Abstract
Peroxisome proliferator-activated receptor-γ (PPARγ) is a ligand-activated transcription factor belonging to the nuclear receptor superfamily, which plays a central role in regulating lipid and glucose metabolism. However, accumulating evidence demonstrates that PPARγ agonists have potential to reduce inflammation, influence the balance of immune cells, suppress oxidative stress, and improve endothelial function, which are all involved in the cellular and molecular mechanisms of cardiac fibrosis. Thus, in this review we discuss the role of PPARγ in various cardiovascular conditions associated with cardiac fibrosis, including diabetes mellitus, hypertension, myocardial infarction, heart failure, ischemia/reperfusion injury, atrial fibrillation, and several other cardiovascular disease (CVD) conditions, and summarize the developmental status of PPARγ agonists for the clinical management of CVD.
1. Introduction
Cardiac fibrosis is an inevitable process of varieties of cardiovascular diseases (CVDs) and is characterized by abnormal accumulation of extracellular matrix (ECM) in the myocardial interstitium. The ECM, composed of collagens, elastic fibers, glycosaminoglycan, and glycoproteins [1], are derived mainly from fibroblasts. Under physiological conditions, ECM is necessary to maintain the normal structure and function of the heart, the formation and degradation of ECM retain in dynamic balance, while in pathological conditions, because of excessive activation of renin-angiotensin-aldosterone system (RAAS), maladjustment of matrix metalloproteinases (MMP), and excessive secretion of some regulation cytokines such as transforming growth factor beta (TGFβ), the dynamic balance would be broken which resulted in ECM deposition and eventually cardiac fibrosis [2]. This pathological process is the beginning of cardiac remodeling and directly leads to arrhythmia [3], impaired cardiac function [4, 5] heart failure (HF), and even sudden cardiac death [6].
Although there are no effective strategies for treatment of cardiac fibrosis right now, it is firmly convinced that inhibition or reversion of myocardial fibrosis will be a promising way for prevention and treatment of HF in the nearby future [7]. Currently, the strategies for treatments of cardiac fibrosis mainly target RAAS system and inflammatory response; however, more and more other molecular mechanisms have been recognized to involve the regulation of cardiac fibrosis [8].
Interestingly, peroxisome proliferator-activated receptor-γ (PPARγ) has been identified to have the function of antimyocardial fibrosis [9–11]. According to published investigations, PPARγ has a wide spectrum of functions in regulating metabolism, attenuating inflammation, maintaining the balance of immune cells, inhibiting apoptosis and oxidative stress, and improving endothelial function [12]. All of these biological functions will be benefit for preventing the cardiac function from deterioration. However, the underling mechanisms of PPARγ in the regulation of cardiac fibrosis are not fully illustrated yet. This review will mainly summarize the reports about PPARγ and its agonist in the regulation of cardiac fibrosis.
2. Structure and Function of PPARγ
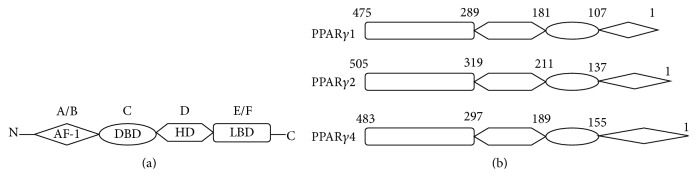
PPARs, belonging to the nuclear hormone receptor superfamily and consisting of three isoforms, PPARα, PPARβ/δ, and PPARγ, are ligand-inducible transcription factors. They are encoded by three separate genes and are distributed in different organs and tissues [14]. Because of the different expression and distribution profile, each of them presents unique biological function [14–16]. Activated by their specific ligands, PPARs can transfer into nucleus and form heterodimers with the retinoid X receptor. The heterodimeric complexes then band to the promoter region of target genes carrying peroxisome proliferator response elements (PPREs) and regulate transcription of target genes [17, 18]. Being similar to other nuclear receptors, PPAR isoforms possess five or six structural regions within four functional domains [14, 17, 19]. Activation function-1 motif (AF-1) locates at the N-terminal and is the target of phosphorylation kinase. The DNA-binding domain (DBD) consists of two highly conserved zinc finger motifs and is responsible for binding to PPRE. The hinge domain (BD) serves as a docking site for cofactors. The ligand bind domain (LBD) located at the C-terminal (E/F domain) is in charge of ligand specificity and activation of PPARs that bind to the PPRE, which increases target gene expression (Figure 1) [14, 17, 19].
Figure 1.
Schematic structure of peroxisome proliferator-activated receptor-γ and its protein isoforms. A/B, C, D, and E/F indicate the N-terminal A/B domain containing a ligand-independent AF-1, the DNA-binding domain, the hinge region, and the C-terminal LBD containing AF-2, respectively. AF-1 is responsible for phosphorylation, while AF-2 promotes the recruitment of coactivators for gene transcription. PPAR: peroxisome proliferator-activated receptor; AF: activation function; DBD: DNA-binding domain; HD: hinge domain; LBD: ligand-binding domain. Figure adapted from [13].
The PPARγ gene is located on human chromosome 3p25 [20]. Seven transcripts have been identified, termed PPARγ1, PPARγ2, PPARγ3, PPARγ4, PPARγ5, PPARγ6, and PPARγ7 [17]. The PPARγ1, PPARγ3, PPARγ5, and PPARγ7 mRNA transcripts translate PPARγ1 protein and PPARγ2 mRNA yields PPARγ2 protein, while PPARγ4 and γ6 mRNA transcripts translate PPARγ4 protein [21–23]. Because of different transcript, translation, and tissue distribution, each protein has different biological functions in a variety of organs and cells (Table 1) [13]. So it is not a surprise that PPARγ plays important roles in CVDs including hypertension [17, 24, 25], atherosclerosis [26], HF [27], diabetic cardiomyopathy [11, 28], angiogenesis [29], valvular calcification [30], aortic aneurysm [31], restenosis following cardiovascular interventions [32], and ischemia/reperfusion (I/R) injury [33, 34].
Table 1.
Tissue and cell distribution of PPARγ mRNA transcripts. Modified from [13].
| PPARγ mRNA transcripts | Tissue and cell distribution |
|---|---|
| PPARγ1 | Cardiac muscle, skeletal muscle, kidney, adrenal, spleen, intestine, pancreatic β-cells, and vascular smooth muscle cells |
| PPARγ2 | Adipose tissue |
| PPARγ3 | Adipose tissue, colon, and macrophages |
| PPARγ4 | Macrophages |
| PPARγ5 | Macrophages |
| PPARγ6 | Macrophages and adipose tissue |
| PPARγ7 | Macrophages and adipose tissue |
3. PPARγ and Cardiac Fibrosis
The primary of activation of PPARγ is to lower serum glucose and improve the insulin sensitivity. In the clinical practice, the specific ligands of PPARγ have been accepted for treatment of diabetes mellitus. However, more and more researches had indicated that activation of PPARγ presents pleiotropic biological effects involving regulation of inflammation and energy metabolism. Because of its pleiotropic effects, PPARγ has been recognized as a target for the treatment of cardiac fibrosis. The characteristics of PPARγ regulate myocardial fibrosis in different CVDs as described below.
3.1. Diabetic Cardiomyopathy
The diabetic cardiomyopathy is accompanied by myocardial hypertrophy, dilated ventricular chamber, and fibrosis [49]. The specific PPARγ ligands, thiazolidinediones (TZDs), are used in clinical practice to improve insulin sensitivity in type 2 diabetes mellitus (T2DM). As shown in Table 2, evidences have demonstrated that TZDs could decrease myocardial fibrosis and improve cardiac dysfunction. In the animal experiment, Ihm and his colleagues found that the PPARγ ligand, rosiglitazone, significantly decreased myocardial fibrosis in the Otsuka Long-Evans Tokushima Fatty (OLETF) rats [35]. The underlying mechanism may be involved in the inhibiting nuclear factor-κB (NF-κB) activation in the myocardium. This biological function directly resulted in downregulation of receptor for advanced glycation end products and connective tissue growth factor (CTGF) expression [35], which have been convinced to play a key role in cardiac fibrosis [50, 51]. As we know, activation of RAAS may also lead to collagen deposition and result in cardiac fibrosis [2, 52]. Research has shown that pioglitazone activation of PPARγ can attenuate cardiac fibrosis in diabetic rats and partly ameliorates cardiac remodeling and function by suppressing activity of RAS [36]. The interesting finding is that rosiglitazone is able to decrease cardiac fibrosis and enhance myocardial vascularization in rat offspring programmed by low protein diet during gestation, which may be implicated in rosiglitazone administration which can decrease angiotensin (Ang) II and endothelin- (ET-) 1 and increase endothelial nitric oxide synthase (eNOS) [37]. Moreover, rosiglitazone reduces atrial interstitial fibrosis and AF promotion in the diabetic rabbits via modulating oxidative stress and inflammation [38]. The selective PPARγ, pioglitazone, could attenuate cardiac fibrosis and collagen concentration by upregulating insulin-like growth factor 1 (IGF-1), phosphorylated Akt, and eNOS in OLETF rats [39]. Furthermore, the PPARγ agonist ciglitazone may alleviate MMP-9 and fibrosis and improve end diastolic diameter in diabetic mice hearts [40]. Unfortunately, a recent study in the same animal model gave a negative conclusion that treatment with rosiglitazone had little cardioprotection and there is no indication for the regulation of NF-κB signaling pathway [41]. But the combination of rosiglitazone and losartan obviously attenuated the interstitial fibrosis and collagen deposition of the heart by inhibiting TGFβ and tumor necrosis factor-α (TNFα), along with the proinflammatory cytokines, interleukin- (IL-) 1β, and IL-6 [41]. Therefore, the authors declared that the benefit may be not derived from the activation of PPARγ. In addition, combination treatment with rosiglitazone and felodipine could improve the metabolic abnormalities and decrease TNFα, TGFβ, collagen I, and collagen III and increased MMP-2, while treatment with rosiglitazone alone had no effect on attenuating the hypertension and only exerted a minimal effect on reducing cardiac fibrosis and improving dyslipidemia and hyperglycemia in diabetic hypertensive rats [28]. Thus, on one hand, whether the activation of PPARγ which could attenuate myocardial fibrosis remains unclear, the improving of cardiac function may not be related to the attenuation of cardiac fibrosis. On the other hand, the discrepancy results may partly be due to the dosage and length of observation time. Thirdly, the selective ligand, rosiglitazone, presents more discrepancy in the published data, so different structure of selective ligand may show different biological function. More investigations are needed to clarify these perplex.
Table 2.
Effects of PPARγ ligands on diabetic related cardiac fibrosis.
| Study model | Dose/duration/route | Major cardiac findings and conclusions | Ref. |
|---|---|---|---|
| Male OLETF rats, LETO rats, 20 weeks old | Rosiglitazone 20 mg/kg/d for 20 weeks, gavage | Suppression of RAGE and CTGF expression in the diabetic myocardium appears to contribute to the antifibrotic effect of rosiglitazone | [35] |
|
| |||
| Male STZ-induced diabetic Sprague-Dawley rats (200 ± 20 g) | Pioglitazone 10 mg/kg/d for 14 weeks, gavage | Activation of the PPARγ signal pathway could repress cardiac fibrosis in diabetic rats and partly improve cardiac remodeling and function by downregulating activity of RAS level | [36] |
|
| |||
| Male offspring of Wistar rats fed NP diet or LP diet, 3 months old | Rosiglitazone 5 mg/kg/d for three months, gavage | Rosiglitazone showed beneficial effects on rat offspring programmed by low protein diet during gestation decreasing cardiac fibrosis and enhancing myocardial vascularization | [37] |
|
| |||
| Alloxan-induced diabetic rabbits 1.8–2.5 Kg | Rosiglitazone 2 mg/kg/d for 4 weeks, unclear | Rosiglitazone attenuates arrhythmogenic atrial structural remodeling and atrial fibrillation promotion | [38] |
|
| |||
| Male OLETF rats, LETO rats, 20 weeks old | Pioglitazone 10 mg/kg/d for 20 weeks, per orem | Activation of PPARγ may decrease collagen concentration and reduce cardiac fibrosis by exerting regulatory effects on cardiac telomere biology | [39] |
|
| |||
| Male WT, CBS+/+, CBS+/−, and Ins2+/−/CBS+/− rats, 20 weeks old | Ciglitazone 3 mg/kg/d for 4 weeks, orally | Treatment with ciglitazone alleviated MMP-9 activity and fibrosis and improved end diastolic diameter | [40] |
|
| |||
| Male OLETF rats, LETO rats, 28 weeks old | Rosiglitazone 3 mg/kg/d and losartan 5 mg/kg/d for 12 weeks, gavage | A combination of rosiglitazone and losartan attenuates myocardial fibrosis and dysfunction | [41] |
|
| |||
| Male diabetic hypertensive rats 179–219 g | Rosiglitazone 3 mg/kg/d or combination of felodipine 5 mg/kg/d for one month, orally | The combined treatment can improve dyslipidemia and decrease TNFα, TGFβ, collagen I, and collagen III, and increased MMP-2 but within a greater effect than treatment with rosiglitazone alone | [28] |
OLETF: Otsuka Long-Evans Tokushima Fatty, LETO: Long-Evans Tokushima Otsuka, RAGE: receptor for advanced glycation end products, CTGF: connective tissue growth factor, WT: wild type, CBS+/−: cystathionine beta synthase mutant, Ins2+/−: insulin 2 mutant, MMP: matrix metalloproteinases, TNF: tumor necrosis factor, TGF: transforming growth factor, NP: normal protein (19% protein), LP: low protein (5% protein), STZ: streptozotocin, and RAS: renin-angiotensin system.
It has been reported recently that the muscle specific ubiquitin ligase muscle ring finger-2 (MuRF2) and MuRF3 regulate PPARγ1 activity to protect against diabetic cardiomyopathy [53, 54]. Although MuRF2−/− hearts have significant increases in fibrosis and PPARγ1-regulated cardiac genes, the expression of PPARγ1 mRNA has no differences in MuRF2−/− hearts and wild-type mice. Unfortunately, only minimal amount of fibrosis was detected in MuRF3−/− hearts and has no differences compared to wild-type controls. Furthermore, PPARγ1 target genes showed increases in both MuRF3−/− and wild-type hearts, but the mRNA expression levels have no differences between the two groups. Thus it can be seen that MuRF2 and MuRF3 inhibit cardiac PPAR isoforms expression to protect against high fat diet-induced diabetic cardiomyopathy, which mainly improve systolic dysfunction and attenuate left ventricular mass and heart weight but do not include cardiac fibrosis. Therefore, more research needs to prove the role of different PPARγ subtypes in myocardial fibrosis.
3.2. Hypertension
There is considerable evidence regarding arterial hypertension which leads to cardiac hypertrophy and myocardial fibrosis [10, 55]. For this reason, it is significant to explore novel strategies to protect the hypertension related cardiac remodeling [56]. Fortunately, despite low expression in the heart, PPARγ acts as a functional antifibrogenic factor in hypertensive heart disease [42]. Recent studies have indicated that treatment with the PPARγ activators resulted in the reduction of ECM deposition and cardiac fibrosis, while PPARγ antagonist GW9662 or T0070907 reversed these changes [10, 42, 43]. In addition, a significant negative correlation was observed between myocardial interstitial fibrosis and mRNA expression of PPARγ [56]. Furthermore, mice with a dominant-negative point mutation in PPARγ (P465L) developed significantly more severe cardiac fibrosis to Ang II-induced hypertension [57].
Despite the fact that the role of PPARγ in chronic pressure overload-induced cardiac fibrosis has been hypothesised previously (details are shown in Table 3), the molecular mechanisms are not fully understood. It has been suggested that activation of PPARγ inhibited both the expressions of TGFβ1 [10, 42–44, 56] and phosphorylation of Smad2/3 [10] in vivo and cultured neonatal rat cardiomyocytes and cardiac fibroblasts. In addition, the PPARγ agonist pioglitazone significantly decreased cardiac inflammatory response by inhibiting NF-κB and activator protein-1 (AP-1) binding activities, the expression of TNFα, and the adhesion of platelet endothelial cell adhesion molecule in stroke-prone spontaneously hypertensive rats (SHRSP) [45]. On the other hand, the downregulation of reactive oxygen species (ROS) mediated by an upregulation PPARγ may play a role in pressure overload-induced cardiac fibrosis [46, 47, 56]. However, Shinzato et al. [44] found that ROS production was not improved in SHRSP treated with pioglitazone. Furthermore, long-term administration of pioglitazone attenuates the development of left ventricular (LV) hypertrophy and fibrosis and inhibited phosphorylation of mTOR and p70S6 kinase in the heart, which are likely attributable to both the activation of AMPK signaling through stimulation of adiponectin secretion and the inhibition of Akt signaling [48].
Table 3.
Effects of PPARγ ligands on hypertension related cardiac fibrosis.
| Study model | Dose/duration/route | Major cardiac findings and conclusions | Ref. |
|---|---|---|---|
| Male SHR and WKY rats, 8 weeks old Cell culture: CFs form SD rats, 1-2 days old |
Curcumin 100 mg/kg/d or curcumin 100 mg/kg/d plus GW9662 10 mg/kg/d for 12 weeks, gavage | Curcumin attenuates cardiac fibrosis in SHRs and inhibits Ang II- induced production of CTGF, PAI-1, ECM, TGFβ1, and phosphorylation of Smad2/3 in CFs in vitro | [10] |
|
| |||
| Male DnTGFβRII and WT C57BL/6 mice, 8–10 weeks old subjected to TAC | Rosiglitazone 10 mg/kg/d or T0070907 1.5 mg/kg/d for 3 weeks, gavage | Downregulation of endogenous PPARγ expression by TGFβ may be involved in pressure overload-induced cardiac fibrosis | [42] |
|
| |||
| Male Wistar rats, weights 250–300 g subjected to abdominal aortic banding at 4 weeks after ligation Cell culture: CFs form Wistar rats, 1–3 days old |
Rosiglitazone 6 g/kg/d or GW9662 0.2 g/kg/d 2 h prior to rosiglitazone 6 g/kg/d for 1 week, intraperitoneal injection | Activation of PPARγ significantly inhibited cardiac remodeling by suppression the expressions of Brq1 and TGFβ1 through the NF-κB pathway | [43] |
|
| |||
| Male SHRSP and WKY rats, 24 weeks old | Pioglitazone 10 mg/kg/d for 8 weeks, mixed with food | Pioglitazone decreased interstitial fibrosis and number of myofibroblasts; mRNA levels of collagen I and BNP; MMP2 activity and protein level of CTGF. However, the mRNA level of collagen III and TGFβ1, MMP9 activity, and ROS production were not improved | [44] |
|
| |||
| Male SHRSP, 6 weeks old | Pioglitazone 10 mg/kg/d for 20 weeks, mixed with food | Subepicardial interstitial fibrosis, left ventricular NF-κB and AP-1 binding activities, the TNFα expression, and the adhesion of PECAM were decreased by pioglitazone treatment | [45] |
|
| |||
| Male SHRSP and WKY rats, 11 weeks old | Pioglitazone 1 mg/kg/d or 2 mg/kg/d, candesartan 0.3 mg/kg/d for 4 weeks, gavage | Pioglitazone suppressed cardiac inflammation and fibrosis and reduced vascular endothelial dysfunction by inhibition of cardiovascular NADPH oxidase, and the combination of pioglitazone and candesartan exerted more beneficial effects | [46] |
|
| |||
| Male C57BL/6J rats, 8 weeks old subjected to abdominal aortic banding | Ciglitazone 2 mg/kg/d for 4 weeks, administered in drinking water | Ciglitazone decreased interstitial and perivascular fibrosis and inhibition of an induction of NOX4, iNOS, MMP-2/MMP-13 expression, and collagen synthesis/degradation | [47] |
|
| |||
| Male inbred Dahl salt- sensitive rats, 7 weeks old | Pioglitazone 2.5 mg/kg/d for 4 weeks, gavage | Pioglitazone treatment ameliorated LV hypertrophy and fibrosis and improved diastolic function by activating AMPK signaling and inhibiting Akt signaling. | [48] |
DnTGFβRII: dominant-negative mutation of the human TGFβ type II receptor, WT: wild type, TGF: transforming growth factor, TAC: transverse aortic constriction, CFs: cardiac fibroblasts, NF-κB: nuclear factor-κB, SHR: spontaneously hypertensive rats, WKY: Wistar Kyoto rats, SD: Sprague-Dawley, CTGF: connective tissue growth factor, PAI-1: Plasminogen activator inhibitor-1, ECM: extracellular matrix, SHRSP: stroke-prone spontaneously hypertensive rats, BNP: brain natriuretic peptide, MMP: matrix metalloproteinases, ROS: reactive oxygen species, NADPH: nicotinamide adenine dinucleotide phosphate, NOX4: nicotinamide adenine dinucleotide phosphate oxidase 4, iNOS: inductive nitric oxide synthase, AP-1: activator protein-1, TNF: tumor necrosis factor, PECAM: platelet endothelial cell adhesion molecule, and AMPK: adenosine monophosphate-activated protein kinas.
3.3. Myocardial Infarction (MI)
Adverse LV remodeling after MI is characterized by myocyte hypertrophy and interstitial fibrosis of the noninfarcted myocardium [58]. Accumulating evidence suggests that angiotensin II receptor blockers (ARBs) induce the activity of PPARγ which inhibit unfavorable LV remodeling [41, 58–60]. PPARγ protein expression is mainly in cardiac myocytes and fibroblasts in the infarcted area three weeks after MI, suggesting the critical role of PPARγ in cardiac fibrosis [59]. A study conducted by Maejima et al. [58] verified that telmisartan effectively inhibits infaust LV remodeling through a reduction of infiltration of macrophages, activation of MMP2 and MMP9, and expression of TGFβ1, CTGF, and osteopontin, while expression of PPARγ and activation of tissue inhibitor of metalloproteinase-1 (TIMP-1) were enhanced in the noninfarcted myocardium of rats. And in in vitro experiments, they got the similar results. Pioglitazone, a PPARγ activator, has been proved to reduce TNFα, TGFβ, and monocyte chemoattractant protein-1 and attenuate myocyte hypertrophy and interstitial fibrosis in MI mice [61]. This indicated that an anti-inflammatory effect mediated by PPARγ activation plays a critical role in post-MI LV remodeling in rats. More recently, a multicenter randomized double-blind study demonstrated that Qiliqiangxin, a traditional Chinese medicine, ameliorates unfavorable myocardial remodeling after acute MI including improved cardiac function, decreased apoptosis, and reduced fibrosis by increasing PPARγ levels. However, the expression of well-known signaling pathways including Akt, SAPK/Jun NH2-terminal kinase phosphorylation (JNK), and ERK was not altered by Qiliqiangxin treatment [62]. Interestingly, Birnbaum et al. showed that pioglitazone is able to limit myocardial infarct size by activating Akt and upregulating cytosolic phospholipase A2 and cyclooxygenase-2 [63]. These suggest that the underlying mechanism may be varied from different drugs, but PPARγ play a critical role in myocardial fibrosis after MI is indisputable. Besides, TZDs also have neutral [64] or detrimental [65] effects on cardiac remodeling or mortality after MI. Therefore, the exact role of TZDs in myocardial remodeling after MI remains controversial and further studies should be done to elucidate the precise effects and mechanisms.
3.4. HF
Although the initial indications for PPAR agonist treatment mainly focus on hyperlipidemia and diabetes, there is a growing body of data which suggest that they maybe improve cardiac function with decreased fibrosis, improved contractility, and endothelial function in animal models of systolic HF [66]. In a rabbit model with nonischemic HF induced by combined aortic regurgitation and aortic stenosis, decreased ejection fraction and unfavorable myocardial remodeling including increased collagen volume fraction were observated. Moreover, the activity and expression of NF-κB subunits p65, RhoA, and Rho GTPase significantly increased. Interestingly, all these changes were reversed and the mRNA and protein expression of PPARγ were significantly increased with simvastatin treatment. Based on these results, the authors declared that simvastatin inhibited RhoA and Rho GTPase signaling to restrain NF-κB activation by the PPARγ-dependent pathway, thus attenuating LV hypertrophy and fibrosis [67]. In addition, pioglitazone treatment reduced the duration of atrial fibrillation (AF) and attenuated atrial structural remodeling including atrial fibrosis via attenuating the expression of TNFα, TGFβ1, and ERK but left unaffected p38 and JNK activation in the rabbit model with congestive heart failure [68]. Therefore, it is conceivable that PPARγ activation suppresses cardiac fibrosis by antagonizing inflammatory and hypertrophic signaling pathways. Likewise, PPARγ acts as a modulator of cardiac fibrosis in human as well. Cardiac remodeling occurring in patients with end-stage heart failure due to ischemic cardiomyopathy is related to PPAR activity, whereby inactivation of PPARα and PPARγ would lead to an increase in the production of ET-1 and the presence of cardiac fibrosis [69]. Nevertheless, rosiglitazone treatment had no significant effects on myocardial fibrosis compared with the vehicle group in MI-induced HF rats [70]. This result should raise questions with regard to these models or the particular species at large. Further studies are needed to test the variety and potential mechanisms.
3.5. I/R Injury
Early reperfusion of ischemic myocardium is necessary to salvage myocardial tissue from ultimate death. Nevertheless, reperfusion always results in cardiomyocyte death, microvasculature injury, and cardiac fibrosis, which ultimately cause myocardial remodeling and dysfunction [71, 72]. Recently, research has shown that rosiglitazone alleviated I/R injury by inhibiting inflammatory, improving endothelial function, reducing oxidative stress, and calcium overload [33]. Likewise, rosiglitazone treatment can effectively suppress the inflammatory induced by I/R injury and promote myocardial functional recovery [73] with an inhibition of JNK, AP-1 DNA-binding activity, and NF-κB signaling pathway [33, 73]. These data demonstrated that rosiglitazone limits postischemic injury, suggesting an important function for PPARγ in the heart.
Snail, a zinc finger transcription factor, activation induces lung, liver, and kidneys fibrosis [74–76]. Recently, its role in cardiac fibrosis after I/R injury and the probable underlying mechanisms had been identified. Lee and her colleagues [77] found that I/R injury to mouse hearts significantly increases the expression of Snail. In addition, the author showed that the cell source of Snail induction is endothelial cells. Moreover, Snail overexpression-mediated endothelial-to-mesenchymal transition-like cells markedly stimulated fibroblasts to myofibroblasts transdifferentiation via secretion of CTGF. What is more, they found that PPARγ agonist rosiglitazone, a selective Snail suppressor, remarkably suppressed cardiac fibrosis, improved cardiac function, and reduced Snail and CTGF expression in vivo. Based on this, the authors suggested that Snail might be a potential target molecule in the treatment of cardiac fibrosis.
3.6. AF
The relevance of atrial fibrosis and AF is well established and the causal relationship between them is interdependent. Atrial fibrosis expedites the development of AF by causing alterations of electrical properties [78]; on the other hand, AF itself promotes atrial fibrosis [79]. Although the underlying mechanisms are not fully understood, inflammation may promote the persistence of AF and atrial remodeling. A study conducted by Chen et al. [80] suggested that the PPARγ mRNA was significantly decreased in the hypertensive AF patients and PPARγ had a negative correlation with inflammatory cytokines TNFα, IL-6, and IL-1. The similar results were observed in elderly patients with AF [81]. In addition, pioglitazone was able to attenuate Ang II-induced electrical and structural remodeling by inhibiting both the TGFβ1/Smad2/3 and the non-Smad TGFβ1/tumor necrosis factor receptor associated factor 6/TGFβ-associated kinase 1 signaling pathways in vitro cellular models [82], which adds further evidence to the benefits of PPARγ agonist for the prevention of AF. Thus, PPARγ is at least partly involved in the pathogenesis of AF by regulation of inflammation through the NF-κB pathway; PPARγ agonist is potential useful in suppressing cardiac fibrosis and preventing AF occurrence.
3.7. Other CVD Conditions
It has been demonstrated that myocardial fibrosis is a common pathological change in radiation-induced heart diseases [83]. In Sprague-Dawley rats receiving chest radiation, the protein expression of TIMP-1 and TGFβ1 was higher than that in rats without radiation in the heart; the PPARγ mRNA and protein expression levels are upregulated in heart injured by radiation as well. However, upregulation of PPARγ failed to inhibit the expression of TIMP-1 and TGFβ1 [84]. Therefore, it is a possible mechanism that PPARγ itself has protective effect in response to radiation-induced heart injury. Regrettably, the authors did not use PPARγ agonists or inhibitors to further discuss its function in radiation-induced heart diseases. Besides, study on experimental animals demonstrated that tenascin-x, an ECM glycoprotein exclusively expressed in fibroblasts, can inhibit myocardial fibrosis via upregulation of TGFβ1 and downregulation of PPARγ in alcoholic cardiomyopathy [85]. These data suggested that PPARγ plays a crucial role in inhibiting cardiac fibrosis; further understanding of cardioprotection properties of PPARγ activator came from the study of pioglitazone influence on experimental autoimmune myocarditis. The authors suggested that pioglitazone could alleviate cardiac inflammation and fibrosis by inhibiting macrophage inflammatory protein-1α expression and modulating the Th1/Th2 balance [86].
Coincidentally, PPARγ shows a pivotal role in multiple other cardiovascular disease states. Singh et al. [87] demonstrated that rosiglitazone relieves cardiac hypertrophy and myocardial fibrosis in a dose-dependent manner possibly through its antioxidant activity in hyperhomocysteinemia rats. Moreover, simvastatin treatment has beneficial effects on augmentation of the PPARγ, PPARα expression, and reducing cardiac interstitial fibrosis biochemical makers including MMP-9 and cathepsin S in apolipoprotein E-deficient mice fed with a high fat diet [88]. More importantly, irbesartan prevents myocardial hypertrophy and fibrosis via activation of the PPARγ and suppression of the TGFβ-CTGF-ERK signaling in angiotensin-converting enzyme 2 knockout mice [9]. Finally, activation of PPARγ inhibits isoprenaline- induced myocardial fibrosis and remodeling via the NF-κB and MAPKs-dependent mechanism in rats [89–92].
3.8. Cardiac Fibroblasts (CFs) Culture In Vitro
Apart from in vivo experiments, PPARγ have been reported to have a number of cardioprotective properties in vivo. Due to a large number of stresses including growth and vasoactive factors, cytokines, and mechanical stimuli [93], fibroblasts proliferate and differentiate into myofibroblasts, a cell type with an increased secretion capacity of ECM [94]. There is convincing evidence that PPARγ ligands, rosiglitazone, pioglitazone, and 15-deoxy-Δ12,14-prostaglandin J2, all inhibit Ang II-induced CFs proliferation and differentiation, collagen synthesis, and ECM production [95–97], which are the critical steps in the pathogenesis of cardiac fibrosis. In addition, rosiglitazone can prevent myocardial fibrosis induced by advanced glycation end products in cultured neonatal rat CFs via inhibiting CFs proliferation, decreasing nitric oxide production, and CTGF expression [98]. Collectively, these data suggest that PPARγ activation has an antifibrotic effect. Despite these findings, the underlying mechanisms for the regulatory effects of PPARγ ligands on cardiac fibrosis are ambiguity and the specific role of PPARγ in this process has not yet been fully elucidated. The molecular mechanisms probably involved NF-κB/TGFβ/Smad2/3 and JNK signaling pathways [95, 99–101].
4. Conclusions and Future Prospects
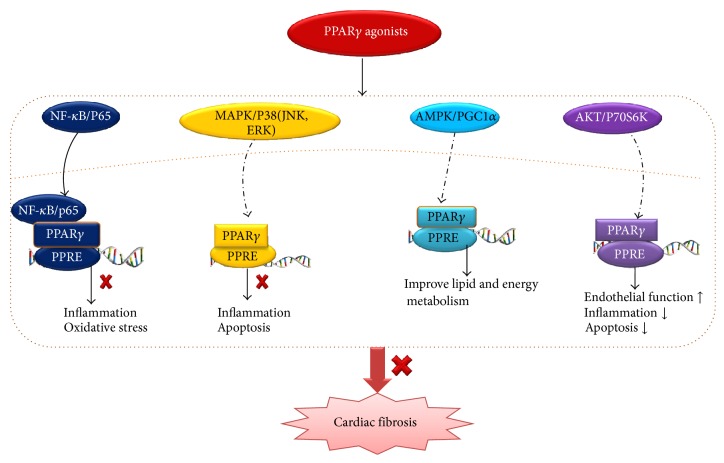
Cardiac fibrosis is associated with varied cardiovascular disease and thus is a pivotal determinant of clinical outcome in heart diseases. Although the last decade has seen enormous progress insight into cardiac fibrosis, there is no precise and effective therapy. At the same time, accumulating evidences demonstrate that PPARγ exerts a broad spectrum of biological functions, including the beneficial effects of alleviating myocardial fibrosis. However, the cardioprotection mechanisms are currently not fully established, and the potential mechanisms were shown in Figure 2. Therefore, in-depth understanding of the potential molecular mechanisms of PPARγ and its ligands in preventing cardiac fibrosis may provide valuable information in the design of novel treatment strategies in HF.
Figure 2.
The possible underlying mechanisms involved in PPARγ agonists alleviate cardiac fibrosis. PPARγ agonists show pleiotropy functions associated with inhibiting cardiac fibrosis via variety of signaling pathways. PPARγ: peroxisome proliferator-activated receptor-γ; PPREs: peroxisome proliferator response elements; NF-κB: nuclear factor-κB; MAPK: mitogen-activated protein kinase; JNK: Jun NH2-terminal kinase phosphorylation; ERK: extracellular signal-regulated kinase; AMPK: adenosine monophosphate-activated protein kinas; PGC1α: peroxisome proliferator-activated receptor gamma coactivator-1α; AKT: also known as protein kinase B.
Unfortunately, despite many beneficial features of PPARγ agonists, they also exhibit adverse effects associated with long-term use. It has been proposed that PPARγ agonists are not free from side effects including edema, headache, hypoglycemia, myalgia, HF, weight gain, bone fractures, increased risk of MI and mortality, and possibly bladder cancer [13, 14, 17, 102–104]. Rosiglitazone, pioglitazone, and troglitazone have been approved for treatment of type 2 diabetes in clinical practice. Contrary to pioglitazone, rosiglitazone and troglitazone were associated with significant tissue toxicities after a relatively short-term exposure [15, 102]. In addition, the dual PPAR agonist ragaglitazar, MK-0767, naveglitazar, tesaglitazar, and muraglitazar for diabetes have failed due to various safety concerns. Aleglitazar, the most recent dual PPARα/γ agonist, has shown a significant dose-dependent reduction in HbA1c and beneficial effects on lipid subfractions [14]. Unfortunately, aleglitazar increased the risks of HF, renal dysfunction, bone fractures, gastrointestinal hemorrhage, and hypoglycemia [105]. Thus, new PPARγ-directed therapeutic modalities should be considered as possible approaches to reducing the adverse events seen with current TZDs. The pan-PRAR agonists bezafibrate, selective PPARγ modulators S26948 and INT131, partial PPARγ agonists balaglitazone, MBX-102, MK-0533, PAR-1622, PAM-1616, KR-62776, and SPPARγ M5, new dual PPARα/γ agonists saroglitazar, have a reduced tendency to cause the adverse effects and might be available in clinical management in the near future [14].
PPARγ agonists convey beneficial effects as therapeutic agents for cardiac fibrosis; however, their functions are not fully established yet. As such, PPARγ agonists possess different properties for different species, and the mechanisms by which they attenuate cardiac fibrosis are required in both experimental animal models and humans [106]. Moreover, the adverse side effects of PPARγ agonists and the potential mechanisms responsible for these effects should be clarified in detail, particularly in humans [106]. Last but not the least, it is necessary to focus on interactions between PPARγ-activating agents and other cardiovascular drugs [106]. Intensive research on these targets should be of great assistance to the development of safety and efficacy PPARγ agonists in the near future.
Acknowledgments
This work is supported by grants from the National Natural Science Foundation of China (81270303 and 81470516), Hubei Province's Outstanding Medical Academic Leader program, and the Fundamental Research Funds for the Central Universities of China (no. 2015301020202).
Abbreviations
- PPARγ:
Peroxisome proliferator-activated receptor-γ
- CVD:
Cardiovascular disease
- ECM:
Extracellular matrix
- RAAS:
Renin-angiotensin-aldosterone system
- MMP:
Matrix metalloproteinases
- TGFβ:
Transforming growth factor beta
- HF:
Heart failure
- PPREs:
Peroxisome proliferator response elements
- AF-1:
Activation function-1 motif
- DBD:
DNA-binding domain
- LBD:
Ligand bind domain
- I/R:
Ischemia/reperfusion
- TZDs:
Thiazolidinediones
- T2DM:
Type 2 diabetes mellitus
- OLETF:
Otsuka Long-Evans Tokushima Fatty
- NF-κB:
Nuclear factor-κB
- CTGF:
Connective tissue growth factor
- Ang II:
Angiotensin II
- ET-1:
Endothelin-1
- IGF-1:
Insulin-like growth factor 1
- eNOS:
Endothelial nitric oxide synthase
- TNFα:
Tumor necrosis factor-α
- IL-1β:
Interleukin-1β
- MuRF2:
Muscle specific ubiquitin ligase muscle ring finger-2
- AP-1:
Activator protein-1
- SHRSP:
Stroke-prone spontaneously hypertensive rats
- ROS:
Reactive oxygen species
- LV:
Left ventricular
- MI:
Myocardial infarction
- ARB:
Angiotensin II receptor blocker
- TIMP-1:
Tissue inhibitor of metalloproteinase-1
- JNK:
Jun NH2-terminal kinase phosphorylation
- AF:
Atrial fibrillation
- CFs:
Cardiac fibroblasts.
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
Huang-Jun Liu and Hai-Han Liao contributed equally to this study.
References
- 1.Cox T. R., Erler J. T. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Disease Models & Mechanisms. 2011;4(2):165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFβ, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circulation Research. 2010;106(11):1675–1680. doi: 10.1161/circresaha.110.217737. [DOI] [PubMed] [Google Scholar]
- 3.Nademanee K., Raju H., de Noronha S. V., et al. Fibrosis, connexin-43, and conduction abnormalities in the Brugada syndrome. Journal of the American College of Cardiology. 2015;66(18):1976–1986. doi: 10.1016/j.jacc.2015.08.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho J. E., Liu C., Lyass A., et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. Journal of the American College of Cardiology. 2012;60(14):1249–1256. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ichiki T., Schirger J. A., Huntley B. K., et al. Cardiac fibrosis in end-stage human heart failure and the cardiac natriuretic peptide guanylyl cyclase system: regulation and therapeutic implications. Journal of Molecular and Cellular Cardiology. 2014;75:199–205. doi: 10.1016/j.yjmcc.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.John B. T., Tamarappoo B. K., Titus J. L., Edwards W. D., Shen W.-K., Chugh S. S. Global remodeling of the ventricular interstitium in idiopathic myocardial fibrosis and sudden cardiac death. Heart Rhythm. 2004;1(2):141–149. doi: 10.1016/j.hrthm.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Krenning G., Zeisberg E. M., Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. Journal of Cellular Physiology. 2010;225(3):631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynn T. A. Cellular and molecular mechanisms of fibrosis. The Journal of Pathology. 2008;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z.-Z., Shang Q.-H., Jin H.-Y., et al. Cardiac protective effects of irbesartan via the PPAR-gamma signaling pathway in angiotensin-converting enzyme 2-deficient mice. Journal of Translational Medicine. 2013;11, article 229 doi: 10.1186/1479-5876-11-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng Z., Yu X.-H., Chen J., Li L., Li S. Curcumin attenuates cardiac fibrosis in spontaneously hypertensive rats through PPAR-γ activation. Acta Pharmacologica Sinica. 2014;35(10):1247–1256. doi: 10.1038/aps.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abou Daya K., Abu Daya H., Nasser Eddine M., Nahhas G., Nuwayri-Salti N. Effects of rosiglitazone (PPARγ agonist) on the myocardium in non-hypertensive diabetic rats. Journal of Diabetes. 2015;7(1):85–94. doi: 10.1111/1753-0407.12140. [DOI] [PubMed] [Google Scholar]
- 12.Ivanova E. A., Parolari A., Myasoedova V., Melnichenko A. A., Bobryshev Y. V., Orekhov A. N. Peroxisome proliferator-activated receptor (PPAR) gamma in cardiovascular disorders and cardiovascular surgery. Journal of Cardiology. 2015;66(4):271–278. doi: 10.1016/j.jjcc.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Azhar S. Peroxisome proliferator-activated receptors, metabolic syndrome and cardiovascular disease. Future Cardiology. 2010;6(5):657–691. doi: 10.2217/fca.10.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee W. S., Kim J. Peroxisome proliferator-activated receptors and the heart: lessons from the past and future directions. PPAR Research. 2015;2015:18. doi: 10.1155/2015/271983.271983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilding J. P. H. PPAR agonists for the treatment of cardiovascular disease in patients with diabetes. Diabetes, Obesity and Metabolism. 2012;14(11):973–982. doi: 10.1111/j.1463-1326.2012.01601.x. [DOI] [PubMed] [Google Scholar]
- 16.Grygiel-Górniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications—a review. Nutrition Journal. 2014;13, article 17 doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Usud D., Kanda T. Peroxisome proliferator-activated receptors for hypertension. World Journal of Cardiology. 2014;6(8):744–754. doi: 10.4330/wjc.v6.i8.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurevich I., Flores A. M., Aneskievich B. J. Corepressors of agonist-bound nuclear receptors. Toxicology and Applied Pharmacology. 2007;223(3):288–298. doi: 10.1016/j.taap.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo L., Tabrizchi R. Peroxisome proliferator-activated receptor gamma as a drug target in the pathogenesis of insulin resistance. Pharmacology and Therapeutics. 2006;111(1):145–173. doi: 10.1016/j.pharmthera.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Dong C., Zhou H., Shen C., et al. Role of peroxisome proliferator-activated receptors gene polymorphisms in type 2 diabetes and metabolic syndrome. World Journal of Diabetes. 2015;6(4):654–661. doi: 10.4239/wjd.v6.i4.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christodoulides C., Vidal-Puig A. PPARs and adipocyte function. Molecular and Cellular Endocrinology. 2010;318(1-2):61–68. doi: 10.1016/j.mce.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y., Jimenez A. R., Medh J. D. Identification and regulation of novel PPAR-γ splice variants in human THP-1 macrophages. Biochimica et Biophysica Acta (BBA)—Gene Structure and Expression. 2006;1759(1-2):32–43. doi: 10.1016/j.bbaexp.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medina-Gomez G., Gray S. L., Yetukuri L., et al. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS genetics. 2007;3(4, article e64) doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarafidis P. A., Lasaridis A. N. Actions of peroxisome proliferator-activated receptors-γ agonists explaining a possible blood pressure-lowering effect. American Journal of Hypertension. 2006;19(6):646–653. doi: 10.1016/j.amjhyper.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Iglarz M., Touyz R. M., Viel E. C., et al. Peroxisome proliferator-activated receptor-α and receptor-γ activators prevent cardiac fibrosis in mineralocorticoid-dependent hypertension. Hypertension. 2003;42(4):737–743. doi: 10.1161/01.hyp.0000083511.91817.b1. [DOI] [PubMed] [Google Scholar]
- 26.Lim S., Lee K.-S., Lee J. E., et al. Effect of a new PPAR-gamma agonist, lobeglitazone, on neointimal formation after balloon injury in rats and the development of atherosclerosis. Atherosclerosis. 2015;243(1):107–119. doi: 10.1016/j.atherosclerosis.2015.08.037.14251 [DOI] [PubMed] [Google Scholar]
- 27.Wojtkowska I., Tysarowski A., Seliga K., et al. PPAR gamma expression levels during development of heart failure in patients with coronary artery disease after coronary artery bypass-grafting. PPAR Research. 2014;2014:5. doi: 10.1155/2014/242790.242790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohamad H. E., Askar M. E., Hafez M. M. Management of cardiac fibrosis in diabetic rats: the role of peroxisome proliferator activated receptor gamma (PPAR-gamma) and calcium channel blockers (CCBs) Diabetology and Metabolic Syndrome. 2011;3(1, article 4) doi: 10.1186/1758-5996-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H., Wei T., Jiang X., et al. PEDF and 34-mer inhibit angiogenesis in the heart by inducing tip cells apoptosis via up-regulating PPAR-γ to increase surface FasL. Apoptosis. 2016;21(1):60–68. doi: 10.1007/s10495-015-1186-1. [DOI] [PubMed] [Google Scholar]
- 30.Chu Y., Lund D. D., Weiss R. M., et al. Pioglitazone attenuates valvular calcification induced by hypercholesterolemia. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(3):523–532. doi: 10.1161/atvbaha.112.300794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motoki T., Kurobe H., Hirata Y., et al. PPAR-γ agonist attenuates inflammation in aortic aneurysm patients. General Thoracic and Cardiovascular Surgery. 2015;63(10):565–571. doi: 10.1007/s11748-015-0576-1. [DOI] [PubMed] [Google Scholar]
- 32.Lim S., Cheng J. J., Kim M., et al. PPARγ gene transfer sustains apoptosis, inhibits vascular smooth muscle cell proliferation, and reduces neointima formation after balloon injury in rats. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(4):808–813. doi: 10.1161/01.atv.0000204634.26163.a7. [DOI] [PubMed] [Google Scholar]
- 33.Hu Q., Chen J., Jiang C., Liu H.-F. Effect of peroxisome proliferator-activated receptor gamma agonist on heart of rabbits with acute myocardial ischemia/reperfusion injury. Asian Pacific Journal of Tropical Medicine. 2014;7(4):271–275. doi: 10.1016/s1995-7645(14)60036-5. [DOI] [PubMed] [Google Scholar]
- 34.Wang H., Zhu Q. W., Ye P., et al. Pioglitazone attenuates myocardial ischemia-reperfusion injury via up-regulation of ERK and COX-2. BioScience Trends. 2012;6(6):325–332. doi: 10.5582/bst.2012.v6.6.325. [DOI] [PubMed] [Google Scholar]
- 35.Ihm S.-H., Chang K., Kim H.-Y., et al. Peroxisome proliferator-activated receptor-γ activation attenuates cardiac fibrosis in type 2 diabetic rats: the effect of rosiglitazone on myocardial expression of receptor for advanced glycation end products and of connective tissue growth factor. Basic Research in Cardiology. 2010;105(3):399–407. doi: 10.1007/s00395-009-0071-x. [DOI] [PubMed] [Google Scholar]
- 36.Zhao S. M. Cardiac fibrosis in diabetic rats: regulation and mechanism of activation of the PPARγ signal pathway. The Chinese Journal of Physiology. 2010;53(4):262–267. doi: 10.4077/cjp.2010.amk076. [DOI] [PubMed] [Google Scholar]
- 37.da Silva Torres T., Aguila M. B., Mandarim-de-Lacerda C. A. Rosiglitazone reverses cardiac adverse remodeling (fibrosis and vascularization) in perinatal low protein rat offspring. Pathology Research and Practice. 2010;206(9):642–646. doi: 10.1016/j.prp.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Liu T., Zhao H., Li J., Korantzopoulos P., Li G. Rosiglitazone attenuates atrial structural remodeling and atrial fibrillation promotion in alloxan-induced diabetic rabbits. Cardiovascular Therapeutics. 2014;32(4):178–183. doi: 10.1111/1755-5922.12079. [DOI] [PubMed] [Google Scholar]
- 39.Makino N., Sasaki M., Maeda T., Mimori K. Telomere biology in cardiovascular disease—role of insulin sensitivity in diabetic hearts. Experimental and Clinical Cardiology. 2010;15(4):e128–e133. [PMC free article] [PubMed] [Google Scholar]
- 40.Mishra P. K., Tyagi N., Sen U., Joshua I. G., Tyagi S. C. Synergism in hyperhomocysteinemia and diabetes: role of PPAR gamma and tempol. Cardiovascular Diabetology. 2010;9, article 49 doi: 10.1186/1475-2840-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shim C. Y., Song B.-W., Cha M.-J., et al. Combination of a peroxisome proliferator-activated receptor-gamma agonist and an angiotensin II receptor blocker attenuates myocardial fibrosis and dysfunction in type 2 diabetic rats. Journal of Diabetes Investigation. 2014;5(4):362–371. doi: 10.1111/jdi.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong K., Chen Y.-F., Li P., et al. Transforming growth factor-β inhibits myocardial PPARγ expression in pressure overload-induced cardiac fibrosis and remodeling in mice. Journal of Hypertension. 2011;29(9):1810–1819. doi: 10.1097/hjh.0b013e32834a4d03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi H.-P., Wang Y., Zhang Q.-H., et al. Activation of peroxisome proliferator-activated receptor γ (PPARγ) through NF-κB/brg1 and TGF-β1 pathways attenuates cardiac remodeling in pressure-overloaded rat hearts. Cellular Physiology and Biochemistry. 2015;35(3):899–912. doi: 10.1159/000369747. [DOI] [PubMed] [Google Scholar]
- 44.Shinzato T., Ohya Y., Nakamoto M., Ishida A., Takishita S. Beneficial effects of pioglitazone on left ventricular hypertrophy in genetically hypertensive rats. Hypertension Research. 2007;30(9):863–873. doi: 10.1291/hypres.30.863. [DOI] [PubMed] [Google Scholar]
- 45.Diep Q. N., Amiri F., Benkirane K., Paradis P., Schiffrin E. L. Long-term effects of the PPARγ activator pioglitazone on cardiac inflammation in stroke-prone spontaneously hypertensive rats. Canadian Journal of Physiology and Pharmacology. 2004;82(11):976–985. doi: 10.1139/y04-094. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura T., Yamamoto E., Kataoka K., et al. Beneficial effects of pioglitazone on hypertensive cardiovascular injury are enhanced by combination with candesartan. Hypertension. 2008;51(2):296–301. doi: 10.1161/HYPERTENSIONAHA.107.099044. [DOI] [PubMed] [Google Scholar]
- 47.Henderson B. C., Sen U., Reynolds C., et al. Reversal of systemic hypertension-associated cardiac remodeling in chronic pressure overload myocardium by ciglitazone. International Journal of Biological Sciences. 2007;3(6):385–392. doi: 10.7150/ijbs.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kato M. F., Shibata R., Obata K., et al. Pioglitazone attenuates cardiac hypertrophy in rats with salt-sensitive hypertension: role of activation of AMP-activated protein kinase and inhibition of Akt. Journal of Hypertension. 2008;26(8):1669–1676. doi: 10.1097/hjh.0b013e328302f0f7. [DOI] [PubMed] [Google Scholar]
- 49.Gray S., Kim J. K. New insights into insulin resistance in the diabetic heart. Trends in Endocrinology and Metabolism. 2011;22(10):394–403. doi: 10.1016/j.tem.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Candido R., Forbes J. M., Thomas M. C., et al. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circulation Research. 2003;92(7):785–792. doi: 10.1161/01.res.0000065620.39919.20. [DOI] [PubMed] [Google Scholar]
- 51.Koitabashi N., Arai M., Kogure S., et al. Increased connective tissue growth factor relative to brain natriuretic peptide as a determinant of myocardial fibrosis. Hypertension. 2007;49(5):1120–1127. doi: 10.1161/HYPERTENSIONAHA.106.077537. [DOI] [PubMed] [Google Scholar]
- 52.Mandavia C. H., Aroor A. R., Demarco V. G., Sowers J. R. Molecular and metabolic mechanisms of cardiac dysfunction in diabetes. Life Sciences. 2013;92(11):601–608. doi: 10.1016/j.lfs.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quintana M. T., He J., Sullivan J., et al. Muscle ring finger-3 protects against diabetic cardiomyopathy induced by a high fat diet. BMC Endocrine Disorders. 2015;15(1, article 36) doi: 10.1186/s12902-015-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He J., Quintana M. T., Sullivan J., et al. MuRF2 regulates PPARγ1 activity to protect against diabetic cardiomyopathy and enhance weight gain induced by a high fat diet. Cardiovascular Diabetology. 2015;14, article 97 doi: 10.1186/s12933-015-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao H., Gu D. W., Li H. T., Ge Q. F., Li G. P. Inhibitory effects of spironolactone on myocardial fibrosis in spontaneously hypertensive rats. Genetics and Molecular Research. 2015;14(3):10315–10321. doi: 10.4238/2015.august.28.17. [DOI] [PubMed] [Google Scholar]
- 56.Zambrano S., Blanca A. J., Ruiz-Armenta M. V., et al. L-Carnitine protects against arterial hypertension-related cardiac fibrosis through modulation of PPAR-γ expression. Biochemical Pharmacology. 2013;85(7):937–944. doi: 10.1016/j.bcp.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 57.Kis A., Murdoch C., Zhang M., et al. Defective peroxisomal proliferators activated receptor gamma activity due to dominant-negative mutation synergizes with hypertension to accelerate cardiac fibrosis in mice. European Journal of Heart Failure. 2009;11(6):533–541. doi: 10.1093/eurjhf/hfp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maejima Y., Okada H., Haraguchi G., et al. Telmisartan, a unique ARB, improves left ventricular remodeling of infarcted heart by activating PPAR gamma. Laboratory Investigation. 2011;91(6):932–944. doi: 10.1038/labinvest.2011.45. [DOI] [PubMed] [Google Scholar]
- 59.Fliegner D., Westermann D., Riad A., et al. Up-regulation of PPARγ in myocardial infarction. European Journal of Heart Failure. 2008;10(1):30–38. doi: 10.1016/j.ejheart.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Schupp M., Janke J., Clasen R., Unger T., Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-γ activity. Circulation. 2004;109(17):2054–2057. doi: 10.1161/01.cir.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- 61.Shiomi T., Tsutsui H., Hayashidani S., et al. Pioglitazone, a peroxisome proliferator-activated receptor-γ agonist, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002;106(24):3126–3132. doi: 10.1161/01.cir.0000039346.31538.2c. [DOI] [PubMed] [Google Scholar]
- 62.Tao L., Shen S., Fu S., et al. Traditional Chinese Medication Qiliqiangxin attenuates cardiac remodeling after acute myocardial infarction in mice. Scientific Reports. 2015;5, article 8374:10. doi: 10.1038/srep08374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Birnbaum Y., Long B., Qian J., Perez-Polo J. R., Ye Y. Pioglitazone limits myocardial infarct size, activates Akt, and upregulates cPLA2 and COX-2 in a PPAR-γ-independent manner. Basic Research in Cardiology. 2011;106(3):431–446. doi: 10.1007/s00395-011-0162-3. [DOI] [PubMed] [Google Scholar]
- 64.Frantz S., Hu K., Widder J., et al. Peroxisome proliferator activated-receptor agonism and left ventricular remodeling in mice with chronic myocardial infarction. British Journal of Pharmacology. 2004;141(1):9–14. doi: 10.1038/sj.bjp.0705585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lygate C. A., Hulbert K., Monfared M., Cole M. A., Clarke K., Neubauer S. The PPARγ-activator rosiglitazone does not alter remodeling but increases mortality in rats post-myocardial infarction. Cardiovascular Research. 2003;58(3):632–637. doi: 10.1016/s0008-6363(03)00289-x. [DOI] [PubMed] [Google Scholar]
- 66.Sarma S. Use of clinically available PPAR agonists for heart failure; do the risks outweigh the potential benefits? Current Molecular Pharmacology. 2012;5(2):255–263. doi: 10.2174/1874467211205020255. [DOI] [PubMed] [Google Scholar]
- 67.Zou C., Qi H., Liu Z.-H., Han L., Zhao C., Yang X. Simvastatin activates the PPARγ-dependent pathway to prevent left ventricular hypertrophy associated with inhibition of RhoA signaling. Texas Heart Institute Journal. 2013;40(2):140–147. [PMC free article] [PubMed] [Google Scholar]
- 68.Shimano M., Tsuji Y., Inden Y., et al. Pioglitazone, a peroxisome proliferator-activated receptor-gamma activator, attenuates atrial fibrosis and atrial fibrillation promotion in rabbits with congestive heart failure. Heart Rhythm. 2008;5(3):451–459. doi: 10.1016/j.hrthm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 69.Gómez-Garre D., Herraíz M., González-Rubio M. L., et al. Activation of peroxisome proliferator-activated receptor-α and -γ in auricular tissue from heart failure patients. European Journal of Heart Failure. 2006;8(2):154–161. doi: 10.1016/j.ejheart.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 70.Wang X., Liu X., Zhan Y., et al. Pharmacogenomic, physiological, and biochemical investigations on safety and efficacy biomarkers associated with the peroxisome proliferator-activated receptor-γ activator rosiglitazone in rodents: a translational medicine investigation. The Journal of Pharmacology and Experimental Therapeutics. 2010;334(3):820–829. doi: 10.1124/jpet.110.167635. [DOI] [PubMed] [Google Scholar]
- 71.Prasad A., Stone G. W., Holmes D. R., Gersh B. Reperfusion injury, microvascular dysfunction, and cardioprotection: the ‘dark side’ of reperfusion. Circulation. 2009;120(21):2105–2112. doi: 10.1161/circulationaha.108.814640. [DOI] [PubMed] [Google Scholar]
- 72.Lee S.-W., Won J.-Y., Lee H.-Y., et al. Angiopoietin-1 protects heart against ischemia/reperfusion injury through VE-cadherin dephosphorylation and myocardiac integrin-β1/ERK/caspase-9 phosphorylation cascade. Molecular Medicine. 2011;17(9-10):1095–1106. doi: 10.2119/molmed.2011.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khandoudi N., Delerive P., Berrebi-Bertrand I., Buckingham R. E., Staels B., Bril A. Rosiglitazone, a peroxisome proliferator-activated receptor-γ, inhibits the Jun NH2-terminal kinase/activating protein 1 pathway and protects the heart from ischemia/reperfusion injury. Diabetes. 2002;51(5):1507–1514. doi: 10.2337/diabetes.51.5.1507. [DOI] [PubMed] [Google Scholar]
- 74.Nagarajan D., Melo T., Deng Z., Almeida C., Zhao W. ERK/GSK3β/Snail signaling mediates radiation-induced alveolar epithelial-to-mesenchymal transition. Free Radical Biology and Medicine. 2012;52(6):983–992. doi: 10.1016/j.freeradbiomed.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rowe R. G., Lin Y., Shimizu-Hirota R., et al. Hepatocyte-derived snail1 propagates liver fibrosis progression. Molecular and Cellular Biology. 2011;31(12):2392–2403. doi: 10.1128/MCB.01218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boutet A., Esteban M. A., Maxwell P. H., Nieto M. A. Reactivation of Snail genes in renal fibrosis and carcinomas: a process of reversed embryogenesis? Cell Cycle. 2007;6(6):638–642. doi: 10.4161/cc.6.6.4022. [DOI] [PubMed] [Google Scholar]
- 77.Lee S.-W., Won J.-Y., Kim W. J., et al. Snail as a potential target molecule in cardiac fibrosis: paracrine action of endothelial cells on fibroblasts through snail and CTGF Axis. Molecular Therapy. 2013;21(9):1767–1777. doi: 10.1038/mt.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krul S. P. J., Berger W. R., Smit N. W., et al. Response to letter regarding article, ‘atrial fibrosis and conduction slowing in the left atrial appendage of patients undergoing thoracoscopic surgical pulmonary vein isolation for atrial fibrillation’. Circulation: Arrhythmia and Electrophysiology. 2015;8(4):p. 997. doi: 10.1161/circep.115.003223. [DOI] [PubMed] [Google Scholar]
- 79.Burstein B., Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. Journal of the American College of Cardiology. 2008;51(8):802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 80.Chen X., Bing Z., He J., et al. Downregulation of peroxisome proliferator-activated receptor-γ expression in hypertensive atrial fibrillation. Clinical Cardiology. 2009;32(6):337–345. doi: 10.1002/clc.20566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin Q., Jia L., Sun Y. A pilot study of circulating PPAR-γ receptor protein in elderly patients with atrial fibrillation. Archives of Medical Science. 2012;8(3):471–476. doi: 10.5114/aoms.2012.29402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gu J., Liu X., Wang Q.-X., et al. Beneficial effects of pioglitazone on atrial structural and electrical remodeling in vitro cellular models. Journal of Molecular and Cellular Cardiology. 2013;65:1–8. doi: 10.1016/j.yjmcc.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 83.Chello M., Mastroroberto P., Romano R., Zofrea S., Bevacqua I., Marchese A. R. Changes in the proportion of types I and III collagen in the left ventricular wall of patients with post-irradiative pericarditis. Cardiovascular Surgery. 1996;4(2):222–226. doi: 10.1016/0967-2109(96)82320-9. [DOI] [PubMed] [Google Scholar]
- 84.Gao S., Wu R., Zeng Y. Up-regulation of peroxisome proliferator-activated receptor gamma in radiation-induced heart injury in rats. Radiation and Environmental Biophysics. 2012;51(1):53–59. doi: 10.1007/s00411-011-0390-9. [DOI] [PubMed] [Google Scholar]
- 85.Jing L., Zhou L.-J., Zhang F.-M., Li W.-M., Sang Y. Tenascin-x facilitates myocardial fibrosis and cardiac remodeling through transforming growth factor-β1 and peroxisome proliferatoractivated receptor β in alcoholic cardiomyopathy. Chinese Medical Journal. 2011;124(3):390–395. doi: 10.3760/cma.j.issn.0366-6999.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 86.Hasegawa H., Takano H., Zou Y., et al. Pioglitazone, a peroxisome proliferator-activated receptor γ activator, ameliorates experimental autoimmune myocarditis by modulating Th1/Th2 balance. Journal of Molecular and Cellular Cardiology. 2005;38(2):257–265. doi: 10.1016/j.yjmcc.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 87.Singh A. P., Kaur T., Dahiya R. S., Singh N., Singh Bedi P. M. Ameliorative role of rosiglitazone in hyperhomocysteinemia-induced experimental cardiac hypertrophy. Journal of Cardiovascular Pharmacology. 2010;56(1):53–59. doi: 10.1097/fjc.0b013e3181de308b. [DOI] [PubMed] [Google Scholar]
- 88.Qin Y.-W., Ye P., He J.-Q., Sheng L., Wang L.-Y., Du J. Simvastatin inhibited cardiac hypertrophy and fibrosis in apolipoprotein E-deficient mice fed a ‘Western-style diet’ by increasing PPAR α and γ expression and reducing TC, MMP-9, and Cat S levels. Acta Pharmacologica Sinica. 2010;31(10):1350–1358. doi: 10.1038/aps.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rani N., Bharti S., Bhatia J., et al. Inhibition of TGF-β by a novel PPAR-γ agonist, chrysin, salvages β-receptor stimulated myocardial injury in rats through MAPKs-dependent mechanism. Nutrition and Metabolism. 2015;12, article 11 doi: 10.1186/s12986-015-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen R., Xue J., Xie M. Puerarin prevents isoprenaline-induced myocardial fibrosis in mice by reduction of myocardial TGF-β1 expression. The Journal of Nutritional Biochemistry. 2012;23(9):1080–1085. doi: 10.1016/j.jnutbio.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 91.Chen R., Xue J., Xie M.-L. Reduction of isoprenaline-induced myocardial TGF-β1 expression and fibrosis in osthole-treated mice. Toxicology and Applied Pharmacology. 2011;256(2):168–173. doi: 10.1016/j.taap.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 92.Guo B.-Y., Li Y.-J., Han R., et al. Telmisartan attenuates isoproterenol-induced cardiac remodeling in rats via regulation of cardiac adiponectin expression. Acta Pharmacologica Sinica. 2011;32(4):449–455. doi: 10.1038/aps.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Porter K. E., Turner N. A. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacology and Therapeutics. 2009;123(2):255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 94.Brown R. D., Ambler S. K., Mitchell M. D., Long C. S. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annual Review of Pharmacology and Toxicology. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 95.Hao G.-H., Niu X.-L., Gao D.-F., Wei J., Wang N.-P. Agonists at PPAR-γ suppress angiotensin II-induced production of plasminogen activator inhibitor-1 and extracellular matrix in rat cardiac fibroblasts. British Journal of Pharmacology. 2008;153(7):1409–1419. doi: 10.1038/bjp.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hou X., Zhang Y., Shen Y. H., et al. PPAR-γ activation by rosiglitazone suppresses angiotensin II-mediated proliferation and phenotypictransition in cardiac fibroblasts via inhibition of activation of activator protein 1. European Journal of Pharmacology. 2013;715(1–3):196–203. doi: 10.1016/j.ejphar.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 97.Zhao S.-M., Shen L.-H., Li H.-W., et al. Down-regulation of the expression of angiotensin II type 1 receptor in neonatal rat cardiac fibroblast by activation of PPARγ signal pathway. Chinese Journal of Physiology. 2008;51(6):357–362. [PubMed] [Google Scholar]
- 98.Li J., Liu N. F., Wei Q. Effect of rosiglitazone on cardiac fibroblast proliferation, nitric oxide production and connective tissue growth factor expression induced by advanced glycation end-products. Journal of International Medical Research. 2008;36(2):329–335. doi: 10.1177/147323000803600216. [DOI] [PubMed] [Google Scholar]
- 99.Martín R., Miana M., Jurado-López R., et al. Diol triterpenes block profibrotic effects of angiotensin II and protect from cardiac hypertrophy. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0041545.e41545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seymour E. M., Bennink M. R., Watts S. W., Bolling S. F. Whole grape intake impacts cardiac peroxisome proliferator-activated receptor and nuclear factor κb activity and cytokine expression in rats with diastolic dysfunction. Hypertension. 2010;55(5):1179–1185. doi: 10.1161/hypertensionaha.109.149393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen R., Xue J., Xie M. Osthole regulates TGF-β1 and MMP-2/9 expressions via activation of PPARα/γ in cultured mouse cardiac fibroblasts stimulated with angiotensin II. Journal of Pharmacy and Pharmaceutical Sciences. 2013;16(5):732–741. doi: 10.18433/j3hk5c. [DOI] [PubMed] [Google Scholar]
- 102.Nissen S. E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. The New England Journal of Medicine. 2007;356(24):2457–2471. doi: 10.1056/nejmoa072761. [DOI] [PubMed] [Google Scholar]
- 103.Nesto R. W., Bell D., Bonow R. O., et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2004;27(1):256–263. doi: 10.2337/diacare.27.1.256. [DOI] [PubMed] [Google Scholar]
- 104.Hampton T. Diabetes drugs tied to fractures in women. The Journal of the American Medical Association. 2007;297(15):p. 1645. doi: 10.1001/jama.297.15.1645. [DOI] [PubMed] [Google Scholar]
- 105.Lincoff A. M., Tardif J.-C., Schwartz G. G., et al. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. The Journal of the American Medical Association. 2014;311(15):1515–1525. doi: 10.1001/jama.2014.3321. [DOI] [PubMed] [Google Scholar]
- 106.Chen R., Liang F., Moriya J., et al. Peroxisome proliferator-activated receptors (PPARs) and their agonists for hypertension and heart failure: are the reagents beneficial or harmful? International Journal of Cardiology. 2008;130(2):131–139. doi: 10.1016/j.ijcard.2008.03.080. [DOI] [PubMed] [Google Scholar]