Abstract
Background
Identifying patients likely to have improved renal function after percutaneous transluminal renal angioplasty and stenting (PTRA) for renal artery stenosis (RAS) is challenging. The purpose of this study was to use a comprehensive multimarker assessment to identify those patients who would benefit most from correction of RAS.
Methods
In 127 patients with RAS and decreased renal function and/or hypertension referred for PTRA, quantification of hemodynamic cardiac stress using B-type natriuretic peptide (BNP), renal function using estimated glomerular filtration rate (eGFR), parenchymal renal damage using resistance index (RI), and systemic inflammation using C-reactive protein (CRP) were performed before intervention.
Results
Predefined renal function improvement (increase in eGFR ≥10%) at 6 months occurred in 37% of patients. Prognostic accuracy as quantified by the area under the receiver-operating characteristics curve for the ability of BNP, eGFR, RI and CRP to predict renal function improvement were 0.59 (95% CI, 0.48–0.70), 0.71 (95% CI, 0.61–0.81), 0.52 (95% CI, 0.41–0.65), and 0.56 (95% CI, 0.44–0.68), respectively. None of the possible combinations increased the accuracy provided by eGFR (lower eGFR indicated a higher likelihood for eGFR improvement after PTRA, P=ns for all). In the subgroup of 56 patients with pre-interventional eGFR <60 mL/min/1.73 m2, similar findings were obtained.
Conclusions
Quantification of renal function, but not any other pathophysiologic signal, provides at least moderate accuracy in the identification of patients with RAS in whom PTRA will improve renal function.
Keywords: Natriuretic peptides, C-reactive protein (CRP), resistance index (RI), renal artery stenosis (RAS), renal function, angioplasty
Introduction
Renal artery stenosis (RAS) is a relatively common problem in patients with systemic atherosclerosis and may lead to uncontrolled arterial hypertension, renal insufficiency, and cardiac disorders, including flush pulmonary edema and heart failure (1). The progressively impaired renal function in patients with RAS is assumed to be caused not only by reduced blood flow to the kidney but also by loss of microvascular renal perfusion induced by hypertensive and ischemic nephropathy (2). The pathophysiological concept is based on the fact that the reduction of perfusion pressure to the kidney activates the renin-angiotensin system, adrenergic stimuli, and volume expansion. Furthermore, the coexistence of hypoperfusion, atherosclerosis, and cardiovascular risk factors activates several additional deleterious proinflammatory and profibrotic pathways that have been implicated in progression of renal damage in hypoperfused kidneys (3).
The effect of percutaneous transluminal renal angioplasty and stenting (PTRA) for hemodynamic relevant RAS on renal function as well as the prediction of patients in whom PTRA improves renal function is a matter of debate (1,4,5).
We aimed to investigate a comprehensive multimarker assessment with quantification of hemodynamic cardiac stress using B-type natriuretic peptide (BNP), quantification of renal function using estimated glomerular filtration rate (eGFR), quantification of parenchymal renal damage using resistance index (RI), and quantification of systemic inflammation using C-reactive protein (CRP) in the prediction of renal function improvement. This is a prospective, two-center cohort study.
Methods
Patient population
This prospective, two-center study included 127 consecutive patients undergoing PTRA for RAS from August 2004 to December 2007 at the University Hospital Basel, Switzerland, and the Herz-Zentrum Bad Krozingen, Germany. Indications for renal arterial endovascular treatment were unilateral or bilateral RAS ≥50% with arterial hypertension (systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or on any antihypertensive drug therapy) (n=71) and/or renal insufficiency (eGFR ≤60 mL/min/1.73 m2) (n=56). Assessment of RAS was based primarily on duplex ultrasound using a Philips ATL, HDI 5000 (Philips, Best, Netherlands). As described previously, RAS was classified as hemodynamically relevant if the renal/aortal velocity ratio was ≥2.5 (6). For unilateral RAS the side-to-side difference in intrarenal RI =1-[end-diastolic velocity/peak systolic velocity] between the 2 kidneys >0.05 was also used to classify hemodynamically relevant RAS. Before intervention, duplex ultrasound was always confirmed by intra-arterial angiography showing a percent diameter stenosis ≥50% by measuring the ratio between the diameter of the narrowest segment of the imaged renal artery and the diameter of a normal segment of the artery proximal to the stenosis or distal to poststenotic dilation. Alternatively, an intra-arterial, trans-lesional systolic pressure gradient of ≥20 mmHg was considered as hemodynamically relevant and was assessed in 31 patients (6). A RAS ≥70% was documented in 84% of all patients and mean systolic pressure gradient was 72±46 mmHg.
The study was carried out according to the principles of the declaration of Helsinki and approved by the local ethics committees. Written informed consent was obtained from all participating patients.
Revascularization procedure
For atherosclerotic renal artery lesions a stent placement procedure with and without pre-dilatation using a guiding catheter technique via the femoral access and a variety of balloon expandable renal stents were used, such as Hippocampus™ (Invatec), Dynamic renal™ (Biotronik) or Palmaz blue™ (J&J Cordis). Procedural success was defined as <30% residual luminal narrowing or residual peak trans-lesional pressure gradient <10 mmHg. Antiplatelet therapy was started at least 1 day before the intervention and routinely consisted of 75 mg of clopidogrel daily for 4 weeks and 100 mg of aspirin indefinitely.
Follow-up and definitions
Baseline evaluation before PTRA and follow-up examinations 6 months after the revascularization procedure included duplex ultrasound with measurement of the renal/aortal velocity ratio and intrarenal RI at both the sides, measurement of serum creatinine, 24-hour ambulatory blood pressure monitoring (BSI, SpaceLab Medical Inc., Issaquah, WA, USA), and documentation of antihypertensive drugs. To estimate the eGFR, we used the formula for creatinine clearance calculated by the abbreviated modification of diet in renal disease study equation (7).
Two patients died during the follow-up period and 10 patients had no follow-up data after PTRA (Figure 1). Therefore, follow-up data was available from 115 patients (91%). Improvement in renal function at 6 months after PTRA was predefined as increase in the absolute value of the eGFR by ≥10% compared to pretreatment values (8). Decrease in renal function at 6 months after treatment was predefined as deterioration in the absolute value of the eGFR by ≥10% compared to the pretreatment value. A change in the absolute value of the eGFR within ±10% was predefined as no change in renal function. Patient with no change or decrease in renal function were summarized as no improvement in renal function.
Figure 1.
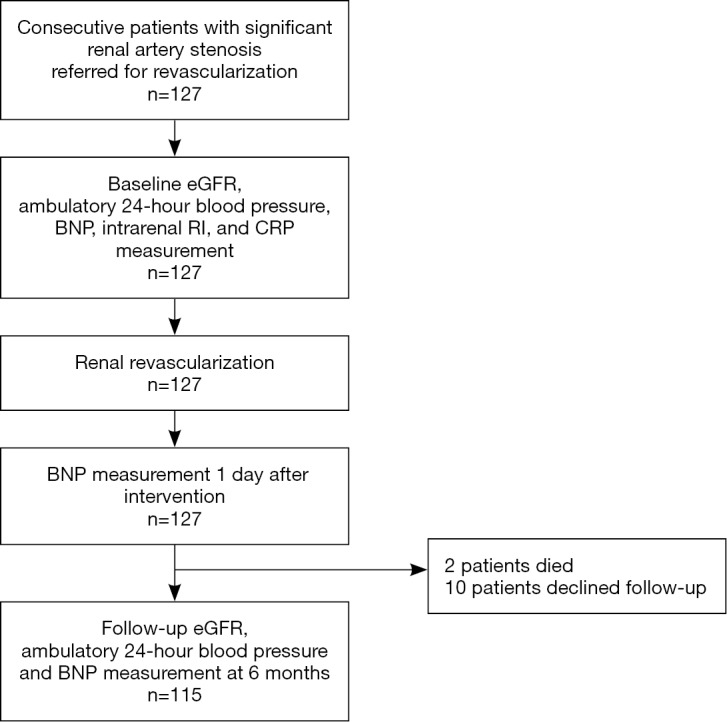
Flow diagram of patients with renal artery stenosis referred for revascularization.
Blood sampling and laboratory methods
A specimen of venous blood for BNP measurement was drawn before the intervention, 1 day and 6 months after the intervention. These samples were collected in plastic tubes containing EDTA and were centrifuged at 3,000 g and analyzed immediately. BNP concentration was determined using the commercially available Biosite assay (Biosite Diagnostics, La Jolla, CA, USA). Precision, analytical sensitivity, and stability characteristics of this fluorescence immunoassay have been previously described (9). In brief, the coefficient of variation for intra-assay precision has been reported to be 9.5%, 12.0%, and 13.9%, and the coefficient of variation for interassay precision is known to be 10.0%, 12.4%, and 14.8% for BNP levels of 28.8, 584.0, and 1,180.0 pg/mL, respectively. The analytic sensitivity was <5.0 pg/mL, with a measurable range of 0 to 5,000 pg/mL. As previously described, age and gender-specific median levels (25th and 75th percentiles) of plasma BNP using the same Biosite assay in 767 normal subjects in sinus rhythmu without cardiovascular disease or cardiac dysfunction were 27 (range, 15–43) pg/mL and 11 (range, 5–20) pg/mL for women and men of 55 to 64 years of age, and 29 (range, 19–52) pg/mL and 18 (range, 7–37) pg/mL for women and men of 65 to 74 years of age, respectively (10).
The laboratory technician who measured BNP was blinded to patient information.
Statistical analysis
The primary objective of this study was to examine whether pre-interventional BNP levels, eGFR, intrarenal RI at the side of the stenosis and CRP predicted improvement in renal function by the 6 months follow-up end point in the overall study cohort and in the subgroup with renal insufficiency at baseline (eGFR ≤ 60 mL/min/1.73 m2). The secondary endpoint was to examine whether the decrease in BNP level one day after intervention predicted improvement in renal function at 6-month follow-up.
Statistical analyses were performed using IBM SPSS/PC (version 19.0, SPSS Inc., Chicago, IL, USA). Discrete variables were expressed as numbers and percentages, continuous variables as mean ± SD or median and interquartile range (25th to 75th percentiles) when the sample data was not normally distributed. Univariate analysis of patients with renal function improvement compared to patients without renal function improvement were made using analysis of variance (ANOVA) or Mann-Whitney U test for continuous factors as appropriate and Chi-square tests for categorical factors. Paired t-test or Wilcoxon signed-rank test as appropriate were used to compare measurements before and after PTRA. Area under the receiver operating characteristic curve was used to estimate the value of baseline BNP, eGFR, intrarenal CRP, and CRP for the prediction of renal function improvement. The comparison of ROC-curves was performed using the method of DeLong on MedCalc (version 11.2.1.0, MedCalc Software, Ostend, Belgium). Multivariable logistic regression analyses were performed to assess the association of renal function improvement with pre-intervention BNP level, eGFR, intrarenal RI, and CRP (adjusted for age and sex).
Results
Baseline characteristics and renal artery intervention
The baseline clinical characteristics are shown in Table 1. Mean baseline eGFR was 65±27 mL/min/1.73 m2 and baseline eGFR <60 mL/min/1.73 m2 was documented in 56 patients (44%). Seven patients (7%) had chronic kidney disease stage 4 or 5 according to KDOQI classification before intervention. Hemodynamically relevant bilateral stenosis was found in 13 patients (10%). The majority of all lesions were atherosclerotic ostial stenoses (78%). PTRA secondary to fibromuscular dysplasia of the renal arteries (11) has been performed in 14% of patients. The overall primary technical success rates for renal revascularization were 100%.
Table 1. Baseline characteristics of all consecutive patients and the subgroup with impaired renal function at baseline undergoing renal artery revascularization with and without renal function improvement during follow up.
| Characteristics | Overall cohort (n=127) | P value | Subgroup with baseline eGFR <60 mL/min/1.73 m2 (n=56) | P value | |||
|---|---|---|---|---|---|---|---|
| All patients (n=127) | Improvement in renal function (n=42) | No improvement in renal function (n=73) | Improvement in renal function (n=27) | No improvement in renal function (n=22) | |||
| Age (mean ± SD), years | 63±13 | 66±11 | 61±14 | 0.06 | 69±11 | 69±11 | 0.94 |
| Female [n, (%)] | 58 [46] | 20 [48] | 32 [44] | 0.70 | 12 [44] | 5 [23] | 0.14 |
| Diabetes mellitus [n, (%)] | 21 [17] | 8 [19] | 9 [12] | 0.41 | 7 [26] | 5 [23] | 1.00 |
| Hypercholesterolemia [n, (%)] | 93 [73] | 27 [66] | 58 [80] | 0.12 | 20 [74] | 20 [91] | 0.16 |
| Smoker [n, (%)] | 52 [41] | 16 [38] | 32 [46] | 0.55 | 10 [37] | 13 [62] | 0.14 |
| Obesity [n, (%)] | 45 [35] | 17 [41] | 24 [33] | 0.43 | 11 [41] | 9 [41] | 1.00 |
| Co-morbidities | |||||||
| CAD [n, (%)] | 48 [38] | 14 [33] | 28 [39] | 0.69 | 11 [41] | 11 [52] | 0.56 |
| Cerebrovascular disease [n, (%)] | 21 [17] | 6 [14] | 12 [17] | 0.79 | 4 [15] | 3 [13] | 1.00 |
| PAD [n, (%)] | 45 [36] | 13 [31] | 30 [41] | 0.32 | 10 [37] | 12 [54] | 0.26 |
| LVEF <40% [n, (%)] | 6 [5] | 1 [3] | 5 [8] | 0.66 | 1 [4] | 4 [18] | 0.14 |
| CRP (mg/L) | 7.0±9.7 | 8.1±8.3 | 6.9±11.0 | 0.53 | 10.5±9.3 | 12.0±17.9 | 0.70 |
| Renal function | |||||||
| Baseline serum creatinine (ìmol/L) | 112±59 | 128±53 | 104±63 | 0.04 | 155±48 | 168±83 | 0.48 |
| Follow-up serum creatinine (ìmol/L) | 110±76 | 101±41 | 116±90 | 0.33 | 121±37* | 192±135 | 0.011 |
| Baseline eGFR (mL/min/1.73 m2) | 65±27 | 54±23 | 73±28 | <0.001 | 40±12 | 41±12 | 0.71 |
| Follow-up eGFR (mL/min/1.73m2) | 69±29† | 70±31† | 68±28 | 0.71 | 53±18* | 39±14† | 0.004 |
| Baseline eGFR <60 mL/min/1.73 m2, n [%] | 56 [44] | 27 [64] | 22 [30] | <0.001 | 27 [100] | 22 [100] | NA |
| CKD stage 1 (GFR >90 mL/min/1.73 m2) | 23 [18] | 2 [5] | 20 [27] | 0.002 | NA | NA | - |
| CKD stage 2 (GFR 60–89 mL/min/1.73 m2) | 48 [38] | 13 [31] | 31 [42] | - | NA | NA | - |
| CKD stage 3 (GFR 30–59 mL/min/1.73 m2) | 47 [37] | 22 [52] | 18 [25] | - | 22 [82] | 18 [82] | 0.49 |
| CKD stage 4 (GFR 15–29 mL/min/1.73 m2) | 8 [6] | 5 [12] | 3 [4] | - | 5 [18] | 3 [14] | - |
| CKD stage 5 (GFR <15mL/min/1.73 m2) | 1 [1] | 0 [0] | 1 [1] | - | 0 [0] | 1 [4] | - |
| Arterial hypertension | |||||||
| SBP at baseline (mmHg) | 147±17 | 151±20 | 146±16 | 0.13 | 152±20 | 150±15 | 0.68 |
| SBP at follow-up (mmHg) | 137±16* | 136±16* | 137±19* | 0.69 | 136±19* | 134±16* | 0.62 |
| DBP at follow-up (mmHg) | 77±11* | 74±9† | 79±12† | 0.02 | 71±9† | 76±13 | 0.13 |
| Number of antihypertensive drugs at baseline | 2.9±1.3 | 3.1±1.2 | 2.8±1.3 | 0.23 | 3.2±1.3 | 3.0±1.4 | 0.70 |
| Number of antihypertensive drugs at follow-up | 2.6±1.4† | 2.7±1.6 | 2.6±1.3 | 0.83 | 2.6±1.5† | 2.9±1.2 | 0.59 |
| Renal anatomy and physiology | |||||||
| Kidney length (mm) | 103±13 | 103±13 | 103±14 | 0.88 | 101±13 | 97±13 | 0.27 |
| Renal/aortal ratio at baseline | 5.3±1.9 | 5.1±1.8 | 5.4±1.9 | 0.49 | 5.4±1.5 | 5.8±1.6 | 0.52 |
| Renal/aortal ratio at follow-up | 1.9±0.9* | 2.0±0.8* | 1.9±1.0* | 0.78 | 1.9±0.8* | 1.7±0.6* | 0.21 |
| Intrarenal RI at baseline | 0.63±0.10 | 0.63±0.11 | 0.62±0.09 | 0.91 | 0.64±0.11 | 0.65±0.11 | 0.74 |
| Contralateral intrarenal RI at baseline | 0.70±0.08¶ | 0.72±0.09¶ | 0.69±0.08¶ | 0.09 | 0.73±0.08¶ | 0.72±0.07¶ | 0.64 |
| Intrarenal RI at follow-up | 0.71±0.08* | 0.72±0.07* | 0.69±0.07* | 0.21 | 0.75±0.10* | 0.73±0.07* | 0.61 |
| Bilateral stenosis [n, (%)] | 13 [10] | 6 [14] | 7 [10] | 0.54 | 6 [22] | 2 [9] | 0.27 |
| ≥ 70% stenosis [n, (%)] | 105 [84] | 38 [90] | 58 [84] | 0.40 | 25 [93] | 21 [91] | 1.00 |
| Ostial stenosis [n, (%)] | 97 [78] | 30 [73] | 58 [80] | 0.49 | 20 [77] | 21 [96] | 0.11 |
| Stent placed [n, (%)] | 106 [85] | 38 [90] | 59 [81] | 0.19 | 27 [100] | 20 [91] | 0.20 |
| Fibromuscular dysplasia [n, (%)] | 18 [14] | 5 [12] | 12 [16] | 0.59 | 1 [5] | 1 [4] | 1.00 |
Data are expressed as mean ± SD, or number (percentage) of patients. *, P<0.001 compared with baseline; †, P<0.05 compared with baseline; ¶, P<0.05 compared with RI on the stenotic side. BP indicates blood pressure; CAD, coronary artery disease; PAD, peripheral artery disease; LVEF, left ventricular ejection fraction, eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; RI, resistance index.
There was no procedure related death. Two patients died from acute myocardial infarction during the follow-up period (Figure 1). We observed four major procedural complications: Intrarenal bleeding successfully treated with embolization; acute occlusion of the main renal artery one week after stent implantation with spontaneous reopening; perforation of the main renal artery treated with extended balloon dilation; dissection of main renal artery distal from stent implantation with occlusion of a segmental arterial branch.
Renal function response
As shown in Table 1, eGFR increased from baseline value of 65±27 mL/min/1.73 m2 before intervention to 69±29 mL/min/1.73 m2 after PTRA at 6-month follow-up (P<0.05). Renal function improvement was documented in 37% of patients (42/115 patients), no change in renal function was found in 44 patients (38%), and decrease in renal function was documented in 29 of patients (25%). In the four patients with major procedural complications only one had a decrease in renal function; two patients had no change and one patient an improvement in renal function. Mean systolic and diastolic blood pressure in all patients decreased from baseline values of 147±17 and 81±13 mmHg before intervention to 137±16 and 77±11 mmHg after renal angioplasty at the 6-month follow-up (P<0.001 for both). The number of antihypertensive agents significantly decreased from 2.9±1.3 to 2.6±1.4 (P=0.009). Differences in baseline characteristics between patients with and without (no change or decrease) renal function improvement are shown in Table 1.
Multimarker assessment
BNP
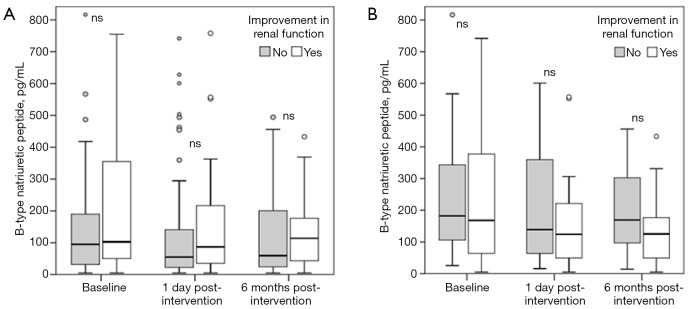
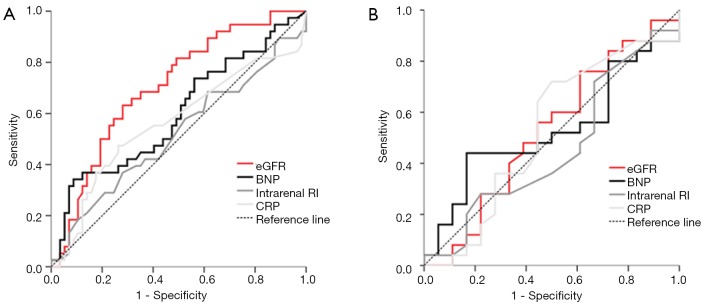
Median BNP before revascularization was 97 pg/mL (IQR, 35–254) and decreased significantly within one day after PTRA to 63 pg/mL (IQR, 24–179) (P<0.001), remaining at 75 pg/mL (IQR, 31–190) at the 6-month follow-up (P=0.03 compared to pre-intervention). BNP levels at baseline, after revascularization, and 6 months post procedure in patients with and without renal function improvement are shown in Table 2 and Figure 2. The AUC for the ability to predict renal function improvement was 0.59 (95%CI, 0.48-0.70; P=0.101) for pre-intervention BNP (Figure 3).
Table 2. B-type natriuretic peptide levels in patients with and without renal function improvement during follow up.
| BNP levels | All patients | Improvement in renal function | No improvement in renal function | P value |
|---|---|---|---|---|
| Overall cohort | [n=127] | [n=42] | [n=73] | |
| BNP pre-intervention (pg/mL) | 97 [35, 254] | 103 [48, 366] | 95 [31, 193] | 0.10 |
| BNP 1 day post-intervention (pg/mL) | 63 [24, 179]* | 87 [35, 217]* | 55 [22, 144]† | 0.15 |
| %BNP decrease | −31 [−57, 6] | −35 [−55, −1] | −29 [−52, 15] | 0.33 |
| BNP 6 months post-intervention (pg/mL) | 75 [31, 190]† | 114 [41, 181]† | 60 [23, 103] | 0.47 |
| Patients with baseline eGFR <60 mL/min/1.73 m2 | [n=56] | [n=27] | [n=22] | |
| BNP pre-intervention (pg/mL) | 164 [65, 352] | 168 [60, 399] | 182 [93, 362] | 0.82 |
| BNP 1 day post-intervention (pg/mL) | 118 [50, 238]† | 124 [48, 235]† | 139 [59, 411] | 0.47 |
| %BNP decrease | −26 [−59,10] | −30 [−60, 0] | 1 [−50, 23] | 0.12 |
| BNP 6 months post-intervention (pg/mL) | 125 [62, 194]† | 125 [46, 181]† | 169 [87, 361] | 0.18 |
*, P<0.001 compared with BNP pre-intervention; †, P<0.05 compared with BNP pre-intervention. BNP, B-type natriuretic peptide; BP, blood pressure; LVEF, left ventricular ejection fraction; RAS, renal artery stenosis. Data are expressed as median [25th and 75th percentiles), or number [percentage) of patients.
Figure 2.
Box plot indicating the median (25th to 75th percentiles) and 5th–95th percentiles of BNP levels before and after percutaneous revascularization for renal artery stenosis in patients with (white) and without (gray bar) renal function improvement. (A) BNP levels in the overall cohort (n=127); (B) BNP levels in patients with impaired pre-interventional renal function (eGFR < 60 mL/min/1.73 m2) (n=56). NS indicates not significant. BNP, B-type natriuretic peptide.
Figure 3.
ROC curves for pre-interventional eGFR, BNP, intrarenal RI, and CRP for the prediction of renal function improvement at 6 months after renal angioplasty and stenting for renal artery stenosis. (A) The area under the ROC curve for the overall cohort (n=127) was 0.71 (95% CI, 0.61–0.81; P<0.001), 0.59 (95% CI, 0.48–0.70; P=0.101), 0.52 (95% CI, 0.41–0.65; P=0.66), and 0.56 (95% CI, 0.44–0.68; P=0.30), respectively; (B) the area under the ROC curve for patients with impaired pre-interventional renal function (eGFR <60 mL/min/1.73 m2) (n=56) was 0.55 (95% CI, 0.39–0.72; P=0.53), 0.48 (95% CI, 0.32–0.65; P=0.817), 0.52 (95% CI, 0.34–0.69; P=0.84), and 0.43 (95% CI, 0.26–0.60; P=0.42), respectively. Diagonal line, no discrimination. ROC, receiver operator characteristic; eGFR, estimated glomerular filtration rate; BNP, B-type natriuretic peptide; RI, resistance index; CRP, C-reactive protein.
eGFR
Mean baseline eGFR was significantly lower in patients with compared to patients without renal function improvement (54±23 vs. 73±28 mL/min/1.73 m2, P<0.001).
The AUC for the ability to predict renal function improvement was 0.71 (95% CI, 0.61–0.81; P<0.001) for pre-intervention eGFR (Figure 3).
RI
Mean intrarenal RI and mean contralateral intrarenal RI at baseline was similar in patients with and without renal function improvement (0.63±0.11 vs. 0.62±0.09, P=0.91and 0.72±0.09 vs. 0.69±0.08, P=0.09). The AUC for the ability to predict renal function improvement was 0.52 (95% CI, 0.41–0.65; P=0.66) for pre-intervention RI (Figure 3) and 0.61 (95% CI, 0.49–0.73; P=0.06) for contralateral RI.
CRP
Mean CRP level at baseline did not significantly differ between patients with than without renal function improvement (8.1±8.3 vs. 6.9±11.0 mg/L; P=0.53). The AUC for the ability to predict renal function improvement was 0.56 (95% CI, 0.44–0.68; P=0.30) for pre-intervention CRP (Figure 3).
Combination of marker
The AUC of the combination of baseline eGFR and BNP or RI or CRP for the ability to predict renal function improvement was 0.71 (95% CI, 0.60–0.79), 0.72 (95% CI, 0.61–0.80), and 0.71 (95% CI, 0.60–0.79), respectively. None of these combinations increased, however, the accuracy provided by eGFR (P=ns for all).
As shown in Table 3, multivariate logistic regression analysis including pre-intervention eGFR, BNP, intrarenal RI, and CRP (adjusted for age and sex) shows that only decreased pre-intervention eGFR was significantly associated with renal function improvement (OR, 0.96; 95% CI, 0.94–0.99; P=0.003).
Table 3. Multivariate analysis for the prediction of renal function improvement during follow up.
| Variables | Odds ratio (95%CI) | P value |
|---|---|---|
| Overall cohort (n=127) | ||
| Age | 0.99 [0.95-1.05] | 0.83 |
| Male sex | 1.72 [0.67-4.42] | 0.26 |
| eGFR pre-intervention (mL/min/1.73 m2) | 0.96 [0.94-0.99] | 0.003 |
| BNP pre-intervention (pg/mL) | 1.00 [0.99-1.01] | 0.22 |
| Intrarenal RI pre-intervention | 0.05 [0.001-14.1] | 0.30 |
| CRP pre-intervention (mg/L) | 0.65 [0.39-1.08] | 0.10 |
| Patients with baseline eGFR < 60 mL/min/1.73 m2 (n=56) | ||
| Age | 0.99 [0.92-1.08] | 0.90 |
| Male sex | 4.04 [0.93-17.66] | 0.06 |
| eGFR pre-intervention (mL/min/1.73 m2) | 1.00 [0.93-1.08] | 0.96 |
| Intrarenal RI pre-intervention | 0.01 [0.00-46.5] | 0.28 |
| BNP pre-intervention (pg/mL) | 1.00 [0.99-1.01] | 0.24 |
| CRP pre-intervention (mg/L) | 0.64 [0.36-1.15] | 0.14 |
CI indicates confidence interval; eGFR, estimated glomerular filtration rate; BNP, B-type natriuretic peptide.
Subgroup analysis in patients with pre-interventional impaired renal function
The clinical characteristics in patients with and without renal function improvement of the subgroup with baseline eGFR <60 mL/min/1.73 m2 are shown in Table 1. In this subgroup renal function improvement was documented in 55% (27/49 patients), no change in renal function was found in 13 patients (27%), and decrease in renal function was documented in 9 patients (18%).
BNP
As shown in Table 2 median BNP before revascularization was elevated at 168 pg/mL (IQR, 63–355) and decreased significantly within one day after PTRA to 121 pg/mL (IQR, 51–244) (P<0.001), remaining at 127 pg/mL (IQR, 60–197) at the 6-month follow-up (P=0.006 compared to pre-intervention) in this subgroup with decreased baseline eGFR.
BNP level was also not significantly different in patient with and without improvement in renal function [168 pg/mL (IQR, 60–399) vs. 182 pg/mL (IQR, 93–362), P=0.82] (Figure 2B). The decrease in BNP one day after revascularization was not significantly different in patient with than without improvement in renal function [−30% (IQR, −60 to 0) vs. 1% (IQR, −50 to 23), P=0.12]. The area under the receiver operating curve for the ability to predict blood renal function improvement was 0.48 (95%CI, 0.32–0.65; P=0.817) for pre-intervention BNP (Figure 3).
eGFR, RI, CRP
In this subgroup mean baseline eGFR, RI, and CRP did not significantly differ between patients with than without renal function improvement (Table 1).
The AUC for the ability to predict renal function improvement for baseline eGFR, RI, and CRP was 0.55 (95% CI, 0.39–0.72; P=0.53), 0.52 (95% CI, 0.34–0.69; P=0.84), and 0.43 (95% CI, 0.26–0.60; P=0.42), respectively (Figure 3).
Combination of marker
As shown in Table 3, multivariate logistic regression analysis in this subgroup including pre-intervention eGFR, BNP, intrarenal RI, and CRP (adjusted for age and sex) shows that none of these parameters are significantly associated with renal function improvement.
Discussion
This prospective study in unselected consecutive patients with hemodynamically relevant RAS and renal insufficiency and/or arterial hypertension referred for PTRA, evaluated the utility of a comprehensive multimarker assessment with quantification of hemodynamic cardiac stress, renal function, parenchymal renal damage, and systemic inflammation using BNP, eGFR, sonographic RI measurement, and CRP in the prediction of renal function improvement after successful revascularization.
We report five major findings. First, renal function improvement 6 months after intervention was documented in 37% of patients in the whole study cohort and in 55% of patients in the subgroup of patients with pre-interventional impaired renal function (eGFR <60 mL/min/1.73 m2). This is in line with previous reports of 11 observational studies which showed improvement of renal function after PTRA in 39% (range, 17–60%) (12). Similarly, Zeller et al. demonstrated in larger study cohort improvement of renal function after stent-supported angioplasty of severe ostial RAS in 52% of patients (13). Most of these studies, however, investigated the effects on renal function measuring serum creatinine rather than eGFR as in our study and as recommended by current guidelines (8).
Second, pre-interventional BNP is elevated in most patients with RAS and decreased significantly one day after revascularization supporting the pathophysiological concept that hemodynamically significant RAS is associated with hemodynamic cardiac stress. Third, pre-interventional BNP and decrease of BNP level after successful revascularization, however, have shown poor accuracy in the prediction of renal function improvement at 6 months. Similarly, other markers as pre-interventional intrarenal RI and CRP are not predictive for renal function improvement. Forth, only decreased pre-interventional eGFR level was associated with renal function improvement at the 6-month follow-up endpoint, independent of other clinical, laboratory and duplex sonographic parameters. Fifth, in the subgroup of patient with pre-interventional impaired renal function none of the investigated markers are predictive for renal function improvement after PTRA.
PTRA is a treatment option for patients with RAS and can be helpful in certain patient populations for improvement of renal function (1). The results of the CORAL study demonstrated no appreciable benefit with regard to the prevention of clinical events in patients with atherosclerotic RAS undergoing renal artery stenting in addition to medical therapy in comparison to those with medical therapy alone (14). In this trial during follow-up only a minimal though significant decrease was demonstrated with regard to systolic blood pressure when performing renal artery stenting in addition to medical therapy as opposed to medical therapy alone. Observational studies and larger controlled trials have shown that up to 50% of patients may have some benefit from RAS treatment with PTRA (12). As these results showed, selection of the appropriate subgroup is key when considering patients for PTRA and unselected PTRA based on the pure detection of RAS is not recommended (4,5,15). It has been suggested that those patients with a decreased renal function may benefit from RAS treatment with PTRA (16). The concept of a variety of markers to help select the appropriate patients with a high likelihood of improving renal function post-intervention is appealing. The results of this prospective non-randomized cohort trial essential reveal that a multimarker assessment with the investigated biomarkers may have only a limited benefit for RAS patient subgroup selection to undergo PTRA.
In fact, eGFR appears to be the most useful prognostic biomarker according to this trial. The patients who harbored a decreased kidney function prior to the procedure, improved markedly in their post-PTRA renal function. Previous studies are in line with these findings and have shown that particularly patients with decrease renal function can benefit from PTRA (17). Zeller et al. already demonstrated that baseline creatinine level and left ventricular function was an independent predictor for improved renal function after PTRA in their large cohort of patient with RAS (18). According to current guidelines eGFR, however, is a more accurate marker then creatinine level alone for renal function assessment as it in-cooperates age, gender, race information and creatinine level (19,20). This result also supports previous hypotheses why particularly patients with kidney injury benefit most from PTRA with regard to renal function improvement. Zeller et al. argued that reversal of RAS is most effective if hemodynamic compromise is severe enough to cause renal dysfunction or if there is a coexisting systemic prerenal component (18).
The neurohormone BNP is, therefore, an interesting biomarker in the setting of RAS. The synthesis and release happens from the bilateral ventricular myocytes secondary to volume expansion or pressure overload (21). BNP has been established in the clinical arena for assessment and follow-up of congestive heart failure patients (22,23). Prior studies demonstrated the value of BNP in RAS for prediction of blood pressure improvement post renal artery revascularization (24,25). Zeller et al. also demonstrated that patients with impaired left ventricular function benefit most from RAS revascularization with regard to renal function improvement (18). In this study BNP revealed not to be an accurate biomarker for renal function prediction post-PTRA. However, BNP decreased significantly in all patients and in the cohort with improved renal function post intervention. This was true for both the overall cohort and the cohort of patients with a baseline eGFR of less than 60 mL/min/1.73 m2. A possible reason while BNP turned out not to be an accurate predictor might be related to the multi-morbid patient population with a variety of confounding factors which may impact ventricular stretch leading to alterations in BNP levels. Essentially BNP is a relative unspecific though sensitive biomarker as demonstrated by the results of this and other studies (26,27).
The RI is another potential sonographic biomarker which did not show significance for prediction of renal function improvement in this study. Previous studies showed that a pre-procedural RI of less than 0.75 enables prediction of superior clinical outcomes post-PTRA (15). Another study found similar results with a RI of more than 0.80 being reliable to determine those patients whose renal function will not be improved post renal revascularization therapy (which was either surgery or renal artery angioplasty) (28). This, however, has been challenged by prospective cohort studies (29). The RI is a biomarker of vascular impedance derived from ultrasound Doppler, hence measuring arterial stiffness (30). Previous studies revealed a correlation of the RI with irreversible renal parenchyma histological changes of the glomerular and tubulo-interstitial system (31). Therefore, people with high RI may already suffer from advanced renal parenchymal disease and most likely do not profit from PTRA (32). One reason why RI did not correlate with clinical outcome in our study could be that patients with very high RI over 0.8 were pre-selected and not referred for PTRA.
CRP turned out to be unhelpful for prediction of renal function improvement post-PTRA in RAS patients. CRP is a highly sensitive but unspecific biomarker for inflammation and ischemia (33). CRP is influenced by a multitude of factors and had be investigated in a variety of clinical settings including the presence of RAS as a serial biomarker for assessing the inflammatory and ischemic status of patients as well as predicting cardiovascular and cerebrovascular events (34-37). CRP was included in the study to evaluate its predictive value in a multimarker assessment approach. However, the inflammatory component measured by serum CRP level seems not to be related with renal function improvement post intervention.
Study limitations
Several limitations apply to this study. In this prospective, non-randomized cohort study all of the patients received percutaneous revascularization of RAS without inclusion of any control group. Therefore, we cannot preclude that factors other than RAS may have contributed to the elevation and decrease of BNP after PTRA. However, median levels of BNP in our cohort with RAS were elevated compared to a previously published healthy control group as well as to a patient population with severe essential hypertension (10,38). Furthermore, we also estimated the eGFR based on creatinine value using an established equation and we did not measure eGFR directly using 125I-iothalamate which has been shown to be more sensitive and reliable for detecting meaningful changes in eGFR after PTRA (39). Furthermore, we did not assess renal mass by ultrasonography as an additional marker for renal function. However, the use of eGFR has been accepted in the guidelines for the assessment of renal function after PTRA and is well established also in larger randomized controlled trials (14).
Another limitation of the prospective study was the follow-up time of 6 months post-PTRA and no standardized optimized medical therapy was performed in all patient universally as recommended by recent trials (4). In future studies longer follow-up times post intervention and standardized optimized medical/conservative therapy are warranted to further validate the results of the current study.
Conclusions
Quantification of renal function, but not any other pathophysiologic signal, provides at least moderate accuracy in the identification of patients with RAS in whom PTRA will improve renal function. Lower eGFR indicates a higher likelihood for eGFR improvement after PTRA. Further studies are needed to improve the selection of patients with RAS who benefit most from PTRA.
Acknowledgements
Funding: D Staub is supported by grants from the Swiss National Science Foundation (Grant PZ00P3_142419 and PBZHB-120997), the Swiss Society of Angiology, and the University of Basel. Dr. Mueller has received research support from the Swiss National Science Foundation, the Swiss Heart Foundation, the Cardiovascular Research Foundation Basel, Abbott, Astra Zeneca, Beckman Coulter, Biomerieux, BRAHMS, Roche, Siemens, Singulex, Sphingotec and the University Hospital Basel, as well as speaker/consulting honoraria and/or travel support from Abbott, ALERE, Astra Zeneca, Bayer, BG Medicine, Biomerieux, BMS, BRAHMS, Cardiorentis, Daiichi Sankyo, Eli-Lilly, Novartis, Roche, Siemens, and Singulex.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Zeller T, Macharzina R, Rastan A, et al. Renal artery stenosis: Up-date on diagnosis and treatment. Vasa 2014;43:27-38. 10.1024/0301-1526/a000325 [DOI] [PubMed] [Google Scholar]
- 2.Garovic VD, Textor SC. Renovascular hypertension and ischemic nephropathy. Circulation 2005;112:1362-74. 10.1161/CIRCULATIONAHA.104.492348 [DOI] [PubMed] [Google Scholar]
- 3.Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 2009;52:196-203. 10.1016/j.pcad.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritchie J, Alderson HV, Kalra PA. Where now in the management of renal artery stenosis? Implications of the ASTRAL and CORAL trials. Curr Opin Nephrol Hypertens 2014;23:525-32. 10.1097/MNH.0000000000000059 [DOI] [PubMed] [Google Scholar]
- 5.Choi SS. Atherosclerotic renal artery stenosis and revascularization. Expert Rev Cardiovasc Ther 2014;12:1419-25. 10.1586/14779072.2014.977258 [DOI] [PubMed] [Google Scholar]
- 6.Staub D, Canevascini R, Huegli RW, et al. Best duplex-sonographic criteria for the assessment of renal artery stenosis--correlation with intra- arterial pressure gradient. Ultraschall Med 2007;28:45-51. 10.1055/s-2007-962881 [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation . K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1-266. [PubMed] [Google Scholar]
- 8.Rundback JH, Sacks D, Kent KC, et al. Guidelines for the reporting of renal artery revascularization in clinical trials. J Vasc Interv Radiol 2003;14:S477-92. 10.1097/01.RVI.0000094621.61428.d5 [DOI] [PubMed] [Google Scholar]
- 9.Cheng V, Kazanagra R, Garcia A, et al. A rapid bedside test for B-type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol 2001;37:386-91. 10.1016/S0735-1097(00)01157-8 [DOI] [PubMed] [Google Scholar]
- 10.Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002;40:976-82. 10.1016/S0735-1097(02)02059-4 [DOI] [PubMed] [Google Scholar]
- 11.Chrysant SG, Chrysant GS. Treatment of hypertension in patients with renal artery stenosis due to fibromuscular dysplasia of the renal arteries. Cardiovasc Diagn Ther 2014;4:36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubel GJ, Murphy TP. The role of percutaneous revascularization for renal artery stenosis. Vasc Med 2008;13:141-56. 10.1177/1358863x07085408 [DOI] [PubMed] [Google Scholar]
- 13.Zeller T, Frank U, Müller C, et al. Stent-supported angioplasty of severe atherosclerotic renal artery stenosis preserves renal function and improves blood pressure control: long-term results from a prospective registry of 456 lesions. J Endovasc Ther 2004;11:95-106. 10.1583/03-1062.1 [DOI] [PubMed] [Google Scholar]
- 14.Cooper CJ, Murphy TP, Cutlip DE, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014;370:13-22. 10.1056/NEJMoa1310753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuksel UC, Anabtawi AG, Cam A, et al. Predictive value of renal resistive index in percutaneous renal interventions for atherosclerotic renal artery stenosis. J Invasive Cardiol 2012;24:504-9. [PubMed] [Google Scholar]
- 16.Beutler JJ, Van Ampting JM, Van De Ven PJ, et al. Long-term effects of arterial stenting on kidney function for patients with ostial atherosclerotic renal artery stenosis and renal insufficiency. J Am Soc Nephrol 2001;12:1475-81. [DOI] [PubMed] [Google Scholar]
- 17.Watson PS, Hadjipetrou P, Cox SV, et al. Effect of renal artery stenting on renal function and size in patients with atherosclerotic renovascular disease. Circulation 2000;102:1671-7. 10.1161/01.CIR.102.14.1671 [DOI] [PubMed] [Google Scholar]
- 18.Zeller T, Frank U, Müller C, et al. Predictors of improved renal function after percutaneous stent-supported angioplasty of severe atherosclerotic ostial renal artery stenosis. Circulation 2003;108:2244-9. 10.1161/01.CIR.0000095786.44712.2A [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lameire N, Adam A, Becker CR, et al. Baseline renal function screening. Am J Cardiol 2006;98:21K-26K. 10.1016/j.amjcard.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 21.Maisel A, Mueller C, Adams K, Jr, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail 2008;10:824-39. 10.1016/j.ejheart.2008.07.014 [DOI] [PubMed] [Google Scholar]
- 22.Mueller C, Scholer A, Laule-Kilian K, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med 2004;350:647-54. 10.1056/NEJMoa031681 [DOI] [PubMed] [Google Scholar]
- 23.Don-Wauchope AC, McKelvie RS. Evidence based application of BNP/NT-proBNP testing in heart failure. Clin Biochem 2015;48:236-46. 10.1016/j.clinbiochem.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 24.Silva JA, Chan AW, White CJ, et al. Elevated brain natriuretic peptide predicts blood pressure response after stent revascularization in patients with renal artery stenosis. Circulation 2005;111:328-33. 10.1161/01.CIR.0000153271.77341.9F [DOI] [PubMed] [Google Scholar]
- 25.Staub D, Zeller T, Trenk D, et al. Use of B-type natriuretic peptide to predict blood pressure improvement after percutaneous revascularisation for renal artery stenosis. Eur J Vasc Endovasc Surg 2010;40:599-607. 10.1016/j.ejvs.2010.07.013 [DOI] [PubMed] [Google Scholar]
- 26.Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J 2006;27:330-7. 10.1093/eurheartj/ehi631 [DOI] [PubMed] [Google Scholar]
- 27.Worster A, Balion CM, Hill SA, et al. Diagnostic accuracy of BNP and NT-proBNP in patients presenting to acute care settings with dyspnea: a systematic review. Clin Biochem 2008;41:250-9. 10.1016/j.clinbiochem.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 28.Radermacher J, Chavan A, Bleck J, et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med 2001;344:410-7. 10.1056/NEJM200102083440603 [DOI] [PubMed] [Google Scholar]
- 29.Zeller T, Müller C, Frank U, et al. Stent angioplasty of severe atherosclerotic ostial renal artery stenosis in patients with diabetes mellitus and nephrosclerosis. Catheter Cardiovasc Interv 2003;58:510-5. 10.1002/ccd.10435 [DOI] [PubMed] [Google Scholar]
- 30.Mostbeck GH, Kain R, Mallek R, et al. Duplex Doppler sonography in renal parenchymal disease. Histopathologic correlation. J Ultrasound Med 1991;10:189-94. [DOI] [PubMed] [Google Scholar]
- 31.Ikee R, Kobayashi S, Hemmi N, et al. Correlation between the resistive index by Doppler ultrasound and kidney function and histology. Am J Kidney Dis 2005;46:603-9. 10.1053/j.ajkd.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 32.Safian RD, Madder RD. Refining the approach to renal artery revascularization. JACC Cardiovasc Interv 2009;2:161-74. 10.1016/j.jcin.2008.10.014 [DOI] [PubMed] [Google Scholar]
- 33.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003;111:1805-12. 10.1172/JCI200318921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winbeck K, Poppert H, Etgen T, et al. Prognostic relevance of early serial C-reactive protein measurements after first ischemic stroke. Stroke 2002;33:2459-64. 10.1161/01.STR.0000029828.51413.82 [DOI] [PubMed] [Google Scholar]
- 35.Sato Y, Takatsu Y, Kataoka K, et al. Serial circulating concentrations of C-reactive protein, interleukin (IL)-4, and IL-6 in patients with acute left heart decompensation. Clin Cardiol 1999;22:811-3. 10.1002/clc.4960221211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlager O, Amighi J, Haumer M, et al. Inflammation and adverse cardiovascular outcome in patients with renal artery stenosis and peripheral artery disease. Atherosclerosis 2009;205:314-8. 10.1016/j.atherosclerosis.2008.12.022 [DOI] [PubMed] [Google Scholar]
- 37.Hommels MJ, van der Ven AJ, Kroon AA, et al. C-reactive protein, atherosclerosis and kidney function in hypertensive patients. J Hum Hypertens 2005;19:521-6. 10.1038/sj.jhh.1001878 [DOI] [PubMed] [Google Scholar]
- 38.Mussalo H, Vanninen E, Ikäheimo R, et al. NT-proANP and BNP in renovascular and in severe and mild essential hypertension. Kidney Blood Press Res 2003;26:34-41. 10.1159/000069763 [DOI] [PubMed] [Google Scholar]
- 39.Crimmins GM, Madder RD, Marinescu V, et al. Validity of estimated glomerular filtration rates for assessment of renal function after renal artery stenting in patients with atherosclerotic renal artery stenosis. JACC Cardiovasc Interv 2014;7:543-9. 10.1016/j.jcin.2013.11.021 [DOI] [PubMed] [Google Scholar]