Abstract
Background
Neuroendocrine tumours (NETs) are rare, heterogeneous group of tumours which usually originate from small, occult primary sites and are characterized by over-expression of somatostatin receptors (SSTRs). Positron emission tomography/computed tomography (PET/CT) using Ga-68-labeled-somatostatin-analogues have shown superiority over other modalities for imaging of NETs. The objective of the study was to retrospectively evaluate the efficacy of Ga-68 DOTANOC PET/CT imaging in detecting the primary site in patients with metastatic NETs of unknown origin and its impact on clinical decision making in such patients.
Methods
Between December 2011 and September 2014, a total of 263 patients underwent Ga-68 DOTANOC PET/CT study in our department for various indications. Out of them, 68 patients (45 males, 23 females; mean age, 54.9±10.7 years; range, 31–78 years) with histopathologically proven metastatic NETs and unknown primary site (CUP-NET) on conventional imaging, who underwent Ga-68 DOTANOC PET/CT scan as part of their clinical work-up were included for analyses. Histopathology (wherever available) and/or follow-up imaging were taken as reference standard. Quantitative estimation of SSTR expression in the form of maximal standardized uptake value (SUVmax) of detected primary and metastatic sites was calculated. Follow-up data of individual patients was collected through careful survey of hospital medical records and telephonic interviews.
Results
Maximum patients presented to our department with hepatic metastasis (50 out of 68 patients) and grade I NETs (>50%). Ga-68 DOTANOC PET/CT scan identified primary sites in 40 out of these 68 patients i.e., in approximately 59% patients. Identified primary sites were: small intestine [19], rectum [8], pancreas [7], stomach [4], lung [1] and one each in rare sites in kidney and prostate. In one patient, 2 primary sites were identified (one each in stomach and duodenum). Mean SUVmax of the detected primary sites was 25.1±18.0 (median: 16.25; range, 2.1–150). Significant positive correlation was found between SUVmax of detected primary site and SUVmax of the histopathologically proven sites of metastasis (r=0.662; P<0.0001). Based on the findings of the Ga-68 DOTANOC PET/CT scan, 3 out of 40 patients underwent definitive treatment for their primary tumour (1 gastric, 1 ileal and 1 prostatic tumour). One patient was being planned for resection of primary rectal lesion at the time of data-collection. Thirty-six out of 68 patients were started on long-acting somatostatin analogues or chemotherapy or targeted therapy. Two patients underwent multiple cycles of peptide receptor radionuclide therapy (PRRNT) using 90Y and 177Lu labeled somatostatin analogues.
Conclusions
Our findings indicate that Ga-68 DOTANOC PET/CT is a promising imaging modality in patients with metastatic NETs of unknown origin for detection of the primary site and in guiding their therapeutic management.
Keywords: Ga-68 DOTANOC PET/CT, neuroendocrine tumours (NETs), carcinoma of unknown primary, somatostatin-receptors, maximal standardized uptake value (SUVmax)
Introduction
Neuroendocrine tumours (NETs) are rare, genetically diverse, predominantly slow-growing tumours with relatively good prognosis (1). NETs predominantly arise from local multipotent gastro-intestinal (GI) stem cells (2) and are either symptomatic (functional) or asymptomatic (non-functional) based on their property of secreting biogenic amines and hormones. NETs account for less than 1% of all malignancies, however, according to Surveillance, Epidemiology and End Results (SEER) program data, their age-adjusted incidence increased 2.7 folds between 1973 and 2004 (3), primarily on account of increased physician awareness and better diagnostic facilities (4). Recent WHO classification allows an optimal prognostic stratification of NETs, which is helpful in deciding the best possible management (5,6).
NETs are characterized by over-expression of somatostatin receptors (SSTRs). SSTRs are G-protein coupled trans-membrane receptors which are internalized after binding to specific ligands. This property of over-expression of SSTRs in NETs has been extremely useful for their detection by functional imaging modalities such as somatostatin-receptor-scintigraphy (SRS) and Ga-68-labeled-somatostatin analogues (DOTA-peptides) positron emission tomography/computed tomography (PET/CT) (7).
Over 90% of all NETs originate in the GI tract, the largest neuroendocrine organ in the body (4). Often the primary site is small, occult with possibility of multiple and variable primary sites which makes their evaluation difficult (7). Patients with metastatic NETs and unknown primary site (CUP-NET) constitute less than 5% of overall carcinomas of unknown primary (CUP) population (8). However, they constitute 10-13% of total NET study populations (9,10). CUP-NET patients have a poorer prognosis than other NET patients (11). Kirshborn et al. reported a 10-year survival rate of just above 20% (12). Surgical resection of primary tumor is curative and should even be considered as a treatment option when resection appears to be difficult and metastasis is present (13-17). Hence, localizing the primary site in these patients has proven benefits.
Conventional anatomical imaging with ultrasound (USG), CT and magnetic resonance imaging (MRI), although recommended in the current management guidelines by National Comprehensive Cancer Network (NCCN) (18) and commonly used for diagnostic work-up, are often unable to characterize or sometimes unable to detect such tumors because of the small lesion size and variable anatomical location (19). EUS has excellent detection rates for tumors located in the head of pancreas (20) but for other sites it is having low sensitivity. Functional imaging like SRS using In-111-DTPA-Octreotide has been explored to detect occult primary sites in patients with metastatic gastro-entero-pancreatic (GEP) NETs with a detection rate of 39% (21). Studies published recently have shown the superiority of Ga-68-DOTA-peptides PET/CT over conventional SRS for imaging various aspects of NETs (22,23).
Ga-68 labeled somatostatin analogues are short peptide analogues of somatostatin which are linked to the positron-emitter Ga-68 by a bifunctional chelate, namely 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA). The Ga-68-DOTA-peptides bind to the SSTRs over-expressed on NET cells. Three major Ga-68-DOTA-peptides currently available for imaging are: Ga-68 DOTA-TOC, Ga-68 DOTA-NOC, and Ga-68 DOTA-TATE. All these agents have been used in PET imaging of SSTR-positive NETs with tremendous success and are comparable in terms of sensitivity and specificity. Hence, the decision to use a particular tracer depends upon cost, availability and other logistical factors. Ga-68 DOTA-peptides PET/CT offers several advantages over conventional SRS like better visualization on account of better spatial resolution of latest PET/CT scanners, low radiation burden for the patient, reduced imaging time and cost-effectiveness (24).
In the largest bicentric study published till date, evaluating the role of Ga-68-DOTANOC PET/CT in the detection of undiagnosed primary sites in patients with metastatic NETs, Prasad et al. (25) found that Ga-68 DOTANOC PET/CT identified primary sites in 59% patients. In a recent study by Screiter et al. (26) published in 2014, the study group found that Ga-68 DOTATOC PET/CT detected primaries in 45.5% patients.
We are one of the largest tertiary care centers in India where Ga-68 DOTANOC PET/CT scans are being conducted since 2011. The primary objective behind our study was to retrospectively evaluate the efficacy of Ga-68 DOTANOC PET/CT imaging in detecting the primary site in our patients presenting with metastatic NETs of unknown origin. The secondary objective was to understand the impact of Ga-68 DOTANOC PET/CT scan on clinical decision making in this subset of patients. The findings of our study will add to the current knowledge pool and thereby help in guiding better overall management of these patients.
Methods
Between December 2011 and September 2014, a total of 263 patients underwent Ga-68 DOTANOC PET/CT scan in our department for various clinical indications. All patients voluntarily consented for the scan after obtaining relevant information including potential-benefits, radiation-exposure and costs.
Inclusion and exclusion criteria
Out of them, 68 (25.8%) consecutive patients referred to us with histopathologically proven metastatic neuroendocrine tumors and unknown primary site on conventional imaging who underwent Ga-68 DOTANOC PET/CT scan as part of their clinical work-up were included for analyses. Patients who underwent Ga-68 DOTANOC PET/CT scan with just clinical, biochemical or radiological suspicion of NETs and without histopathological evidence were excluded from analyses. Out of 68 patients, 8 patients had already received treatment i.e., long acting octreotide analogues, chemotherapy or targeted therapy for their metastatic lesions prior to undergoing the Ga-68 DOTANOC PET/CT scan. These patients were not excluded from the analyses based on our experience and available literature (27) that primary sites continue to be visualized with minor alterations of intensity of tracer uptake on Ga-68 DOTANOC PET/CT scans even after receiving these systemic treatments.
These 68 patients (patient characteristics; Table 1) were investigated retrospectively and various parameters like age, sex, site of metastatic neuroendocrine lesion, histopathological grades & proliferation indexes (Ki67), sites of detected primary lesions, quantitative estimation of SSTR expression in the form of maximal standardized uptake value (SUVmax) in metastatic as well as detected primary sites were noted. This review was done through a detailed study of their hospital medical records and careful re-analysis of their Ga-68 DOTANOC PET/CT scans by two experienced nuclear medicine physicians. Follow-up data was collected from individual patients at the time of their subsequent visits to the department for follow-up Ga-68 DOTANOC PET/CT scans. In patients where no subsequent visits were recorded, follow-up information was obtained through detailed telephonic interviews, recording data such as details of further treatment received, details of surgery (if any), histopathological reports and subsequent Ga-68 DOTANOC scans. This data was recorded in individual patient-case-sheet. Relationship between SUVmax of histopathologically proven metastatic site and SUVmax of detected primary site was studied using bi-variate analysis.
Table 1. Table showing patient characteristics: patient demographics, sites of histopathologically proven metastases and their histopathological grades.
| Characteristics | n (%) |
|---|---|
| Total patients | 68 |
| Males | 45 (66.2) |
| Females | 23 (33.8) |
| Age (in years) | |
| Mean | 54.9±10.7 |
| Median | 55 |
| Range | 31–78 |
| Histopathologically proven metastatic sites | |
| Liver | 50/68 (73.5) |
| Lymph node | 10/68 (14.7) |
| Mesentery | 6/68 (8.8) |
| Bone | 1/68 (1.5) |
| Orbit (soft tissue) | 1/68 (1.5) |
| Histopathological grades of NET | Available in 31/68 patients |
| Grade 1 | 16/31 (51.6) |
| Grade 2 | 10/31 (32.2) |
| Grade 3 | 5/31 (16.1) |
NET, neuroendocrine tumour.
Ga-68 DOTANOC positron emission tomography (PET)/computed tomography (CT)
Synthesis of Ga-68 DOTANOC
Ga-68 DOTANOC is prepared in our department by experienced radiochemist using standard company-provided protocol. Ga-68 is eluted from a commercially available Ge-68/Ga-68 generator. At our center we have a 1,025 MBq Ge-68/Ga-68 generator (itG, Isotope Technologies Garching GmbH). Ga-68 DOTANOC was prepared as described by Zhernesekov et al. (28) using manual synthesizer (Eckert & Ziegler, Eurotrope). Radiolabeling yields of >95% were usually achieved within 15 min. Care was taken to ensure that the radiation exposure to the radiochemist falls within prescribed limits (29).
Imaging protocol
Ga-68 DOTANOC PET/CT imaging was performed at our centre according to the recommended guidelines (30). Fasting was not required. Patients were usually administered 3–4 mCi (111–148 MBq) of Ga-68 DOTANOC intravenously through an indwelling catheter. They were then instructed to remain seated or recumbent after Ga-68 DOTANOC administration in isolation room. Patients were asked to void just prior to start of image acquisition to reduce the radiation exposure. Image acquisition was done approximately 60±15 minutes after Ga-68 DOTANOC administration from vertex to mid-thigh [a transmission scan using CT (120 kV, 200 mA) followed by 4–5 min per bed emission scan for five to eight bed positions using dedicated Discovery STE BGO advanced PET/CT scanner (GE healthcare, USA)]. The system consists of a 16-slice, spiral CT and is optimized for use in whole-body oncology. PET data were obtained in 3D mode, with attenuation-correction calculated from co-registered CT images. Additional breath-hold CT for evaluation of the lungs and three-phase CT abdomen was also acquired for every patient as per the institutional protocol. Regional/delayed views were taken as and when required. A total of 100 mL intravenous CT contrast was administered to every patient (through an automated injection pump), unless contra-indicated due to deranged renal function or history of allergic reaction to intravenous contrast. The PET/CT scanner was subjected to daily quality-control (QC) evaluation before the start of acquisition. The studies were reconstructed using a default vendor-implemented iterative reconstruction algorithm (Ordered-subset-expectation-maximization, OSEM) using standard protocols. The reconstructed data was available in maximal intensity projection (MIP), coronal, saggital and axial slices.
Image analysis
Ga-68 DOTANOC PET/CT scan images were interpreted by two experienced nuclear medicine physicians. The intensity level was set manually. First, the MIP images were visually inspected in varying scales. Thereafter, axial slices were viewed in combination with the corresponding CT image and the fused images. Physiological sites of DOTANOC uptake such as the pituitary gland, thyroid, bilateral adrenal glands, uncinate process of the pancreas, spleen, kidneys and gastrointestinal tract were carefully distinguished from abnormal sites of DOTANOC uptake. Tracer accumulations in structures that do not take up the tracer physiologically, or accumulations higher than background activity, were considered as abnormal. Anatomical localization of areas of abnormal DOTANOC uptake was then confirmed on axial CT images or fused PET/CT images. Regional one- or two-bed delayed views were acquired in equivocal cases. For detection of primary site, special attention was paid to analyzing the stomach, pancreas and small intestine in patients presenting with hepatic or abdominal lymph-nodal metastasis. Sites of focal abnormal DOTANOC uptake were noted. Whether the focal abnormal uptake on the PET/CT scan corresponds to the likely primary site was decided by consensus of both the physicians. Uptake in the metastatic sites and detected primary site was measured semi-quantitatively as SUVmax corrected for body weight. The SUV is the decay-corrected ratio between measured uptake in the region of interest and the expected uptake if Ga-68 DOTANOC were distributed evenly throughout the body. A circular region-of-interest (ROI) with a fixed diameter was placed over the region of highest intensity in the metastatic lesions as well as in lesions labeled as primary sites of disease, and uptake was automatically quantified as SUVmax by the vendor-provided software.
Reference standard
For patients with detected primary sites who subsequently underwent biopsy/resection of their primary sites, histopathology of surgical specimens was used as the standard of reference. In rest of the patients, visualization of lesion on follow-up examinations using Ga-68 DOTANOC PET/CT and other imaging modalities such as USG, CT scan and endoscopy was used as reference. For confirmation of true positive primaries in patients without a histopathologic examination, the mean follow-up period was 15.2±9.0 months (median: 13.3 months; range, 2.3–38.6 months).
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD), and categorical variables are presented as absolute numbers and percentages. The correlation between SUVmax of histologically proven metastatic sites and SUVmax of the detected primary sites was determined using Pearson’s correlation coefficient and P<0.05 were considered as statistically significant. Microsoft Office Excel 2007 and MedCalc commercial software package (MedCalc Statistical software version 14.12.0–32 bit, Belgium) (31) were used for all the statistical analyses and preparing data sheets.
Results
Ga-68 DOTANOC PET/CT scan was performed in 68 consecutive patients with histopathologically proven metastatic NETs and unknown primary site (CUP-NET). Forty-five of them were males (66.2%) and twenty-three females (33.8%). The incidence of CUP-NET was significantly higher in males (male: female ratio of 1.95:1) (Table 1).
Of these sixty-eight patients, 73.5% patients presented with hepatic metastasis (50 out of 68 patients). Rest of the patients presented with lymph nodal [10], mesenteric [6], skeletal [1] and orbital soft tissue [1] metastasis. On carefully re-examining all the histopathological reports, grades of NETs were documented in only 31 out of 68 patients (Table 1). Of these 31 patients, more than fifty-percent presented with grade I NETs. Grades of NETs have been defined in latest WHO classification of NETs (6) based on number of mitoses per 10 high power fields and proliferation-index (Ki67/MiB index).
Ga-68 DOTANOC PET/CT scan identified primary sites in 40 out of these 68 patients i.e., in approximately 59% patients. Maximum primary sites were identified in small intestine (46%) comprising of duodenum, jejunum and ileum. Other identified primary sites were: rectum, pancreas, stomach, lung and rare sites in kidney and prostate (Figures 1,2,3,4,5,6). In one patient, two primary sites were identified, one each in stomach and duodenum. Identified primary sites were reported on Ga-68 DOTANOC PET/CT scan as “likely to be the primary mitotic site”. In 8 patients, who had received long acting octreotide/systemic chemotherapy/targeted treatment like Sorafenib prior to undergoing Ga-68 DOTANOC PET/CT scan, primary sites were detected in 5 (62.5%) patients.
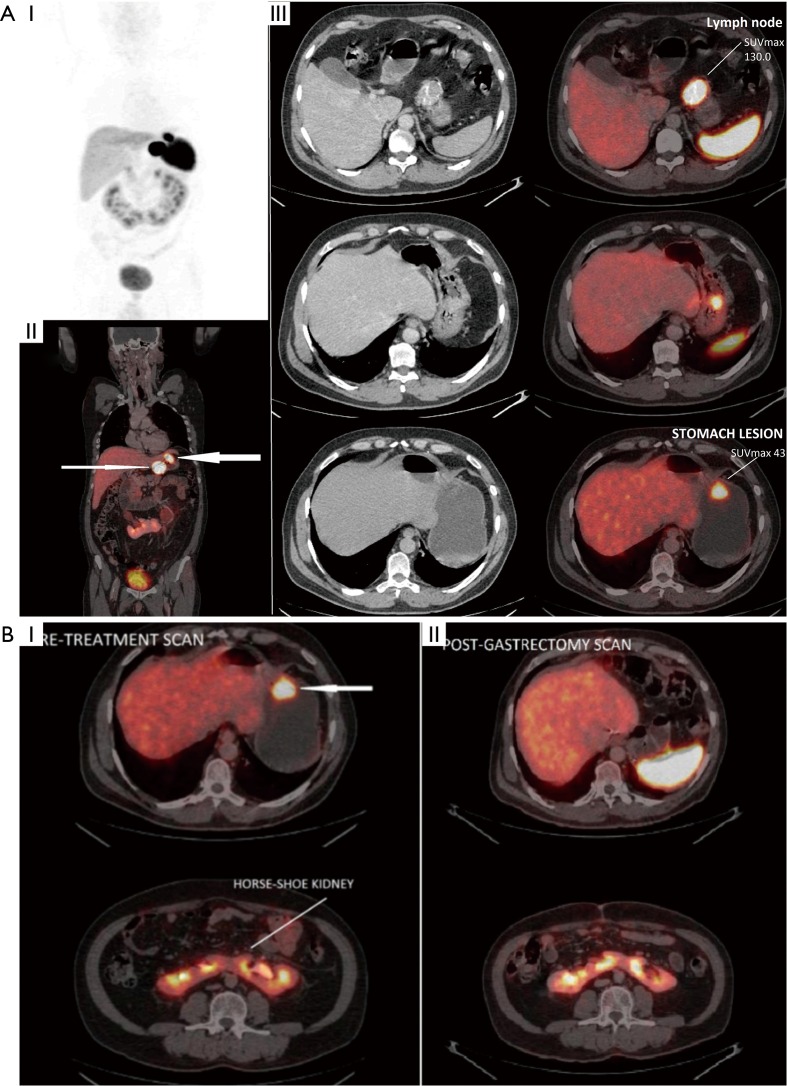
Figure 1.
Figure demonstrating utility of Ga-68 DOTANOC PET/CT scans in diagnosing primary site in CUP-NET. (A) A 55-year-old male presented with metastatic gastro-hepatic lymph node (CUP-NET). Ga-68 DOTANOC PET/CT scan demonstrated primary site in greater curvature of the stomach (thicker arrow) (SUVmax, 43.0) along with gastro-hepatic lymph node (thin arrow) (SUVmax, 130.0): (I) PET MIP image; (II) fused PET/CT coronal image; (III) fused PET/CT trans-axial images. (B) Patient later underwent total gastrectomy. Histopathology was reported as well-differentiated NET, Ki67 <2%. Note is made of incidentally diagnosed Horse-shoe kidney: (I) pre-treatment scan; (II) post-gastrectomy scan. NET, neuroendocrine tumour; PET/CT, positron emission tomography/computed tomography; SUVmax, maximal standardized uptake value; MIP, maximal intensity projection.
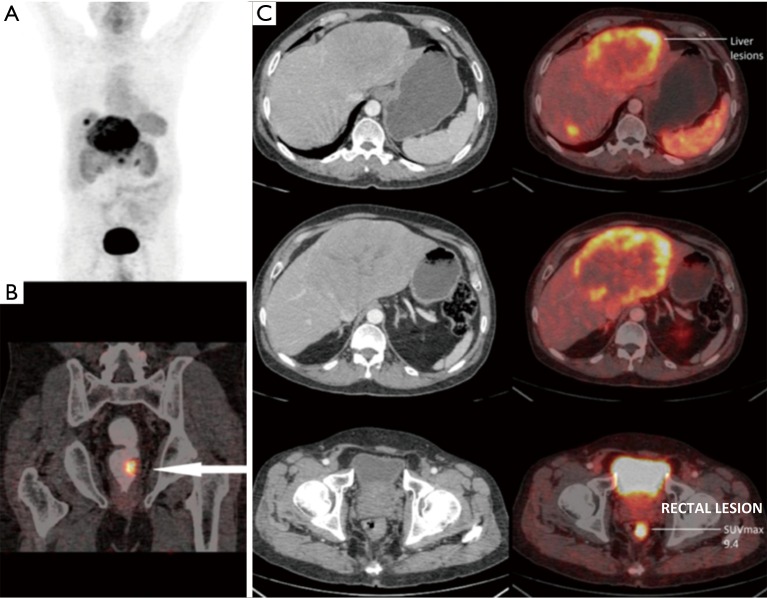
Figure 2.
A 58-year-old male presented with metastatic neuroendocrine liver lesions (unknown primary). Ga-68 DOTANOC PET/CT scan revealed primary site in the left lateral wall of the rectum (arrow) (SUVmax, 9.4) which was later confirmed on colonoscopic biopsy. Patient underwent left liver lobectomy and was being planned for rectal surgery at the time of data collection for this study. (A) PET MIP image; (B) fused PET/CT coronal image; (C) fused PET/CT trans-axial images. PET/CT, positron emission tomography/computed tomography; SUVmax, maximal standardized uptake value; MIP, maximal intensity projection.
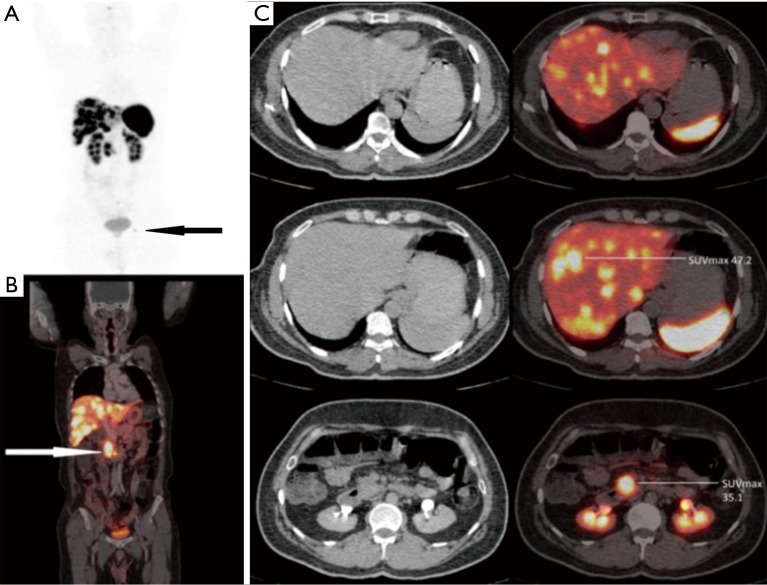
Figure 3.
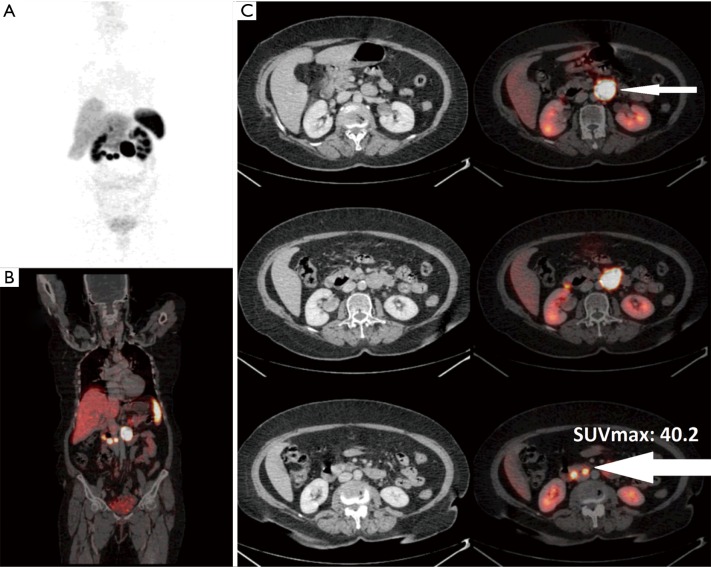
A 48-year-old male presented with metastatic neuroendocrine liver lesions (unknown primary). Ga-68 DOTANOC PET/CT scan revealed primary site in the head and uncinate process of the pancreas (white arrow) (SUVmax, 35.1) along with hepatic, lymphnodal and solitary skeletal metastasis in left acetabulum (black arrow). (A) PET MIP image; (B) fused PET/CT coronal image; (C) fused PET/CT trans-axial images. PET/CT, positron emission tomography/computed tomography; SUVmax, maximal standardized uptake value; MIP, maximal intensity projection.
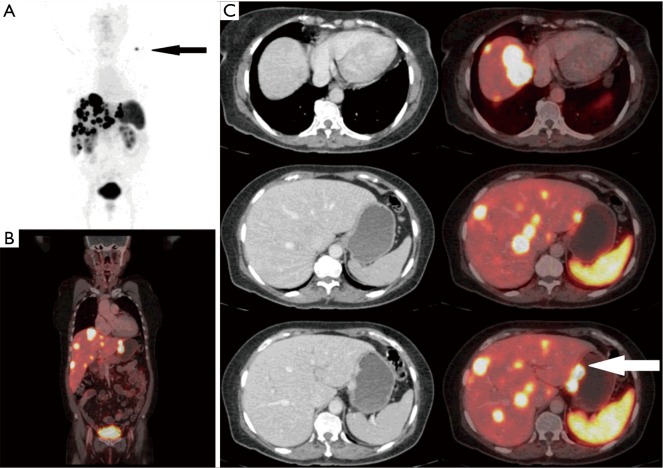
Figure 4.
A 61-year-old female with metastatic NET liver lesions (unknown primary). Ga-68 DOTANOC scan identified primary site in lesser curvature of stomach (white arrow) (SUVmax, 37.3) along with additional lymph nodal and soft tissue metastasis (black arrow). (A) PET MIP image; (B) fused PET/CT coronal image; (C) fused PET/CT trans-axial images. NET, neuroendocrine tumour; SUVmax, maximal standardized uptake value; PET/CT, positron emission tomography/computed tomography; MIP, maximal intensity projection.
Figure 5.
A 67-year-old female presented with metastatic mesenteric lymph node (CUP-NET). Ga-68 DOTANOC PET/CT scan revealed at least three avid nodular lesions in third part of duodenum (thicker arrow) (site of primary) with lymph nodal metastases (thin arrow). No surgery was performed in this patient. (A) PET MIP image; (B) fused PET/CT coronal image; (C) fused PET/CT trans-axial images. NET, neuroendocrine tumour; PET/CT, positron emission tomography/computed tomography; MIP, maximal intensity projection.
Figure 6.
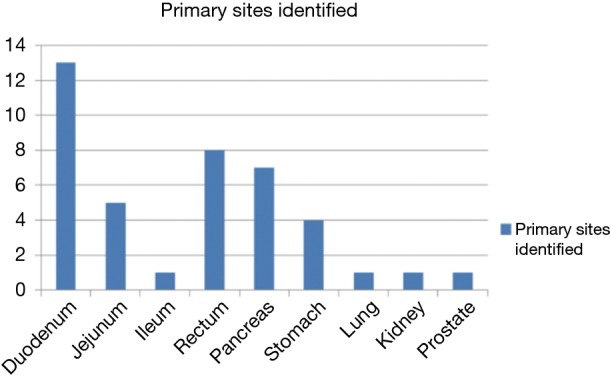
Bar diagram showing individual detected primary sites. Maximum primary sites were detected in small intestine comprising of duodenum, jejunum and ileum (n=19).
Mean SUVmax of the detected primary sites was 25.1±18.0 (median: 16.25; range, 2.1–150). The mean SUVmax of detected primary sites was the highest for pancreas (30.98±20.34) and lowest for rectum (10.11±5.87) (Table 2). Mean SUVmax of the histopathologically proven metastatic sites was 38.6±39 (median, 26; range, 4.9–170.5). Significant positive correlation was found between SUVmax of detected primary sites and SUVmax of the histopathologically proven sites of metastasis (r=0.662; P<0.0001).
Table 2. Table showing SUVmax of the primary sites calculated on Ga-68 DOTANOC PET/CT scan.
| Detected primary sites | SUVmax (mean ± SD) |
|---|---|
| Small intestine (SI) | 30.66±34.6 (median, 22) |
| Pancreas | 30.98±20.34 (median, 25.66) |
| Stomach | 29.87±18.31 (median, 37.3) |
| Rectum | 10.11±5.87 (median, 8.75) |
SUVmax, maximal standardized uptake value; PET/CT, positron emission tomography/computed tomography; SD, standard deviation.
Based on the findings of the Ga-68 DOTANOC PET/CT scan, 3 out of 40 patients underwent definitive treatment for their primary tumour (1 gastric, 1 ileal and 1 prostatic tumour). One patient was being planned for resection of primary rectal lesion at the time of data-collection. Thirty-six out of 68 patients were started on long-acting somatostatin-analogues or chemotherapy or targeted therapy based on tumor’s differentiation status and somatostatin-receptor expression on Ga-68 DOTANOC PET/CT scans. Two patients underwent multiple cycles of peptide receptor radionuclide therapy (PRRNT) using Y-90 and Lu-177 labeled-somatostatin analogues.
Eight patients died during follow-up. In 3 out of these 8 patients, primary site was detected on Ga-68 DOTANOC PET/CT scan, whereas, no primary site was identified in 5 patients.
Discussion
The expanding use of Ga-68 labeled somatostatin analogues PET/CT imaging in clinical evaluation of NETs underlines the need to evaluate its appropriate role in the management of these tumours. Till date, various aspects of Ga-68 DOTANOC PET/CT imaging have been studied. The studies date back to last decade and most of the work has been done in European nations. Ambrosini et al. (32) and Naswa et al. (33) in their studies evaluating role of Ga-68 DOTANOC PET/CT in initial staging, found sensitivity in the range of 78–92% and specificity of 92.5–98% for detection of NETs.
Patients with metastatic NETs and unknown primary site (CUP-NET) constitute 10–13% of total NET study populations (9,10) and have a relatively poorer prognosis than other NET patients (11). Catena et al. (10) reported that majority of CUP-NETs are well-differentiated. In our study, we found that majority of the patients (>50%) presented with well-differentiated (grade I) metastatic neuroendocrine lesions. Catena et al. (10) also found that patients with well-differentiated neuroendocrine tumor of unknown primary site usually have liver metastases. A total of 73.5% of our patients presented with hepatic metastasis (50 out of 68 patients). Rest of the patients presented with lymph nodal [10], mesenteric [6], skeletal [1], orbital soft tissue [1] metastasis.
Role of Ga-68-DOTA-peptides PET/CT in localizing the undiagnosed primary sites in patients with metastatic NETs has been studied earlier. In the largest bicentric study published till date, done on 59 patients, Prasad et al. (25) found that Ga-68 DOTANOC PET/CT identified primary sites in 35 out of 59 patients (59%) and concluded that Ga-68 DOTANOC PET/CT is superior to In-111-OctreoScan in this aspect. In their study, most commonly identified primary site was pancreas followed by small intestine. In a similar study by Naswa et al. (34), the group identified primary site in 12 out of 20 patients (60%). In a recent study by Screiter et al. (26) published in 2014, the study group found that Ga-68 DOTATOC PET/CT detected primary mitotic site in 45.5% (15 out of 33 patients) with most common site being the small intestine. In our study, we were able to identify the primary mitotic site in 40 out of 68 patients i.e., in approximately 59% patients. The results of our study are concordant with the studies done by Prasad et al. (25) and Naswa et al. (34). In our study, rate of detection of primary sites was higher compared to study done by Screiter et al. (26). Possible reasons could be differences in study-population characteristics and tumour-heterogeneity. As histopathological grades were not available in all the patients, we are unable to comment upon the histopathological grade-wise identification rate in our study population.
Most commonly detected primary site was small intestine (19 patients) including duodenum, jejunum and ileum. In fact, 73% of all primary sites were detected in GEP region (Figure 6), which is consistent with the observations of the past (3). In 5 out of 6 patients who presented with mesenteric metastases, primary site was diagnosed in small intestine. Small bowel NETs are known to metastasize first to mesentery, followed by hepatic metastases. Few rare primary sites were also identified; one each in prostate gland and kidney. Patient identified with primary site in prostate gland presented with para-rectal lymph nodal metastases. Ga-68 DOTANOC scan identified not only the primary site in prostate gland but also identified additional skeletal metastases in this patient. Histopathological examination from prostate was suggestive of poorly-differentiated NET. This patient received definitive chemo-radiation for his primary lesion.
In 8 patients, who had received long-acting somatostatin analogues or systemic chemotherapy or targeted treatment like sorafenib, prior to undergoing Ga-68 DOTANOC PET/CT scan, primary sites were detected in 5 (62.5%) patients. There was no significant difference in the detection rate between this subset and the entire study population. This confirmed our experience that primary sites continue to be visualized with minor alterations of intensity of radiotracer uptake post somatostatin-analogue or chemotherapy or targeted-therapy administration.
Mean SUVmax of the detected primary sites was 25.1±18.0 (median: 16.25; range, 2.1–150). The mean SUVmax of detected primary sites was the highest for pancreas and lowest for rectum (Table 2). Standardized uptake value (SUV) on Ga-68 DOTANOC PET/CT scan has a direct positive correlation with somatostatin-receptor (SSTR) density on NETs cells (35,36). Low-grade NETs have a higher SSTR expression and hence demonstrate higher SUVmax on Ga-68 DOTANOC PET/CT scans and as NETs becomes more aggressive, they lose their property of SSTR expression (37). In our study, pancreatic and small intestinal primary sites demonstrated higher SUVmax and hence, higher differentiation status compared to primaries detected in rectum. GEP NETs are found to express SSTRs in 80–100% of cases (38,39). Significant positive correlation was found between SUVmax of detected primary sites and SUVmax of the histopathologically proven sites of metastasis (r=0.662; P<0.0001). This observation is suggestive of concordant somatostatin-receptor expression and differentiation status in primary site and its metastases i.e., similar phenotype.
Based on the findings of the Ga-68 DOTANOC PET/CT scan, 3 out of 40 patients underwent definitive treatment for their primary tumour (1 gastric, 1 ileal and 1 prostatic tumour). Histopathological analyses of all the resected specimens demonstrated NETs in the diagnosed primary sites i.e., true-positives. One patient was being planned for resection of primary rectal lesion at the time of data-collection for this study. Additional histopathological sampling was available from three patients (through endoscopy) which demonstrated NETs in their detected primary sites in rectum, duodenum and jejunum respectively i.e., true-positives. In the remaining patients, primary tumors were not operated, probably due to their advanced disease/distant metastases.
Thirty-six out of 68 patients were started on long-acting somatostatin analogues or chemotherapy or targeted therapy based on scan findings. Two patients underwent multiple cycles of PRRNT using 90Y and 177Lu labeled somatostatin analogues. Ga-68 DOTANOC PET/CT scan is a whole-body examination which gives information regarding the site and extent of primary NET and distant metastasis, along with, somatostatin-receptor expression in individual lesions. Hence, Ga-68 DOTANOC PET/CT scan is a useful tool for the treating physician in deciding appropriate management for NET patients. Also, this modality can be examined for its role in assessing therapeutic response to various standard and newer treatments.
Eight patients died due to cancer-related causes during follow-up. In 3 out of these 8 patients, primary site was detected on Ga-68 DOTANOC PET/CT scan, whereas, no primary site was identified in five patients. Whether patients with undiagnosed primary sites on Ga-68 DOTANOC PET/CT scans have a poorer prognosis compared to the patients with diagnosed primary sites, is difficult to comment upon, based on the findings of our retrospective study. A dedicated prospective study with longer follow-up period is warranted to study this phenomenon.
Ours is the largest study till-date as far as number of study participants is concerned. The results of our study are in concordance with the available literature. The findings of our study reiterate the fact that Ga-68 DOTANOC PET/CT imaging is a very promising modality for the detection of unknown primary sites in metastatic neuroendocrine carcinomas of unknown origin and should be considered as first-line investigation in such cases. Even in cases, where it is unable to detect the primary site, it provides useful information for guiding clinical management of such patients. Tumor grade/differentiation status currently is an important determinant of the personalized management of these patients (8).
Our study is limited by its retrospective design, which is less accurate than a prospective study in terms of the data obtained, date of first diagnosis, or histologic grades. An advantage of the retrospective design is the inclusion of a relatively large number of patients with this rare tumor entity. Another drawback was that histology was not available for all patients, which was common to all other studies performed till date in this regard (25,26,34) and was due to the fact that patients were not operated by their treating physicians without an apparent clinical benefit and for the sole reason of obtaining biopsy material.
Conclusions
The findings of our study reiterate the fact that Ga-68 DOTANOC PET/CT imaging is very promising modality for the detection of primary site in metastatic neuroendocrine carcinomas of unknown origin and should be considered as first-line investigation in such cases. Even in cases, where it is unable to detect the primary site, it provides useful information for guiding clinical management of such patients.
Acknowledgements
Pradeep Kumar, Yogendra Rawat, Jitendra Kumar & Govind Singh.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumors. Lancet Oncol 2008;9:61-72. 10.1016/S1470-2045(07)70410-2 [DOI] [PubMed] [Google Scholar]
- 2.Pictet RL, Rall LB, Phelps P, et al. The neural crest and the origin of the insulin-producing and other gastrointestinal hormone-producing cells. Science 1976;191:191-2. 10.1126/science.1108195 [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
- 4.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934-59. 10.1002/cncr.11105 [DOI] [PubMed] [Google Scholar]
- 5.Anlauf M. Neuroendocrine neoplasms of the gastroenteropancreatic system: pathology and classification. Horm Metab Res 2011;43:825-31. 10.1055/s-0031-1291307 [DOI] [PubMed] [Google Scholar]
- 6.Rindi G, Arnold R, Bosman FT, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman F, Carneiro F, Hruban R, et al, editors. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC Press, 2010. [Google Scholar]
- 7.Reubi JC, Waser B, Schaer JC, et al. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med 2001;28:836-46. 10.1007/s002590100541 [DOI] [PubMed] [Google Scholar]
- 8.Spigel DR, Hainsworth JD, Greco FA. Neuroendocrine carcinoma of unknown primary site. Semin Oncol 2009;36:52-9. 10.1053/j.seminoncol.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 9.Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
- 10.Catena L, Bichisao E, Milione M, et al. Neuroendocrine tumors of unknown primary site: gold dust or misdiagnosed neoplasms? Tumori 2011;97:564-7. [DOI] [PubMed] [Google Scholar]
- 11.Hauso O, Gustafsson BI, Kidd M, et al. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer 2008;113:2655-64. 10.1002/cncr.23883 [DOI] [PubMed] [Google Scholar]
- 12.Kirshbom PM, Kherani AR, Onaitis MW, et al. Carcinoids of unknown origin: comparative analysis with foregut, midgut, and hindgut carcinoids. Surgery 1998;124:1063-70. 10.1067/msy.1998.93105 [DOI] [PubMed] [Google Scholar]
- 13.Fendrich V, Bartsch DK. Surgical treatment of gastrointestinal neuroendocrine tumors. Langenbecks Arch Surg 2011;396:299-311. 10.1007/s00423-011-0741-7 [DOI] [PubMed] [Google Scholar]
- 14.Fischer L, Mehrabi A, Buchler MW. Neuroendocrine tumors of the duodenum and pancreas. Surgical strategy. Chirurg 2011;82:583-90. 10.1007/s00104-011-2069-9 [DOI] [PubMed] [Google Scholar]
- 15.Norton JA, Fraker DL, Alexander HR, et al. Surgery increases survival in patients with gastrinoma. Ann Surg 2006;244:410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plockinger U, Rindi G, Arnold R, et al. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours. A consensus statement on behalf of the European Neuroendocrine Tumour Society (ENETS). Neuroendocrinology 2004;80:394-424. 10.1159/000085237 [DOI] [PubMed] [Google Scholar]
- 17.Plockinger U, Wiedenmann B, De Herder WW. ENETS consensus guidelines for the standard of care in neuroendocrine tumors. Neuroendocrinology 2009;90:159-61. 10.1159/000225945 [DOI] [PubMed] [Google Scholar]
- 18.NCCN Guidelines version 1. 2015 Neuroendocrine tumours of unknown primary. Available online: http://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- 19.Ramage JK, Davies AH, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumors. Gut 2005;54 Suppl 4:iv1-16. 10.1136/gut.2004.053314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khashab MA, Yong E, Lennon AM, et al. EUS is still superior to multidetector computerized tomography for detection of pancreatic neuroendocrine tumors. Gastrointest Endosc 2011;73:691-6. 10.1016/j.gie.2010.08.030 [DOI] [PubMed] [Google Scholar]
- 21.Savelli G, Lucignani G, Seregni E, et al. Feasibility of somatostatin receptor scintigraphy in the detection of occult primary gastro-entero-pancreatic (GEP) neuroendocrine tumors. Nucl Med Commun 2004;25:445-9. 10.1097/00006231-200405000-00004 [DOI] [PubMed] [Google Scholar]
- 22.Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J Nucl Med 2007;48:508-18. 10.2967/jnumed.106.035667 [DOI] [PubMed] [Google Scholar]
- 23.Kowalski J, Henze M, Schuhmacher J, et al. Evaluation of positron emission tomography imaging using [68Ga]-DOTA-D Phe(1)-Tyr(3)-octreotide in comparison to [111In]-DTPAOC SPECT. First results in patients with neuroendocrine tumors. Mol Imaging Biol 2003;5:42-8. 10.1016/S1536-1632(03)00038-6 [DOI] [PubMed] [Google Scholar]
- 24.Antunes P, Ginj M, Zhang H, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging 2007;34:982-93. 10.1007/s00259-006-0317-x [DOI] [PubMed] [Google Scholar]
- 25.Prasad V, Ambrosini V, Hommann M, et al. Detection of unknown primary neuroendocrine tumours (CUP-NET) using (68) Ga-DOTA-NOC receptor PET/CT. Eur J Nucl Med Mol Imaging 2010;37:67-77. 10.1007/s00259-009-1205-y [DOI] [PubMed] [Google Scholar]
- 26.Schreiter NF, Bartels AM, Froeling V, et al. Searching for primaries in patients with neuroendocrine tumors (NET) of unknown primary and clinically suspected NET: Evaluation of Ga-68 DOTATOC PET/CT and In-111 DTPA octreotide SPECT/CT. Radiol Oncol 2014;48:339-47. 10.2478/raon-2014-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haug AR, Rominger A, Mustafa M, et al. Treatment with octreotide does not reduce tumor uptake of 68Ga-DOTATATE as measured by PET/CT in patients with neuroendocrine tumors. J Nucl Med 2011;52:1679-83. 10.2967/jnumed.111.089276 [DOI] [PubMed] [Google Scholar]
- 28.Zhernosekov KP, Filosofov DV, Baum RP, et al. Processing of generator-produced 68Ga for medical application. J Nucl Med 2007;48:1741-8. 10.2967/jnumed.107.040378 [DOI] [PubMed] [Google Scholar]
- 29.Dwivedi DK, Snehlata, Dwivedi AK, et al. Radiation exposure to nuclear medicine personnel handling positron emitters from Ge-68/Ga-68 generator. Indian J Nucl Med 2011;26:86-90. 10.4103/0972-3919.90258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Virgolini I, Ambrosini V, Bomanji JB, et al. Procedure guidelines for PET/CT tumor imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging 2010;37:2004-10. 10.1007/s00259-010-1512-3 [DOI] [PubMed] [Google Scholar]
- 31.Download MedCalc Version 14.12.0. MedCalc-easy-to-use Statistical Software. Available online: http://www.medcalc.org/download.php
- 32.Ambrosini V, Campana D, Tomassetti P, et al. 68Ga-labelled peptides for diagnosis of gastroenteropancreatic NET. Eur J Nucl Med Mol Imaging 2012;39 Suppl 1:S52-60. 10.1007/s00259-011-1989-4 [DOI] [PubMed] [Google Scholar]
- 33.Naswa N, Sharma P, Kumar A, et al. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: A prospective single-center study. AJR Am J Roentgenol 2011;197:1221-8. 10.2214/AJR.11.7298 [DOI] [PubMed] [Google Scholar]
- 34.Naswa N, Sharma P, Kumar A, et al. 68Ga-DOTANOC PET/CT in patients with carcinoma of unknown primary of neuroendocrine origin. Clin Nucl Med 2012;37:245-51. 10.1097/RLU.0b013e31823ea730 [DOI] [PubMed] [Google Scholar]
- 35.Boy C, Heusner TA, Poeppel TD, et al. 68Ga-DOTATOC PET/CT and somatostatin receptor (sst1-sst5) expression in normal human tissue: Correlation of sst2 mRNA and SUV max. Eur J Nucl Med Mol Imaging 2011;38:1224-36. 10.1007/s00259-011-1760-x [DOI] [PubMed] [Google Scholar]
- 36.Kaemmerer D, Peter L, Lupp A, et al. Molecular imaging with 68Ga- SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumors. Eur J Nucl Med Mol Imaging 2011;38:1659-68. 10.1007/s00259-011-1846-5 [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues M, Gabriel M, Heute D, et al. Concordance between results of somatostatin receptor scintigraphy with 111In-DOTA-DPhe 1-Tyr 3-octreotide and chromogranin A assay in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2008;35:1796-802. 10.1007/s00259-008-0794-1 [DOI] [PubMed] [Google Scholar]
- 38.Kubota A, Yamada Y, Kagimoto S, et al. Identification of somatostatin receptor subtypes and an implication for the efficacy of somatostatin analogue SMS 201-995 in treatment of human endocrine tumors. J Clin Invest 1994;93:1321-5. 10.1172/JCI117090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reubi JC. Somatostatin and other peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology 2004;80 Suppl 1:51-6. 10.1159/000080742 [DOI] [PubMed] [Google Scholar]