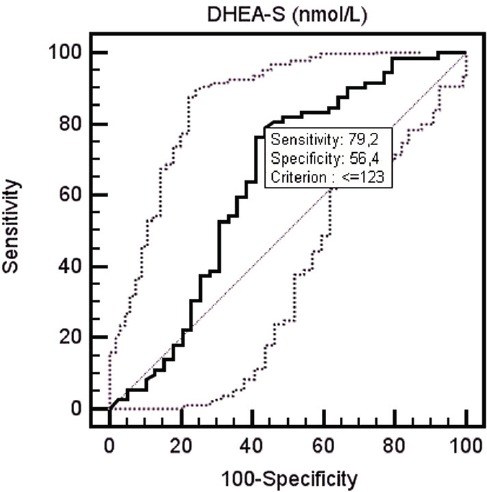
Receiver operating characteristic curve showing the performance of dehydroepiandesterone-sulphate in patients with fibromyalgia.
No significant correlation was determined between disease period, sensitive spot number, FIQ score, serum cortisol, and serum DHEA-S values.
Relation between clinical characteristics and serum cortisol and DHEA-S
A negative (r=-0.01) correlation coefficient was determined between FIQ and cortisol results before treatment, also a negative (r=-0.03) correlation coefficient was determined between FIQ and cortisol results after treatment. These correlation coefficients were not statistically significant (p=0.244). No statistically significant correlation was determined between disease period and sensitive spot number, and between serum cortisol and serum DHEA-S values (p=0.327).
Discussion
Anomalies in the HPA axis may play a central role in the etiology and pathogenesis of many diseases. Studies performed recently have shown that FMS may be related to hypo-functional stress systems and particularly the autonomous nervous system and HPA axis.11,12 In the study of Calis et al,11 performed with 22 FMS patients and 15 controls, it was proven by CT that long term adrenocorticotropic hormone (ACTH)/corticotropin releasing hormone (CRH) deficiency causes reduced adrenal gland size in FMS with CT. Similarly, cortisol levels were determined to be reduced compared with the controls study of McLean et al.12 In the 8-week yoga study of Curtis et al13 on 22 FMS patients, reduced patient pain, increased disease awareness, recovered clinic condition, and significant recovery in cortisol levels compared with the levels before treatment were observed at the end of treatment. Genc et al5 reported that a 6-week EP can influence symptoms and affect the HPA axis hormones. Similar to other studies,11 serum cortisol levels were determined to be lower than healthy controls in our study. While the cause and effect relation cannot be expressed precisely due to the cross-sectional design of our study, our findings and data obtained from previous studies and reduced levels of serum cortisol were suggested to be associated with the physiopathology of fibromyalgia.
It was demonstrated that chronic pain stimulants in FMS patients cause neuroplasticity and dysfunction in pain generating sections of the central nervous system, and the brain’s response to pain was different compared with the healthy controls.14 This was due to an active hormone interacting with DHEA-S, NMDA, and GABA receptors, which helped regulate neuronal excitability and plasticity, and also acted as a neuroprotective agent with those properties.15 A study by De Abreu Freitas et al7 determined that serum DHEA-S levels of FMS patients were lower compared with the healthy controls, and serum DHEA-S levels were negatively correlated with pain. It was also reported that antianabolic effects, such as pain and fatigue, in FMS patients may be associated with decreases in serum DHEA-S levels. Dessein et al’s study16 found that serum DHEA-S levels of fibromyalgia patients were significantly lower than the healthy controls. Similarly, our study determined that serum DHEA-S levels of fibromyalgia patients were significantly lower than in the healthy controls. Kuratsune et al’s17 study on patients with chronic fatigue syndrome found serum DHEA-S levels to be low in those patients. It was reported that both physical and psychological symptoms in those patients may have had an association with DHEA-S deficiency may be associated with DHEA-S deficiency. Another study,18 determined that DHEA-S prevents the neurotoxic effect of corticosterone on hippocampus cells. In a study of Finckh et al,18 which investigated lack of adrenal response on 52 FMS patients, daily 50 mg DHEA treatment, or placebo was administered for 4 months. According to the information obtained from the literature, no studies have examined the effect of BT on serum DHEA-S levels. While serum DHEA-S levels measured before treatment were low, after DHEA treatment, the DHEA-S levels were significantly higher. Our findings and data from previous studies11,12 suggest that DHEA-S may play a part in the physiopathology of fibromyalgia. In our study, fibromyalgia patients were divided into 2 groups in terms of administered treatment: the group receiving BT and PT compared with the group receiving only PT. The effects of therapy administered to the 2 groups on serum cortisol and serum DHEA-S levels were investigated, and it was observed that therapy had no significant effect on the levels of these hormones. In a study by Kuczera et al,19 which investigated the effect of spa therapy on serum ACTH and cortisol levels, serum levels of those hormones had increased. A study by Bellometti et al,20 which was performed on fibromyalgia patients with mud packs and antidepressant trazodone, showed that this therapy may affect the HPA axis and stimulate ACTH, cortisol, and beta-endorphine levels.21 There is limited data in the literature on the effect of BT on serum cortisol levels. In a study by Aktaş et al,22 when fibromyalgia patients were administered BT and an insignificant increase was reported in serum cortisol levels after treatment, it was suggested that increases in serum cortisol levels may be the indication of response. Our study determined that serum cortisol levels did not change significantly with treatment, and there was an insignificant decrease in serum cortisol levels. The common reasons for the presentation of our patients to the clinic are acute pain conditions, notably, cortisol is a hormone that increases in acute pain conditions. Increases in serum cortisol levels due to acute stress may be reduced after BT and PT. A study by Cleare et al21 stated that serum DHEA-S levels of patients with chronic fatigue syndrome were lower than in the control group before treatment, and serum DHEA-S levels were significantly decreased after treatment. It was also suggested that decreases in serum DHEA-S levels may be an indicator of recovery. In accordance with the literature, our study also found that BT and PT cause an insignificant decrease in serum DHEA-S levels.
Study limitations
There are some limitations to our study. Firstly, it can be said that study pattern was cross-sectional and that serum cortisol and serum DHEA-S levels were not observed over the long term may have affected the findings. Forming a cause and effect relation is more difficult in cross-sectional studies. The American College of Sports Medicine suggest13 that the chronicle effects of physical exercise (including many hormonal changes) appear after 8 weeks of training. Perhaps, the intensity of exercises or duration of BT applied in this study did not have sufficient time to promote alterations in DHEA-S levels. We suggest that long term follow-up of patients and numerous blood samples may be beneficial in future studies. Cortisol release shows a specific diurnal change. Secretion rate of cortisol is faster in the mornings while it is lower late in the evenings. We have taken the blood samples from all groups in the same time period, between hours 08:00-09:00 a.m. in the morning, in order to subject our study to minimal effect from the diurnal rhythm of cortisol Considering that the secretion of cortisol changes within the day, several blood samples may be taken at different time periods in future studies. Another limitation of our study was that a significant number of the patients were female (95%), and no balanced distribution is available in terms of gender. For preventing the effect of gender difference on serum cortisol and serum DHEA-S levels, we formed a control group with similar properties.
Further studies are needed on this subject including long-term follow-up of patients and numerous measurements of serum cortisol and DHEA-S levels. Clinical characteristics of fibromyalgia patients and the relation between serum cortisol and serum DHEA-S levels were also investigated in many studies. Yoshikawa et al23 found no significant correlation between serum DHEA-S level and sensitive spot number, depression scale point, and visual analogue scale values. Similarly, Sturgeon et al24 reported that DHEA-S did not correlate with pain intensity, or psychological or biological variables in women with fibromyalgia. The findings of our study have shown serum cortisol and serum DHEA-S level were not associated with FIQ and the sensitive spot number.
As a result, serum cortisol and serum DHEA-S levels of fibromyalgia patients were determined to be significantly lower than healthy controls in our study. No significant changes were determined in either serum cortisol and serum DHEA-S levels when measured again after BT and PT. While the cause and effect relation cannot be expressed precisely due to the cross-sectional design of our study, our findings, along with the data obtained from previous studies, revealed that low levels of serum cortisol and DHEA-S may be associated with the physiopathology of fibromyalgia. We suggest that the serum cortisol and DHEA-S levels should be evaluated in FMS treatment. Future studies with more frequent militarization have been made and longer term follow-up of the patients are needed to assess the impact of the treatment on serum cortisol and DHEA-S levels.
Footnotes
References
- 1.Theoharides TC, Tsilioni I, Arbetman L, Panagiotidou S, Stewart JM, Gleason RM, et al. Fibromyalgia syndrome in need of effective treatments. J Pharmacol Exp Ther. 2015;355:255–263. doi: 10.1124/jpet.115.227298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazzichi L, Sernissi F, Consensi A, Giacomelli C, Sarzi-Puttini P, Sernissi F, et al. Fibromyalgia: a critical digest of the recent literature. Clin Exp Rheumatol. 2011;29(6 Suppl 69):S1–S11. [PubMed] [Google Scholar]
- 3.Naumann J, Sadaghiani C. Therapeutic benefit of balneotherapy and hydrotherapy in the management of fibromyalgia syndrome: a qualitative systematic review and meta-analysis of randomized controlled trials. Arthritis Res Ther. 2014;16:R141. doi: 10.1186/ar4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas EN, Blotman F. Aerobic exercise in fibromyalgia: A practical review. Rheumatol Int. 2010;30:1143–1150. doi: 10.1007/s00296-010-1369-6. [DOI] [PubMed] [Google Scholar]
- 5.Genc A, Tur BS, Aytur YK, Oztuna D, Erdogan MF. Does aerobic exercise affect the hypothalamic-pituitary-adrenal hormonal response in patients with fibromyalgia syndrome? J Phys Ther Sci. 2015;27:2225–2231. doi: 10.1589/jpts.27.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Švob Štrac D, Jazvinšćak Jembrek M, Erhardt J, MirkovićKos K, Peričić D. Modulation of Recombinant GABA(A) receptors by neurosteroid dehydroepiandrosterone sulfate. Pharmacology. 2012;89:163–171. doi: 10.1159/000336058. [DOI] [PubMed] [Google Scholar]
- 7.De Abreu Freitas RP, Lemos TMAM, Spyrides MHC, De Sousa MBC. Influence of cortisol and DHEA-S on pain and other symptoms in post menopausal women with fibromyalgia. J Back Musculoskelet Rehabil. 2012;25:245–252. doi: 10.3233/BMR-2012-0331. [DOI] [PubMed] [Google Scholar]
- 8.Peixoto C, Devicari Cheda JN, Nardi AE, Veras AB, Cardoso A. The effects of dehydroepiandrosterone (DHEA) in the treatment of depression and depressive symptoms in other psychiatric and medical illnesses: a systematic review. Curr Drug Targets. 2014;15:901–914. doi: 10.2174/1389450115666140717111116. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 10.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18:728–733. [PubMed] [Google Scholar]
- 11.Calis M, Gökçe C, Ates F, Ulker S, Izgi HB, Demir H, et al. Investigation of the hypothalamo-pituitary-adrenal axis (HPA) by 1 microg ACTH test and metyrapone test in patients with primary fibromyalgia syndrome. J Endocrinol Invest. 2004;27:42–46. doi: 10.1007/BF03350909. [DOI] [PubMed] [Google Scholar]
- 12.McLean SA, Williams DA, Harris RE, Kop WJ, Groner KH, Ambrose K, et al. Momentary relationship between cortisol secretion and symptoms in patients with fibromyalgia. Arthritis Rheum. 2005;52:3660–3669. doi: 10.1002/art.21372. [DOI] [PubMed] [Google Scholar]
- 13.Curtis K, Osadchuk A, Katz J. An eight-week yoga intervention is associated with improvements in pain, psychological functioning and mindfulness, and changes in cortisol levels in women with fibromyalgia. J Pain Res. 2011;4:189–201. doi: 10.2147/JPR.S22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovengo SL, Russell IJ, Larson AA. Increased concentrations of nerve growth factor in cerebrospinal fluid of patients with fibromyalgia. J Rheumatol. 1999;26:1564–1569. [PubMed] [Google Scholar]
- 15.Mortaud S, Nicolas L, Pinoteau W, Tordjman S, Carlier M, Roubertoux PL. Brain pathways mediating the pro-aggressive effect of the steroid sulfatase (Sts) gene. Behav Genet. 2010;40:211–219. doi: 10.1007/s10519-010-9340-6. [DOI] [PubMed] [Google Scholar]
- 16.Dessein PH, Shipton EA, Joffe BI, Hadebe DP, Stanwix AE, Van der Merwe BA. Hyposecretion of adrenal androgens and the relation of serum adrenal steroids, serotonin and insulin-like growth factor-1 to clinical features in women with fibromyalgia. Pain. 1999;83:313–319. doi: 10.1016/s0304-3959(99)00113-x. [DOI] [PubMed] [Google Scholar]
- 17.Kuratsune H, Yamaguti K, Sawada M, Kodate S, Machii T, Kanakura Y, et al. Dehydroepiandrosterone sulfate deficiency in chronic fatigue syndrome. Int J Mol Med. 1998;1:143–146. doi: 10.3892/ijmm.1.1.143. [DOI] [PubMed] [Google Scholar]
- 18.Finckh A, Berner IC, Aubry-Rozier B, So AK. A randomized controlled trial of dehydroepiandrosterone in postmenopausal women with fibromyalgia. J Rheumatol. 2005;32:1336–1340. [PubMed] [Google Scholar]
- 19.Kuczera M, Kokot F. [Effect of spa therapy on the endocrine system. I. Stress reaction hormones] Pol Arch Med Wewn. 1996;95:11–20. [PubMed] [Google Scholar]
- 20.Bellometti S, Galzigna L. Function of the hypothalamic adrenal axis in patients with fibromyalgia syndrome undergoing mud-pack treatment. Int J Clin Pharmacol Res. 1999;19:27–33. [PubMed] [Google Scholar]
- 21.Cleare AJ, O’Keane V, Miell JP. Levels of DHEA and DHEAS and responses to CRH stimulation and hydrocortisone treatment in chronic fatigue syndrome. Psychoneuroendocrinology. 2004;29:724–732. doi: 10.1016/S0306-4530(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 22.Aktaş N. The effectiveness of physical therapy with balneotherapy in patients with fibromyalgia and its relationship with serum cortisol levels of the results. Specialist thesis, Cumhuriyet University School of Medicine; 2008. [Google Scholar]
- 23.Yoshikawa GT, Heymann RE, Helfenstein M, Pollak DF. A comparison of quality of life, demographic and clinical characteristics of Brazilian men with fibromyalgia syndrome with male patients with depression. Rheumatol Int. 2010;30:473–478. doi: 10.1007/s00296-009-0994-4. [DOI] [PubMed] [Google Scholar]
- 24.Sturgeon JA, Darnall BD, Zwickey HL, Wood LJ, Hanes DA, Zava DT, et al. Proinflammatory cytokines and DHEA-S in women with fibromyalgia: impact of psychological distress and menopausal status. J Pain Res. 2014;7:707–716. doi: 10.2147/JPR.S71344. [DOI] [PMC free article] [PubMed] [Google Scholar]


