Abstract
Platinum-containing molecules are widely used as anticancer drugs. These molecules exert cytotoxic effects by binding to DNA through various mechanisms. The binding between DNA and platinum-based drugs hinders the opening of DNA, and therefore, DNA duplication and transcription are severely hampered. Overall, impeding the above-mentioned important DNA mechanisms results in irreversible DNA damage and the induction of apoptosis. Several molecules, including multinuclear platinum compounds, belong to the family of platinum drugs, and there is a body of research devoted to developing more efficient and less toxic versions of these compounds. In this study, we combined different biophysical methods, including single-molecule assays (magnetic tweezers) and bulk experiments (ultraviolet absorption for thermal denaturation) to analyze the differential stability of double-stranded DNA in complex with either cisplatin or multinuclear platinum agents. Specifically, we analyzed how the binding of BBR3005 and BBR3464, two representative multinuclear platinum-based compounds, to DNA affects its stability as compared with cisplatin binding. Our results suggest that single-molecule approaches can provide insights into the drug-DNA interactions that underlie drug potency and provide information that is complementary to that generated from bulk analysis; thus, single-molecule approaches have the potential to facilitate the selection and design of optimized drug compounds. In particular, relevant differences in DNA stability at the single-molecule level are demonstrated by analyzing nanomechanically induced DNA denaturation. On the basis of the comparison between the single-molecule and bulk analyses, we suggest that transplatinated drugs are able to locally destabilize small portions of the DNA chain, whereas other regions are stabilized.
Introduction
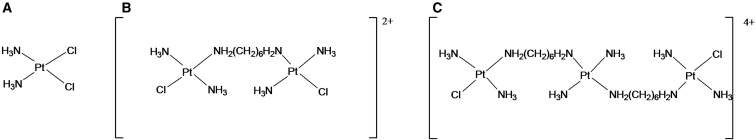
Cisplatin (CIS; cis-diamminedichloroplatinum(II)) (Fig. 1 A) forms the foundation of the numerous platinum-based compounds that have been reported thus far (1). CIS has been referred to as the penicillin of cancer because of its efficacy and versatility in the treatment of several solid tumors (2). The main cellular target of this drug is DNA. Similar to bifunctional alkylating agents, CIS interferes with DNA function by forming cross-links within DNA and between DNA and DNA-associated proteins (e.g., DNA polymerases, DNA topoisomerases, and histones) (3, 4). Cellular studies have shown that the most frequent bivalent DNA adducts produced by CIS cross-link adjacent purines within one DNA strand, with a preference for guanines (5, 6). Intramolecular links involving guanine (N7) are also formed, as are less frequent intermolecular links, representing an obstacle for DNA replication and transcription; the intramolecular links are relevant to drug cytotoxicity (7). CIS and related drugs (e.g., oxaliplatin and carboplatin) are widely prescribed in the clinic as antitumor drugs. Despite the importance of these drugs in clinical practice, several drawbacks have been observed since the discovery of CIS and the later development of second- and third-generation platinum compounds (8).
Figure 1.
Chemical structures of CIS (A), BBR3005 (B), and BBR3464 (C).
Currently, the development of new platinum-containing compounds is an active area of research (9, 10, 11). In addition to monofunctional Pt complexes, multinuclear Pt(II) agents have been developed. These derivatives form long-range (Pt, Pt) inter- and intrastrand cross-links that lead to the formation of a range of DNA adducts; thus, they may improve the spectrum of antitumor activity and confer the ability to overcome CIS resistance (3, 12). A few nanomechanical methods have been used so far to analyze the interactions between DNA and platinum-based drugs. For example, atomic force microscopy measurements highlight the changes in DNA topography (DNA shortening, compaction, loop formation, and persistence-length reduction) as a consequence of DNA-CIS interaction (13, 14, 15, 16, 17). In force-spectroscopy mode, atomic force microscopy allows discrimination of the binding characteristics of platinum-based drugs by measuring single-molecule DNA nanomechanical properties (18). Other studies have demonstrated the capability of optical tweezers techniques to measure the persistence length of the CIS-DNA complex and have shown that CIS binds cooperatively to the DNA molecule (19, 20). In the same vein, using optical tweezers, still other authors have observed a reduction of DNA persistence length and DNA extension in the presence of intercalants (21, 22, 23), noting also that the measured values of the persistence length depend on the maximum stretching force (24). Finally, magnetic tweezers (MT) measurements have revealed the effect of drug binding on DNA nanomechanics by detecting persistence-length variations (15, 25), or the presence of two different persistence lengths depending on the applied force (9), or the various effects of combining CIS and transplatin (26).
In this study, we use a single-molecule assay based on the MT technique to analyze how DNA is affected by the binding of three platinum-containing anticancer drugs, CIS and the multinuclear platinum drugs BBR3005 and BBR3464.
BBR3005 is a dinuclear platinum compound that contains two monofunctional platinum groups (Fig. 1 B) separated by six methylene groups. These features confer high flexibility to the Pt nuclei; therefore, the molecule is free to adopt the best geometry for DNA interaction (27). BBR3005 forms interstrand cross-links with high efficiency (∼70–90% of the total DNA adducts), whereas its ability to twist and unwind DNA is similar to that of CIS (3).
BBR3464 is a trinuclear platinum complex containing two monofunctional platinum groups (Fig. 1 C). Compared with CIS, this compound displays faster DNA binding (likely because of its greater positive charge and higher flexibility) and forms more DNA adducts. Although CIS forms rigid, short-range, intrastrand adducts, BBR3464 forms adducts more commonly described as flexible and long range, with a high percentage of interstrand cross-links (3). Compared with BBR3005, BBR3464 forms interstrand cross-links at a lower frequency (∼20% of the total DNA adducts) but with significantly faster reaction kinetics (3). Although these features represent promising antitumor properties against both CIS-sensitive and CIS-resistant tumors, the efficiency of BBR3464 during preclinical studies has been disappointing (10).
Since their invention (28, 29, 30), MT techniques have often been used to analyze the interactions between DNA and DNA ligands, including small drug molecules (25, 31, 32) and proteins (33, 34, 35, 36, 37, 38). MT can be used to apply controlled force and torsion to single DNA molecules and enables measurement of the influence of such nanomechanical stresses on the DNA structure, primarily through the assessment of end-to-end distance at nanometric resolution. The force and torsion are transmitted to the DNA via a magnetic microbead that is attached to the DNA. An MT apparatus essentially consists of a microscope that detects the movements of the bead and a magnet for nanomanipulation of the bead. Here, we used MT to detect small modifications in DNA extension, with high sensitivity at the single-molecule level. Moreover, MT allowed us to induce controlled torsional stresses on single double-stranded DNA molecules, which in turn caused unwinding and triggered the formation of melting bubbles. In particular, for negative turns in the intermediate force regime (), a region exhibiting enhanced fluctuations in measured DNA extension has recently been demonstrated (39, 40, 41, 42, 43, 44, 45). These fluctuations result from DNA extension randomly oscillating between denaturation conditions (characterized by a long extension) and plectonemic conditions (characterized by a reduced extension because of the formation of plectonemes). By quantitatively evaluating such extension fluctuations (an index of DNA supercoiling and/or denaturation) via assessments of the movement of the magnetic bead along the z axis, we can extract the so-called characteristic force () of the DNA (39, 40, 41). At , the measured standard deviation, , of the fluctuations in DNA extension reaches a maximum. Analogously, this phenomenon is also reflected in an increment of the temporal fluctuations of . At , the DNA extension fluctuations show slower time behavior, as quantitatively demonstrated by studying the time correlation function of the data and the characteristic time, as a function of the applied force (39, 40, 41). The maximum of is reached when the value of applied force corresponds to . In analyzing , we can obtain precise information regarding DNA nanomechanical stability (i.e., the DNA denaturation transition induced by nanomechanical stresses) (39, 40, 41).
Because DNA-drug interactions can result in an imbalance in DNA thermal stability and shift the DNA denaturation profile, we focused on the tendency of DNA-drug complexes to denature. Additionally, we compared the nanomechanical stability results obtained using MT with information obtained using traditional biophysical tools, namely, spectrophotometric melting assays. Melting profiles were obtained based on the well-known increment in DNA ultraviolet (UV) absorption upon denaturation and by performing temperature ramps in the range . We consider the similarities and differences between denaturation induced via nanomechanics and that induced via thermal heating, and we explore how these two procedures can produce complementary information.
MT technology was a good complement to the ensemble biophysical tools in providing a better understanding of the molecular behavior of platinum-based agents and other compounds that interact with DNA. These assessments may be useful for the improvement of currently available drugs and for the development of a new generation of anticancer molecules.
Materials and Methods
Activating solution
Platinum compounds were kept in an inactive state using high concentrations of chloride ions (46). To maintain a reasonable concentration of (50 mM) with a low concentration of ions, we diluted all platinum compounds in an activating buffer composed of 50 mM (47). is required to avoid nonspecific attachment of the magnetic beads to the glass surface.
Drug-compound preparation
The compounds were diluted in a physiological solution, 154 mM NaCl, and stored in the dark (CIS at room temperature and BBR3464 and BBR3005 at ). Immediately before injection into the MT cell, the drugs were diluted in a 50 mM NaClO4 buffer to a final concentration of . This concentration greatly exceeds the basepair concentration (well below 1 nM) of the DNA present in the capillary after washing, and considering the reported binding kinetics (3, 46, 47), it is thus sufficient to almost saturate all cross-linking sites. Representative data at different drug concentrations are reported in Figs. S1–S3 in the Supporting Material.
It is important to note that, as explained above, we follow protocols (3, 46, 47) that allow the drug to incubate in its most reactive form (in the absence of chloride ions). Other authors (9, 19) have performed similar experiments with MT, but they used incubation buffers that inactivate the platinum drugs, and as a consequence, they needed larger drug concentrations to achieve saturation of the DNA binding sites.
Cross-linking reaction
DNA (immobilized in the capillary) was incubated with the drugs overnight (14–16 h), i.e., for a waiting time much longer than the cross-linking kinetics half-period. On the basis of the literature, in our incubation conditions, such a half-period is 1.5 h for CIS and BBR3005 (48, 49) and 15 min for BBR3464 (3). As a consequence, we assumed that for all the drugs, the reaction with DNA reached saturation within our incubation time. To prevent attachment of the bead to the capillary surface, the incubation was performed exerting a pulling force of . After incubation, the capillary was washed using 6–8 mL of PTE buffer (phosphate-buffered saline (PBS), pH 7.4, containing 1 mM EDTA and 0.1% Tween) to remove ions and any drugs remaining in the solution. All measurements were performed under pseudophysiological conditions (in PTE buffer), as previously described (25, 41, 50).
DNA for MT measurements
The DNA sample used for the MT experiments included a 6964-bp-long sequence containing biotin- and digoxigenin-modified tails and was prepared as previously described (25). Briefly, the central sequence (5780 bp) was obtained from NotI/SacII restriction digests of the pCMV6-neo plasmid (Origene, Rockville, MD). The tails (537 bp and 647 bp in length) were obtained by polymerase chain reaction using the pBR322 plasmid (Stratagene, San Diego, CA) as a template and primers carrying consensus sites for NotI or SacII at their 5′ ends [REF]. Polymerase chain reaction was performed using biotin-16-dUTP-labeled nucleotides (Roche, Basel, Switzerland) for the 537 bp sequence or digoxigenin-11-dUTP-labeled nucleotides (Roche) for the 647 bp sequence in the reaction mixture. After amplification, the sticky ends were obtained by digesting with either NotI or SacII at 37°C for 2 h. The central sequence (5780 bp) obtained by NotI/SacII digestion of pCMV6-neo plasmid (Origene) was ligated to the tails overnight at 16°C with T4 DNA-ligase (Promega, Madison, WI) with a 5:1 excess of the ends with respect to the central sequence. The effectiveness of the ligation reaction was verified using gel agarose electrophoresis. The ligation products were diluted to a final concentration of 0.2 ng/μL in TE buffer, aliquoted in volumes of 10 μL, and stored at −20°C. Labeled DNA was conjugated to streptavidin-coated magnetic beads (Dynabeads MyOne C1, cat. no. 650.01, Invitrogen, Carlsbad, CA) as previously described (25). After conjugation, the mixture was brought to a final volume of 500 μL by the addition of PTE buffer. The entire solution was flowed into a capillary (50 μL capacity) with an inner surface that had been functionalized with an anti-digoxigenin antibody (Roche). The protocol for capillary functionalization and the storage conditions were previously described (25). The DNA/bead mixture was incubated in the capillary for 1 h at room temperature, allowing the digoxigenin-modified tails to interact with the anti-digoxigenin-functionalized glass surface. Finally, the capillary was washed with 8 mL PTE buffer to remove excess microbeads and unbound DNA.
DNA for UV absorption measurements
For spectrophotometric thermal melting studies on DNA and DNA-drug complexes, we used calf-thymus DNA (Sigma Aldrich, St. Louis, MO) without any further purification. In these bulk studies, we used a DNA concentration of 1.5 mM incubated for 1 day in the presence of a drug molecule/DNA basepair ratio of 0.5 in 50 mM of sodium perchlorate. After that, the DNA was diluted with concentrated PBS to reach a final salt concentration of 150 mM PBS and a final DNA concentration of 150 μM.
Magnetic tweezers
The MT apparatus is fully described elsewhere (25). Briefly, the MT setup consists of an inverted optical microscope coupled to a system of magnets that can be moved vertically by a mechanical arm to apply a stretching force to DNA. Furthermore, the magnets can be rotated to exert torque on the DNA. The MT cell is placed over the microscope objective. The cell is composed of a capillary with an inner surface functionalized with the anti-digoxigenin antibody. The above-described DNA sample is flowed through the capillary. The result is a DNA segment (∼2 μm in length) that is attached to the glass surface at the lower end and to a magnetic bead at the upper end. The sample is illuminated with an LED light, and images of the beads magnified at 75× are recorded in real time with a camera coupled to the objective lens (60 frames/s at a resolution of 134 nm × 134 nm pixels). The images of the diffraction fringes generated by the bead were analyzed, and an appropriate correction for the camera integration time was applied (51); thus, the three-dimensional vertical position of the bead could be calculated, and the DNA extension and the force applied to the bead could be measured.
UV melting measurements
UV measurements were performed using a Thermo Scientific (Waltham, MA) Evolution 300 spectrophotometer with a resolution of 0.1°C. The temperature was varied at a rate of 0.2°C/min. At this rate, the melting transitions showed no appreciable hysteresis.
Results
Single-molecule MT measurements
In Fig. 2 A, we show typical data representing DNA extension, , measured as a function of the number of imposed turns, , for different fixed values of the applied force, F, between pN and pN from top to bottom. Analogously, in Fig. 2 B, we present measurements of as a function of F for different fixed values of between nt = 0 and nt = −400 from left to right. These measurements were taken for bare DNA under physiological conditions, and similar measurements have been reported several times in the past (29, 39, 52, 53). These data illustrate the effects on DNA extension of imposing turns (denaturation or plectoneme formation) or applying forces to the bead. A wealth of quantitative information can be extracted from such data. The buckling transitions for the different forces are indicated by the short vertical segments in Fig. 2 A. The buckling number, , corresponds to the positive value of nt at which the formation of plectonemes begins. Furthermore, in Fig. 2 A, the slopes, of the -versus- data at positive values of are determined for different values of F. We recall that is proportional to the plectoneme size (52). The linear fits used to obtain are indicated by the longer black segments in Fig. 2 A. From Fig. 2 B, the values of the DNA contour length, , and persistence length, , as fitting parameters of the so-called wormlike chain (WLC) model for the versus-F data measured at can be extracted (52). The black line in Fig. 2 B shows the appropriateness of the WLC model and the fit accuracy.
Figure 2.
Single-molecule MT measurements of bare DNA at physiological conditions. (A) DNA extension, , measured as a function of the number of turns, , for fixed values of applied force, F, for (top to bottom) , , , , and . The vertical short black lines indicate the positions of the buckling transitions, , at which plectoneme formation begins. The long black lines illustrate the linear fit used to extract the slopes, , of the -versus- data. (B) DNA extension, , measured as a function of the applied force, F, for fixed values of the number of turns, (left to right): , , , , , and . The continuous black line represents a fit to the wormlike chain (WLC) model of the -versus-F data taken at . The resulting fitting parameters are and . To see this figure in color, go online.
In Fig. 2 B, we also show the -versus-F data obtained at . The DNA extension is still an increasing function of the force, but for large negative turns, a latency region ( for pN) is observed in which DNA extension is negligible because of the formation of plectonemes, which greatly reduce DNA extension. In the latency region, the applied force is not sufficient to uncoil the plectonemes. At higher forces, the plectonemes are relaxed, and complete DNA extension is obtained. This behavior at was recently described by likening it to a phase transition between plectonemic and denatured DNA phases (39, 40, 41). For pN, an overwinding () of the double helix cannot foster denaturation (29). For this reason, the versus-F data for are not meaningful in the force regime explored here, because DNA extension was greatly reduced due to the formation of plectonemes.
This type of measurement was applied to the DNA-drug complexes to determine whether DNA-drug interactions influence the values of the nanomechanical parameters , , , and . The results of this analysis are presented in Figs. 3, 4, and 5 for both bare DNA and DNA in the presence of drugs. Fig. 3 shows the behavior of the buckling number, , as a function of the applied force, F. The data are presented in double-logarithmic plots to emphasize the existence of power-law dependences. Similarly, Fig. 4 shows the double-logarithmic behavior of values as a function of F for both bare DNA and DNA-drug complexes. Under all conditions shown in Figs. 3 and 4, the existence of power-law dependences are confirmed by the small dispersion around the red lines (the red lines represent the linear best fit of the double-logarithmic representation of the data). This presentation of the measurements shows that the data can be described by the expressions and and, furthermore, that the linear fitting procedure enables the extraction of the specific values of γ and β under the different conditions. In Figs. 3 and 4, the obtained values of γ and β are indicated in the inset label together with their corresponding fitting uncertainties, obtained as the standard deviation of the MATLAB internal fitting procedure. In the explored conditions, the measured values of γ are similar () between bare DNA and the CIS-DNA complex, whereas the values seem reduced () for the DNA-BBR complexes, indicating that DNA-BBR binding affects by reducing its dependence on the applied force. Considering the measured values of β, we observe a negative dependence of on the force under all conditions, in agreement with previous reports (52). In addition, the values of β corresponding to bare DNA and DNA+drug complexes are similar ().
Figure 3.
Data extracted from MT measurements similar to those shown in Fig. 2A. Double-logarithmic plot of the buckling transition values, , measured as a function of the applied force, F, for bare DNA and for DNA-drug complexes: (A) bare DNA, (B) cisplatin, (C) BBR3464, (D) BBR3005. The continuous lines represent a linear fit on the double-logarithmic plots. This fit extracts the values of the exponent, γ, when assuming the power-law behavior (see labels for the fitting values of γ obtained for the various drugs). To see this figure in color, go online.
Figure 4.
Data extracted from MT measurements similar to those in Fig. 2A, shown as double-logarithmic plots of the slopes, , measured as a function of the applied force, F, for bare DNA and for DNA-drug complexes: (A) bare DNA, (B) cisplatin, (C) BBR3464, (D) BBR3005. The continuous lines represent linear fits of the double-logarithmic plot that were performed to determine the values of the exponent β when assuming the power-law behavior (see labels for the fitting values of β obtained for the various drugs). To see this figure in color, go online.
Figure 5.
Parameters resulting from the fit of the WLC model to the -versus-F MT measurements. Data were taken at , similar to those shown by the black line in Fig. 2B. (A) Persistence length, . (B) Contour length, , measured for the different DNA-drug complexes (see labels for the drug names). To see this figure in color, go online.
In Fig. 5, we show the values of and extracted by fitting the data at zero turns to the WLC model. The mean values of and are obtained as the average over tenths of DNA filaments, each measured approximately five times, and the error bars are the corresponding standard deviations. In these measurements, we note that the values of are not strongly influenced by the presence of the drugs, with the exception of the data taken with the BBR3464, where we observe a significant decrement of . Furthermore, we observe from the data a general decrease of the fitting values of for DNA with drugs with respect to the data taken for bare DNA. Overall, the BBR3464 is the most effective drug in reducing both and .
More essential differences between the behavior of bare DNA and that of DNA in the presence of the analyzed drugs are revealed during the nanomechanically induced denaturation transition. Fig. 6, A and B, shows the DNA extension fluctuations, , and their autocorrelation characteristic time, , plotted as a function of the pulling force. The maxima of and define the value, which represents the force value at which plectonemes and melting bubbles are equally probable (39, 40, 41). As is apparent from Fig. 6, A and B, strongly depends on a specific drug interaction with the DNA. Although the bare DNA and the DNA+CIS complex share similar values in characteristic force ( pN), the values of the characteristic force for DNA-BBR3464 and DNA-BBR3005 are lower ( pN for BBR3005 and pN for BBR3464), indicating that nanomechanical melting is notably favored when DNA is bound to either of the two BBR drugs.
Figure 6.
(A) DNA extension fluctuations, . (B) Characteristic time, , of the DNA extension fluctuations measured as a function of the applied force, F, for the various DNA-drug complexes (see labels for the drug names). The force at which and attain a maximum value represents the characteristic force, , at which the nanomechanical denaturation begins. To see this figure in color, go online.
Bulk (UV absorption) denaturation measurements
The results obtained from the single-molecule analysis were compared with those from the bulk measurements. Fig. 7 A shows the thermal melting profiles of the DNA and DNA+drug complexes obtained by varying the temperature and measuring the UV absorption at 260 nm. Under all conditions, either complete or partial denaturation at high temperatures was detected. However, different behaviors were observed depending on the various DNA-drug complexes. In general, melting transitions can be described by the melting temperatures and the transition range (54). Normalized UV absorption data can be fit with the equation (55) (represented in Fig. 7 A as solid lines)
| (1) |
where is the normalized difference between the 260 nm absorption at low and high temperatures, is the melting temperature, and is the range of the transition. Under the studied conditions, the presence of the drugs induced shifts in values: a small negative shift for DNA+CIS complexes, positive shifts for BBR3464 compounds, and no shift for BBR3005 (bare, Tm = 79.6°C; CIS, Tm = 77.8°C; BBR3464, Tm = 82.5°C; and BBR3005, Tm = 79.9°C). Furthermore, increased for all complexes with respect to for bare DNA, indicating that the transitions occurred over a larger temperature range (bare, ΔT = 2.3°C; CIS, ΔT = 2.8°C; BBR3464, ΔT = 6.9°C; and BBR3005, ΔT = 5.1°C). This broadening of the transition is also shown in Fig. 7 B, which displays the derivatives of the fitting functions presented in Fig. 7 A. The widths of the bell-shaped derivatives depend on the presence of the drugs and are larger for the BBR complexes. The temperatures at which the transition began, (indicated by the arrows in Fig. 7 A), were lower for the drug complexes than for bare DNA. We defined as the temperature at which only 3% of the DNA is melted. The lower values for the DNA-drug complexes suggest that, under the studied conditions, some DNA regions of the drug-complexed DNA were more easily denatured by thermal heating with respect to the bare DNA.
Figure 7.
Bulk measurements of thermal DNA denaturation. (A) Normalized 260 nm UV absorption variation, , measured as a function of temperature, T, for bare DNA and the indicated DNA-drug complexes. The continuous lines represent the fits described by Eq. 1. The vertical arrows indicate the temperatures () at which 3% of DNA was melted. (B) Temperature derivatives of the fitting curves of (A). To see this figure in color, go online.
Discussion
With regard to the single-molecule measurements corresponding to bare DNA, the data in Fig. 2 A provide key information that is directly extracted from standard MT measurements for positive , namely, the values of the buckling transition, , and the slope, (56). In considering the behavior of bare DNA in this study, according to a standard model for bare DNA (56), and are predicted to display power-law dependence as a function of the imposed force, F. In the standard model, double-stranded DNA can be simply represented as an elastic rod with a torsional constant, C, and a bending constant, . By minimizing the elastic energy, the model predicts the following expressions for and (56):
| (2) |
| (3) |
Given the above expressions, the standard model predicts and with and (52). For the bare DNA, considering the data shown in Figs. 3 A and 4 A and the values and derived by fitting the experimental measurements to a power-law fitting function, the predicted -versus-F behavior is reasonably confirmed (), but the theoretical relationship between and F has not been adequately established (). However, quantitative discrepancies between theory and experiment have been observed for bare DNA several times in past research (29, 57, 58, 59), and more sophisticated models have been presented (60, 61, 62, 63, 64) to describe the experimental findings with better accuracy. Here, we focus on the dependence of and on the binding of the evaluated platinum-based drugs. The values of that were measured for the DNA-platinum-compound complexes appear to be only slightly different from those measured for bare DNA, whereas the values of seem reduced for both BBR compounds. However, given the large experimental uncertainties of and , it is difficult to draw any reliable conclusion about the influence of platinum compounds on these parameters.
As shown in Fig. 2 B, the relationship between DNA extension and force at is well described by the WLC model, expressed by the equation
| (4) |
The good agreement between the WLC predictions and the experiments is confirmed not only by the data at corresponding to the bare DNA (see solid line in Fig. 2 B) but also by the data at corresponding to the DNA-drug complexes (see Figs. S4–S18). The nanomechanical effects on the DNA elastic rod properties (torsional and bending constant values) of the drug interactions are reported in Fig. 5. In the case of CIS, we detect a reduction of the persistence-length values, as observed in the past and explained by kink formation (13, 20, 65). It should be noted that with respect to our measurements, such previous studies reported a more relevant reduction of the persistence length for DNA in the presence of CIS. This difference between our data () and literature data ( values ranging between 10 and 25 nm) is presumably due to the specific experimental procedure we used to perform the drug incubation. Namely, during incubation, we kept the DNA under a tension of >1 pN. In the case of BBR drugs, this procedure is mandatory to avoid systematic bead adhesion to the capillary due to progressive DNA collapse. When cross-linking DNA with CIS, applying the same procedure, although it would not be strictly necessary, allows an easier comparison of the effects of the different drugs on DNA. On the other hand, it may be responsible for the systematic differences between our data and those in the quoted literature. In BBR compounds, we also note a reduction in which is more pronounced for BBR3464. This sizeable decrease might be linked to the superior ability of BBR3464 to form long-range bridging interactions. These interactions are known to induce both enhanced compaction of DNA and local denaturation, leading to unusual flexibility within the double helix. Furthermore the long-range interactions can induce loop formation along the chain, which may account for the larger measured error bars in the BBR3464-DNA extension (see Fig. 5 B).
As shown in Fig. 6, the influence of drug binding is demonstrated by the DNA extension fluctuations at intermediate values of applied force and at negative values of imposed turns. At the characteristic force, the DNA oscillates between plectonemic and denaturation-bubble regimes, producing increments in and . According to previous studies (39, 41), the characteristic force depends on the DNA stability and on the DNA persistence length through the relationship (39)
| (5) |
where α is the binding energy of the number of basepairs necessary for relaxing one turn. It should be observed that the values of α are extracted from Eq. 5, which is obtained as an extension (39, 41) of the standard model (52). Such a model is approximated, since it disregards several contributions (entropic terms, electrostatic interactions, etc.) that have been inserted in more recent approaches (63). However we assume that, at least qualitatively, Eq. 5 can address the main features of the mechanical denaturation phenomenon. Accordingly, we plotted the values of as a function of and we found that the data for the DNA and the three DNA-drug complexes cluster in different regions of the (, ) space (Fig. 8 A). As shown in Fig. 8 A, these regions are almost superimposed for bare DNA and DNA+CIS, whereas the regions are clearly separated for DNA+BBR3005 and DNA+BBR3464. Therefore, the values of α calculated from the data in Fig. 8 A, which are shown in Fig. 8 B, are similar for bare DNA and DNA-CIS and, notably, decrease for DNA-BBR3005 and DNA-BBR3464. This result indicates quantitatively that multinuclear platinum drugs are more efficient at locally destabilizing, and likely damaging, DNA. A lower α value reflects that DNA is more easily denatured under nanomechanical stress and that BBR-bound DNA can be more susceptible to weakening and destruction. This suggests that the DNA basepairs that are involved in the initial phases of denaturation are those between the two bonds of each single drug molecule. Indeed the smallest molecule (CIS), which binds to adjacent bases, does not significantly perturb the DNA local nanomechanical stability. Conversely, BBR3464, the drug displaying the largest binding-site length, also induces the most severe destabilization.
Figure 8.
Magnetic tweezers data concerning the nanomechanical melting transition of DNA. (A) Characteristic force, , plotted as a function of the persistence length, , for different DNA-drug complexes (see labels for the drug names). (B) The nanomechanical parameter α representing the binding energy between the single strands of DNA for the indicated DNA-drug complexes. To see this figure in color, go online.
The values of α, and measured by the single-molecule nanomanipulation system indicated that multinuclear platinum drugs are more efficient at inducing local perturbations in the native structure of DNA (they display lower α values). Moreover, of the multinuclear platinum compounds that were evaluated, only BBR3464 induced significant variations in overall DNA structural and mechanical properties (i.e., reduced and values). This behavior likely depends on different interactions with double-stranded DNA and realistically accounts for the enhanced cytotoxicity in CIS-resistant cells (12, 66, 67, 68).
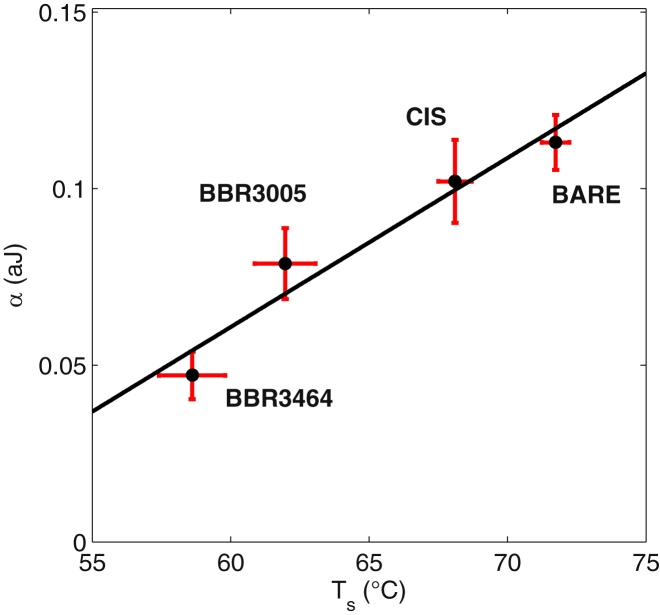
Finally, with regard to the similarities and differences between nanomechanical DNA denaturation and thermal DNA melting, the bulk experiments show that both of the tested BBR compounds produced an overall stabilizing effect on DNA (higher melting temperatures). However, the single-molecule experiments suggest the opposite behavior, because the values of the parameter α are decreased in the presence of the BBR drugs. This apparent inconsistency can be resolved by considering the fact that the nanomechanical opening involves only a small fraction of basepairs. According to previously published estimates (39, 41), the number of open basepairs is a linear function of the values at which the force-versus-extension experiments are performed. For each turn, approximately six or seven basepairs are involved in bubble formation (39, 41). The data shown in Fig. 6 were acquired at , corresponding to a few hundred open basepairs over a total number of kbp of DNA used for the MT experiments. Consequently, the nanomechanical parameter α should be compared to the temperature , at which only 3% of the DNA is denatured (see Fig. 6 A) rather than to the melting temperature, at which 50% is denatured. As shown in Fig. 9, we note a qualitative linear dependence between α and . This dependence demonstrates that although DNA resistance to denaturation can be probed by both nanomechanical and thermal assays, nanomechanics is maximally sensitive to the initial stages of denaturation, whereas thermal melting explores the bulk behavior over a larger denaturation range. In the case presented here, the two analyses even yield qualitatively different information. The diverse nature of the interactions that can be established between DNA and the evaluated BBR drugs can lead to both local disruptions between the two bonds of a single drug and stabilization of the double-helical structure on average. The cumulative effect of such interactions, which is best accounted for by the thermal melting analyses, seems to be an overall increase in the stability of the double strand, highlighted by the notable increase in the melting temperatures of both BBR3005 and BBR3464. The described heterogeneity of the drug binding effects along the chains can be revealed by comparing the bulk and single-molecule experiments only.
Figure 9.
Summary of the DNA single-molecule nanomechanical melting and bulk thermal denaturation data. The nanomechanical parameter α (shown in Fig. 8B and representing the binding energy between the number of bases of DNA necessary for relaxing one turn) is plotted as a function of the starting melting temperature, (indicated by the arrows in Fig. 7A and defined as the temperature for which only 3% of DNA is melted), for the indicated DNA-drug complexes. The black lines represent the linear fits. To see this figure in color, go online.
Conclusions
DNA is recognized as a primary target of many antitumor agents, including platinum-containing drugs. Our results indicate that inducing nanomechanical melting by exerting negative twists allows for evaluation of the resistance of DNA to the formation of initial denaturation bubbles, complementing the information regarding the melting-transition midpoint, which is obtainable using bulk thermal denaturation. The single-molecule analysis indicates the presence of destabilized DNA regions, whereas the bulk measurements suggest that the average stability is increased. Simultaneously, the decrease in the starting points of the melting transitions of the transplatinated drugs agrees with the single-molecule observations.
Small values of the parameter α or the relevant decrement are indicators of a drug’s action on DNA. Moreover, although of course the efficacy of a Pt-containing agent is not simply related to its ability to bind DNA, but also depends on drug uptake, cellular localization, and detoxification, the pharmacodynamic properties also reflect a drug’s DNA binding mode and its influence on DNA structure (e.g., DNA damage), likely accounting for its cytotoxicity (69). On the basis of these findings, we suggest that both single-molecule and bulk biophysical methods could represent a starting point for guiding the design and development of more efficient platinum-based drugs.
Author Contributions
D.S. designed research, performed research, analyzed data, and wrote the article; G.L.B. and L.N. designed research and wrote the article; G.Z. and S.B. performed research and wrote the article; M.C., V.C., A.T., and N.M. performed research; R.G., M.G.C., T.B., and N.Z. designed research; F.M. designed research, analyzed data, and wrote the article.
Acknowledgments
We thank D. Brogioli for assisting in the assembly of the magnetic tweezers setup, D. Barisani for useful advice about the functionalization of the DNA constructs, and C. Sissi for stimulating discussion about DNA melting.
Funding for the open-access charge was from the University of Milano-Bicocca, Italy. G.Z. and T.B. acknowledge support from the Italian Ministry of Education and Research, PRIN project 2010LKE4CC.
Editor: Antoine van Oijen.
Footnotes
Eighteen figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)00218-6.
Supporting Material
References
- 1.Coluccia M., Natile G. Trans-platinum complexes in cancer therapy. Anticancer. Agents Med. Chem. 2007;7:111–123. doi: 10.2174/187152007779314080. [DOI] [PubMed] [Google Scholar]
- 2.Wheate N.J., Walker S., Oun R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010;39:8113–8127. doi: 10.1039/c0dt00292e. [DOI] [PubMed] [Google Scholar]
- 3.Brabec V., Kaspárková J., Farrell N. DNA modifications by a novel bifunctional trinuclear platinum phase I anticancer agent. Biochemistry. 1999;38:6781–6790. doi: 10.1021/bi990124s. [DOI] [PubMed] [Google Scholar]
- 4.Chválová K., Brabec V., Kaspárková J. Mechanism of the formation of DNA-protein cross-links by antitumor cisplatin. Nucleic Acids Res. 2007;35:1812–1821. doi: 10.1093/nar/gkm032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahara P.M., Rosenzweig A.C., Lippard S.J. Crystal structure of double-stranded DNA containing the major adduct of the anticancer drug cisplatin. Nature. 1995;377:649–652. doi: 10.1038/377649a0. [DOI] [PubMed] [Google Scholar]
- 6.Cepeda V., Fuertes M.A., Pérez J.M. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer. Agents Med. Chem. 2007;7:3–18. doi: 10.2174/187152007779314044. [DOI] [PubMed] [Google Scholar]
- 7.Deans A.J., West S.C. DNA interstrand crosslink repair and cancer. Nat. Rev. Cancer. 2011;11:467–480. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen D.W., Pouliot L.M., Gottesman M.M. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol. Rev. 2012;64:706–721. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee N.K., Park J.S., Hong S.C. Elasticity of cisplatin-bound DNA reveals the degree of cisplatin binding. Phys. Rev. Lett. 2008;101:248101. doi: 10.1103/PhysRevLett.101.248101. [DOI] [PubMed] [Google Scholar]
- 10.Olszewski U., Hamilton G. A better platinum-based anticancer drug yet to come? Anticancer. Agents Med. Chem. 2010;10:293–301. doi: 10.2174/187152010791162306. [DOI] [PubMed] [Google Scholar]
- 11.Almaqwashi A.A., Paramanathan T., Williams M.C. Strong DNA deformation required for extremely slow DNA threading intercalation by a binuclear ruthenium complex. Nucleic Acids Res. 2014;42:11634–11641. doi: 10.1093/nar/gku859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts J.D., Peroutka J., Farrell N. Cellular pharmacology of polynuclear platinum anti-cancer agents. J. Inorg. Biochem. 1999;77:51–57. doi: 10.1016/s0162-0134(99)00147-6. [DOI] [PubMed] [Google Scholar]
- 13.Onoa G.B., Cervantes G., Prieto M.J. Study of the interaction of DNA with cisplatin and other Pd(II) and Pt(II) complexes by atomic force microscopy. Nucleic Acids Res. 1998;26:1473–1480. doi: 10.1093/nar/26.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onoa G.B., Moreno V. Study of the modifications caused by cisplatin, transplatin, and Pd(II) and Pt(II) mepirizole derivatives on pBR322 DNA by atomic force microscopy. Int. J. Pharm. 2002;245:55–65. doi: 10.1016/s0378-5173(02)00332-0. [DOI] [PubMed] [Google Scholar]
- 15.Hou X.M., Zhang X.H., Wang P.Y. Cisplatin induces loop structures and condensation of single DNA molecules. Nucleic Acids Res. 2009;37:1400–1410. doi: 10.1093/nar/gkn933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z., Tan S., Xing Z. The interactions of cisplatin and DNA studied by atomic force microscopy. Micron. 2010;41:833–839. doi: 10.1016/j.micron.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Cassina V., Seruggia D., Mantegazza F. Atomic force microscopy study of DNA conformation in the presence of drugs. Eur. Biophys. J. 2011;40:59–68. doi: 10.1007/s00249-010-0627-6. [DOI] [PubMed] [Google Scholar]
- 18.Krautbauer R., Pope L.H., Gaub H.E. Discriminating small molecule DNA binding modes by single molecule force spectroscopy. FEBS Lett. 2002;510:154–158. doi: 10.1016/s0014-5793(01)03257-4. [DOI] [PubMed] [Google Scholar]
- 19.Crisafuli F., Cesconetto E., Rocha M. DNA-cisplatin binding mechanism peculiarities studied with single molecule stretching experiments. Appl. Phys. Lett. 2012;100:083701. doi: 10.1039/c2ib00183g. [DOI] [PubMed] [Google Scholar]
- 20.Crisafuli F.A., Cesconetto E.C., Rocha M.S. DNA-cisplatin interaction studied with single molecule stretching experiments. Integr. Biol. (Camb.) 2012;4:568–574. doi: 10.1039/c2ib00183g. [DOI] [PubMed] [Google Scholar]
- 21.Cheng C.M., Lee Y.J., Yang T.S. Determining the binding mode and binding affinity constant of tyrosine kinase inhibitor PD153035 to DNA using optical tweezers. Biochem. Biophys. Res. Commun. 2011;404:297–301. doi: 10.1016/j.bbrc.2010.11.110. [DOI] [PubMed] [Google Scholar]
- 22.Rocha M.S., Cavalcante A.G., Ramos E.B. On the effects of intercalators in DNA condensation: a force spectroscopy and gel electrophoresis study. J. Phys. Chem. B. 2014;118:4832–4839. doi: 10.1021/jp501589d. [DOI] [PubMed] [Google Scholar]
- 23.Biebricher A.S., Heller I., Wuite G.J. The impact of DNA intercalators on DNA and DNA-processing enzymes elucidated through force-dependent binding kinetics. Nat. Commun. 2015;6:7304. doi: 10.1038/ncomms8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bazoni R.F., Lima C.H., Rocha M.S. Force-dependent persistence length of DNA-intercalator complexes measured in single molecule stretching experiments. Soft Matter. 2015;11:4306–4314. doi: 10.1039/c5sm00706b. [DOI] [PubMed] [Google Scholar]
- 25.Salerno D., Brogioli D., Mantegazza F. Magnetic tweezers measurements of the nanomechanical properties of DNA in the presence of drugs. Nucleic Acids Res. 2010;38:7089–7099. doi: 10.1093/nar/gkq597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W., Sun Z.-Q., Wang P.-Y. Elastic response and length change of single DNA molecules induced by a combination of cisplatin and transplatin. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2012;85:021918. doi: 10.1103/PhysRevE.85.021918. [DOI] [PubMed] [Google Scholar]
- 27.Wheate N.J., Collins G. Multi-nuclear platinum complexes as anti-cancer drugs. Coord. Chem. Rev. 2003;241:133–145. [Google Scholar]
- 28.Smith S.B., Finzi L., Bustamante C. Direct mechanical measurements of the elasticity of single DNA molecules by using magnetic beads. Science. 1992;258:1122–1126. doi: 10.1126/science.1439819. [DOI] [PubMed] [Google Scholar]
- 29.Strick T.R., Allemand J.F., Croquette V. The elasticity of a single supercoiled DNA molecule. Science. 1996;271:1835–1837. doi: 10.1126/science.271.5257.1835. [DOI] [PubMed] [Google Scholar]
- 30.Gosse C., Croquette V. Magnetic tweezers: micromanipulation and force measurement at the molecular level. Biophys. J. 2002;82:3314–3329. doi: 10.1016/S0006-3495(02)75672-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipfert J., Kerssemakers J.W., Dekker N.H. Magnetic torque tweezers: measuring torsional stiffness in DNA and RecA-DNA filaments. Nat. Methods. 2010;7:977–980. doi: 10.1038/nmeth.1520. [DOI] [PubMed] [Google Scholar]
- 32.Celedon A., Wirtz D., Sun S. Torsional mechanics of DNA are regulated by small-molecule intercalation. J. Phys. Chem. B. 2010;114:16929–16935. doi: 10.1021/jp107541q. [DOI] [PubMed] [Google Scholar]
- 33.Koster D.A., Croquette V., Dekker N.H. Friction and torque govern the relaxation of DNA supercoils by eukaryotic topoisomerase IB. Nature. 2005;434:671–674. doi: 10.1038/nature03395. [DOI] [PubMed] [Google Scholar]
- 34.Charvin G., Strick T.R., Croquette V. Tracking topoisomerase activity at the single-molecule level. Annu. Rev. Biophys. Biomol. Struct. 2005;34:201–219. doi: 10.1146/annurev.biophys.34.040204.144433. [DOI] [PubMed] [Google Scholar]
- 35.Abels J.A., Moreno-Herrero F., Dekker N.H. Single-molecule measurements of the persistence length of double-stranded RNA. Biophys. J. 2005;88:2737–2744. doi: 10.1529/biophysj.104.052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koster D.A., Palle K., Dekker N.H. Antitumour drugs impede DNA uncoiling by topoisomerase I. Nature. 2007;448:213–217. doi: 10.1038/nature05938. [DOI] [PubMed] [Google Scholar]
- 37.Seol Y., Neuman K.C. Single-molecule measurements of topoisomerase activity with magnetic tweezers. Methods Mol. Biol. 2011;778:229–241. doi: 10.1007/978-1-61779-261-8_15. [DOI] [PubMed] [Google Scholar]
- 38.Ding Y., Manzo C., Finzi L. DNA supercoiling: a regulatory signal for the λ repressor. Proc. Natl. Acad. Sci. USA. 2014;111:15402–15407. doi: 10.1073/pnas.1320644111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salerno D., Tempestini A., Mantegazza F. Single-molecule study of the DNA denaturation phase transition in the force-torsion space. Phys. Rev. Lett. 2012;109:118303. doi: 10.1103/PhysRevLett.109.118303. [DOI] [PubMed] [Google Scholar]
- 40.Marko J.F., Neukirch S. Global force-torque phase diagram for the DNA double helix: structural transitions, triple points, and collapsed plectonemes. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2013;88:062722. doi: 10.1103/PhysRevE.88.062722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tempestini A., Cassina V., Mantegazza F. Magnetic tweezers measurements of the nanomechanical stability of DNA against denaturation at various conditions of pH and ionic strength. Nucleic Acids Res. 2013;41:2009–2019. doi: 10.1093/nar/gks1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng H., Bosman J., van Noort J. Coexistence of twisted, plectonemic, and melted DNA in small topological domains. Biophys. J. 2014;106:1174–1181. doi: 10.1016/j.bpj.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galburt E.A., Tomko E.J., Ruiz Manzano A. Force-dependent melting of supercoiled DNA at thermophilic temperatures. Biophys. Chem. 2014;187–188:23–28. doi: 10.1016/j.bpc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Matek C., Ouldridge T.E., Louis A.A. Plectoneme tip bubbles: coupled denaturation and writhing in supercoiled DNA. Sci. Rep. 2015;5:7655. doi: 10.1038/srep07655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doye J.P., Ouldridge T.E., Smith W.P. Coarse-graining DNA for simulations of DNA nanotechnology. Phys. Chem. Chem. Phys. 2013;15:20395–20414. doi: 10.1039/c3cp53545b. [DOI] [PubMed] [Google Scholar]
- 46.Jamieson E.R., Lippard S.J. Structure, recognition, and processing of cisplatin-DNA adducts. Chem. Rev. 1999;99:2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- 47.Boudný V., Vrána O., Brabec V. Biophysical analysis of DNA modified by 1,2-diaminocyclohexane platinum(II) complexes. Nucleic Acids Res. 1992;20:267–272. doi: 10.1093/nar/20.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bancroft D.P., Lepre C.A., Lippard S.J. PT-195 NMR kinetic and mechanistic studies of cis- and trans-diamminedichloroplatinum(II) binding to DNA. J. Am. Chem. Soc. 1990;112:6860–6871. [Google Scholar]
- 49.Zaludová R., Zákovská A., Brabec V. DNA interactions of bifunctional dinuclear platinum(II) antitumor agents. Eur. J. Biochem. 1997;246:508–517. doi: 10.1111/j.1432-1033.1997.00508.x. [DOI] [PubMed] [Google Scholar]
- 50.Nelson P.C., Zurla C., Dunlap D. Tethered particle motion as a diagnostic of DNA tether length. J. Phys. Chem. B. 2006;110:17260–17267. doi: 10.1021/jp0630673. [DOI] [PubMed] [Google Scholar]
- 51.Brogioli D., Croccolo F., Mantegazza F. Nano-particle characterization by using Exposure Time Dependent Spectrum and scattering in the near field methods: how to get fast dynamics with low-speed CCD camera. Opt. Express. 2008;16:20272–20282. doi: 10.1364/oe.16.020272. [DOI] [PubMed] [Google Scholar]
- 52.Strick T., Allemand J., Bensimon D. Twisting and stretching single DNA molecules. Prog. Biophys. Mol. Biol. 2000;74:115–140. doi: 10.1016/s0079-6107(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 53.Sheinin M.Y., Forth S., Wang M.D. Underwound DNA under tension: structure, elasticity, and sequence-dependent behaviors. Phys. Rev. Lett. 2011;107:108102. doi: 10.1103/PhysRevLett.107.108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calladine C.R., Drew H.R., Luisi B.F., Travers A.A. Elesevier Academic Press; San Diego, CA: 2004. Understanding DNA. [Google Scholar]
- 55.Phillips R., Kondev J., Theriot J., Garcia H.G. Garland Science; San Diego, CA: 2013. Physical Biology of the Cell. [Google Scholar]
- 56.Strick T.R., Dessinges M.N., Croquette V. Stretching of macromolecules and proteins. Rep. Prog. Phys. 2003;66:1–45. [Google Scholar]
- 57.Strick T.R., Allemand J.F., Croquette V. Behavior of supercoiled DNA. Biophys. J. 1998;74:2016–2028. doi: 10.1016/S0006-3495(98)77908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarkar A., Léger J.F., Marko J.F. Structural transitions in DNA driven by external force and torque. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2001;63:051903. doi: 10.1103/PhysRevE.63.051903. [DOI] [PubMed] [Google Scholar]
- 59.Mosconi F., Allemand J.F., Croquette V. Measurement of the torque on a single stretched and twisted DNA using magnetic tweezers. Phys. Rev. Lett. 2009;102:078301. doi: 10.1103/PhysRevLett.102.078301. [DOI] [PubMed] [Google Scholar]
- 60.Goyal S., Perkins N.C., Lee C.L. Nonlinear dynamics and loop formation in Kirchhoff rods with implications to the mechanics of DNA and cables. J. Comput. Phys. 2005;209:371–389. [Google Scholar]
- 61.Purohit P.K. Plectoneme formation in twisted fluctuating rods. J. Mech. Phys. Solids. 2008;56:1715–1729. [Google Scholar]
- 62.Brutzer H., Luzzietti N., Seidel R. Energetics at the DNA supercoiling transition. Biophys. J. 2010;98:1267–1276. doi: 10.1016/j.bpj.2009.12.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neukirch S., Marko J.F. Analytical description of extension, torque, and supercoiling radius of a stretched twisted DNA. Phys. Rev. Lett. 2011;106:138104. doi: 10.1103/PhysRevLett.106.138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marko J.F., Neukirch S. Competition between curls and plectonemes near the buckling transition of stretched supercoiled DNA. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2012;85:011908. doi: 10.1103/PhysRevE.85.011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee N.K., Park J.S., Hong S.C. Investigation of the elasticity of a cisplatin-DNA adduct via single-molecule measurements and bimodal modeling. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2009;79:041921. doi: 10.1103/PhysRevE.79.041921. [DOI] [PubMed] [Google Scholar]
- 66.Di Blasi P., Bernareggi A., Formento M.L. Cytotoxicity, cellular uptake and DNA binding of the novel trinuclear platinun complex BBR 3464 in sensitive and cisplatin resistant murine leukemia cells. Anticancer Res. 1998;18(4C):3113–3117. [PubMed] [Google Scholar]
- 67.Perego P., Caserini C., Zunino F. A novel trinuclear platinum complex overcomes cisplatin resistance in an osteosarcoma cell system. Mol. Pharmacol. 1999;55:528–534. [PubMed] [Google Scholar]
- 68.Perego P., Gatti L., Zunino F. The cellular basis of the efficacy of the trinuclear platinum complex BBR 3464 against cisplatin-resistant cells. J. Inorg. Biochem. 1999;77:59–64. doi: 10.1016/s0162-0134(99)00142-7. [DOI] [PubMed] [Google Scholar]
- 69.Wang D., Lippard S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.