Abstract
Background
The purpose of this study was to determine whether short-term supplementation of a powdered tart cherry supplement prior to and following stressful endurance exercise would affect markers of muscle damage, inflammation, oxidative stress, and/or muscle soreness.
Methods
27 endurance-trained runners or triathlete (21.8 ± 3.9 years, 15.0 ± 6.0 % body fat, 67.4 ± 11.8 kg) men (n = 18) and women (n = 9) were matched based on average reported race pace, age, body mass, and fat free mass. Subjects were randomly assigned to ingest, in a double-blind manner, capsules containing 480 mg of a rice flour placebo (P, n = 16) or powdered tart cherries [CherryPURE®] (TC, n = 11). Subjects supplemented one time daily (480 mg/day) for 10-d, including race day, up to 48-hr post-run. Subjects completed a half-marathon run (21.1 km) under 2-hr (111.98 ± 11.9 min). Fasting blood samples and quadriceps muscle soreness ratings using an algometer with a graphic pain rating scale were taken pre-run, 60-min, 24 and 48-h post-run and analyzed by MANOVA with repeated measures.
Results
Subjects in the TC group averaged 13 % faster half-marathon race finish times (p = 0.001) and tended to have smaller deviations from predicted race pace (p = 0.091) compared to P. Attenuations in TC muscle catabolic markers were reported over time for creatinine (p = 0.047), urea/blood urea nitrogen (p = 0.048), total protein (p = 0.081), and cortisol (p = 0.016) compared to P. Despite lower antioxidant activity pre-run in TC compared to P, changes from pre-run levels revealed a linear increase in antioxidant activity at 24 and 48-h of recovery in TC that was statistically different (16–39 %) from P and pre-run levels. Inflammatory markers were 47 % lower in TC compared to P over time (p = 0.053) coupled with a significant difference between groups (p = 0.017). Soreness perception between the groups was different over time in the medial quadriceps (p = 0.035) with 34 % lower pre-run soreness in TC compared to P. Over the 48-h recovery period, P changes in medial quadriceps soreness from pre-run measures were smaller compared to TC.
Conclusion
Results revealed that short-term supplementation of Montmorency powdered tart cherries surrounding an endurance challenge attenuated markers of muscle catabolism, reduced immune and inflammatory stress, better maintained redox balance, and increased performance in aerobically trained individuals.
Keywords: Recovery, Antioxidants, Anti-inflammatory, Muscle damage
Background
Acute bouts of strenuous aerobic exercise facilitate a stress response characterized by mechanical muscle damage, oxidative stress, and inflammation that parallels the physiological stress response associated with many adverse traumatic cardiovascular events and illnesses [1–3]. As a result, this type of long duration mechanical muscle stress and high oxidative metabolic demand [4], significantly increases free radical production beyond the capacity of the endogenous antioxidant systems. Ultimately, this increase facilitates excessive cell damage, altered cell signaling [5–7], decreased cellular performance [5–8], lipid peroxidation, oxidation of proteins and glutathione, and subsequent DNA damage [3, 9]. Exercise-induced muscle soreness is indirectly related to inflammation as a product of high nociceptor and mechanoreceptor sensitivity to potent metabolites released during muscular degeneration [10, 11].
The use of antioxidant supplements, such as vitamins C [12–15] and E [4, 14, 15], in athletic applications to help fortify the body’s endogenous antioxidant response has spurred some success. However, vitamins C and E (independently or in combination with N-acetylcysteine, β-carotene, or α-lipoic acid) remain controversial due to conflicting reports of effectiveness [3, 16–19] with potential post-exercise pro-oxidant effects on muscle protein anabolism [20–22], endogenous antioxidant capacity [22], and mitochondrial biogenesis [23].
More recent nutritional research has focused on the antioxidant effects of functional foods containing high concentrations of phenolic compounds such as flavonoids and anthocyanins. It is proposed that these may act synergistically with other compounds contained within the food to provide an overall aerobic exercise recovery benefit [4, 24]. A wide variety of antioxidant and polyphenol-containing functional foods such as grape extract [25], chokeberries [26], and blueberries [8] have shown performance-enhancing and exercise recovery benefits. Exercise-based research with similar functional foods spurred investigation with tart (e.g. Mortmorency) cherry concentrate and juice supplementation to help increase performance by theoretically attenuating muscle damage, oxidative stress, and inflammation associated with aerobic challenges [7].
There are a few studies that have evaluated the effects of tart cherry supplementation on responses to endurance-based exercise. The first endurance-based study investigated the effects of 8-d tart cherry cultivar-blended juice supplementation on exercise-induced muscle pain surrounding an endurance relay race event (running distance = 22.5–31.4 km) [27]. Exercise-induced muscle pain was reduced as a result of tart cherry supplementation, but the findings were not confirmed by subsequent blood marker analysis [27]. Following a similar 8-d tart cherry juice supplementation protocol, a second study reported greater lower body isometric strength and quicker restoration of muscular function with reduced blood markers of muscle damage, oxidative stress, and inflammation in response to a marathon run [28]. A third endurance study examined the effects of 7-d tart cherry concentrate supplementation on physiological markers of muscle damage, oxidative stress, and inflammation surrounding 3-d of simulated high-intensity road cycling [4]. Similar to the second study, reductions of oxidative and inflammatory responses were the primary findings, thereby demonstrating a potential acute recovery-enhancing effect between bouts of high-intensity aerobic exercise with tart cherry supplementation [4].
The primary objective of this study was to determine whether short-term (10-d) supplementation with a powdered form of tart cherry skins would facilitate greater aerobic performance through attenuation of oxidative stress, inflammation, muscle damage, and muscle soreness.
Methods
Subjects
Twenty-seven male (n = 18) and female (n = 9) endurance-trained runners or triathletes (21.8 ± 3.9 years, 67.4 ± 11.8 kg, 15.0 ± 6.0 % body fat, 51.2 ± 11.4 kg free fat mass) participated as subjects in this study. Subjects were recruited through paper and electronically distributed flyers at Texas A&M University. Entrance criteria required the runners or triathletes to have been involved in a consistent running program for at-least 1-year and able to run a half-marathon (21.1 km) in less than 2 h. Figure 1 provides a breakdown of the subject population. Subject discontinuation of participation was not related to any aspect of the supplementation or testing protocol.
Fig. 1.
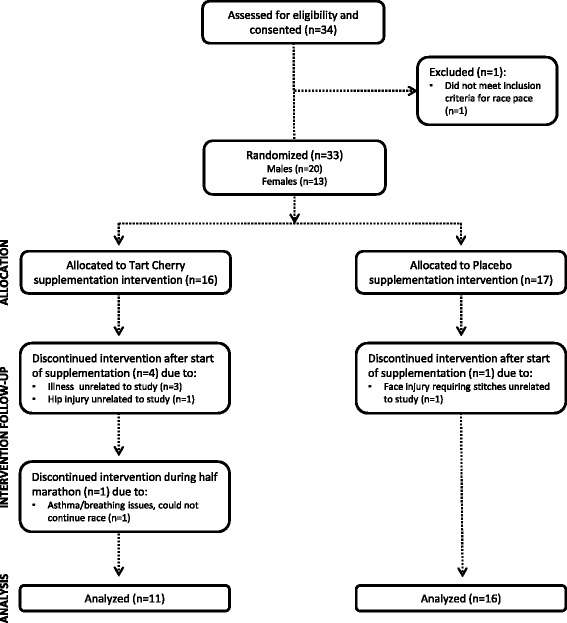
Consort diagram breakdown of the subject population from recruitment to data analysis
All subjects signed informed consent documents and the study was approved by the Texas A&M University Institutional Review Board prior to any data collection. Subjects were not allowed to participate in this study if they reported any of the following: 1) metabolic disorders or taking any thyroid, hyperlipidemic, hypoglycemic, anti-hypertensive, anti-inflammatory (e.g. NSAIDs), and/or androgenic medications; 2) history of hypertension, hepatorenal, musculoskeletal, autoimmune, and/or neurological disease(s); and 3) allergy to cherries or any cherry components (e.g. polyphenols, anthocyanins, anthocyanidins).
Experimental design
The study was conducted in a randomized, double-blind, and placebo-controlled manner (see Fig. 2). All subjects completed a morning familiarization (FAM) session where they were provided detailed information regarding the study design, testing procedures, and supplementation protocols. Informed consent, medical history, and endurance training history questionnaires were also completed during the FAM session. A nurse reviewed medical history documents and performed a physical exam (resting vital signs and lung auscultation) on each subject to ensure participation eligibility. A fasting blood sample was taken at the end of the FAM session. Approximately 10-d prior to the endurance exercise intervention, subjects returned to the lab for a morning baseline testing session to determine body mass, height, and body composition. Following baseline measurements subjects were matched based on average reported race pace, fat free mass, body mass, and age and randomly separated into two groups: 1) a placebo group or 2) a powdered tart cherry group. Subjects were instructed to not change their dietary habits in any way throughout the study. Nutritional habits were monitored through self-dietary recall for 4-d (3 weekdays and 1 weekend day) of the first seven supplementation days.
Fig. 2.
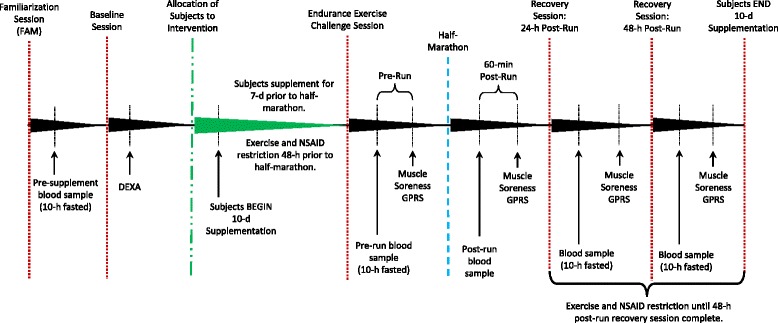
Experimental study design. DEXA dual-energy X-Ray absorptiometer, MVC maximal voluntary contraction, 1-RM 1-repetition maximum, NSAID non-steroidal anti-inflammatory drugs, GPRS graphic pain rating scale, 7-d 7-day, 48-h 48-hour
Subjects were instructed to begin supplementation 7-d prior to the endurance exercise challenge (Day 0). Subjects were asked to fast overnight for 10-h to account for diurnal variation as well as abstain from exercise and consumption of non-steroidal anti-inflammatory medications (NSAIDs) for 48-h prior to all testing days. On the day of the endurance exercise challenge, the subjects reported to the lab where body mass, resting heart rate, and resting blood pressure were measured. Subjects then donated a fasting venous blood sample (approximately 20 ml) using standard clinical procedures and rated perceptions of muscle soreness to a standardized application of pressure on their dominant thigh at three designed locations using a graphic pain rating scale (GPRS). Twenty minutes prior to the start of the half-marathon race, subjects were allowed to warm-up as they normally would before running a road race. Subjects completed a half-marathon (21.1 km) run outdoors at their normal race/competition pace. Both water and glucose-electrolyte drinks were provided ad libitum to the subjects at regular intervals during the race. Fasting (except 60-min post-run) blood samples and GPRS ratings of quadriceps muscle soreness were completed at 60-min, 24 and 48-h of post-run recovery. The last or tenth day of supplementation correlated with 48-hours post-run recovery.
Exercise protocol
Half-marathon (21.1 km) run
On the morning of supplementation day 8, all subjects performed an outdoor half-marathon run (21.1 km) for best time on a closed course under simulated race day conditions. Race start (0800) conditions were: ambient temperature = 22.8 °C, wind = 14.5 kph, humidity = 90 %, dew point = 21.1 °C. Conditions at the race finish (1030) were: ambient temperature = 25.0 °C, wind = 14.5 kph, humidity = 86 %. The race was run completely on concrete and pavement surfaces. All subjects were given 20-minutes for individual warm-up routines. At regular intervals (4 total locations) throughout the race, fluids (water and/or glucose-electrolyte beverages) were made available ad libitum to the subjects. Each subject had their own water and glucose-electrolyte beverage bottle labeled with a number that corresponded to their race number. All fluid bottles were weighed before and after the race to determine fluid consumption for each subject. Official race splits and finish times were recorded by designated lab staff. Following the race, subjects were not allowed to run to cool down, only stretching and minimal ambulation was permitted until the 60-min post-run testing session.
Supplementation protocol
Subjects were assigned in a double-blinded and randomized manner to ingest a rice flour placebo (P, n = 16) or powdered tart cherry (TC, n = 11). Subjects were matched into one of the two groups according to average reported race pace from previous (within the last 1 year) race events, fat free mass, body mass, and age. Subjects were instructed to ingest one 480 mg supplement capsule one time daily directly after breakfast at 0800 for 7-d prior to, the day of, and for 2-days following the half-marathon race for a total supplementation timeline of 10-d. The tart cherry supplements contained 480 mg of freeze dried Montmorency tart cherry skin powder derived from tart cherry skins obtained after juicing (CherryPURE™ Freeze Dried Tart Cherry Powder, Shoreline Fruit, LLC, Transverse City, MI, USA). Prior analytical testing conducted in 2012 by Advanced Laboratories (Salt Lake City, UT, USA) demonstrated that 31 mL (10.5 fl oz) of tart cherry juice provides approximately 600 mg of phenolic compounds and 40 mg of anthocyanins, which is equivalent to consuming 290 mg of CherryPURE™. Using the same comparison, the 480 mg CherryPURE™ supplement provided in the current study would be equivalent to 51.3 mL (17.4 fl oz) of tart cherry juice providing 991 mg of phenolic compounds and 66 mg of anthocyanins. The supplements were prepared for distribution by Shoreline Fruit, LLC and sent to Advanced Laboratories (Salt Lake City, UT, USA) to quantify the nutritional contents of the powdered tart cherry supplements. Both supplements were prepared in capsules identical in taste and appearance. The supplements were packaged in generic bottles by Shoreline Fruit, LLC for double blind administration.
Procedures
Dietary inventories
Within the first 7-d of supplementation, subjects were instructed to record all food and fluid intake over a 4-d period (3 weekdays, 1 weekend day). Dietary inventories were then reviewed by a registered dietician and analyzed for average daily energy (total kilocalories), macronutrient (protein, fat, and carbohydrates), and dietary antioxidant (vitamins C and E, and β-carotene) intake using ESHA Food Processor (Version 8.6) Nutritional Analysis software (ESHA Research Inc., Salem, OR, USA).
Anthropometrics and Body composition
At the beginning of every testing session, subjects had their height and body mass measured according to standard procedures using a Healthometer Professional 500KL (Pelstar LLC, Alsip, IL, USA) self-calibrating digital scale with an accuracy of ±0.02 kg. Whole body bone density and body composition measures (excluding cranium) were determined with a Hologic Discovery W Dual-Energy X-ray Absorptiometer (DEXA; Hologic Inc., Waltham, MA, USA) equipped with APEX Software (APEX Corporation Software, Pittsburg, PA, USA) by using procedures previously described [29]. Mean test-retest reliability studies performed on male athletes in our lab with this DEXA machine have revealed mean coefficients of variation for total bone mineral content and total fat free/soft tissue mass of 0.31–0.45 % with a mean intraclass correlation of 0.985 [30]. On the day of each test, the equipment was calibrated following the manufacturer’s guidelines for quality assurance.
Muscle soreness perception assessment
Pressure application to the three specified areas of the quadriceps muscle group on each subject’s dominant leg was standardized to 50 N of pressure using a handheld Commander Algometer (JTECH Medical, Salt Lake City, UT, USA). The standard amount of pressure was applied to the vastus lateralis at both 25 and 50 % of the distance between the superior border of the patella to the greater trochanter of the femur at the hip and to the vastus medalis at 25 % of the distance between the aforementioned landmarks. These three specific locations were measured and marked with a permanent marker on each subject during the baseline muscle soreness perception measurement before the half-marathon race. The subjects were asked to maintain these three marked locations between testing sessions to avoid error with secondary measurement. The subject was seated in a reclined supine position and given the algometer GPRS sheet to evaluate the perception of muscle soreness at each of the three quadriceps locations. The order of pressure application was standardized across all sessions and subjects: 25 % VM, 25 % VL, and 50 % VL. The 50 N of pressure was applied to a relaxed quadriceps at each of the three locations using the algometer for a period of 3-sec to give the subject enough time to record their soreness evaluation on the GPRS. Perceptions of muscle soreness were recorded by measuring the distance (centimeters) of the participant mark on the GPRS from 0 cm (no pain). Reliability statistical analyses revealed a mean intraclass correlation of 0.909.
Blood collection
Subjects donated approximately four teaspoons (20 mL) of venous blood after a 10-h fast from an antecubital vein using standard phlebotomy procedures. Blood samples were collected in two 7.5 mL BD Vacutainer® serum separation tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), left at room temperature for 15-min, and then centrifuged at 3500 rpm for 10-min using a standard, refrigerated (4 °C) bench top Thermo Scientific Heraeus MegaFuge 40R Centrifuge (Thermo Electron North America LLC, West Palm Beach, FL, USA). Serum supernatant was removed and stored at −80 °C in polypropylene microcentrifuge tubes for later analysis. The multiple serum microcentrifuge tubes for each subject was allocated for a specific group of assays and thawed only once during analysis. Blood was also collected in a single 3.5 mL BD Vacutainer® containing K2 EDTA (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), left at room temperature for 15-min, and refrigerated for approximately 3–4 h before complete blood count analysis.
Clinical chemistry analysis
Whole blood samples were analyzed for complete blood count with platelet differentials (hemoglobin, hematocrit, red blood cell counts (RBC), white blood cell counts (WBC), lymphocytes, granulocytes (GRAN), and mid-range absolute count (MID) using a Abbott Cell Dyn 1800 (Abbott Laboratories, Abbott Park, IL, USA) automated hematology analyzer. The internal quality control for Abbott Cell Dyn 1800 was performed using three levels of manufacturer control fluids to calibrate acceptable standard deviation (SD) and coefficients of variation (CV) values for all aforementioned analytes. Samples were re-run if the observed values were outside control values and/or clinical norms according to standard procedures. Reliability statistical analyses revealed a mean intraclass correlation of 0.729 across all measures. Serum samples were analyzed using a Cobas c111 (Roche Diagnostics GmbH, Indianapolis, IN, USA) automated clinical chemistry analyzer that was calibrated according to manufacturer guidelines. This analyzer has been known to be highly valid and reliable in previously published reports [31]. Each serum sample was assayed for a standard partial metabolic panel [(aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin)] and clinical markers of protein and fatty acid metabolism [(uric acid, creatinine, blood urea nitrogen (BUN), BUN:creatinine ratio, total protein, and creatine kinase (CK)]. The internal quality control for the Cobas c111 was performed using two levels of manufacturer control fluids to calibrate acceptable SD and CV values for all aforementioned assays. Samples were re-run if the observed values were outside control values and/or clinical norms according to standard procedures. Reliability statistical analyses revealed a mean intraclass correlation of 0.793 across all measures.
Markers of anabolic/catabolic hormone status
Serum samples were assayed using standard commercially available enzyme-linked immunosorbent assay kits (ELISAs) for cortisol and testosterone (ALPCO Diagnostics, Salem, NH, USA). Serum concentrations were determined calorimetrically using a BioTek ELX-808 Ultramicroplate reader (BioTek Instruments Inc., Winooski, VT, USA) at an optical density of 450 nm against a known standard curve using manufacturer recommended procedures. Samples were run in duplicate according to standard procedures. Test to test variability of performing these assays yielded average CV values for the aforementioned markers of: CORT (±6.85 %), and TEST (±4.47 %) with a test retest correlation for the same markers of: CORT (r = 0.92), TEST (r = 0.98).
Markers of oxidative stress
Serum samples were assayed using standard commercially available ELISA kits for Superoxide Dismutase (SOD Activity Assay kit), Total Antioxidant Status (TAS, Antioxidant Assay kit), Thiobarbituric Acid Reactive Substance (TBARS, Malondialdehyde-MDA, TCA method kit) (Cayman Chemical Company, Ann Arbor, MI, USA), and Nitrotyrosine (ALPCO Diagnostics, Salem, NH, USA). Serum concentrations for SOD and Nitrotyrosine were determined calorimetrically using a BioTek ELX-808 Ultramicroplate reader (BioTek Instruments Inc., Winooski, VT, USA) at an optical density of 450 nm against a known standard curve using standard procedures, while TAS serum concentrations were analyzed calorimetrically at 405 nm. Lastly, serum concentrations for TBARS were determined fluorometrically using a SpectraMax Gemini multimode plate reader (Molecular Devices LLC, Sunnyvale, CA, USA) at an excitation wavelength of 530 nm and an emission wavelength of 550 nm against a known standard curve using standard procedures. Samples were run in duplicate according to standard procedures. Test to test variability of performing these assays yielded average CV values for the aforementioned markers of: SOD (±8.35 %), TAS (±14.24 %), TBARS (±8.30 %), and NT (±10.03 %) with a test retest correlation for the same markers of: SOD (r = 0.83), TAS (r = 0.85), TBARS (r = 0.94), and NT (r = 0.99).
Cytokine/Chemokine markers of inflammation
Serum markers of inflammation [(interleukin-1β (IL-1β), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and granulocyte-macrophage colony-stimulating factor (GM-CSF)] were measured by using a commercially available Milliplex MAP 13-Plex Human High Sensitivity T-Cell Magnetic Bead Panel kit (EMD Millipore Corporation, St. Charles, MO, USA). A minimum of 100 positive beads for each cytokine/chemokine was acquired with a Luminex MagPix instrument (Luminex Corporation, Austin, TX, USA). Samples were run in duplicate according to standard procedures. Test to test variability of performing these assays yielded an average CV value range of ±4.26 to ±6.05 % for the aforementioned markers with an average test retest correlation of r = 0.99 for the same markers.
Statistical analysis
Individual group and time data are presented throughout as means (± SD), while group effects are presented as means (± SEM). All related variables were grouped and analyzed using repeated measures MANOVA in IBM SPSS Statistics Software version 22.0 for Windows (IBM Corporation, Armonk, NY, USA). Half-marathon finish time was also used as a covariate in subsequent ANCOVA analyses to determine if previously reported statistical outcomes were attributed to running intensity or to supplementation. Post-hoc LSD pairwise comparisons were used to analyze any significance among groups where needed with Cohen’s d calculations employed to determine effect magnitude. Data were considered statistically significant when the probability of error was less than 0.05 and considered to be trending when the probability of error was between 0.05 and 0.10.
Results
Subject characteristics
A total of 27 healthy, endurance trained or triathlete men (n = 18) and women (n = 9) completed the study protocol. Participant demographic data are presented in Table 1. One-way ANOVA revealed no significant differences (p >0.05) in baseline demographic or anthropometric markers.
Table 1.
Demographics by study group
| Variable | Group | Mean | Group (SEM) | p-value |
|---|---|---|---|---|
| N | P | 16 | n/a | n/a |
| TC | 11 | n/a | ||
| Total | 27 | n/a | ||
| Age | P | 22.44 ± 4.86 | 1.214 | 0.305 |
| TC | 20.82 ± 1.89 | 0.569 | ||
| Total | 21.78 ± 3.95 | 0.761 | ||
| Height (cm) | P | 173 ± 11.43 | 2.851 | 0.592 |
| TC | 175 ± 8.59 | 2.589 | ||
| Total | 174 ± 10.25 | 1.972 | ||
| Body Mass (kg) | P | 65.48 ± 12.07 | 3.018 | 0.317 |
| TC | 70.17 ± 11.25 | 3.392 | ||
| Total | 67.39 ± 11.76 | 2.263 | ||
| Baseline HR (bpm) | P | 58.50 ± 9.02 | 2.255 | 0.703 |
| TC | 59.64 ± 4.46 | 1.343 | ||
| Total | 58.96 ± 58.96 | 1.426 | ||
| BMD (g/cm2) | P | 1.04 ± 0.11 | 0.028 | 0.458 |
| TC | 1.08 ± 0.13 | 0.038 | ||
| Total | 1.06 ± 0.12 | 0.023 | ||
| FFM (kg) | P | 48.67 ± 11.32 | 2.830 | 0.171 |
| TC | 54.85 ± 11.01 | 3.319 | ||
| Total | 51.19 ± 11.41 | 2.195 | ||
| FM (kg) | P | 9.81 ± 3.20 | 0.801 | 0.085§ |
| TC | 7.76 ± 2.44 | 0.735 | ||
| Total | 2.44 ± 3.04 | 0.585 | ||
| Body Fat (%) | P | 16.87 ± 6.40 | 1.599 | 0.051§ |
| TC | 12.31 ± 4.42 | 1.333 | ||
| Total | 15.01 ± 6.03 | 1.160 |
Mean data expressed as means ± SD. Data represents general study population demographics and anthropometric measures. One-way ANOVA p-levels listed for each variable: § represents p <0.10 difference between groups. HR heart rate, BMD bone mineral density, LM lean mass, FFM free-fat mass, FM fat mass
Nutritional intake and compliance
Table 2 lists relevant nutrition components analyzed in the 4-d dietary recall. P tended to consume a smaller amount of average daily calories compared to TC (31.0 kcal/kg vs. 37.4 kcal/kg, p = 0.094). This differential is likely due dropped subjects (see Fig. 1) causing a greater proportion of females in P (nf = 3/11, 27.3 %) versus TC (nf = 6/16, 37.5 %). When stratifying the statistical dietary analysis by gender within each group, average daily calorie (p = 0.44) and dietary carbohydrate (p = 0.64) consumption was the same across groups. No other statistically significant interactions were observed across groups with respect to dietary intake.
Table 2.
Relative dietary analysis by study group
| Variable | Group | Mean | Group (SEM) | p-value |
|---|---|---|---|---|
| Average Daily Caloric Consumption (kcal/kg) | P | 30.89 ± 8.75 | 2.19 | 0.094§ |
| TC | 37.71 ± 11.65 | 3.51 | ||
| Total | 33.67 ± 10.40 | 2.00 | ||
| Dietary Protein (g/kg) | P | 1.29 ± 0.56 | 0.14 | 0.146 |
| TC | 1.62 ± 0.58 | 0.17 | ||
| Total | 1.42 ± 0.58 | 0.11 | ||
| Dietary Carbohydrates (g/kg) | P | 3.54 ± 1.50 | 0.37 | 0.138 |
| TC | 4.57 ± 2.00 | 0.60 | ||
| Total | 3.96 ± 1.76 | 0.34 | ||
| Dietary Fat (g/kg) | P | 1.24 ± 0.49 | 0.12 | 0.886 |
| TC | 1.27 ± 0.72 | 0.22 | ||
| Total | 1.25 ± 0.58 | 0.11 | ||
| Dietary Beta-Carotene (mcg/kg) | P | 38.01 ± 71.15 | 17.79 | 0.611 |
| TC | 54.22 ± 92.29 | 27.83 | ||
| Total | 44.62 ± 79.13 | 15.23 | ||
| Dietary Vitamin C [Ascorbic Acid] (mg/kg) | P | 0.92 ± 0.69 | 0.17 | 0.277 |
| TC | 1.46 ± 1.78 | 0.54 | ||
| Total | 1.14 ± 1.25 | 0.24 | ||
| Dietary Vitamin E [Alpha-Tocopherol] (mg/kg) | P | 0.099 ± 0.095 | 0.024 | 0.853 |
| TC | 0.106 ± 0.107 | 0.032 | ||
| Total | 0.102 ± 0.098 | 0.019 |
Mean data expressed as means ± SD. Data represents nutritional analysis from subject 4-d dietary records accounting for subject body mass as a computation of relative dietary components. One-way ANOVA p-levels listed for each variable: § represents p <0.10 difference between groups
Half-marathon performance measures
Table 3 presents half-marathon split and finish times in addition to projected versus actual average race paces. There was no difference in projected race finish times between groups (p = 0.304). TC subjects had faster half-marathon split (p = 0.002) and race finish times (p = 0.001) corresponding to a quicker overall race pace compared to P. The actual race pace was slower compared to the projected race pace in both groups (p <0.001), but the difference tended to be smaller (p = 0.091) in TC compared to P. Due to the significant difference in race performance, half-marathon finish time was used as a covariate in subsequent ANCOVA analyses to determine if other statistical outcomes were attributed to running intensity or to supplementation.
Table 3.
Running performance by study group
| Variable | Group | Mean | Group (SEM) | p-value |
|---|---|---|---|---|
| ½ Marathon Split Time (min) | P | 54.30 ± 4.18 | 1.045 | 0.002* |
| TC | 49.03 ± 3.65 | 1.099 | ||
| Total | 52.15 ± 4.71 | 0.906 | ||
| ½ Marathon Finish Time (min) | P | 118 ± 9.72 | 2.429 | 0.001* |
| TC | 103 ± 9.28 | 2.798 | ||
| Total | 112 ± 11.86 | 2.283 | ||
| ½ Marathon Projected Race Pace (min/km) | P | 12.00 ± 1.28 | 0.338 | 0.304 |
| TC | 11.45 ± 1.45 | 0.407 | ||
| Total | 11.77 ± 1.35 | 0.264 | ||
| ½ Marathon Actual Race Pace (min/km) | P | 14.48 ± 1.19 | 0.293 | 0.002* |
| TC | 12.70 ± 1.14 | 0.354 | ||
| Total | 13.76 ± 1.45 | 0.230 |
Mean data expressed as means ± SD. Data represents the half-marathon performance measures. Half-marathon projected race pace figures were calculated based upon subjects’ self-reported previous endurance running race performances. The overall MANOVA analysis revealed overall Wilks’ Lambda time (p <0.001) and group x time (p = 0.091). Univariate ANOVA p-levels from the MANOVA analysis are presented for both pacing variables. One-way ANOVA p-levels listed for each timing variable: * represents p <0.05 difference between groups
Markers of mechanical damage and physiological stress
Table 4 presents the serum mechanical damage and physiological stress marker data. Serum creatinine and urea/BUN makers increased on average 19 and 21 %, respectively, over pre-run values during the recovery in P, but only 6 and 3 % in TC. Serum total protein content increased on average 4 % over pre-run values during the recovery in P, but decreased 3 % below pre-run in TC. Significant (or trends approaching significance) changes across groups and group differences over time for creatinine (p = 0.047, group p = 0.007), urea/BUN (p = 0.048, group p = 0.004), and total protein (p = 0.081, group p = 0.060) were further supported by ANCOVA analyses accounting for running intensity. Subsequent post-hoc analysis indicated a significantly attenuated serum creatinine level 60-min post-run and a mitigated urea/BUN response in TC compared to P 24-h post-run (see Fig. 3). The total protein response never increased above pre-run levels over the 48-h recovery in TC compared to significant elevations 60-min and 48-h post-run in P (see Fig. 4).
Table 4.
Markers of muscle catabolism, Secondary muscle damage, and Physiological stress
| Variable | Group | Baseline | Pre-Run | 60-min Post | 24-hr Post | 48-hr Post | Group Mean | p-value (GG) | p-value (WSC) | RFT Covariate p-value (WSC) |
|---|---|---|---|---|---|---|---|---|---|---|
| AST (U/L) | P | 32.69 ± 33.67 | 26.62 ± 14.97 | 37.20 ± 17.36 | 50.52 ± 22.96 | 43.33 ± 16.68 | 38.07 ± 3.98 | G = 0.911 | G = 0.402 | |
| TC | 29.46 ± 10.54 | 26.30 ± 7.48 | 36.96 ± 9.68 | 49.97 ± 31.12 | 44.14 ± 24.60 | 37.37 ± 4.80 | T = 0.002* | T L = 0.005* | T q = 0.593 | |
| Time Mean | 31.08 ± 5.27 | 26.46 ± 2.45 | 37.08 ± 2.89†Ψ | 50.25 ± 5.20†Ψ◊ | 43.74 ± 3.96†Ψ# | G X T = 0.859 | G X T q = 0.740 | G X T L = 0.707 | ||
| ALT (U/L) | P | 22.09 ± 13.55 | 20.32 ± 7.15 | 23.65 ± 8.04 | 26.42 ± 7.36 | 28.53 ± 8.02 | 24.20 ± 2.15 | G = 0.576 | G = 0.057§ | |
| TC | 23.06 ± 8.22 | 23.13 ± 10.39 | AM ± 10.22 | 27.94 ± 14.22 | 30.07 ± 14.80 | 26.11 ± 2.59 | T = 0.008* | T L = 0.006* | T q = 0.677 | |
| Time Mean | 22.57 ± 2.29 | 21.73 ± 1.68 | 25.01 ± 1.76Ψ | 27.18 ± 2.09Ψ◊ | 29.30 ± 2.20†Ψ◊# | G X T = 0.842 | G X T q = 0.603 | G X T L = 0.869 | ||
| Total Billirubin (umol/L) | P | 9.01 ± 2.87 | 8.15 ± 3.03 | 13.03 ± 4.68 | 8.44 ± 4.06 | 7.65 ± 3.45 | 9.25 ± 0.76 | G = 0.756 | G = 0.614 | |
| TC | 8.71 ± 4.64 | 7.60 ± 3.75 | 11.65 ± 4.50 | 8.99 ± 4.22 | 7.46 ± 2.75 | 8.88 ± 0.91 | T <0.001* | T q = 0.001* | T q = 0.484 | |
| Time Mean | 8.86 ± 0.72 | 7.88 ± 0.65 | 12.34 ± 0.90†Ψ | 8.71 ± 0.81◊ | 7.55 ± 0.62◊ | G X T = 0.699 | G X T L = 0.694 | G X T q = 0.591 | ||
| Urea/BUN (mmol/L) | P | 4.75 ± 1.08 | 5.45 ± 1.36 | 6.36 ± 1.14 | 7.13 ± 1.15 | 6.33 ± 1.44 | 6.00 ± 0.21 | G = 0.857 | G = 0.426 | |
| TC | 5.11 ± 0.69 | 6.02 ± 1.17 | 6.16 ± 1.01 | 6.40 ± 1.44 | 6.03 ± 1.58 | 5.94 ± 0.26 | T <0.001* | T L <0.001* | T L = 0.026* | |
| Time Mean | 4.93 ± 0.19 | 5.74 ± 0.25† | 6.26 ± 0.21†Ψ | 6.77 ± 0.25†Ψ | 6.18 ± 0.29†# | G X T = 0.144 | G X T L = 0.095§ | G X T L = 0.014* | ||
| Creatinine (umol/L) | P | 71.71 ± 15.07 | 74.29 ± 11.54 | 106.56 ± 15.45†Ψ | 79.85 ± 10.88†◊ | 78.20 ± 11.17† | 82.12 ± 3.12 | G = 0.651 | G = 0.522 | |
| TC | 77.20 ± 15.93 | 82.29 ± 13.96 | 100.44 ± 25.52†Ψ | 81.54 ± 15.08◊ | 80.34 ± 12.72 | 84.36 ± 3.77 | T <0.001* | T q <0.001* | T L = 0.007* | |
| Time Mean | 74.45 ± 3.02 | 78.29 ± 2.46 | 103.50 ± 3.94†Ψ | 80.70 ± 2.49†◊ | 79.27 ± 2.31◊ | G X T = 0.087§ | G X T L = 0.246 | G X T L = 0.010* | ||
| BUN/Creatinine Ratio |
P | 16.60 ± 3.05 | 18.58 ± 5.58 | 15.22 ± 4.38 | 22.49 ± 4.61 | 20.36 ± 5.29 | 18.65 ± 0.90 | G = 0.589 | G = 0.329 | |
| TC | 16.97 ± 3.87 | 18.33 ± 3.50 | 15.80 ± 3.53 | 19.50 ± 3.61 | 18.79 ± 5.26 | 17.88 ± 1.09 | T <0.001* | T L = 0.001* | T q = 0.240 | |
| Time Mean | 16.75 ± 3.34 | 18.48 ± 4.77 | 15.46 ± 3.99Ψ | 21.27 ± 4.41†Ψ◊ | 19.72 ± 5.23†◊# | G X T = 0.158 | G X T L = 0.140 | G X T L = 0.103§ | ||
| Uric Acid (umol/L) | P | 271 ± 46 | 292 ± 44 | 373 ± 48 | 321 ± 48 | 308 ± 47 | 313 ± 11 | G = 0.789 | G = 0.724 | |
| TC | 290 ± 50 | 308 ± 52 | 364 ± 64 | 326 ± 82 | 302 ± 65 | 318 ± 14 | T <0.001* | T q <0.001* | T L = 0.014* | |
| Time Mean | 280 ± 9 | 300 ± 9† | 369 ± 11†Ψ | 324 ± 13†Ψ◊ | 305 ± 11†◊# | G X T = 0.444 | G X T L = 0.188 | G X T L = 0.015* | ||
| CK (U/L) | P | 606 ± 1696 | 276 ± 510 | 532 ± 627 | 907 ± 683 | 593 ± 525 | 583 ± 144 | G = 0.626 | G = 0.806 | |
| TC | 298 ± 317 | 228 ± 191 | 474 ± 253 | 870 ± 771 | 490 ± 395 | 472 ± 173 | T = 0.036* | T L = 0.139 | T q = 0.836 | |
| Time Mean | 452 ± 260 | 252 ± 81 | 503 ± 100Ψ | 889 ± 141Ψ◊ | 541 ± 93Ψ# | G X T = 0.680 | G X T q = 0.416 | G X T q = 0.599 | ||
| Total Protein (mmol/L) | P | 67.51 ± 8.56 | 72.93 ± 5.41† | 76.78 ± 4.95† | 70.77 ± 5.35◊ | 80.21 ± 6.48†Ψ# | 73.64 ± 0.87 | G = 0.746 | G = 0.846 | |
| TC | 68.70 ± 8.06 | 76.28 ± 4.78† | 74.94 ± 8.85† | 67.83 ± 4.52◊ | 78.20 ± 6.84†# | 73.19 ± 1.05 | T < 0.001* | T L <0.001* | T L = 0.008* | |
| Time Mean | 68.11 ± 1.64 | 74.61 ± 1.01† | 75.86 ± 1.33† | 69.30 ± 0.99Ψ◊ | 79.20 ± 1.30†Ψ◊# | G X T = 0.316 | G X T L = 0.066§ | G X T L = 0.004* |
Individual group and time data expressed as means ± SD, while group effects are presented as means ± SEM. Data represents the response to muscle catabolism, mechanical damage, and physiological stress at each testing session during the 10 day intervention. MANOVA analysis revealed overall Wilks’ Lambda time (p <0.001) and group x time (p = 0.504). Univariate ANOVA p-levels from MANOVA analysis are presented for each variable. Univariate ANOVA p-levels are listed first by the Greenhouse-Geisser (GG) analysis and then by the within-subjects contrasts (WSC) to demonstrate the potential shape of the time or group x time interaction with significance indicated by the following super/subscripts: *indicates p <0.05 p-level significance, §indicates p <0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: † represents p <0.05 difference from baseline value, Ψ represents p <0.05 difference from pre-run, ◊ represents p <0.05 difference from 60-min post, # represents p <0.05 difference from 24-hr post. AST aspartate aminotransferase, ALT alanine aminotransferase, BUN blood urea nitrogen, CK creatine kinase, RFT race finish time, G group p-level, T time p-level, G x T interaction, q quadratic p-level, L linear p-level
Fig. 3.
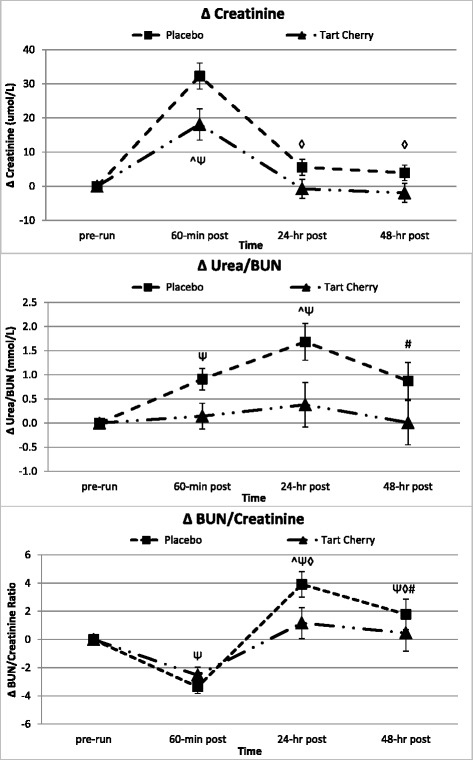
Secondary indices of muscle damage and protein catabolism. Data expressed as means ± SE and significance indicated by the following super/subscripts: * indicates p <0.05 p-level significance, § indicates p <0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p <0.05 difference between groups, Ψ represents p <0.05 difference from pre-run, ◊ represents p <0.05 difference from 60-min post, # represents p <0.05 difference from 24-hr post
Fig. 4.
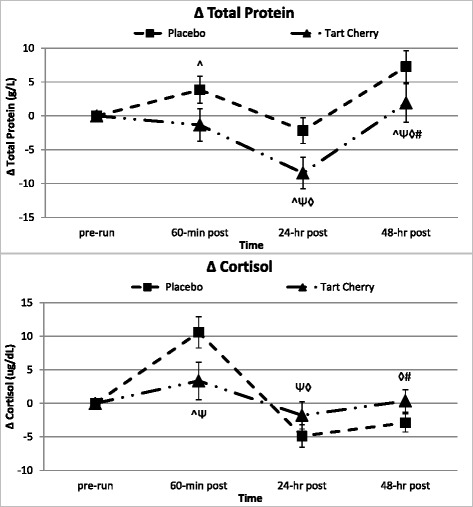
Markers of protein catabolism and physiological stress. Data expressed as means ± SE and significance indicated by the following super/subscripts: * indicates p <0.05 p-level significance, § indicates p <0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p <0.05 difference between groups, Ψ represents p <0.05 difference from pre-run, ◊ represents p <0.05 difference from 60-min post, # represents p <0.05 difference from 24-hr post
Anabolic/catabolic hormone response markers
Table 5 demonstrates the serum testosterone and cortisol hormone marker response. Testosterone and cortisol demonstrated significant changes over time and from baseline, peaking (elevated or depressed) 60-min post-run. Significant group differences over time and from pre-run levels were reported for serum cortisol (p = 0.012, delta p = 0.016). Serum cortisol levels 60-min post-run increased 44 % over pre-run values in P, but only 15 % in TC. Subsequent post-hoc analysis indicated significantly attenuated serum cortisol levels in TC compared to P 60-min and 24-h post-run (see Fig. 4). These results were supported when accounting for differences in running intensity.
Table 5.
Anabolic/Catabolic hormone response
| Variable | Group | Baseline | Pre-Run | 60-min Post | 24-hr Post | 48-hr Post | Group Mean | p-value (GG) | p-value (WSC) | RFT Covariate p-value (WSC) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cortisol (ug/dL) | P | 20.92 ± 7.21 | 24.04 ± 5.16 | 34.63 ± 10.14^†Ψ | 19.17 ± 4.87Ψ◊ | 21.14 ± 5.83◊ | 23.98 ± 1.23 | G = 0.408 | G = 0.868 | |
| TC | 21.34 ± 6.68 | 22.14 ± 7.35 | 25.48 ± 7.45 | 20.36 ± 5.93◊ | 22.50 ± 4.77 | 22.36 ± 1.48 | T <0.001* | T q <0.001* | T L = 0.259 | |
| Time Mean | 21.13 ± 1.37 | 23.09 ± 1.20 | 30.06 ± 1.80†Ψ | 19.76 ± 1.04Ψ◊ | 21.82 ± 1.06◊# | G X T = 0.005* | G X T q = 0.012* | G X T q = 0.030* | ||
| Testosterone (ng/mL) | P | 7.22 ± 3.90 | 6.77 ± 3.55 | 6.34 ± 3.55 | 6.48 ± 3.32 | 6.74 ± 3.41 | 6.71 ± 0.88 | G = 0.745 | G = 0.497 | |
| TC | 6.65 ± 4.24 | 6.52 ± 3.89 | 5.59 ± 3.48 | 6.21 ± 3.75 | 6.31 ± 3.69 | 6.26 ± 1.06 | T = 0.058§ | T q = 0.042* | T q = 0.026* | |
| Time Mean | 6.94 ± 0.79 | 6.64 ± 0.72 | 5.96 ± 0.69†Ψ | 6.34 ± 0.69 | 6.53 ± 0.69 | G X T = 0.848 | G X T L = 0.881 | G X T L = 0.171 | ||
| Test/Cort Ratio | P | 0.037 ± 0.026 | 0.030 ± 0.018 | 0.019 ± 0.010 | 0.035 ± 0.024 | 0.034 ± 0.020 | 0.031 ± 0.005 | G = 0.874 | G = 0.546 | |
| TC | 0.035 ± 0.023 | 0.035 ± 0.023 | 0.026 ± 0.023 | 0.034 ± 0.024 | 0.030 ± 0.021 | 0.032 ± 0.006 | T = 0.001* | T q = 0.009* | T q = 0.588 | |
| Time Mean | 0.036 ± 0.024 | 0.032 ± 0.020 | 0.022 ± 0.016†Ψ | 0.035 ± 0.023◊ | 0.032 ± 0.020◊ | G X T = 0.327 | G X T q = 0.101§ | G X T q = 0.290 |
Individual group and time data expressed as means ± SD, while group effects are presented as means ± SEM. Data represents the stress and sex hormone response at each testing session during the 10 day intervention. MANOVA analysis revealed overall Wilks’ Lambda time (p <0.001) and group x time (p = 0.102). Univariate ANOVA p-levels from MANOVA analysis are presented for each variable. Univariate ANOVA p-levels are listed first by the Greenhouse-Geisser (GG) analysis and then by the within-subjects contrasts (WSC) to demonstrate the potential shape of the time or group x time interaction with significance indicated by the following super/subscripts: * indicates p <0.05 p-level significance, § indicates p <0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p <0.05 difference between groups, † represents p <0.05 difference from baseline value, Ψ represents p <0.05 difference from pre-run, ◊ represents p <0.05 difference from 60-min post, # represents p <0.05 difference from 24-hr post. Cort/Test Cortisol/Testosterone ratio, RFT race finish time, G group p-level, T time p-level, G x T interaction, q quadratic p-level, L linear p-level
Markers of free radical production and oxidative stress
Table 6 shows the response of free radical production and oxidative stress markers. None of the measures for free radical production or oxidative stress demonstrated significant changes over time. Serum TAS levels tended to be different between groups over time (p = 0.089), which was supported when accounting for running intensity differences. Serum TAS levels decreased 1–8 % from pre-run levels in P over the 48-h recovery, but increased 15–31 % in TC (p = 0.046). Post-hoc analysis revealed a linear increase in TC serum TAS activity from pre-run levels that was statistically different from P and pre-run values at 48-h of recovery (see Fig. 5).
Table 6.
Markers of free radical production and oxidative stress
| Variable | Group | Baseline | Pre-Run | 60-min Post | 24-hr Post | 48-hr Post | Group Mean | p-value (GG) | p-value (WSC) | RFT Covariate p-value (WSC) |
|---|---|---|---|---|---|---|---|---|---|---|
| Nitrotyrosine (nM) | P | 279 ± 305 | 214 ± 164 | 231 ± 180 | 193 ± 139 | 198 ± 156 | 223 ± 71 | G = 0.493 | G = 0.852 | |
| TC | 305 ± 425 | 284 ± 382 | 319 ± 436 | 260 ± 352 | 302 ± 435 | 294 ± 85 | T = 0.177 | T L = 0.183 | T q = 0.667 | |
| Time Mean | 292 ± 70 | 249 ± 53 | 275 ± 60Ψ | 227 ± 48Ψ◊ | 250 ± 58◊ | G X T = 0.699 | G X T L = 0.619 | G X T L = 0.597 | ||
| TBARS (uM) | P | 8.24 ± 4.62 | 7.59 ± 2.54 | 7.01 ± 4.24 | 7.35 ± 3.52 | 7.73 ± 3.39 | 7.59 ± 0.71 | G = 0.484 | G = 0.840 | |
| TC | 8.34 ± 4.06 | 7.94 ± 4.08 | 7.91 ± 3.46 | 8.93 ± 4.52 | 8.79 ± 4.09 | 8.38 ± 0.86 | T = 0.690 | T q = 0.332 | T q = 0.462 | |
| Time Mean | 8.29 ± 0.86 | 7.77 ± 0.64 | 7.46 ± 0.77 | 8.14 ± 0.77 | 8.26 ± 0.72 | G X T = 0.783 | G X T L = 0.547 | G X T L = 0.520 | ||
| TAS (mM) | P | 3.13 ± 0.85 | 3.20 ± 0.88^ | 3.08 ± 0.89 | 3.18 ± 1.08 | 2.96 ± 0.95 | 3.11 ± 0.17 | G = 0.476 | G = 0.381 | |
| TC | 2.99 ± 0.84 | 2.50 ± 0.97 | 2.87 ± 1.07 | 2.96 ± 1.26 | 3.27 ± 0.87Ψ | 2.92 ± 0.20 | T = 0.713 | T q = 0.321 | T L = 0.436 | |
| Time Mean | 3.06 ± 0.17 | 2.85 ± 0.18 | 2.98 ± 0.19 | 3.07 ± 0.23 | 3.12 ± 0.18 | G X T = 0.239 | G X T q = 0.089§ | G X T q = 0.089§ | ||
| SOD (U/mL) | P | 0.49 ± 0.09 | 0.52 ± 0.11 | 0.50 ± 0.12 | 0.49 ± 0.08 | 0.49 ± 0.14 | 0.50 ± 0.02 | G = 0.198 | G = 0.285 | |
| TC | 0.45 ± 0.10 | 0.46 ± 0.16 | 0.47 ± 0.15 | 0.42 ± 0.15 | 0.48 ± 0.10 | 0.46 ± 0.02 | T = 0.687 | T L = 0.756 | T L = 0.155 | |
| Time Mean | 0.47 ± 0.02 | 0.49 ± 0.03 | 0.49 ± 0.03 | 0.46 ± 0.02 | 0.48 ± 0.03 | G X T = 0.808 | G X T L = 0.564 | G X T L = 0.656 |
Individual group and time data expressed as means ± SD, while group effects are presented as means ± SEM. Data represents the response to reactive oxygen and nitrogen species production in addition to antioxidant activity at each testing session during the 10 day intervention. MANOVA analysis revealed overall Wilks’ Lambda time (p <0.001) and group x time (p = 0.684). Univariate ANOVA p-levels from MANOVA analysis are presented for each variable. Univariate ANOVA p-levels are listed first by the Greenhouse-Geisser (GG) analysis and then by the within-subjects contrasts (WSC) to demonstrate the potential shape of the time or group x time interaction with significance indicated by the following super/subscripts: § indicates p <0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p <0.05 difference between groups, Ψ represents p <0.05 difference from pre-run, ◊ represents p <0.05 difference from 60-min post. TBARS thiobarbituric acid reactive substances, TAS total antioxidant status, SOD superoxide dismutase, RFT race finish time, G group p-level, T time p-level, G x T interaction, q quadratic p-level, L linear p-level
Fig. 5.
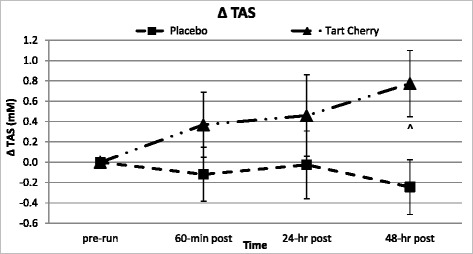
Changes in antioxidant activity with supplementation and endurance exercise. Data expressed as means ± SE and significance indicated by the following super/subscripts: * indicates p <0.05 p-level significance, § indicates p <0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p <0.05 difference between groups, Ψ represents p <0.05 difference from pre-run, ◊ represents p <0.05 difference from 60-min post, # represents p <0.05 difference from 24-hr post
Inflammatory response markers
Table 7 shows the serum inflammatory cytokine and chemokine marker response. Accounting for running intensity differences, both IL-2 (p = 0.089) and IL-6 (p = 0.064) measures tended to be different between groups over time. Serum IL-2 levels increased 0.2 % from pre-run levels throughout recovery in P, but decreased 28 % in TC. Further, serum IL-6 levels increased 64 % from pre-run in P, but only 17 % in TC. Delta post-hoc analyses demonstrated significant attenuation of serum IL-6 measures 60-min post-run in TC compared to P (104 % TC increase vs. 210 % P increase). Serum IL-2 significantly decreased in TC compared to P and pre-run measures over the 48-h recovery (see Fig. 6).
Table 7.
Pro-inflammatory cytokines and chemokines
| Variable | Group | Baseline | Pre-Run | 60-min Post | 24-hr Post | 48-hr Post | Group Mean | p-value (GG) | p-value (WSC) | RFT Covariate p-value (WSC) |
|---|---|---|---|---|---|---|---|---|---|---|
| TNF-α (pg/mL) | P | 2.34 ± 1.23 | 2.74 ± 1.22 | 3.02 ± 1.55 | 2.48 ± 1.04 | 2.49 ± 0.90 | 2.61 ± 0.26 | G = 0.922 | G = 0.654 | |
| TC | 2.49 ± 0.98 | 2.82 ± 0.90 | 2.93 ± 1.36 | 2.60 ± 0.91 | 2.42 ± 0.82 | 2.65 ± 0.31 | T = 0.003* | T q = 0.003* | T L = 0.156 | |
| Time Mean | 2.40 ± 1.12 | 2.77 ± 1.08† | 2.98 ± 1.45† | 2.53 ± 0.97Ψ◊ | 2.46 ± 0.85Ψ◊ | G X T = 0.779 | G X T L = 0.504 | G X T L = 0.151 | ||
| IFN-γ (pg/mL) | P | 7.67 ± 6.52 | 8.02 ± 7.77 | 8.73 ± 9.49 | 7.36 ± 7.15 | 7.75 ± 7.78 | 7.91 ± 2.55 | G = 0.382 | G = 0.570 | |
| TC | 10.96 ± 11.44 | 10.71 ± 9.14 | 11.51 ± 12.50 | 12.47 ± 17.61 | 11.68 ± 15.42 | 11.47 ± 3.08 | T = 0.604 | T q = 0.184 | T L = 0.424 | |
| Time Mean | 9.01 ± 8.81 | 9.12 ± 8.29 | 9.86 ± 10.67 | 9.44 ± 12.46 | 9.35 ± 11.41 | G X T = 0.388 | G X T L = 0.456 | G X T q = 0.461 | ||
| IL-1β (pg/mL) | P | 0.67 ± 0.15 | 0.85 ± 0.21 | 0.84 ± 0.19 | 0.75 ± 0.20 | 0.69 ± 0.16 | 0.76 ± 0.08 | G = 0.059§ | G = 0.058§ | |
| TC | 0.93 ± 0.51 | 1.12 ± 0.58 | 1.05 ± 0.39 | 0.98 ± 0.44 | 0.93 ± 0.40 | 1.00 ± 0.09 | T <0.001* | T q <0.001* | T L = 0.559 | |
| Time Mean | 0.78 ± 0.36 | 0.96 ± 0.42† | 0.92 ± 0.30† | 0.85 ± 0.33Ψ◊ | 0.79 ± 0.30Ψ◊ | G X T = 0.860 | G X T q = 0.662 | G X T L = 0.465 | ||
| IL-2 (pg/mL) | P | 1.18 ± 0.96 | 1.33 ± 1.10 | 1.51 ± 1.38 | 1.23 ± 0.85 | 1.27 ± 0.94 | 1.30 ± 0.28 | G = 0.939 | G = 0.786 | |
| TC | 1.17 ± 1.20 | 1.64 ± 2.18 | 1.32 ± 0.99 | 1.12 ± 1.01 | 1.11 ± 1.06 | 1.27 ± 0.34 | T = 0.070§ | T q = 0.001* | T L = 0.293 | |
| Time Mean | 1.17 ± 1.04 | 1.46 ± 1.60† | 1.43 ± 1.22† | 1.18 ± 0.90◊ | 1.21 ± 0.97◊ | G X T = 0.290 | G X T L = 0.195 | G X T L = 0.089§ | ||
| IL-6 (pg/mL) | P | 0.63 ± 0.54^ | 0.75 ± 0.53^ | 2.33 ± 1.38†Ψ | 0.69 ± 0.44^◊ | 0.68 ± 0.43 | 1.02 ± 0.19 | G = 0.017* | G = 0.509 | |
| TC | 0.94 ± 1.00 | 1.14 ± 1.27† | 2.32 ± 1.69†Ψ | 0.89 ± 0.97Ψ◊ | 0.79 ± 0.76Ψ | 1.21 ± 0.23 | T <0.001* | T q <0.001* | T q = 0.648 | |
| Time Mean | 0.76 ± 0.76 | 0.91 ± 0.90† | 2.33 ± 1.48†Ψ | 0.77 ± 0.69Ψ◊ | 0.72 ± 0.58Ψ◊ | G X T = 0.550 | G X T L = 0.053§ | G X T L = 0.064§ | ||
| IL-8 (pg/mL) | P | 2.74 ± 1.58 | 2.84 ± 1.29 | 6.21 ± 3.51 | 2.94 ± 1.24 | 2.52 ± 1.01 | 3.45 ± 0.4 | G = 0.002* | G = 0.637 | |
| TC | 3.31 ± 1.87 | 3.24 ± 1.71 | 5.39 ± 2.78 | 3.26 ± 2.08 | 2.74 ± 1.47 | 3.59 ± 0.49 | T <0.001* | T q <0.001* | T q = 0.185 | |
| Time Mean | 2.98 ± 1.69 | 3.00 ± 1.46 | 5.88 ± 3.20† | 3.07 ± 1.61◊ | 2.61 ± 1.20Ψ◊# | G X T = 0.287 | G X T q = 0.269 | G X T q = 0.166 | ||
| IL-12p70 (pg/mL) |
P | 1.79 ± 1.83 | 1.84 ± 1.75 | 1.95 ± 1.93 | 1.70 ± 1.56 | 1.81 ± 1.93 | 1.82 ± 0.42 | G = 0.009* | G = 0.706 | |
| TC | 1.54 ± 1.78 | 1.59 ± 1.52 | 1.75 ± 1.93 | 1.34 ± 1.29 | 1.27 ± 1.12 | 1.50 ± 0.51 | T = 0.012* | T q = 0.008* | T q = 0.893 | |
| Time Mean | 1.69 ± 1.78 | 1.74 ± 1.63 | 1.87 ± 1.89† | 1.55 ± 1.44Ψ◊ | 1.59 ± 1.65Ψ◊ | G X T = 0.310 | G X T q = 0.124 | G X T q = 0.167 |
Individual group and time data expressed as means ± SD, while group effects are presented as means ± SEM. Data represents the pro-inflammatory response at each testing session during the 10 day intervention. MANOVA analysis revealed overall Wilks’ Lambda time (p <0.001) and group x time (p = 0.302). Univariate ANOVA p-levels from MANOVA analysis are presented for each variable. Univariate ANOVA p-levels are listed first by the Greenhouse-Geisser (GG) analysis and then by the within-subjects contrasts (WSC) to demonstrate the potential shape of the time or group x time interaction with significance indicated by the following super/subscripts: * indicates p <0.05 p-level significance, § indicates p <0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p <0.05 difference between groups, † represents p <0.05 difference from baseline value, Ψ represents p <0.05 difference from pre-run, ◊ represents p <0.05 difference from 60-min post, # represents p <0.05 difference from 24-hr post. TNF-α tumor necrosis factor alpha, IFN-γ interferon gamma, IL interleukin, RFT race finish time, G group p-level, T time p-level, G x T interaction, q quadratic p-level, L linear p-level
Fig. 6.
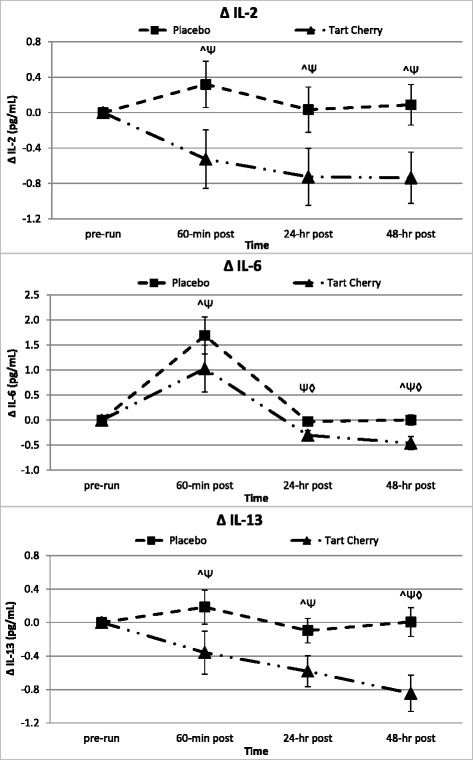
Influence of supplementation and endurance exercise on markers of the inflammatory and anti-inflammatory response. Data expressed as means ± SE and significance indicated by the following super/subscripts: * indicates p <0.05 p-level significance, § indicates p <0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p <0.05 difference between groups, Ψ represents p <0.05 difference from pre-run, ◊ represents p <0.05 difference from 60-min post, # represents p <0.05 difference from 24-hr post
Anti-inflammatory response markers
Table 8 presents the serum anti-inflammatory cytokine marker results. Accounting for running intensity, serum IL-13 levels were significantly different across groups (p = 0.031) and tended to be different between groups over time (p = 0.053). Serum IL-13 markers decreased 6 % on average from pre-run over the 48-h recovery in P, compared to a 13 % decrease in TC. Specifically, at 60-min post-run, serum IL-13 markers increased 5 % over pre-run values in P, but actually decreased 7 % below pre-run in TC. The serum IL-13 group (p = 0.029) and group by time (p = 0.014) differences from pre-run levels over the recovery period were also significant (see Fig. 6).
Table 8.
Anti-inflammatory cytokines
| Variable | Group | Baseline | Pre-Run | 60-min Post | 24-hr Post | 48-hr Post | Group Mean | p-value (GG) | p-value (WSC) | RFT Covariate p-value (WSC) |
|---|---|---|---|---|---|---|---|---|---|---|
| IL-4 (pg/mL) | P | 4.57 ± 2.02 | 5.51 ± 2.31 | 5.20 ± 2.15 | 4.02 ± 1.49 | 4.21 ± 1.63 | 4.70 ± 1.28 | G = 0.304 | G = 0.165 | |
| TC | 6.93 ± 8.98 | 8.71 ± 11.72 | 6.65 ± 6.37 | 5.41 ± 5.13 | 6.38 ± 7.03 | 6.82 ± 1.55 | T = 0.012* | T q = 0.012* | T L = 0.309 | |
| Time Mean | 5.53 ± 5.89 | 6.82 ± 7.64 | 5.79 ± 4.34 | 4.59 ± 3.45Ψ◊ | 5.09 ± 4.66Ψ◊# | G X T = 0.320 | G X T q = 0.189 | G X T L = 0.189 | ||
| IL-5 (pg/mL) | P | 0.57 ± 0.37 | 0.63 ± 0.42 | 0.66 ± 0.47 | 0.66 ± 0.46 | 0.61 ± 0.40 | 0.62 ± 0.14 | G = 0.102§ | G = 0.013* | |
| TC | 0.96 ± 0.75 | 1.01 ± 0.73 | 1.07 ± 0.76 | 1.01 ± 0.80 | 0.87 ± 0.59 | 0.98 ± 0.16 | T = 0.093§ | T q = 0.013* | T q = 0.452 | |
| Time Mean | 0.73 ± 0.58 | 0.78 ± 0.59 | 0.82 ± 0.62 | 0.80 ± 0.63 | 0.71 ± 0.49Ψ◊# | G X T = 0.542 | G X T L = 0.185 | G X T L = 0.205 | ||
| IL-7 (pg/mL) | P | 3.75 ± 1.63 | 3.37 ± 1.57 | 4.66 ± 2.61 | 3.72 ± 1.81 | 3.47 ± 1.15 | 3.80 ± 0.43 | G = 0.295 | G = 0.427 | |
| TC | 4.66 ± 2.54 | 4.27 ± 1.84 | 5.12 ± 2.22 | 4.55 ± 1.69 | 4.01 ± 1.42 | 4.52 ± 0.52 | T <0.001* | T q = 0.010* | T L = 0.845 | |
| Time Mean | 4.12 ± 2.06 | 3.74 ± 1.71† | 4.85 ± 2.42†Ψ | 4.06 ± 1.78◊ | 3.69 ± 1.27◊# | G X T = 0.708 | G X T L = 0.492 | G X T L = 0.485 | ||
| IL-10 (pg/mL) | P | 2.91 ± 3.13 | 2.77 ± 1.89 | 24.17 ± 26.99 | 2.77 ± 1.78 | 2.68 ± 1.68 | 7.06 ± 1.36 | G = 0.683 | G = 0.948 | |
| TC | 3.31 ± 2.74 | 3.99 ± 5.07 | 17.16 ± 18.60 | 3.66 ± 3.69 | 2.79 ± 1.98 | 6.18 ± 1.64 | T = 0.001* | T q = 0.001* | T q = 0.691 | |
| Time Mean | 3.07 ± 2.93 | 3.27 ± 3.51 | 21.31 ± 23.79†Ψ | 3.13 ± 2.70◊ | 2.72 ± 1.77◊ | G X T = 0.421 | G X T q = 0.495 | G X T L = 0.509 | ||
| IL-13 (pg/mL) | P | 1.52 ± 0.95 | 1.77 ± 1.14 | 1.86 ± 1.27 | 1.55 ± 1.00 | 1.58 ± 0.86 | 1.65 ± 0.36 | G = 0.091§ | G = 0.031* | |
| TC | 2.56 ± 2.01 | 2.96 ± 2.37 | 2.74 ± 1.75 | 2.56 ± 2.00 | 2.40 ± 1.66 | 2.65 ± 0.43 | T = 0.006* | T q = 0.003* | T q = 0.027* | |
| Time Mean | 1.94 ± 1.53 | 2.25 ± 1.80† | 2.22 ± 1.52† | 1.96 ± 1.54Ψ◊ | 1.91 ± 1.29Ψ◊ | G X T = 0.538 | G X T L = 0.236 | G X T q = 0.053§ |
Individual group and time data expressed as means ± SD, while group effects are presented as means ± SEM. Data represents the anti-inflammatory response at each testing session during the 10 day intervention. MANOVA analysis revealed overall Wilks’ Lambda time (p <0.001) and group x time (p = 0.447). Univariate ANOVA p-levels from MANOVA analysis are presented for each variable. Univariate ANOVA p-levels are listed first by the Greenhouse-Geisser (GG) analysis and then by the within-subjects contrasts (WSC) to demonstrate the potential shape of the time or group x time interaction with significance indicated by the following super/subscripts: * indicates p <0.05 p-level significance, § indicates p <0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: † represents p <0.05 difference from baseline value, Ψ represents p <0.05 difference from pre-run, ◊ represents p <0.05 difference from 60-min post, # represents p <0.05 difference from 24-hr post. IL interleukin, RFT race finish time, G group p-level, T time p-level, G x T interaction; q quadratic p-level, L linear p-level
Clinical markers of immune-related complete blood counts
Table 9 demonstrates the immune response-related complete blood count marker results. All immune-related complete blood counts demonstrated significant changes over time (p <0.001), but no significant changes between groups.
Table 9.
Markers of immune-related complete blood counts
| Variable | Group | Baseline | Pre-Run | 60-min Post | 24-hr Post | 48-hr Post | Group Mean | p-value (GG) | p-value (WSC) | RFT Covariate p-value (WSC) |
|---|---|---|---|---|---|---|---|---|---|---|
| Lymphocytes (K/uL) | P | 1.83 ± 0.58 | 2.50 ± 0.81 | 1.47 ± 0.54 | 2.04 ± 0.69 | 2.26 ± 0.35 | 2.02 ± 0.11 | G = 0.732 | G = 0.414 | |
| TC | 1.82 ± 0.53 | 2.63 ± 0.72 | 1.43 ± 0.25 | 1.75 ± 0.45 | 2.18 ± 0.46 | 1.96 ± 0.14 | T <0.001* | T q = 0.013* | T q = 0.509 | |
| Time Mean | 1.83 ± 0.11 | 2.56 ± 0.15† | 1.45 ± 0.09†Ψ | 1.90 ± 0.12Ψ◊ | 2.22 ± 0.08†Ψ◊# | G X T = 0.404 | G X T L = 0.905 | G X T L = 0.200 | ||
| WBC (K/uL) | P | 5.93 ± 1.45 | 6.54 ± 1.71 | 12.61 ± 3.39^†Ψ | 6.64 ± 1.84◊ | 5.14 ± 0.84 | 7.37 ± 0.29 | G = 0.314 | G = 0.398 | |
| TC | 5.83 ± 1.50 | 6.85 ± 1.61 | 10.80 ± 3.40†Ψ | 5.73 ± 1.20◊ | 5.33 ± 1.05 | 6.91 ± 0.35 | T <0.001* | T q <0.001* | T L = 0.613 | |
| Time Mean | 5.88 ± 0.29 | 6.70 ± 0.33† | 11.71 ± 0.66†Ψ | 6.18 ± 0.32◊ | 5.24 ± 0.18†Ψ◊# | G X T = 0.223 | G X T q = 0.162 | G X T q = 0.500 | ||
| MID (K/uL) | P | 0.45 ± 0.14 | 0.53 ± 0.20 | 0.67 ± 0.23† | 0.49 ± 0.19◊ | 1.10 ± 0.25†Ψ◊# | 0.65 ± 0.03 | G = 0.477 | G = 0.607 | |
| TC | 0.43 ± 0.11 | 0.61 ± 0.19† | 0.54 ± 0.15 | 0.40 ± 0.10Ψ | 1.10 ± 0.32†Ψ◊# | 0.61 ± 0.04 | T <0.001* | T L = 0.022* | T q = 0.495 | |
| Time Mean | 0.44 ± 0.03 | 0.57 ± 0.04† | 0.60 ± 0.04† | 0.44 ± 0.03Ψ◊ | 1.10 ± 0.06†Ψ◊# | G X T = 0.276 | G X T q = 0.388 | G X T q = 0.562 | ||
| GRAN (K/uL) | P | 3.59 ± 1.07 | 3.53 ± 1.04 | 10.49 ± 3.49^†Ψ | 4.09 ± 1.28◊ | 1.76 ± 0.59†Ψ◊# | 4.69 ± 0.24 | G = 0.349 | G = 0.566 | |
| TC | 3.59 ± 1.41 | 3.64 ± 0.86 | 8.83 ± 3.35†Ψ | 3.58 ± 0.98◊ | 2.05 ± 1.00◊ | 4.34 ± 0.29 | T < 0.001* | T q <0.001* | T L = 0.326 | |
| Time Mean | 3.59 ± 0.24 | 3.58 ± 0.19 | 9.66 ± 0.67†Ψ | 3.84 ± 0.23◊ | 1.90 ± 0.15†Ψ◊# | G X T = 0.259 | G X T q = 0.168 | G X T q = 0.493 | ||
| GM-CSF (pg/mL) | P | 26.38 ± 41.92 | 25.49 ± 31.29 | 26.08 ± 34.72 | 22.64 ± 30.21 | 22.41 ± 29.72 | 24.60 ± 9.15 | G = 0.485 | G = 0.696 | |
| TC | 42.26 ± 47.28 | 37.37 ± 45.40 | 35.27 ± 46.54 | 29.52 ± 38.02 | 29.41 ± 36.03 | 34.77 ± 11.04 | T = 0.056§ | T L = 0.022* | T q = 0.794 | |
| Time Mean | 34.32 ± 8.64 | 31.43 ± 7.36 | 30.68 ± 7.81 | 26.08 ± 6.57†Ψ◊ | 25.91 ± 6.34†Ψ◊ | G X T = 0.407 | G X T L = 0.221 | G X T L = 0.319 |
Individual group and time data expressed as means ± SD, while group effects are presented as means ± SEM. Data represents the complete blood count immune response markers at each testing session during the 10 day intervention. MANOVA analysis revealed overall Wilks’ Lambda time (p <0.001) and group x time (p = 0.684). Univariate ANOVA p-levels from MANOVA analysis are presented for each variable. Univariate ANOVA p-levels are listed first by the Greenhouse-Geisser (GG) analysis and then by the within-subjects contrasts (WSC) to demonstrate the potential shape of the time or group x time interaction with significance indicated by the following super/subscripts: * indicates p <0.05 p-level significance, § indicates p <0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p <0.05 difference between groups, † represents p <0.05 difference from baseline value, Ψ represents p <0.05 difference from pre-run, ◊ represents p <0.05 difference from 60-min post, # represents p <0.05 difference from 24-hr post. WBC white blood cell, MID mid-range absolute count, GRAN granulocyte absolute count, GM-CSF granulocyte-macrophage colony-stimulating factor, RFT race finish time, G group p-level, T time p-level, G x T interaction, q quadratic p-level, L linear p-level
Muscle soreness perception assessment
Table 10 presents perceptions of muscle soreness. Perceptions were not measured at baseline. All locations of muscle soreness measurement demonstrated significant changes over time (p <0.001), peaking 60-min post-run. Significant differences between groups over time were found in vastus medalis (¼) soreness perception (p = 0.035) that was confirmed when accounting for running intensity discrepancies. Subsequent post-hoc analysis indicated significantly attenuated (34 %) pre-run vastus medalis (¼) soreness in TC compared to P with no differences in soreness perception between groups over the recovery (see Fig. 7). The change from pre-run vastus medalis (¼) soreness was smaller in P soreness perception compared to TC (p = 0.035) over the 48-h recovery. The other two locations of quadriceps soreness perception testing did not reveal any significant differences between supplementation groups.
Table 10.
Quadriceps muscle soreness perception
| Variable | Group | Pre-Run | 60-min Post | 24-hr Post | 48-hr Post | Group Mean | p-value (GG) | p-value (WSC) | RFT Covariate p-value (WSC) |
|---|---|---|---|---|---|---|---|---|---|
| Algo I (cm) | P | 5.99 ± 2.94^ | 6.98 ± 3.35 | 6.44 ± 2.69 | 5.95 ± 3.01 | 6.34 ± 0.66 | G = 0.523 | G = 0.660 | |
| TC | 3.96 ± 2.15 | 6.43 ± 3.15Ψ | 6.31 ± 3.43Ψ | 5.98 ± 3.04Ψ | 5.67 ± 0.80 | T = 0.003* | T q = 0.002* | T L = 0.440 | |
| Time Mean | 4.98 ± 0.52 | 6.71 ± 0.64Ψ | 6.38 ± 0.59Ψ | 5.96 ± 0.59Ψ | G X T = 0.110 | G X T L = 0.035* | G X T L = 0.028* | ||
| Algo II (cm) | P | 5.02 ± 2.55 | 5.77 ± 2.91 | 5.58 ± 2.56 | 4.78 ± 3.02 | 5.29 ± 0.60 | G = 0.393 | G = 0.847 | |
| TC | 4.15 ± 2.66 | 5.12 ± 3.01 | 4.51 ± 3.10 | 4.08 ± 2.30 | 4.47 ± 0.72 | T = 0.122 | T q = 0.013* | T L = 0.229 | |
| Time Mean | 4.59 ± 0.51 | 5.45 ± 0.58 | 5.05 ± 0.55 | 4.43 ± 0.54 | G X T = 0.921 | G X T q = 0.889 | G X T q = 0.458 | ||
| Algo III (cm) | P | 4.65 ± 3.04^ | 6.41 ± 2.91^Ψ | 6.04 ± 2.90^Ψ◊ | 4.41 ± 2.90◊# | 5.38 ± 0.67 | G = 0.098§ | G = 0.226 | |
| TC | 2.59 ± 2.45 | 4.43 ± 2.89Ψ | 3.48 ± 2.92 | 3.84 ± 3.11Ψ | 3.58 ± 0.80 | T < 0.001* | T q < 0.001* | T L = 0.508 | |
| Time Mean | 3.62 ± 0.55 | 5.42 ± 0.57Ψ | 4.76 ± 0.57Ψ | 4.12 ± 0.59◊ | G X T = 0.058§ | G X T q = 0.053§ | G X T q = 0.257 |
Individual group and time data expressed as means ± SD, while group effects are presented as means ± SEM. Data represents the participant soreness perception in the quadriceps muscle group at each testing session during the 10 day intervention. MANOVA analysis revealed overall Wilks’ Lambda time (p <0.001) and group x time (p = 0.199). Univariate ANOVA p-levels from MANOVA analysis are presented for each variable. Univariate ANOVA p-levels are listed first by the Greenhouse-Geisser (GG) analysis and then by the within-subjects contrasts (WSC) to demonstrate the potential shape of the time or group x time interaction with significance indicated by the following super/subscripts: * indicates p <0.05 p-level significance, § indicates p <0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p <0.05 difference between groups, Ψ represents p <0.05 difference from pre-run, ◊ represents p <0.05 difference from 60-min post, # represents p <0.05 difference from 24-hr post. Algo I Algometer location #1: Vastus Medalis 1/4, Algo II Algometer location #2: Vastus Lateralis 1/4, Algo III Algometer location #3: Vastus Lateralis 1/2, RFT race finish time, G group p-level, T time p-level, G x T interaction, q quadratic p-level, L linear p-level
Fig. 7.
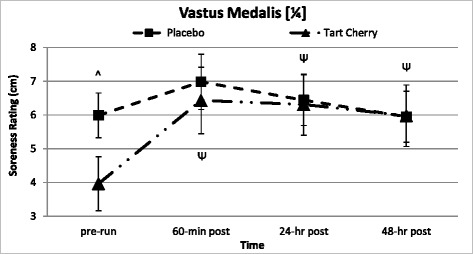
Perceptions of muscle soreness. Data expressed as means ± SE and significance indicated by the following super/subscripts: * indicates p <0.05 p-level significance, § indicates p <0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p <0.05 difference between groups, Ψ represents p <0.05 difference from pre-run, ◊ represents p <0.05 difference from 60-min post, # represents p <0.05 difference from 24-hr post
Discussion
Previous research has investigated the efficacy of tart cherry supplementation surrounding bouts of endurance exercise, however, this is the first study to investigate the effect of Montmorency tart cherry skin powder on acute endurance performance recovery. It was hypothesized that supplementation with this novel powdered tart cherry skin supplement prior to a single bout of endurance exercise would attenuate markers of muscle damage, oxidative stress, inflammation, and perceptions of muscle soreness in facilitation of faster recovery. Tart cherry ingestion reduced post-run serum markers of muscle catabolism, secondary muscle damage, and physiological stress over the 48-h recovery period compared to the placebo. Decreased muscle catabolism and stress are indicative of the attenuated recovery inflammatory response reported with tart cherry supplementation versus placebo. Antioxidant activity in those who ingested tart cherry was greater than the placebo, particularly 24 and 48-h post-run. Despite increases in actual over projected race pace times in both groups, the tart cherry group demonstrated smaller pace differences compared to placebo. Medial quadriceps soreness in tart cherry supplementers was significantly lower pre-run compared to those ingesting the placebo. However, results indicated a smaller change from pre-run medial quadriceps soreness in placebo supplementers over the 48-h recovery period compared to the tart cherry group.
Examining subject endurance performance, the increase in actual versus projected race pace irrespective of group is likely attributed to the 10-h fast and blood draw on the day of the endurance exercise challenge that would have not been experienced prior to any other race. The apparent beneficial effect of tart cherry powder supplementation on endurance performance through a decrease in race completion time is consistent with some of the previously published findings. Nieman et al. conducted a study in young, healthy males with 2-wk of quercetin supplementation (1000 mg/day) versus placebo [32]. Following a 12-min treadmill running trial at a 15 % grade and self-selected speed, Nieman et al. reported a significantly greater pre-supplementation versus post-supplementation change in distance covered with quercetin supplementation versus placebo [32]. The polyphenol content of a fruit-derived supplement similar to tart cherry was proven beneficial after extended supplementation in a study conducted by Kang et al. on regular endurance exercisers [33]. Kang et al. demonstrated that 30-d supplementation of oligomerized lychee fruit extract significantly elevated both submaximal running time and anaerobic threshold compared to a vitamin C/E mixture and a placebo [33].
Attenuation of muscle catabolism and secondary markers of muscle damage following prolonged endurance exercise physiologically provides the body optimal conditions for quicker recovery in preparation for subsequent performance bouts. Studying trained endurance runners, Kratz et al. [34] analyzed hemodynamic clinical chemistry makers before, 4 and 24-h post-Boston marathon. The results of demonstrated significant increases in total CK, AST, ALT, total protein, uric acid, total bilirubin, BUN, and creatinine 4-h post-race and confirmed significant elevations in CK, BUN, creatinine, uric acid, ALT, and AST over pre-race values 24-h post-race [34]. Bell et al. [35], in an acute endurance study following a combination of cycling sprints and time trials, reported results similar to the current study, demonstrating no differences in the CK response between Montmorency tart cherry concentrate and placebo supplementation. Unlike the current study, Howatson et al. [28] following marathon running demonstrated a trend of lower post-run CK levels when supplementing with tart cherry juice compared to placebo up to 48-h of recovery. Despite conflicting evidence among previous endurance-based tart cherry research, the post-run collective attenuation of these markers in the current study demonstrates a beneficial effect of powdered tart cherry supplementation on indices of muscle catabolism.
Previous research in the literature seems to conclude that the inflammatory process is mediated by both pro-inflammatory cytokines [36] and neuroendocrinological factors [37]. However, it has also been demonstrated that as major players in the development of secondary muscle damage, neutrophils, may also amplify the release of inflammatory cytokines [38, 39]. Nieman et al. [40] supplemented trained cyclists with quercetin, quercetin-EGCG (epigallocatechin 3-gallate), or placebo soft chews for 24-d surrounding 3-d of consecutive bouts of 3-h submaximal cycling. Nieman et al. [40] reported a significant decreases in plasma concentrations IL-6 immediately post-exercise on the third exercise day in the quercetin-EGCG group compared to placebo [40]. Howatson et al. [28] reported significantly lower inflammation immediately post-marathon through analysis of IL-6 that coincided with quicker recovery of knee extensor maximal strength following the marathon in Montmorency tart cherry juice supplemented subjects compared to placebo. Coinciding with the reduced inflammatory findings of Nieman et al. [40] and Howatson et al. [28] following endurance challenges, the current half-marathon study also reported a post-run attenuation in IL-6 with powdered tart cherry supplementation versus placebo. Similar to the post-exercise reduction in anti-inflammatory response (IL-10) in the quercetin-EGCG group published by Nieman et al. [40], the powdered tart cherry group in the current study also demonstrated similar changes via an attenuated IL-13 response over the 48-h recovery compared to placebo.
Glucocorticoids, specifically cortisol, released due to activation of the stress response through muscle mechanical microtrauma and ROS production have demonstrated an immunosuppressive influence. Davison and Gleeson [41], in an investigation of moderately trained males during 2.5-h moderate intensity cycling compared the effects of a beverage containing a vitamin C supplement with and without carbohydrate before and during endurance exercise. This study by Davison and Gleeson [41] revealed a significant increase in plasma cortisol levels immediately and 1-h post-exercise in both the placebo and vitamin C only supplemented groups with no significant difference between these two groups at 1-h post-exercise [41]. The addition of carbohydrates (alone or with vitamin C) significantly lowered the cortisol response during the exercise recovery up to 1-h post-exercise [41]. This result potentially demonstrates lower physiological stress post-exercise due to a higher energy state. The results of the current study revealed a similar cortisol response 60-min and 24-h post-run as both fasted groups demonstrated an increase from pre-run values, but the placebo group response from pre-run was significantly greater than the tart cherry group. This cortisol response group difference 60-min post-run may be due to a combination of anti-inflammatory and antioxidant effects of tart cherry anthocyanins.
Due to the reduced cortisol response 60-min post-run following tart cherry supplementation in the current study, it is likely that this anthocyanin-rich supplementation may modulate endogenous cytokine secretion following stressful exercise challenges. An acute supplementation study providing moderately active subjects with 48 g of anthocyanin-rich black currant extract immediately surrounding a single bout of high-intensity rowing conducted by Lyall et al. [42] demonstrated a significant post-exercise attenuation of pro-inflammatory cytokine production from LPS-stimulated cells. Lyall et al. [42] postulated from subsequent in vitro experimentation that this reduced cytokine production may have resulted from anthocyanin-based inhibition of NF-κB-mediated mechanisms. In the current study, attenuated IL-6 and serum cortisol levels during post-run recovery in tart cherry supplementers compared to placebo, demonstrates a potential relationship between the perception of physiological stress, regulation of anti-inflammatory cytokines, and cortisol release through NF-κB-mediation.
The attenuation muscle catabolic indices in the current study may also be partially attributed to an improved post-run redox balance with tart cherry supplementation compared to placebo. The greater antioxidant bioavailability from functional foods, such as tart cherries, containing high levels of flavonoids and anthocyanins [43], has been hypothesized to beneficially support endogenous antioxidant systems following strenuous exercise and excessive ROS-production. Howatson et al. [44] analyzed plasma TAS following a full marathon in trained endurance runners, and found that TAS was significantly greater in the tart cherry supplemented group compared to control up to 48-h post-race [7, 28, 38]. Unlike the tart cherry group, TAS levels dropped below baseline 48-h following endurance exercise in the placebo group as they failed to maintain redox balance. This discrepancy between supplementation groups demonstrates possible tart cherry antioxidant effectiveness on excessive ROS production during bouts of endurance exercise [7]. Similar to the recovery findings of Howatson et al. [44], the current study revealed a linear increase in TAS activity culminating in a 48-h recovery TAS activity that was greater in the tart cherry group compared to placebo. This demonstrates a potential short-term antioxidant effect of powdered tart cherry consumption surrounding a single endurance challenge with better achievement of redox balance compared to placebo supplementation.
Additional redox research has reported changes in exercise-induced oxidative stress utilizing TBARS analyses to measure lipid hydroperoxidation decomposition products over time. Supplementing with a tart cherry juice blend or placebo for 8-d surrounding a marathon run, Howatson et al. [28] demonstrated significantly lower TBARS levels 48-h post-marathon in the tart cherry supplemented group versus placebo. In coordination with Howatson et al. [28], Pilaczynska-Szczesniak et al. [26] reported significantly attenuated serum TBARS levels at 1-min and 24-h post-2,000 m incremental rowing test following 4-wk of chokeberry supplementation in trained rowing athletes compared to those supplemented with a placebo. In two more recent studies within the same trained rowing athlete population used by Pilaczynska-Szczesniak et al. [26], Skarpanska-Stejnborn et al. [45, 46] reported no differences in post-2,000 m incremental rowing test TBARS levels following 4-5-wk of supplementation with either Rhodiola rosea L. extract or artichoke extract. Contradictory outcomes across several studies may be due to mode of exercise, training and nutrition status, and duration of supplementation. Further, evidence in the literature utilizing lipid peroxidation (TBARS assays) analysis has presented a potential lack of oxidative damage detection specificity in human studies that may also explain the variability in results between the current and previous studies [4, 7, 35, 47].
As a highly reactive oxide metabolite of nitric oxide, peroxynitrate-bound tyrosine residues forming nitrotyrosine (NT) was measured by Sureda et al. [48] following supplementation of vitamin C + vitamin E surrounding a half-marathon. Suerda et al. [48] reported a significant increase in NT immediately post-race and 3-h post-race in the placebo group compared to the vitamin C + vitamin E supplemented group, indicating that antioxidant supplementation may have a dampening effect on oxidation of nitrogen-containing compounds with endurance exercise. The current study, however, reported no differences in NT levels over the study protocol or between supplementation groups. The outcome inconsistency may also be attributable to the differences in the antioxidant supplement and bioavailability, thus exhibiting a potential mechanistic variability in whole fruit-derived versus extracted antioxidant supplements (e.g. vitamins C and E).
The effect of phytochemical or vitamin containing anti-inflammatory supplements on the perception of muscle soreness after an endurance exercise challenge is inconsistent within the literature. Close et al. [20] acutely supplemented subjects with either ascorbic acid or a placebo surrounding an eccentrically braked endurance trial, where physically active subjects ran downhill continuously for 30-min. Close et al. [20] reported no significant differences in VAS pain ratings nor pressure algometry between groups up to 14-d post-exercise on six lower extremity locomotion muscle groups. With no effect on post-aerobic exercise delayed onset muscle soreness (DOMS) in addition to previous evidence in the literature, Close et al. [20] suggested a dissociation between post-exercise ROS production and DOMS. Following a marathon running event and a bout of high intensity stochastic cycling using a similar 200 mm VAS protocol, Howatson et al. [28] and Bell et al. [35] respectively, reported no difference in DOMS ratings between Montmorency tart cherry juice supplementation and placebo up to 72-h post-exercise. Within the current study, no quadriceps soreness differences were detected between groups over the three recovery time points. Due to the significant difference in pre-run medial quadriceps soreness between supplementation groups, delta changes calculated from pre-run revealed greater recovery medial muscle soreness with tart cherry supplementation compared to placebo. Without a baseline measure of quadriceps soreness perception and a subject training load record surrounding data collection, it is difficult to rationalize the pre-run muscle soreness discrepancy. However, the variability in soreness perceptions across muscle groups within the current study compared to previous research may also be due to the disparity in measurement protocol, supplements, exercise modality, and/or subject pool training status. Measurement of muscle soreness perception in the present study utilizing both an algometer and a GRPS was implemented to help ameliorate the purely subjective nature of a VAS as the only measure of pain or soreness.
The strengths of the current study revolve around the cohort of soreness measures and hemodynamic markers that contribute a more comprehensive analysis to the existing body of published literature. Some of the more recent endurance-based tart cherry supplementation research studies have investigated phytonutrient effectiveness on a comprehensive panel of hemodynamic markers, which will allow for parallels to be drawn to the powdered tart cherry supplement used in the current study. The current study did not enter the study cohort into a previously established race competition, but rather created a half-marathon race exclusively for the study. The utilization of this supplement within a trained population demonstrated its effectiveness under normative training, diet, and performance conditions. Potential limitations and weakness of the current study should also be considered. The placebo-control matched design of this study was effective in equalizing study subject exposure to the conditions of the half-marathon irrespective of supplement group. However, compared to previous research implementing a cross-over design, some variability associated with subject pairing may have been possible. Differences in aerobic state of training beyond the study inclusion/exclusion criteria may also have been a source of variability in study cohort recruitment. Due to the large number of hemodynamic markers measured in this study, the five selected time points of blood draws over the course of the experimental period may have not fully represented the pharmacokinetic profile of each marker. The major overriding strength of the current study is that this is the first study to be conducted utilizing a powdered form of tart cherries rather than a juice or concentrate.
Conclusions
The findings of the current study revealed that consumption of a Montmorency powdered tart cherry supplement 7-d before, the day of, and 2-d after completing an endurance running challenge, appears to be an effective dietary supplement that may help attenuate post-run markers of muscle catabolism and physiological stress in aerobically trained individuals. Attenuation of inflammatory markers over the 48-h recovery also demonstrates significant promise with powdered tart cherry supplementation. Coupled with the dampening of the inflammatory response, the powdered tart cherry subjects seemed to better maintain post-run redox balance compared to placebo supplemented subjects. The initial effectiveness on aerobic performance, serum markers of muscle catabolism, physiological stress, and inflammatory mechanisms coupled with a more stable post-run redox balance potentially indicates a reduction in secondary muscle damage as a result of powdered tart cherry supplementation. Despite inconclusive evidence surrounding the perceptions of medial quadriceps soreness, the primary findings of the current study demonstrate that powdered tart cherry supplementation in endurance-trained individuals provides similar benefits as previously studied tart cherry juices or concentrates following acute bouts of aerobic-based exercise.
Acknowledgements
We would like to thank the subjects that participated in this study as well as all the students and laboratory assistants, particularly Nicholas Barringer, Jeremy Carter, Yanghoon Peter Jung, Adriana Coletta, Brittany Sanchez, Minye Cho, and Majid Koozehchian, in the Exercise & Sport Nutrition Laboratory at Texas A&M University who assisted with data collection. We would also like to thank Mike Vaughn and Katherine Kelly for their valuable assistance in sample analysis.
Authors’ contributions
KL assisted in designing the study, writing the grant, study coordination, data collection, data analysis, and manuscript preparation. RD, CG, AO, and SS assisted in data collection and sample analysis. EG assisted with data collection, sample analysis, and review of dietary inventories as the RD on staff. CR is the director of the exercise and sport nutrition lab. MG assisted in research design and consultation. SMT, SR, and SC assisted in study consultation. RBK (corresponding author) assisted in the design of the study, data analysis, manuscript preparation, obtained the grant, and served as study PI. All authors read and approved the final manuscript.
Competing interests
This study was funded by Anderson Global Group, LLC (Irvine, CA, USA) and Shoreline Fruit, LLC (Traverse City, MI, USA) through an unrestricted research grant to Texas A&M University. All researchers involved independently collected, analyzed, and interpreted the results from this study and have no financial interests concerning the outcome of this investigation. The results from this study do not constitute endorsement by the authors and/or the institution concerning the nutrients investigated.
References
- 1.Cannonl JG, Blumberg JB. Acute phase immune responses in exercise. 2000. p. 177. [Google Scholar]
- 2.Vider J, Lehtmaa J, Kullisaar T, Vihalemm T, Zilmer K, Kairane Č, et al. Acute immune response in respect to exercise-induced oxidative stress. Pathophysiology. 2001;7(4):263–70. doi: 10.1016/S0928-4680(00)00057-2. [DOI] [PubMed] [Google Scholar]
- 3.Mastaloudis A, Morrow JD, Hopkins DW, Devaraj S, Traber MG. Antioxidant supplementation prevents exercise-induced lipid peroxidation, but not inflammation, in ultramarathon runners. Free Radic Biol Med. 2004;36(10):1329–41. doi: 10.1016/j.freeradbiomed.2004.02.069. [DOI] [PubMed] [Google Scholar]
- 4.Bell PG, Walshe IH, Davison GW, Stevenson E, Howatson G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients. 2014;6(2):829–43. doi: 10.3390/nu6020829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol. 2010;95(1):1–9. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243–76. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell PG, McHugh MP, Stevenson E, Howatson G. The role of cherries in exercise and health. Scand J Med Sci Sports. 2014;24(3):477–90. doi: 10.1111/sms.12085. [DOI] [PubMed] [Google Scholar]
- 8.McAnulty LS, Nieman DC, Dumke CL, Shooter LA, Henson DA, Utter AC, et al. Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running. Appl Physiol Nutr Metab. 2011;36(6):976–84. doi: 10.1139/h11-120. [DOI] [PubMed] [Google Scholar]
- 9.Moflehi D, Kok L-Y, Tengku-Kamalden T-F, Amri S. Effect of single-session aerobic exercise with varying intensities on lipid peroxidation and muscle-damage markers in sedentary males. Glob J Health Sci. 2012;4(4):48. doi: 10.5539/gjhs.v4n4p48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoenfeld BJ. The use of Nonsteroidal anti-inflammatory drugs for exercise-induced muscle damage. Sports Med. 2012;42(12):1017–28. doi: 10.1007/BF03262309. [DOI] [PubMed] [Google Scholar]
- 11.Proske U, Morgan D. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537(2):333–45. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson D, Williams C, McGregor SJ, Nicholas CW, McArdle F, Jackson MJ, et al. Prolonged vitamin C supplementation and recovery from demanding exercise. Int J Sport Nutr Exerc Metab. 2001;11(4):466. doi: 10.1123/ijsnem.11.4.466. [DOI] [PubMed] [Google Scholar]
- 13.Bryer S, Goldfarb A. Effect of high dose vitamin C supplementation on muscle soreness, damage, function, and oxidative stress to eccentric exercise. Int J Sport Nutr Exerc Metab. 2006;16(3):270. doi: 10.1123/ijsnem.16.3.270. [DOI] [PubMed] [Google Scholar]
- 14.Fischer CP, Hiscock NJ, Penkowa M, Basu S, Vessby B, Kallner A, et al. Supplementation with vitamins C and E inhibits the release of interleukin-6 from contracting human skeletal muscle. J Physiol. 2004;558(2):633–45. doi: 10.1113/jphysiol.2004.066779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGinley C, Shafat A, Donnelly AE. Does antioxidant vitamin supplementation protect against muscle damage? Sports Med. 2009;39(12):1011–32. doi: 10.2165/11317890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Cabrera M-C, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87(1):142–9. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 17.Yfanti C, Akerström T, Nielsen S, Nielsen AR, Mounier R, Mortensen OH, et al. Antioxidant supplementation does not alter endurance training adaptation. Med Sci Sports Exerc. 2010;42(7):1388–95. doi: 10.1249/MSS.0b013e3181cd76be. [DOI] [PubMed] [Google Scholar]
- 18.Beaton LJ, Allan DA, Tarnopolsky MA, Tiidus PM, Phillips SM. Contraction-induced muscle damage is unaffected by vitamin E supplementation. Med Sci Sports Exerc. 2002;34(5):798–805. doi: 10.1097/00005768-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Theodorou AA, Nikolaidis MG, Paschalis V, Koutsias S, Panayiotou G, Fatouros IG, et al. No effect of antioxidant supplementation on muscle performance and blood redox status adaptations to eccentric training. Am J Clin Nutr. 2011;93(6):1373–83. doi: 10.3945/ajcn.110.009266. [DOI] [PubMed] [Google Scholar]
- 20.Close GL, Ashton T, Cable T, Doran D, Holloway C, McArdle F, et al. Ascorbic acid supplementation does not attenuate post-exercise muscle soreness following muscle-damaging exercise but may delay the recovery process. Br J Nutr. 2006;95(05):976–81. doi: 10.1079/BJN20061732. [DOI] [PubMed] [Google Scholar]
- 21.Teixeira VH, Valente HF, Casal SI, Marques AF, Moreira PA. Antioxidants do not prevent postexercise peroxidation and may delay muscle recovery. Med Sci Sports Exerc. 2009;41(9):1752–60. doi: 10.1249/MSS.0b013e31819fe8e3. [DOI] [PubMed] [Google Scholar]
- 22.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci. 2009;106(21):8665–70. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strobel NA, Peake JM, Matsumoto A, Marsh SA, Coombes JS, Wadley GD. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med Sci Sports Exerc. 2011;43(6):1017–24. doi: 10.1249/MSS.0b013e318203afa3. [DOI] [PubMed] [Google Scholar]
- 24.Peternelj T-T, Coombes JS. Antioxidant supplementation during exercise training. Sports Med. 2011;41(12):1043–69. doi: 10.2165/11594400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Lafay S, Jan C, Nardon K, Lemaire B, Ibarra A, Roller M, et al. Grape extract improves antioxidant status and physical performance in elite male athletes. J Sports Sci Med. 2009;8(3):468. [PMC free article] [PubMed] [Google Scholar]
- 26.Pilaczynska-Szczesniak L, Skarpanska-Steinborn A, Deskur E, Basta P, Horoszkiewicz-Hassan M. The influence of chokeberry juice supplementation on the reduction of oxidative stress resulting from an incremental rowing ergometer exercise. Int J Sport Nutr Exerc Metab. 2005;15(1):48–58. doi: 10.1123/ijsnem.15.1.48. [DOI] [PubMed] [Google Scholar]
- 27.Kuehl KS, Perrier ET, Elliot DL, Chesnutt JC. Efficacy of tart cherry juice in reducing muscle pain during running: a randomized controlled trial. J Int Soc Sports Nutr. 2010;7:17. doi: 10.1186/1550-2783-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howatson G, McHugh MP, Hill JA, Brouner J, Jewell AP, van Someren KA, et al. Influence of tart cherry juice on indices of recovery following marathon running. Scand J Med Sci Sports. 2010;20(6):843–52. doi: 10.1111/j.1600-0838.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- 29.Klesges RC, Ward KD, Shelton ML, Applegate WB, Cantler ED, Palmieri GM, et al. Changes in bone mineral content in male athletes: mechanisms of action and intervention effects. JAMA. 1996;276(3):226–30. doi: 10.1001/jama.1996.03540030060033. [DOI] [PubMed] [Google Scholar]
- 30.Almada A, Kreider R, Ransom J, Rasmussen C. Comparison of the reliability of repeated whole body DEXA scans to repeated spine and hip scans. J Bone Miner Res. 1999;14(9):S369.
- 31.Bowling JL, Katayev A. An evaluation of the Roche Cobas c 111. Lab Med. 2010;41(7):398–402. doi: 10.1309/LM6T8D1LKQXVNCAC. [DOI] [Google Scholar]
- 32.Nieman DC, Williams AS, Shanely RA, Jin F, McAnulty SR, Triplett NT, et al. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med Sci Sports Exerc. 2010;42(2):338–45. doi: 10.1249/MSS.0b013e3181b18fa3. [DOI] [PubMed] [Google Scholar]
- 33.Kang SW, Hahn S, Kim J-K, Yang S-M, Park B-J, Lee SC. Oligomerized lychee fruit extract (OLFE) and a mixture of vitamin C and vitamin E for endurance capacity in a double blind randomized controlled trial. J Clin Biochem Nutr. 2012;50(2):106. doi: 10.3164/jcbn.11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kratz A, Lewandrowski KB, Siegel AJ, Chun KY, Flood JG, Van Cott EM, et al. Effect of marathon running on hematologic and biochemical laboratory parameters, including cardiac markers. Am J Clin Pathol. 2002;118(6):856–63. doi: 10.1309/14TY-2TDJ-1X0Y-1V6V. [DOI] [PubMed] [Google Scholar]
- 35.Bell PG, Walshe IH, Davison GW, Stevenson EJ, Howatson G. Recovery facilitation with Montmorency cherries following high-intensity, metabolically challenging exercise. Appl Physiol Nutr Metab. 2015;40(4):414–23. doi: 10.1139/apnm-2014-0244. [DOI] [PubMed] [Google Scholar]
- 36.Gleeson M. Immune function in sport and exercise. J Appl Physiol. 2007;103(2):693–9. doi: 10.1152/japplphysiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- 37.Pedersen BK, Toft AD. Effects of exercise on lymphocytes and cytokines. Br J Sports Med. 2000;34(4):246–51. doi: 10.1136/bjsm.34.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sousa M, Teixeira VH, Soares J. Dietary strategies to recover from exercise-induced muscle damage. Int J Food Sci Nutr. 2013;65(2):151–63. doi: 10.3109/09637486.2013.849662. [DOI] [PubMed] [Google Scholar]
- 39.Cannon JG, Pierre BAS. Cytokines in exertion-induced skeletal muscle injury. Mol Cell Biochem. 1998;179(1–2):159–68. doi: 10.1023/A:1006828425418. [DOI] [PubMed] [Google Scholar]
- 40.Nieman DC, Henson DA, Maxwell KR, Williams AS, McAnulty SR, Jin F, et al. Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Med Sci Sports Exerc. 2009;41(7):1467–75. doi: 10.1249/MSS.0b013e318199491f. [DOI] [PubMed] [Google Scholar]
- 41.Davison G, Gleeson M. Influence of acute vitamin C and/or carbohydrate ingestion on hormonal, cytokine, and immune responses to prolonged exercise. Int J Sport Nutr Exerc Metab. 2005;15(5):465. doi: 10.1123/ijsnem.15.5.465. [DOI] [PubMed] [Google Scholar]
- 42.Lyall KA, Hurst SM, Cooney J, Jensen D, Lo K, Hurst RD, et al. Short-term blackcurrant extract consumption modulates exercise-induced oxidative stress and lipopolysaccharide-stimulated inflammatory responses. Am J Physiol Regul Integr Comp Physiol. 2009;297(1):R70–R81. doi: 10.1152/ajpregu.90740.2008. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Nair MG, Strasburg GM, Chang Y-C, Booren AM, Gray JI, et al. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J Nat Prod. 1999;62(2):294–6. doi: 10.1021/np980501m. [DOI] [PubMed] [Google Scholar]
- 44.Janaszewska A, Bartosz G. Assay of total antioxidant capacity: comparison of four methods as applied to human blood plasma. Scand J Clin Lab Investig. 2002;62(3):231–6. doi: 10.1080/003655102317475498. [DOI] [PubMed] [Google Scholar]
- 45.Skarpanska-Stejnborn A, Pilaczynska-Szczesniak L, Basta P, Deskur-Smielecka E. The influence of supplementation with Rhodiola rosea L. extract on selected redox parameters in professional rowers. Int J Sport Nutr. 2009;19(2):186. doi: 10.1123/ijsnem.19.2.186. [DOI] [PubMed] [Google Scholar]
- 46.Skarpanska-Stejnborn A, Pilaczynska-Szczesniak L, Basta P, Deskur-Smielecka E, Horoszkiewicz-Hassan M. The influence of supplementation with artichoke (Cynara scolymus L.) extract on selected redox parameters in rowers. Int J Sport Nutr Exerc Metab. 2008;18(3):313. doi: 10.1123/ijsnem.18.3.313. [DOI] [PubMed] [Google Scholar]
- 47.Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189(1):41–54. doi: 10.1016/S0300-483X(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 48.Sureda A, Ferrer MD, Mestre A, Tur JA, Pons A. Prevention of neutrophil protein oxidation with vitamins C and e diet supplementation without affecting the adaptive response to exercise. Int J Sport Nutr Exerc Metab. 2013;23(1):31–9. doi: 10.1123/ijsnem.23.1.31. [DOI] [PubMed] [Google Scholar]


