Abstract
BACKGROUND/OBJECTIVES
The incidence of thyroid cancer has increased in many countries, including Korea. International differences in the incidence of thyroid cancer may indicate a role of diet, but findings from previous studies are inconclusive. Therefore, we aimed to investigate the roles of nutrients in thyroid cancer risk in Korean women.
SUBJECTS/METHODS
We conducted a case-control study comprising 113 cases and 226 age-matched controls. Nutrient intake was assessed using a validated food frequency questionnaire, and the association between nutrient intake and thyroid cancer risk was estimated using a logistic regression model.
RESULTS
We found that high calcium intake was associated with a reduced risk of thyroid cancer (OR [95% CI] = 0.55 [0.35-0.89]). Significant associations were observed among subjects who were older than 50 years, had low BMI, and had low calorie intake. However, other nutrients included in this study did not show any significant associations with thyroid cancer risk.
CONCLUSIONS
This study suggested a possible protective effect of calcium on thyroid cancer risk. Well-designed prospective studies are required to confirm these findings.
Keywords: Thyroid cancer, nutrient, calcium, Korean
INTRODUCTION
The incidence of thyroid cancer is increasing worldwide, and thyroid cancer represents the most common type of endocrine tumor [1]. The incidence of thyroid cancer in Korea has increased rapidly in women and has become one of the highest in the world [2]. The increased incidence rate of thyroid cancer may be partially attributed to the increased detection of subclinical cancer due to advanced diagnostic technologies and frequent medical examinations [3]. Although the risk of thyroid cancer has increased, relatively little is known about the etiology of this disease. Established risk factors include ionizing radiation and a history of benign proliferative thyroid diseases, including goiter and thyroid nodules. However, international differences in the incidence of thyroid cancer suggest that lifestyle or environmental factors (including diet) may play a role in thyroid carcinogenesis [4].
Several epidemiologic studies have indicated that dietary habits may play a relevant role in the development of thyroid cancer. Iodine is the most commonly investigated factor because of its role in the formation of thyroid hormones in the thyroid gland and in benign thyroid disorders [5,6,7]. Some dietary factors may be related to thyroid cancer risk, including the consumption of fish [4,8,9], fruits and vegetables, particularly cruciferous vegetables [10,11,12]. It is assumed that specific nutrients and components in these foods (e.g., iodine, vitamin C, vitamin E, and β-carotene) may contribute to thyroid carcinogenesis. A few studies have investigated the roles of specific nutrients, but the findings from these studies are still inconclusive [13,14].
The present study investigated the roles of nutrients in thyroid cancer risk in Korean women. We also examined whether these associations differed with respect to other possible risk factors of thyroid cancer.
SUBJECTS AND METHODS
Study population
We conducted a case-control study on participants in the Cancer Screening Program at the National Cancer Center in South Korea [15] between October 2007 and July 2014. Participants were aged 30 years or older and underwent screening examinations for overall health and for selected cancers. All of the participants were asked to complete a self-administered questionnaire during the baseline evaluation. The data collected in the baseline evaluation included socio-demographic characteristics, personal and family medical history, lifestyle factors, and reproductive factors. Among 13,646 participants, 9,146 provided FFQ and were used as potential cases and controls.
Potential cases of thyroid cancer (ICD10 code C73) were ascertained by linkage to the Korea Central Cancer Registry (KCCR) database, which was used to determine the incidence of cancer in Korea. One hundred thirteen women thyroid cancer patients were identified as thyroid cancer after a baseline examination. Controls were selected from participants who were free from all cancers. Two controls for each case were matched by age. A total of 113 thyroid cancer cases and 226 controls were used for the final analysis. The study protocol was approved by the Institutional Review Board of the National Cancer Center (NCCNCS13698), and written informed consent was obtained from each participant.
Data collection and dietary assessment
All of the participants were asked to complete a self-administered questionnaire, which included socio-demographic characteristics, personal and family medical history, and lifestyle and reproductive factors. Participants were also asked about their average frequency of intake and portion size of specific foods eaten during the previous year using the validated food frequency questionnaire (FFQ), which covers 106 food items [16]. The detailed procedure used to design the FFQ is described by Ahn et al. [17] and the validity and reproducibility of the FFQ used in the current study have been tested using the three-day dietary record as a gold standard [16]. Nine frequency categories (i.e., never or rarely, once a month, two or three times a month, once or twice a week, three or four times a week, five or six times a week, once a day, twice a day, and three times a day) and three portion sizes (i.e., small, medium, and large) were included in the FFQ.
Nutrient intake (e.g., macronutrients, vitamins, and minerals) was computed by multiplying the frequency of consumption of each unit of food with the nutrient content of the specified portions. Dietary protein, fat, calcium, and iron intake were each calculated separately by their sources (animal vs. plant).
Statistical analysis
All statistical analyses were performed using the SAS 9.3 software (SAS Institute Inc., Cary, NC). A two-sided P-value less than 0.05 was considered statistically significant.
The chi-square (χ2) test (for categorical variables) and t-test (for continuous variables) were used to compare general characteristics between cases and controls. Energy-adjusted nutrient intakes were computed as the residuals from the regression model, with total caloric intake as the independent variable and absolute nutrient intake as the dependent variable [18]. We categorized participants into two groups (high/low) based on median nutrient intake levels in the control groups. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using a logistic regression model. Multivariate models were adjusted for body mass index (BMI; calculated as weight in kilograms divided by the square of the height in meters; < 23, 23-25, and ≥ 25) and smoking status (nonsmoker, former smoker, and current smoker) based on a literature search [19,20]. Protein, fat, calcium, and iron intake were analyzed according to their sources (animal or plant). We also conducted subgroup analyses of the association between dietary calcium intake and thyroid cancer risk by age, BMI, and the level of total caloric intake.
RESULTS
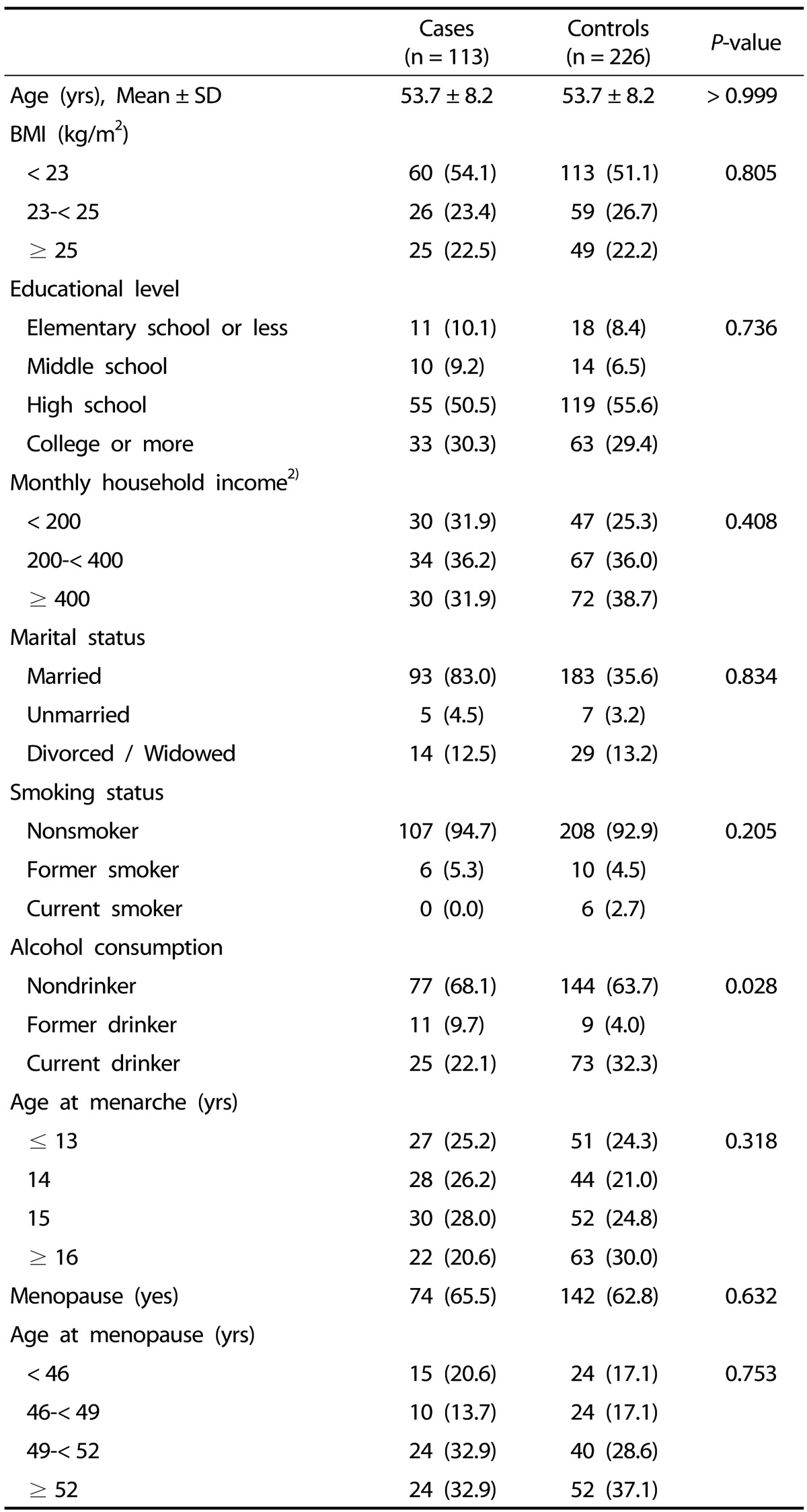
The general characteristics of the study participants are shown in Table 1. The mean age of the study participants was 53.7 years, and only women were included. No differences in age, BMI, education level, income, marital status, smoking status, or reproductive factors were observed between the cases and the controls. Cases were less likely to drink alcohol than controls.
Table 1. General characteristics of the study subjects1).
1) Data were analyzed using chi-square test (for categorical variables) and t-test (for continuous variables).
Data are presented as n (%) unless otherwise indicated.
2) Unit is 10,000 Korean won.
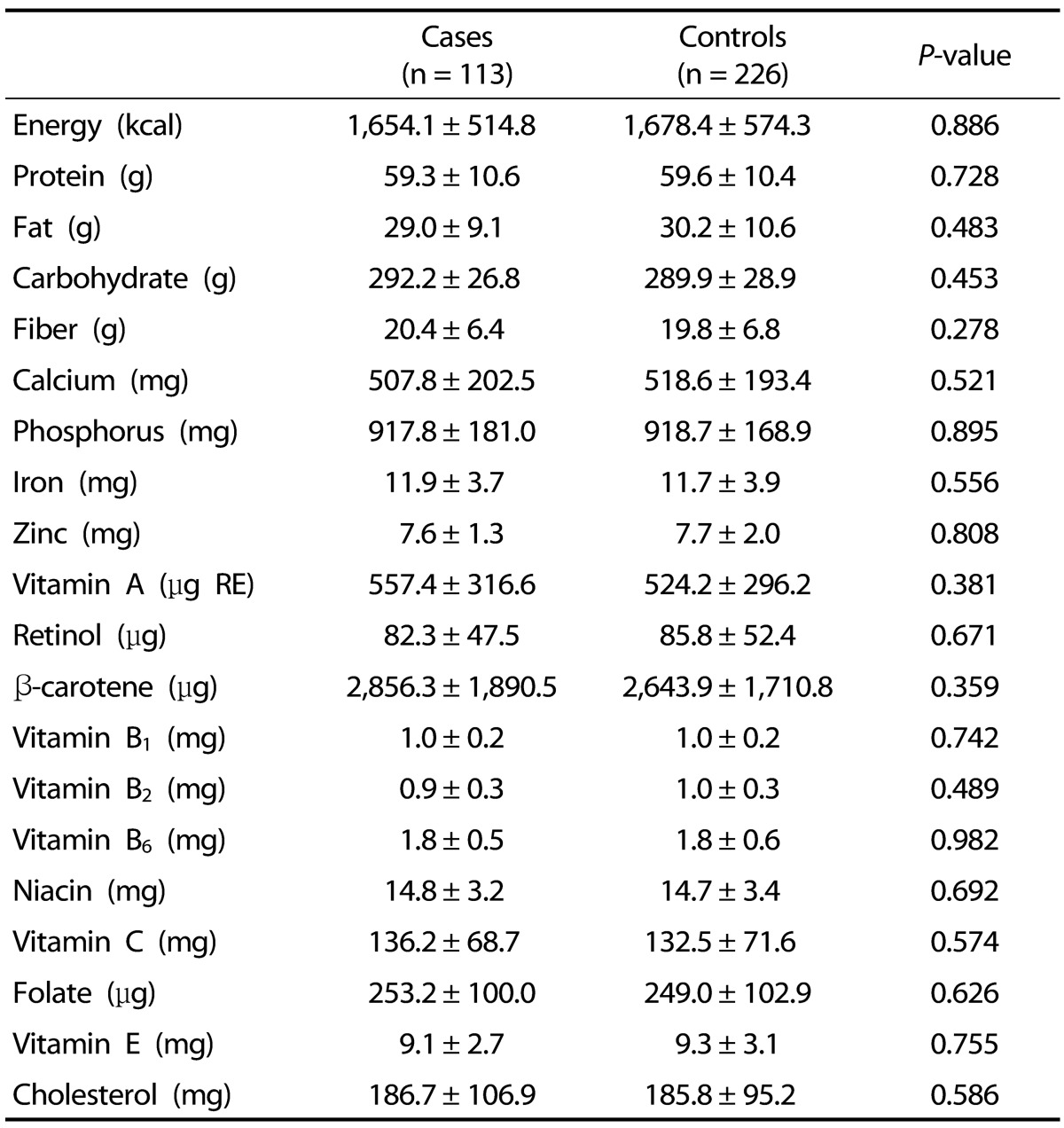
Table 2 shows the nutrient intakes of the subjects. The estimated mean intake of energy in this study was 1,670 kcal/day. We also compared the intake of macronutrients (protein, fat, and carbohydrates), vitamins (vitamin A, retinol, β-carotene, vitamin B1, vitamin B2, vitamin B6, niacin, and vitamin C) and minerals (calcium, phosphorus, iron, zinc) between cases and controls. However, we did not find any differences between cases and controls with respect to these dietary factors.
Table 2. Comparison of nutrient intake among study participants1).
1) Data were analyzed using t-test and are presented as mean ± SD.
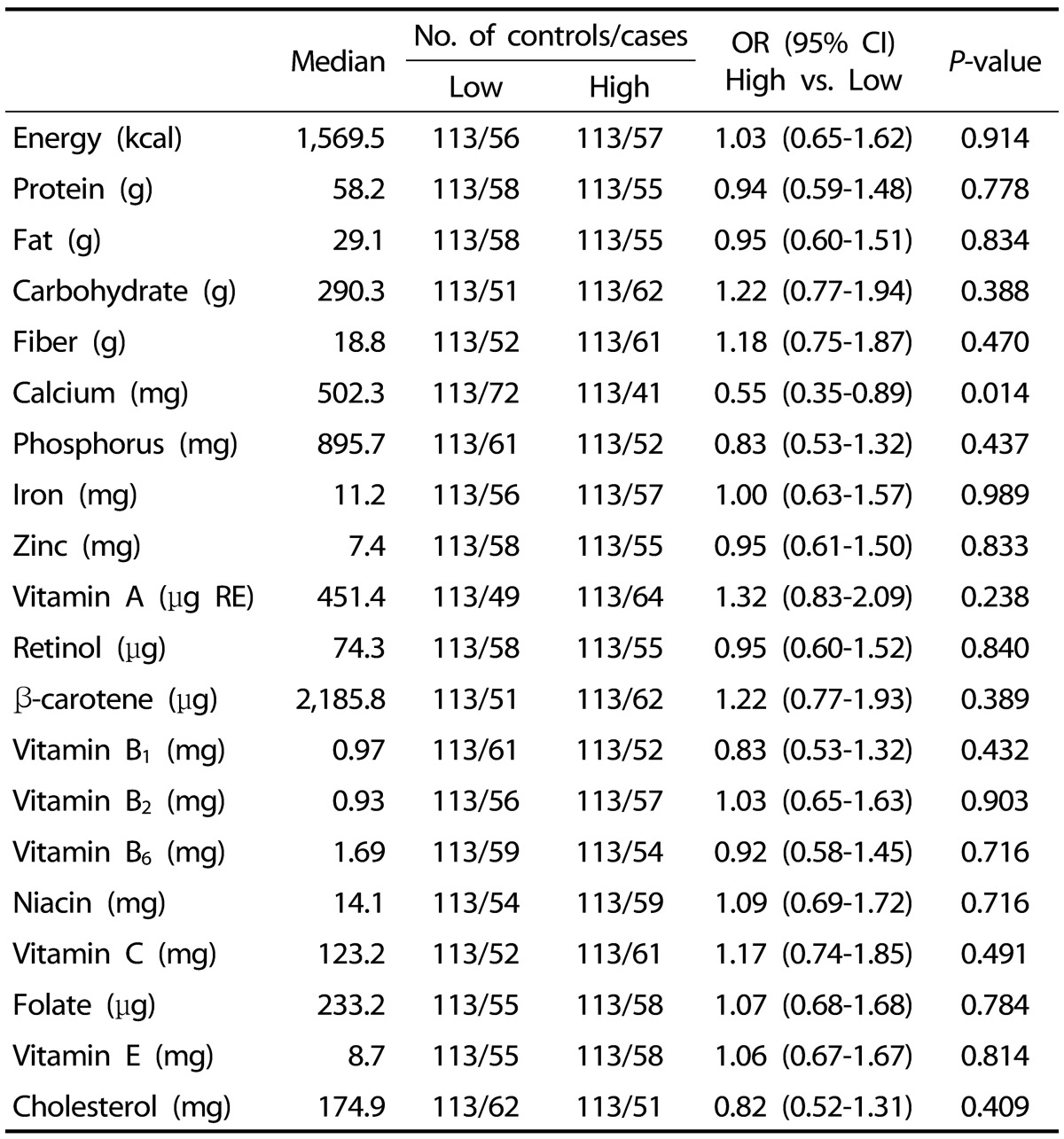
Table 3 shows the ORs and 95% CIs of thyroid cancer risk for selected nutrients in women. We found that a high consumption of calcium was associated with a reduced risk of thyroid cancer (OR [95% CI] = 0.55 [0.35-0.89]; P = 0.014). We did not observe any significant associations between thyroid cancer risk and the other nutrients.
Table 3. Association between the level of calcium intake and thyroid cancer risk1).
1) Data were categorized into two groups (high/low) based on median nutrient intake levels in the control group and were analyzed using a logistic regression model.
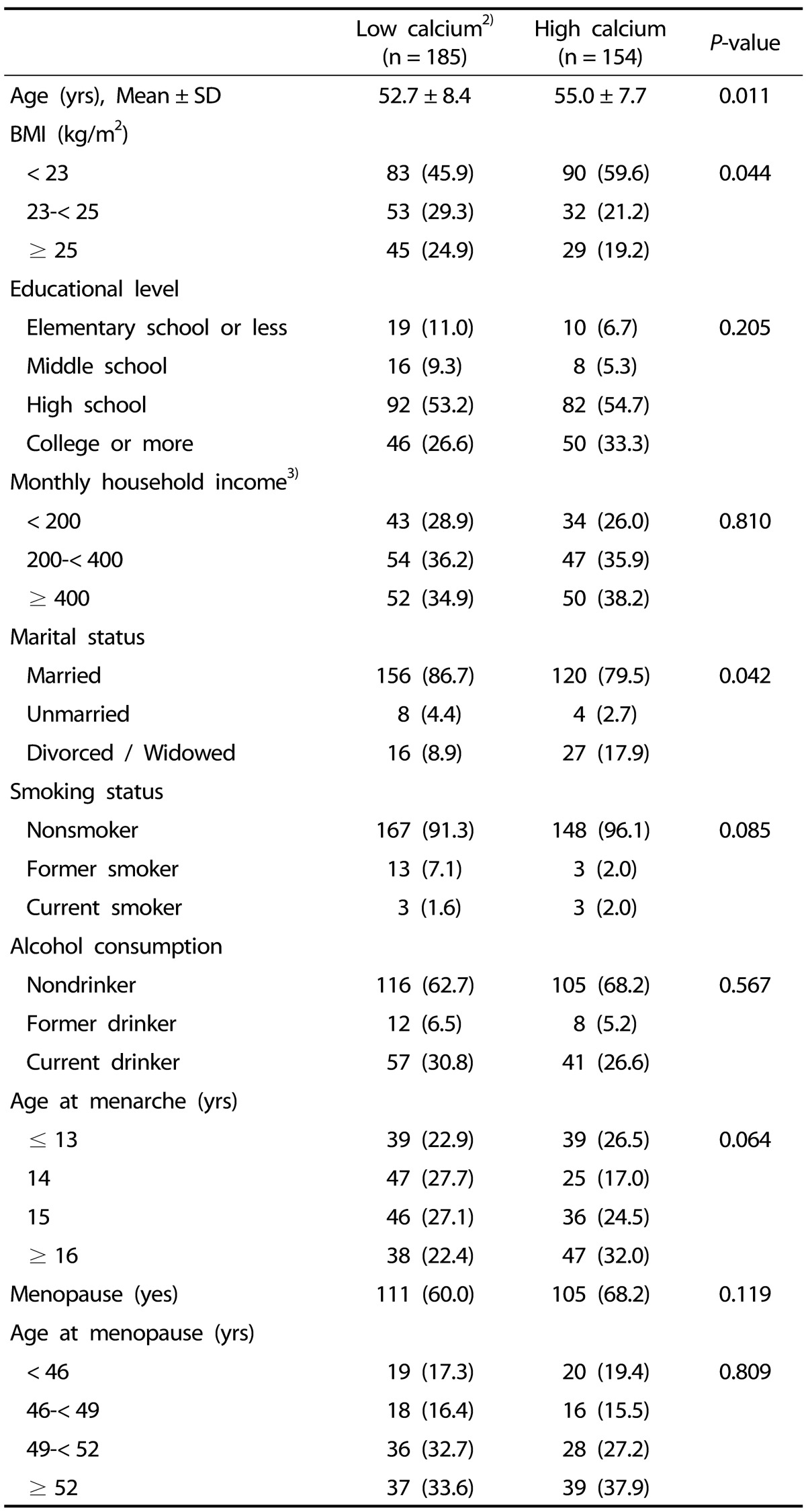
We therefore categorized the participants into two groups according to their levels of calcium intake and examined whether the general characteristics of the participants differed by calcium intake levels. The general characteristics of the participants were not significantly different in the high calcium and low calcium intake groups (Table 4).
Table 4. Participant characteristics by calcium intake1).
1) Data were analyzed using chi-square test (for categorical variables) and t-test (for continuous variables).
Data are presented as n (%) unless otherwise indicated.
2) Data were categorized into two groups (high/low) based on median nutrient intake levels in the control group.
3) Unit is 10,000 Korean won.
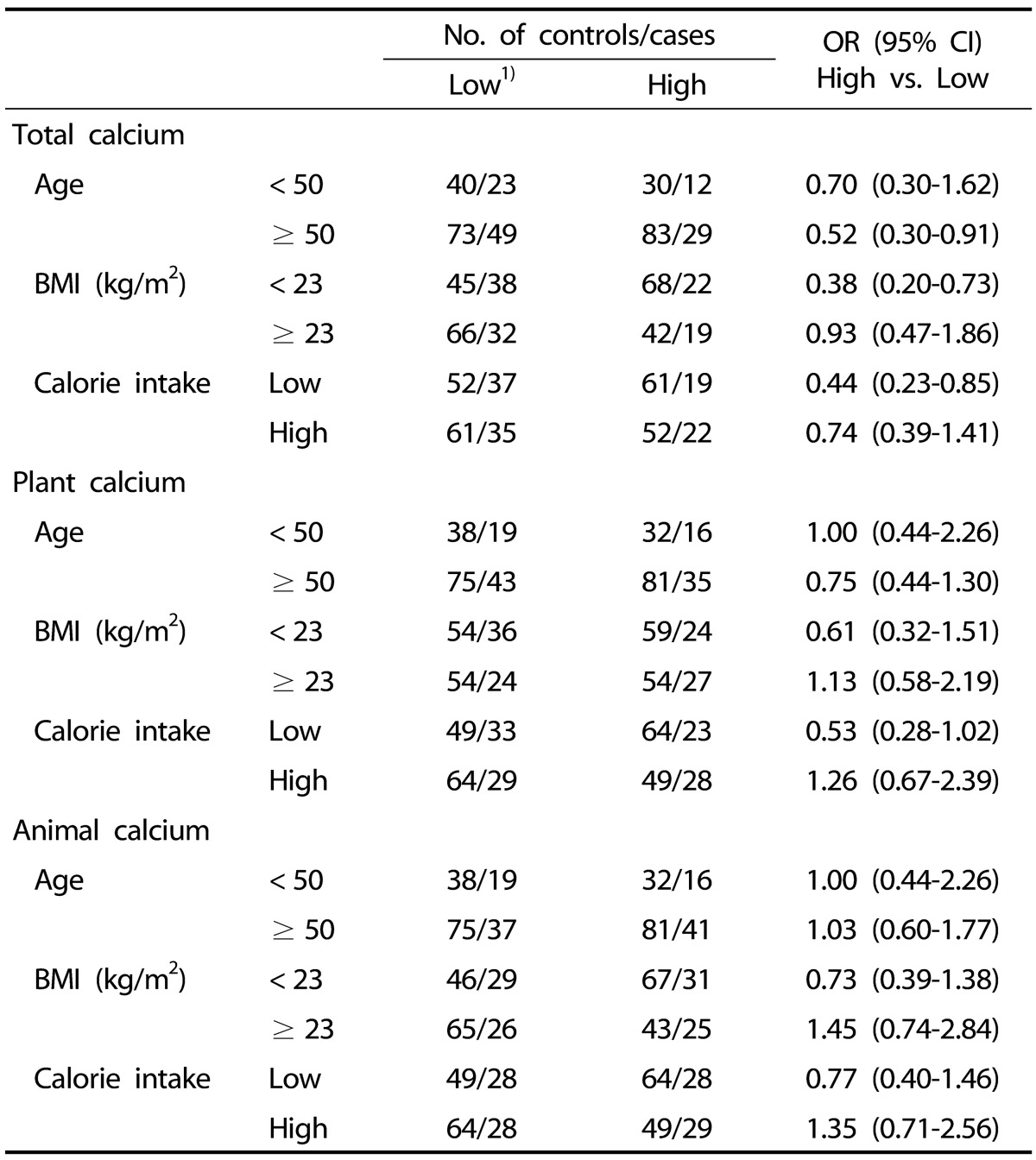
To examine the effects of other risk factors on the association between calcium intake and thyroid cancer risk, we conducted subgroup analyses by age, BMI, smoking status, and the level of total caloric intake (Table 5). Significant associations were observed in subjects who were older than 50 (OR [95% CI] = 0.52 [0.30-0.91]), had low BMI (OR [95% CI] = 0.38 [0.20-0.73]), and had low calorie intake (OR [95% CI] = 0.44 [0.23-0.85]). However, these significant associations were not observed in a subgroup analysis differentiating the source of calcium (plant vs. animal). Multivariate logistic regressions adjusted by BMI and smoking status showed similar associations. Therefore, we only report the results from crude analyses.
Table 5. Association between calcium intake and thyroid cancer risk.
1) Data were categorized into two groups (high/low) based on median nutrient intake levels in the control group.
DISCUSSION
This case-control study found that total calcium intake was inversely associated with the incidence of thyroid cancer. This association differed by age, BMI, and total calorie intake. However, other nutrients included in this study were not associated with the incidence of thyroid cancer.
Several epidemiological studies have investigated the role of specific nutrients, including calcium, with respect to cancer risk. One case-control study in the United States investigated the association between thyroid cancer and calcium, zinc, magnesium, and iron supplements but reported no significant associations [21]. Two cohort studies in the United States assessed the risk of thyroid cancer by levels of calcium and other nutrients, but these studies found no significant differences in risk [14,22]. However, the chemo-preventive effect of calcium on other types of cancer was reported in previous experimental and epidemiological studies. Evidence from animal studies suggested that a high calcium intake reduces colonic carcinogenesis [23]. Calcium supplements also reduce colonic epithelial cell proliferation in humans [24]. In a randomized clinical trial, Lappe et al. [25] found that improving calcium nutritional status substantially decreased the risk of cancer in postmenopausal women. A pooled analysis of 10 cohort studies [26] also reported that calcium intake is associated with a 15% to 20% reduced risk of colorectal cancer. A significant inverse association of calcium intake was also observed with breast cancer risk [27]. The present study did not identify associations between thyroid cancer risk and other nutrients, including antioxidants. Previous studies reported a potential inverse association of β-carotene, vitamins C and E, and selenium with thyroid cancer risk [28,29]. The role of these nutrients in cancer prevention stems from their antioxidant properties. The role of antioxidants in thyroid carcinogenesis requires further investigation.
The mechanism by which calcium affects thyroid carcinogenesis is not clear. Calcium is an important micronutrient that controls numerous intracellular and extracellular processes [30]. Calcium reduces proliferation, stimulates differentiation, and induces apoptosis [31,32], which may play a role in carcinogenesis [33,34]. Evidence suggests that calcium has direct effects in both normal and tumor cells [31], and may improve signaling within cells, thereby causing cancer cells to differentiate or die [31,35]. An experimental study demonstrated that elevated calcium concentrations decrease cell proliferation and induce the differentiation of mammary cells [36]. However, the role of calcium in carcinogenesis may differ by cancer site [22]. In the gastrointestinal tract, calcium binds to bile and fatty acids to form insoluble complexes, which may reduce the proliferative stimuli of these compounds [31]. Calcium is hypothesized to reduce fat-induced cell proliferation in breast cancer by maintaining intracellular calcium concentrations [32]. However, the exact mechanisms by which calcium helps reduce the risk of thyroid cancer are unclear due to a lack of evidence, and these mechanisms should be investigated further.
Several factors may affect the role of calcium in thyroid carcinogenesis. We conducted subgroup analyses of known risk factors of thyroid cancer, such as age, sex, BMI, and high caloric intake [37]. The incidence of thyroid cancer is higher in women than in men, and smoking is inversely associated with thyroid cancer risk [20]. Previous studies indicated that obesity was weakly associated with an elevated risk of thyroid cancer [37], and data from the Korea National Health and Nutrition Examination Survey reported that a higher calcium intake was associated with a decreased prevalence of obesity in Koreans [38]. We found a significant inverse association only in subjects with lower BMIs. Other nutrients, such as vitamin D, may also affect the role of calcium in carcinogenesis [26]. The mean age of the study population was 53.7 years, and the study participants consumed more calcium from non-dairy sources than from dairy products, which is in contrast to Western populations. The population characteristics in this study may also affect the associations found. Calcium intake in Korean populations is lower than in Western populations [38]. An understanding of how these factors modify the association between calcium and thyroid cancer risk may help elucidate the etiology of thyroid cancer.
However, the findings from this study should be interpreted with caution because of several limitations. First, this was a case-control study and had its own limitations (e.g., recall bias, selection bias). Recall of diet may be influenced by disease status. Second, the sample size of the study was relatively small, and some significant associations may have been missed. In addition, due to the large number of nutrients included in this analysis, the association between calcium and thyroid cancer risk could be a chance finding. Third, the data from the FFQ may limit our estimations of nutritional effects on thyroid cancer risk because self-reporting is subject to measurement error. The FFQ surveyed information from the previous year, but dietary intake varies from day to day and over the course of a lifetime. Further errors may also have been introduced by the conversion of food data into nutrient data using tables detailing the chemical compositions of foods. Finally, we did not examine whether associations with calcium intake differed by tumor subtype or tumor aggressiveness. Therefore, we may have missed some associations that exist only for certain tumor subtypes or for more or less aggressive tumors.
In conclusion, this study suggested a possible protective effect of calcium on thyroid cancer risk in Korean women. However, further large prospective studies are required to confirm this finding.
Footnotes
This work was supported by grants from the National Research Foundation of Korea (NRF-2015R1D1A1A09058684) and from the National Cancer Center of Korea (NCC1510040).
References
- 1.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Oh CM, Kong HJ, Cho H, Lee DH, Lee KH. Prediction of cancer incidence and mortality in Korea, 2015. Cancer Res Treat. 2015;47:142–148. doi: 10.4143/crt.2015.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verkooijen HM, Fioretta G, Pache JC, Franceschi S, Raymond L, Schubert H, Bouchardy C. Diagnostic changes as a reason for the increase in papillary thyroid cancer incidence in Geneva, Switzerland. Cancer Causes Control. 2003;14:13–17. doi: 10.1023/a:1022593923603. [DOI] [PubMed] [Google Scholar]
- 4.Dal Maso L, Bosetti C, La Vecchia C, Franceschi S. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control. 2009;20:75–86. doi: 10.1007/s10552-008-9219-5. [DOI] [PubMed] [Google Scholar]
- 5.Harach HR, Ceballos GA. Thyroid cancer, thyroiditis and dietary iodine: a review based on the Salta, Argentina model. Endocr Pathol. 2008;19:209–220. doi: 10.1007/s12022-008-9038-y. [DOI] [PubMed] [Google Scholar]
- 6.Horn-Ross PL, Morris JS, Lee M, West DW, Whittemore AS, McDougall IR, Nowels K, Stewart SL, Spate VL, Shiau AC, Krone MR. Iodine and thyroid cancer risk among women in a multiethnic population: the Bay Area Thyroid Cancer Study. Cancer Epidemiol Biomarkers Prev. 2001;10:979–985. [PubMed] [Google Scholar]
- 7.Blomberg M, Feldt-Rasmussen U, Andersen KK, Kjaer SK. Thyroid cancer in Denmark 1943-2008, before and after iodine supplementation. Int J Cancer. 2012;131:2360–2366. doi: 10.1002/ijc.27497. [DOI] [PubMed] [Google Scholar]
- 8.Cléro É, Doyon F, Chungue V, Rachédi F, Boissin JL, Sebbag J, Shan L, Bost-Bezeaud F, Petitdidier P, Dewailly E, Rubino C, de Vathaire F. Dietary iodine and thyroid cancer risk in French Polynesia: a case-control study. Thyroid. 2012;22:422–429. doi: 10.1089/thy.2011.0173. [DOI] [PubMed] [Google Scholar]
- 9.Wingren G, Hatschek T, Axelson O. Determinants of papillary cancer of the thyroid. Am J Epidemiol. 1993;138:482–491. doi: 10.1093/oxfordjournals.aje.a116882. [DOI] [PubMed] [Google Scholar]
- 10.Jung SK, Kim K, Tae K, Kong G, Kim MK. The effect of raw vegetable and fruit intake on thyroid cancer risk among women: a case-control study in South Korea. Br J Nutr. 2013;109:118–128. doi: 10.1017/S0007114512000591. [DOI] [PubMed] [Google Scholar]
- 11.Bosetti C, Negri E, Kolonel L, Ron E, Franceschi S, Preston-Martin S, McTiernan A, Dal Maso L, Mark SD, Mabuchi K, Land C, Jin F, Wingren G, Galanti MR, Hallquist A, Glattre E, Lund E, Levi F, Linos D, La Vecchia C. A pooled analysis of case-control studies of thyroid cancer. VII. Cruciferous and other vegetables (International) Cancer Causes Control. 2002;13:765–775. doi: 10.1023/a:1020243527152. [DOI] [PubMed] [Google Scholar]
- 12.Truong T, Baron-Dubourdieu D, Rougier Y, Guénel P. Role of dietary iodine and cruciferous vegetables in thyroid cancer: a countrywide case-control study in New Caledonia. Cancer Causes Control. 2010;21:1183–1192. doi: 10.1007/s10552-010-9545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang LR, Sawka AM, Adams L, Hatfield N, Hung RJ. Vitamin and mineral supplements and thyroid cancer: a systematic review. Eur J Cancer Prev. 2013;22:158–168. doi: 10.1097/CEJ.0b013e32835849b0. [DOI] [PubMed] [Google Scholar]
- 14.O'Grady TJ, Kitahara CM, DiRienzo AG, Gates MA. The association between selenium and other micronutrients and thyroid cancer incidence in the NIH-AARP Diet and Health Study. PLoS One. 2014;9:e110886. doi: 10.1371/journal.pone.0110886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J. Cancer screenee cohort study of the National Cancer Center in South Korea. Epidemiol Health. 2014;36:e2014013. doi: 10.4178/epih/e2014013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, Park C, Kim DH. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr. 2007;61:1435–1441. doi: 10.1038/sj.ejcn.1602657. [DOI] [PubMed] [Google Scholar]
- 17.Ahn Y, Lee JE, Paik HY, Lee HK, Jo I, Kimm K. Development of a semi-quantitative food frequency questionnaire based on dietary data from the Korea National Health and Nutrition Examination Survey. Nutr Sci. 2003;6:173–184. [Google Scholar]
- 18.Willett WC. Nutritional Epidemiology. 3rd ed. Oxford: Oxford University Press; 2013. [Google Scholar]
- 19.Han JM, Kim TY, Jeon MJ, Yim JH, Kim WG, Song DE, Hong SJ, Bae SJ, Kim HK, Shin MH, Shong YK, Kim WB. Obesity is a risk factor for thyroid cancer in a large, ultrasonographically screened population. Eur J Endocrinol. 2013;168:879–886. doi: 10.1530/EJE-13-0065. [DOI] [PubMed] [Google Scholar]
- 20.Cho YA, Kim J. Thyroid cancer risk and smoking status: a meta-analysis. Cancer Causes Control. 2014;25:1187–1195. doi: 10.1007/s10552-014-0422-2. [DOI] [PubMed] [Google Scholar]
- 21.Ron E, Kleinerman RA, Boice JD, Jr, LiVolsi VA, Flannery JT, Fraumeni JF., Jr A population-based case-control study of thyroid cancer. J Natl Cancer Inst. 1987;79:1–12. [PubMed] [Google Scholar]
- 22.Park Y, Leitzmann MF, Subar AF, Hollenbeck A, Schatzkin A. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch Intern Med. 2009;169:391–401. doi: 10.1001/archinternmed.2008.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipkin M. Preclinical and early human studies of calcium and colon cancer prevention. Ann N Y Acad Sci. 1999;889:120–127. doi: 10.1111/j.1749-6632.1999.tb08729.x. [DOI] [PubMed] [Google Scholar]
- 24.Lipkin M, Newmark H. Effect of added dietary calcium on colonic epithelial-cell proliferation in subjects at high risk for familial colonic cancer. N Engl J Med. 1985;313:1381–1384. doi: 10.1056/NEJM198511283132203. [DOI] [PubMed] [Google Scholar]
- 25.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 26.Cho E, Smith-Warner SA, Spiegelman D, Beeson WL, van den Brandt PA, Colditz GA, Folsom AR, Fraser GE, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Miller AB, Pietinen P, Potter JD, Rohan TE, Terry P, Toniolo P, Virtanen MJ, Willett WC, Wolk A, Wu K, Yaun SS, Zeleniuch-Jacquotte A, Hunter DJ. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst. 2004;96:1015–1022. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 27.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J Natl Cancer Inst. 2002;94:1301–1311. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 28.D'Avanzo B, Ron E, La Vecchia C, Francaschi S, Negri E, Zleglar R. Selected micronutrient intake and thyroid carcinoma risk. Cancer. 1997;79:2186–2192. [PubMed] [Google Scholar]
- 29.Touvier M, Kesse E, Clavel-Chapelon F, Boutron-Ruault MC. Dual Association of beta-carotene with risk of tobacco-related cancers in a cohort of French women. J Natl Cancer Inst. 2005;97:1338–1344. doi: 10.1093/jnci/dji276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 31.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3:601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson EA, James KA, Newmark HL, Carroll KK. Effects of dietary fat, calcium, and vitamin D on growth and mammary tumorigenesis induced by 7,12-dimethylbenz(a)anthracene in female Sprague-Dawley rats. Cancer Res. 1989;49:6300–6303. [PubMed] [Google Scholar]
- 33.Whitfield JF, Bird RP, Chakravarthy BR, Isaacs RJ, Morley P. Calcium-cell cycle regulator, differentiator, killer, chemopreventor, and maybe, tumor promoter. J Cell Biochem Suppl. 1995;22:74–91. [PubMed] [Google Scholar]
- 34.Sergeev IN. Calcium as a mediator of 1,25-dihydroxyvitamin D3-induced apoptosis. J Steroid Biochem Mol Biol. 2004;89-90:419–425. doi: 10.1016/j.jsbmb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Milner JA, McDonald SS, Anderson DE, Greenwald P. Molecular targets for nutrients involved with cancer prevention. Nutr Cancer. 2001;41:1–16. doi: 10.1080/01635581.2001.9680606. [DOI] [PubMed] [Google Scholar]
- 36.Xue L, Lipkin M, Newmark H, Wang J. Influence of dietary calcium and vitamin D on diet-induced epithelial cell hyperproliferation in mice. J Natl Cancer Inst. 1999;91:176–181. doi: 10.1093/jnci/91.2.176. [DOI] [PubMed] [Google Scholar]
- 37.Peterson E, De P, Nuttall R. BMI, diet and female reproductive factors as risks for thyroid cancer: a systematic review. PLoS One. 2012;7:e29177. doi: 10.1371/journal.pone.0029177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee HJ, Cho JI, Lee HS, Kim CI, Cho E. Intakes of dairy products and calcium and obesity in Korean adults: Korean National Health and Nutrition Examination Surveys (KNHANES) 2007-2009. PLoS One. 2014;9:e99085. doi: 10.1371/journal.pone.0099085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HJ, Cho JI, Lee HS, Kim CI, Cho E. Intakes of dairy products and calcium and obesity in Korean adults: Korean National Health and Nutrition Examination Surveys (KNHANES) 2007-2009. PLoS One. 2014;9:e99085. doi: 10.1371/journal.pone.0099085. [DOI] [PMC free article] [PubMed] [Google Scholar]