Abstract
Background
The Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) was established in 2008 to answer strategic questions about schistosomiasis control. For programme managers, a high-priority question is: what are the most cost-effective strategies for delivering preventive chemotherapy (PCT) with praziquantel (PZQ)? This paper describes the process SCORE used to transform this question into a harmonized research protocol, the study design for answering this question, the village eligibility assessments and data resulting from the first year of the study.
Methods
Beginning in 2009, SCORE held a series of meetings to specify empirical questions and design studies related to different schedules of PCT for schistosomiasis control in communities with high (gaining control studies) and moderate (sustaining control studies) prevalence of Schistosoma infection among school-aged children. Seven studies are currently being implemented in five African countries. During the first year, villages were screened for eligibility, and data were collected on prevalence and intensity of infection prior to randomisation and the implementation of different schemes of PZQ intervention strategies.
Results
These studies of different treatment schedules with PZQ will provide the most comprehensive data thus far on the optimal frequency and continuity of PCT for schistosomiasis infection and morbidity control.
Conclusions
We expect that the study outcomes will provide data for decision-making for country programme managers and a rich resource of information to the schistosomiasis research community.
Trial registration
The trials are registered at International Standard Randomised Controlled Trial registry (identifiers: ISRCTN99401114, ISRCTN14849830, ISRCTN16755535, ISRCTN14117624, ISRCTN95819193 and ISRCTN32045736).
Keywords: Schistosomiasis, Schistosoma haematobium, Schistosoma mansoni, Control, Preventive chemotherapy, Praziquantel, Côte d’Ivoire, Kenya, Mozambique, Niger, Tanzania
Background
The Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) was established through a grant provided to the University of Georgia (UGA) Research Foundation from the Bill & Melinda Gates Foundation in December 2008, to answer strategic questions about schistosomiasis control and elimination. SCORE’s focus is on operational research that will enhance the effectiveness of current and future schistosomiasis control programmes [1]. The SCORE portfolio includes evaluation of screening tests for Schistosoma mansoni [2, 3]; development of a gold-standard diagnostic test for S. mansoni and Schistosoma haematobium [4, 5]; assessment of the impact of drug pressure on parasite genetics [6]; research related to snail control [7]; the “rapid answers project” (RAP), which synthesises existing data to answer important programmatic questions; and studies on elimination of S. mansoni and S. haematobium in areas of very low prevalence [8, 9]. It also includes large field studies to compare multi-year strategies for preventive chemotherapy (PCT) in areas with moderate and high prevalence of schistosomiasis [1, 10]. This paper describes the decision-making process that informed the study design for these gaining and sustaining schistosomiasis control programmes that are facilitated by a series of cluster randomised trials with different PCT schemes. We present the results of eligibility assessments and year 1 data collection within 825 villages in five African countries where the studies are being conducted.
The prevalence of schistosome infection is usually highest in school-aged children (SAC) [11–15]. More than 90 % of schistosome infections occur in sub-Saharan Africa [16–18], resulting in at least 3.3 million disability-adjusted life years (DALYs) due to schistosomiasis-associated clinical and subtle morbidities [16, 19]. The World Health Organization (WHO) treatment guidelines call for PCT mainly targeting SAC in endemic areas through periodic administration of praziquantel (PZQ) [20], either through school-based treatment (SBT) or community-wide treatment (CWT) [1, 21–25]. The SCORE studies of gaining and sustaining control of schistosomiasis will compare the impacts and costs of different multi-year strategies involving CWT, SBT and “drug holidays” (i.e. years without PCT). Information from this research is expected to inform programme managers of national disease control programmes about the most effective strategies for gaining and sustaining the control of schistosomiasis and might also inform strategic shifts from morbidity control to interruption of transmission, therefore local elimination.
Methods
Process for designing SCORE’s operational research on gaining and sustaining control of schistosomiasis
In April 2009, SCORE convened a meeting involving researchers, programme managers and representatives of WHO to design the gaining and sustaining control studies. Discussions included the role of mapping, the need to integrate SCORE operational research with ongoing government programmes, integration of schistosomiasis control with other neglected tropical disease (NTD) prevention and control efforts (mainly soil-transmitted helminthiasis and lymphatic filariasis), data management for multi-country studies and concerns about ensuring the effectiveness and sustainability of PCT. It was decided that the studies would be large-scale cluster randomised trials in areas with starting prevalence of ≥10 % in SAC. Interventions would occur once a year, except in villages on “PZQ holiday” years, during which no drug administration will occur. Data would be collected annually, except in villages on “drug holiday” that year, starting before the first round of PCT and ending in the year after the fourth treatment round.
Based on this input, SCORE developed a request for proposals, which was disseminated to teams with a history of conducting relevant population-based helminthiasis research in Africa. Teams could propose to conduct studies of gaining control of S. haematobium (Sh2) or S. mansoni (Sm2), respectively, in areas with prevalence among SAC of ≥25 % or sustaining control (Sh1 or Sm1 studies, respectively) in areas with prevalence ranging between 10 and 24 %. Five study teams, each consisting of a principal investigator (PI) from the country where the study would take place and a northern partner, were selected to participate (Table 1). Criteria for selecting teams included likelihood that the proposed study areas would have sufficient numbers of villages meeting inclusion criteria, experience and track record of the team and proposed approach to ensuring high coverage of PCT.
Table 1.
Study sites, study teams and numbers of villages screened in the eligibility surveys
| Type of study | Country | Region of the country | Lead African partner institution | Lead Northern partner institution | # villages screened | # (%) villages that met criteria |
|---|---|---|---|---|---|---|
| Sustaining control | Côte d’Ivoire (Sm1) | Région des Montagnes and Région du Moyen Cavally | Université Félix Houphouët-Boigny; Abidjan, Côte d’Ivoire | Swiss Tropical and Public Health Institute; Basel, Switzerland | 263 | 77 (29.3) |
| Kenya (Sm1) | Kisumu region in western Kenya bordering Lake Victoria | Center for Global Health Research, Kenya Medical Research Institute (KEMRI); Nairobi, Kenya | Centers for Disease Control and Prevention (CDC); Atlanta, USA | 150 | 75 (50.0) | |
| Niger (Sh1) | Dosso and Tillaberi regions in western Niger | Réseau International Schistosomoses, Environnement, Aménagement et Lutte (RISEAL-Niger), Niamey, Niger | Schistosomiasis Control Initiative (SCI), Imperial College London; London, UK | 150 | 75 (50.0) | |
| Gaining control | Kenya (Sm2) | Kisumu region in western Kenya bordering Lake Victoria | Center for Global Health Research, KEMRI; Nairobi, Kenya | CDC; Atlanta, USA | 320 | 150 (46.9) |
| Mozambique (Sh2) | Cabo Delgado province in northern Mozambique | Catholic University of Mozambique; Beira, Mozambique | SCI, Imperial College London; London, UK | 150 | 150 (100.0) | |
| Niger (Sh2) | Dosso and Tillaberi regions in western Niger | National NTD Programme, Ministry of Health, Niamey, Niger | SCI, Imperial College London; London, UK | 248 | 150 (60.5) | |
| Tanzania (Sm2) | Mwanza region bordering Lake Victoria | Mwanza Research Center, National Institute for Medical Research (NIMR); Mwanza, Tanzania | University of Copenhagen; Copenhagen, Denmark | 308 | 167 (50.9) | |
| Total | 1,569 | 767 (48.3) |
Sh1 sustaining control study in S. haematobium moderate endemicity villages, Sh2 gaining control study in S. haematobium high endemicity settings, Sm1 sustaining control study in S. mansoni moderate endemicity villages, Sm2 gaining control study in S. mansoni high endemicity villages
Mozambique and Niger were funded to conduct studies of S. haematobium, while Côte d’Ivoire, Kenya and Tanzania were funded to conduct studies of S. mansoni. Two teams (in Kenya and Niger) were funded to conduct studies of both gaining and sustaining control; in both countries gaining and sustaining studies occurred in separate areas. Côte d’Ivoire was funded to conduct a sustaining control study [26], and Mozambique and Tanzania were each funded to conduct gaining control studies (Table 1).
After being selected, the study teams participated in a “harmonization meeting” to review and refine the protocol. Protocols were standardised and plans were developed to ensure that data from comparable sites could be combined in subsequent analyses. In addition to finalising the study design and sample sizes, investigators agreed to a number of process measures and activities that were deemed essential for a successful study. These included robust plans for community sensitization, a commitment to measure coverage after each round of PCT and a willingness to revisit communities that did not achieve the coverage target to treat people who had been missed. Major discussion points and decisions from the initial and harmonization meetings are described in Table 2.
Table 2.
Key decisions related to design of the gaining and sustaining control of schistosomiasis studies, and their rationale
| Decision | Rationale |
|---|---|
| The study arms would not necessarily align with WHO recommendations for PCT | The WHO recommendations are not solidly evidence-based, and studying them would not answer most pressing questions |
| Sustaining schistosomiasis control studies would only involve SBT, and not adults or CWT | Existing data indicate adults are not major sources of transmission when prevalence of infection is <25 %, making testing and interventions for adults not cost-effective when resources are limited |
| Sustaining and gaining control of schistosomiasis studies would involve places with prevalence 10–24 % and ≥25 % in children aged 9–12 year, respectively | The cutoff of 10 % for sustaining studies was based on the idea that below that, one is moving towards elimination, and this will require additional interventions besides PCT. The choice of 25 % prevalence to divide gaining and sustaining studies was based on expert opinion |
| Sustaining control of schistosomiasis studies would include three arms, gaining studies would have six arms | SCORE would have preferred to test many more combinations of interventions, however this was not practical. The numbers of arms, numbers of villages per arm and number of children per village were an attempt to balance scientific, resource-related and practical considerations |
| Children aged 13–14 years would be tested to determine eligibility of a village for the sustaining or gaining control of schistosomiasis studies | Children who test positive must be treated. Testing children aged 9–12 years and treating those infected could affect the year 1 and subsequent study results, especially if prevalence is high. A very high prevalence could necessitate treating the entire village |
| “Drug holidays” would be included in study arms | The cost and impact of “drug holidays” is not known. If holidays have minimal negative effects on prevalence and intensity of Schistosoma infection in villages that have been targetted by PCT, holidays could allow for more widespread treatment |
| In all studies, first-year students would be tested at the beginning and end of the study | First-year students provide a measure of new infections in the community. If transmission is decreasing, prevalence and intensity in these children should fall |
| A convenience sample of adults would be tested in gaining control of schistosomiasis studies | Although initial plans called for a more systematic approach to identifying adults for testing, this proved impractical given the resources, so convenience samples were allowed |
| SCORE would provide mobile data collection software | Information provided at the harmonization meeting indicated that the software being used in lymphatic filariasis research could be readily adapted for SCORE use. This turned out not to be the case, but SCORE’s commitment to standardising data collection, providing support for data cleaning and storage and supporting mobile technology remained |
| SCORE-supported research needed to be conducted in close collaboration with Ministries of Health and Education | This was deemed essential both to ensure that PCT in SCORE study areas were conducted per protocol and to encourage the Ministries to use the results. In addition, it was assumed that PZQ access and use would work best when coordinated with the national schistosomiasis control programme |
| Study villages would need to achieve high levels of coverage; if these were not achieved during PCT, a team would need to return to the village to provide additional treatment | It was recognised that high coverage levels are not always achieved by PCT programmes. However, comparison of effectiveness among arms would require that treatments be delivered and actually consumed. Investigators were encouraged to have treatments directly observed to assure compliance |
| Investigators would be encouraged to publish their countries’ results; the SCORE secretariat would take responsibility for publishing combined results | In addition to encouraging widespread dissemination of the results of research, data sharing approaches that would allow investigators to use the data for modelling and other purposes were to be developed |
CWT community-wide treatment, PCT preventive chemotherapy, PZQ praziquantel, SBT school-based treatment, SCORE schistosomiasis consortium for operational research and evaluation, WHO World Health Organization
Study design
Figure 1 shows the treatment arms for the gaining (n = 6 arms) and sustaining (n = 3 arms) control studies. The most intensive intervention arm in the gaining control studies involves four years of CWT, while the least intensive intervention arm involves two years of SBT alternating with “PZQ holiday” years. For sustaining control, the most intensive intervention arm involves four years of SBT, while the least intensive arms involve two years of SBT followed by two “PZQ holiday” years. The pattern of interventions in the three arms in the sustaining control studies matches that of arms 4, 5 and 6 in the gaining control studies.
Fig. 1.
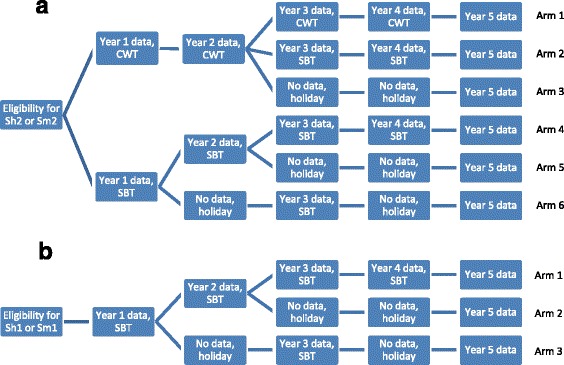
a Study arms and timeline for the studies of gaining control of schistosomiasis in Africa. CWT, community-wide treatment; SBT, school-based treatment; Sm2, gaining control study in S. mansoni endemic villages; Sh2, gaining control study in S. haematobium endemic villages. b Study arms and timeline for the studies of sustaining control of schistosomiasis in Africa. SBT, school-based treatment; Sh1, sustaining control study in S. haematobium endemic villages; Sm1, sustaining control study in S. mansoni endemic villages
A simple random allocation approach was used to assign all eligible villages to study arms except in Niger, where, in contradiction to the harmonized protocols, geographically clustered groups of 25 villages were randomly assigned to study arms.
The number of villages per arm and number of 9- to 12-year-old children tested per village were based on sample size calculations and practical considerations. Power computations were based on generalized estimating equations used to fit a logistic regression model that included treatment arm and time effects and treatment-by-time interaction, and assumed an over-dispersion parameter of φ = 5.0. Year 1 infection prevalence was assumed to be 50 % for gaining control studies and 25 % for sustaining studies. We assumed that the most intense treatment (arm 1) would reduce prevalence to a specific target level (15 and 10 % for gaining and sustaining control studies, respectively) by study end. We computed minimum effect sizes (difference between arm 1 and an alternative treatment arm at study conclusion) that could be detected with 90 % power for a two-sided α = 0.05 level test. Separate computations were carried out using various numbers of villages per treatment arm and children sampled per village. We assumed negligible correlation between measurements in year 1 and at study conclusion. Based on these assumptions, we estimated that 25 villages per treatment arm and 100 children aged 9–12 years per village were sufficient to detect absolute treatment differences of 11.4 and 9.4 % at the conclusion of the gaining and sustaining studies, respectively.
In addition to children aged 9–12 years, in the first and final fifth year, the protocol includes testing up to 100 children aged 5–8 years in both gaining and sustaining studies, and testing of 50 adults (aged 20–55 years) in the first and fifth years of the gaining studies. Populations to be tested in the different study years and methods of testing are shown in Table 3. No testing was to be conducted during “PZQ holiday” years because children found positive would need to be treated, and if the village prevalence was very high, it might be unethical to not treat the whole village.
Table 3.
Populations tested in gaining and sustaining control of schistosomiasis studies, and microscopy performed, by year of the study
| Intended population tested per village | Microscopy performed | Year 1 | Years 2–4 | Year 5 |
|---|---|---|---|---|
| 100 children aged 9–12 years | One mid-day urine specimen subjected to two filtrations; or three stool specimens subjected to duplicate Kato-Katz thick smears | Gaining and sustaining | Gaining and sustaining | Gaining and sustaining |
| 100 (or as many as possible) children aged 5–8 years | One mid-day urine specimen subjected to two filtrations; or one stool specimen subjected to duplicate Kato-Katz thick smears | Gaining and sustaining | Gaining and sustaining | |
| Adults | One mid-day urine specimen subjected to two filtrations; or one stool specimen subjected to duplicate Kato-Katz thick smears | Gaining | Gaining |
Eligibility surveys
To be eligible for inclusion in the five-year study, a village had to have a primary school (so that it could be randomised to SBT), at least 100 children aged 9–12 years and a starting prevalence of Schistosoma infection in the appropriate range (≥25 % for gaining and 10–24 % for sustaining control studies). Fifty children aged 13–14 years were tested in potentially eligible villages until the required number of villages (150 for gaining and 75 for sustaining control) were found. S. mansoni was assessed in these eligibility surveys by examining two slides from one stool sample per child using the Kato-Katz method [27], while S. haematobium was evaluated by microscopic examination of filtered urine or reagent strip testing for microhaematuria on a single mid-day urine specimen [13–15].
Laboratory methods
For the eligibility surveys that involved children aged 13–14 years and for prevalence and intensity evaluations in adults and children 5–8 years of age, S. mansoni infection was based on microscopic examination of duplicate Kato-Katz thick smears from a single stool specimen [27]. For the annual cross-sectional prevalence and intensity assessment of S. mansoni in 9- to 12-year-old children, stool specimens were collected on three consecutive days from each child, and eggs enumerated on duplicate Kato-Katz thick smears per specimen.
The eligibility surveys assessed S. haematobium infection by either urine filtration microscopy [28, 29] or a reagent strip assay for microhaematuria [30] on a single urine specimen. For the cross-sectional prevalence and intensity surveys, two 10-ml aliquots from a single mid-day urine specimen were filtered, and the filters examined quantitatively under a microscope by two independent experienced laboratory technicians for S. haematobium eggs.
Data collection and management
SCORE provided a list of variables to be collected on individual participants, as well as standard operating procedures (SOPs) for diagnosing schistosomiasis. Forms for collecting village-level data on such factors as water and sanitation, occupations and other contextual factors that might affect study results were also provided.
Most year 1 data were collected on paper. Kenya, Mozambique and Tanzania used a mobile-based system (EpiCollect®) developed at Imperial College London for data collection. Côte d’Ivoire used a mobile-based system (LINKS®) developed at the Task Force for Global Health for some data collection beginning in year 2. These systems involve data entry on-site, synching of data to a central server and downloading of data for cleaning and analysis.
Statistical analysis
Simple univariate and bivariate analyses were used to describe study participant demographics. Differences in infection prevalence and intensity by study arm were assessed using logistic and Poisson regression models, respectively, with adjustment for over-dispersion. Calculations of infection intensity included only those individuals who had at least one positive slide; for those, intensity was the geometric mean on all their slides, including those without Schistosoma eggs detected.
Ethics statement
Written informed consent was obtained from adults (including parents/legal guardians of children in the study) and assent was obtained from children less than 18 years old, except in places where village-level consent is the standard, in which case local requirements were met. Ethical review of research protocol was implemented by human subjects committee in each African country and by the institutional review board (IRB) of their respective northern partners. Sm1 and Sm2 studies in Kenya were reviewed and approved by the National Ethics Review Committee of the Kenyan Medical Research Institute (KEMRI; approval numbers SCC 1800 and SCC 1820, respectively) and by the IRB of the Centers for Disease Control and Prevention (CDC; approval #: 1661). For the Sm1 study in Côte d’Ivoire, ethical approval was obtained from the ethics committees in Côte d’Ivoire (reference no. 1994MSHP/CNER) and Basel (reference no. EKBB 279/10). In Niger, ethical approval was obtained from the Niger Republic National Consulate for ethical review (reference no. 012/2010/CCNE) and from the Imperial College Research Ethic Committee (ICREC_8_2_2). In Mozambique, ethical approval was received from the Ministry of Health (reference no. 235/CNBS/10) and the Imperial College Research Ethic Committee (ICREC_10_8_2). In Tanzania, ethical approval was obtained from the National Institute for Medical Research (NIMR; reference no. NIMR/HQ/R.8a/Vol. IX/1022). In addition to these, the UGA IRB implemented an administrative human subjects review and issued additional approval per country’s protocol as follows: 10021–0, 10221–0, 10267–0, 10353–0, 10431–0 and 10533–0 for Côte d’Ivoire, Kenya Sm1, Kenya Sm2, Tanzania, Niger and Mozambique, respectively.
The trials have been registered with the International Standard Randomised Controlled Trial registry under ISRCT numbers 99401114 (Côte d’Ivoire), 14849830 (Kenya Sm1), 16755535 (Kenya Sm2), 95819193 (Tanzania), 32045736 (Niger), and 14117624 (Mozambique).
Results
Eligibility surveys
Investigators in the gaining control studies (Fig. 1a) had to test between 150 and 320 villages to identify the 150 eligible villages; for sustaining studies, between 150 and 263 villages were surveyed to find 75 eligible villages (Table 1). The most common reason for excluding villages was that the prevalence among 13- to 14-year-old children was out of range. In addition, 20 villages in the Tanzania gaining studies were excluded because they had high rates (>10 %) of mixed S. haematobium and S. mansoni infections. Based on the added complexity of analysis and significant increases in funding required for testing to include areas with predominantly mixed infections, such settings were considered beyond the scope of the current SCORE study design.
Year 1 surveys
Studies of gaining control (Kenya, Tanzania, Mozambique and Niger)
Studies of gaining control (Fig. 1b) in S. mansoni areas bordering Lake Victoria in the Kisumu region of western Kenya and Mwanza region of Tanzania included 11,541 and 14,620 children aged 9–12 years, respectively (Table 4). The prevalence of S. mansoni infection among children aged 9–12 years was 62.7 % in Kenya and 55.5 % in Tanzania. Among children aged 9–12 years, 7.3 % of villages in Kenya and 20.3 % of villages in Tanzania had a prevalence less than 25 %, despite prevalence ≥25 % in the eligibility survey. Numbers of children between 9 and 12 year of age enrolled per village ranged from 12 to 101 in Kenya, and from 11 to 122 in Tanzania. There were no significant differences in prevalence of infection or intensity among 9- to 12-year-old children by study arm in either country (Fig. 2).
Table 4.
Numbers of participants and prevalence in the year 1 survey, by study type and country
| Age group | Variable | Study - country | ||||||
|---|---|---|---|---|---|---|---|---|
| Sm2 - Kenya | Sm2 - Tanzania | Sh2 – Mozambique | Sh2 – Niger | Sm1 - Côte d’Ivoire | Sm1 - Kenya | Sh1 - Niger | ||
| 5–8 years | Number enrolled | 4,725 | 12,359 | 7,463 | 13,553 | 4,812 | 1,609 | 6,667 |
| Prevalence (%) | 35.7 | 38.5 | 63.1 | 24.0 | 5.3 | 5.8 | 3.3 | |
| 9–12 years | Number enrolled | 11,541 | 14,620 | 7,317 | 14,249 | 7,410 | 4,614 | 6,682 |
| Prevalence (%) | 62.7 | 55.5 | 66.6 | 21.3 | 20.9 | 17.7 | 4.2 | |
| Adults (20–55 years) | Number enrolled | 7,107 | 4,922 | 4,259 | 7,041 | N/A | N/A | N/A |
| Prevalence (%) | 44.7 | 28.1 | 44.8 | 11.3 | N/A | N/A | N/A | |
| Total | Number enrolled | 23,373 | 31,901 | 19,039 | 34,843 | 12,222 | 6,223 | 13,349 |
N/A not assessed, Sh1 sustaining control study in S. haematobium endemic villages, Sh2 gaining control study in S. haematobium endemic villages, Sm1 sustaining control study in S. mansoni endemic villages, Sm2 gaining control study in S. mansoni endemic villages
Fig. 2.

Baseline infection prevalence and intensity for gaining control of schistosomiasis studies, by study arm. Figures depict box plots. Horizontal lines in box interiors indicate medians. Box lengths represent the interquartile range (i.e. amount of data between the 75th and 25th percentile), + signs in boxes represent mean infection intensity (in eggs per gram of faeces (for S. mansoni) or per 10 ml of urine (for S. haematobium)) or prevalence in the respective arms 1–6 and the whiskers represent the minimum and maximum infection prevalence or intensity. Sh2, gaining control study in S. haematobium villages; Sm2, gaining control study in S. mansoni endemic villages
Studies of gaining control of S. haematobium in Cabo Delgado province of northern Mozambique and the Dosso and Tillaberi regions of western Niger enrolled 7317 and 14,249 children aged 9–12 years, respectively (Table 4). The prevalence of S. haematobium infection was 66.6 % in Mozambique and 21.3 % in Niger. Approximately 5.3 % of villages in Mozambique and 67.3 % in Niger had prevalence less than 25 % in this age group, despite having had a prevalence ≥25 % among 13–14 year olds in the eligibility survey. The number of 9- to 12-year-old children enrolled per village ranged from 10 to 139 and from 51 to 149 in Mozambique and Niger, respectively. Both the prevalence and intensity of infection were similar among study arms (Fig. 2) in Mozambique. However, the S. haematobium infection prevalence was significantly different by study arms in Niger (p <0.001) (Fig. 2). Infection prevalence was lower among children aged 5–8 years and among adults, compared to children aged 9–12 years, in Kenya, Tanzania, Mozambique and Niger. However, in Niger Sh2 (Table 4), infection prevalence was slightly higher among younger children (24.0 %) than among those aged 9–12 years (21.3 %).
Studies of sustaining control (Côte d’Ivoire, Kenya and Niger)
Studies of sustaining control in S. mansoni areas (Fig. 1b) included 7410 children aged 9–12 from Région des Montagnes and Région du Moyen Cavally in western Côte d’Ivoire and 4614 from Kenya (Table 4). The prevalence among these children was 20.9 % in Côte d’Ivoire and 17.7 % in western Kenya, near Lake Victoria, in an area distinct from that where the gaining control study was being conducted. In the baseline data on 9- to 12-year-old children, 43 % (n = 32) of villages in Côte d’Ivoire and 39 % (n = 29) in Kenya had year 1 prevalence outside the desired range of 10–24 %. Nine (12 %) Ivorian and 14 (19 %) Kenyan villages had infection prevalence <10 % and 23 (31 %) Ivorian and 15 (20 %) Kenyan villages had infection prevalence ≥25 %. The number of children aged 9–12 years enrolled per village ranged from 77 to 115 in Côte d’Ivoire and from 26 to 123 in Kenya. There were no significant differences in prevalence or intensity of S. mansoni infection by study arm in either country (Fig. 3). Adults were not recruited into studies of sustaining control.
Fig. 3.
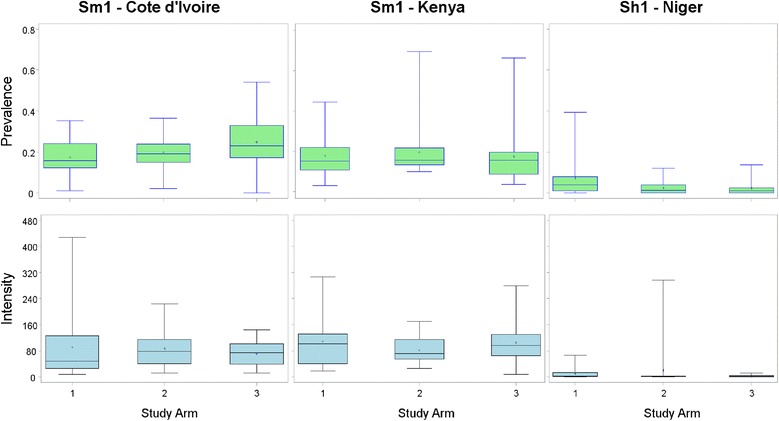
Infection prevalence and intensity for sustaining control of schistosomiasis studies, by study arm. Figures depict box plots. Horizontal lines in box interiors indicate medians. Box lengths represent the interquartile range (i.e. amount of data between the 75th and 25th percentile), + signs in boxes represent mean infection intensity (eggs per gram of faeces (for S. mansoni) or eggs per 10 ml of urine (for S. haematobium)) or prevalence in the respective arms 1–3 and the whiskers represent the minimum and maximum infection prevalence or intensity. Sh1, sustaining study in S. haematobium endemic villages; Sm1, sustaining study in S. mansoni endemic village
Sustaining control studies of S. haematobium in Niger enrolled 6682 children aged 9–12 years from the Dosso and Tillaberi regions of western Niger, with a prevalence of infection of 4.2 % (Table 4). About 92 % of villages in Niger had year 1 prevalence outside the desired range of 10–24 %, mostly less than 10 %. The number of children enrolled per village ranged from 24 to 102 with 46 (61 %) of villages achieving the protocol enrollment target of 100. There were no significant differences in prevalence and intensity of S. haematobium infection by study arm in Niger (Fig. 3) (p = 0.122 and p = 0.111, respectively). Prevalence of infection among children aged 5–8 years was 3.3 % (Table 4).
Discussion
The SCORE gaining and sustaining control studies are large operational intervention studies, involving complex coordination among multiple investigators from many countries, between research projects and national schistosomiasis control programmes (often integrated within a broader NTD master plan), and among national and subnational governmental levels and in-country community-based and non-governmental organisations. In year 1 alone, the project enrolled 66,433 children aged 9–12 years across 825 villages in five countries, in addition to large numbers of younger children (5–8 years) and adults (20–55 years). Despite these large numbers, enrolment in the year 1 study was somewhat lower than the expected 82,500. Possible reasons for this discrepancy include low school enrolment, lack of parental consent, limited pre-study sensitization in some areas, and flooding, political unrest and other contextual issues extraneous to the study. In addition to the logistical challenges of visiting 75 or 150 villages in each study, consenting and enrolling large numbers of people, and the demands of laboratory testing were considerable. The gaining and sustaining control surveys of S. mansoni were particularly difficult, requiring duplicate assessment of three consecutive stool specimens from children in the 9–12 year age group, as well as duplicate testing of a single stool specimen in young children and adults. For example, in the first year alone 214,122 Kato-Katz thick smears were read for the 38,185 enrolled children aged 9–12 years. Technicians read an additional 77,351 Kato-Katz thick smears from the 35,555 enrolled young children and adults. By design, the number of stools used for infection assessment differed by age-group and may in part explain the difference in the prevalence of infection between age-groups as previously reported [31].
The SCORE gaining and sustaining schistosomiasis control project made a dedicated effort as part of study planning activities to identify eligible villages with sufficient numbers of children for the respective studies. In all countries but Mozambique, many more villages had to be visited than anticipated to identify the number of potentially eligible villages for this study. This suggests that the historical data used to identify study areas was outdated or unreliable, which might be partially explained by escalating control efforts [32]. Despite eligibility survey prevalence in 13- to 14-year-old children that met study criteria (range: 10–24 %), on testing in year 1 of the actual study, 57.8 % of villages of eligible villages in the sustaining studies had prevalence below 10 % or above 24 % among children aged 9–12 years. In the gaining control studies, 26 % of villages with prevalence ≥25 % in the eligibility survey had prevalence levels <25 % in 9- to 12-year-old children in year 1 of the study. Possible explanations for the discordance between the eligibility and the year 1 survey results include that (i) the prevalence of Schistosoma infection in children aged 13–14 years was substantially higher than that among 9- to 12-year-old children in the same communities; (ii) differences in sampling methodology between eligibility and study sample stool assessments; and (iii) possible random inclusion of atyptical sample of 13- to 14-year-old children with high infection burden in the eligibility survey but infection level among children in the study sample were more typical in the 9- to 12-year-old children leading to regression of average infection levels towards the mean.
The harmonized protocol for gaining and sustaining control of schistosomiasis was successfully implemented in four of the five funded countries. Niger did not allocate interventions to villages as required by the protocol, but instead grouped study villages by their proximity to one another – three groups in the sustaining study and six in the gaining study. These geographically clustered groups were then randomly assigned to study arms. The differences in prevalence and intensity in the arms of the gaining study is a result of this failure to properly randomise at the village level.
Because the data being collected by the Niger team could not be used to answer the overarching gaining and sustaining questions, new research questions and a new study protocol were devised three years into the investigation, to compare the benefits of twice a year PCT compared with once a year (Fig. 4). In year 3, villages within each of the original study arms were randomly assigned to receive PZQ treatment either once or twice a year in an effort to make use of the two years of data collection and intervention that had already occurred.
Fig. 4.

Revised study design for Niger. CWT, community-wide treatment; SBT, school-based treatment
Harmonization of data collection and management efforts across study teams and regions proved a formidable challenge. Although SCORE provided a list of variables to be collected and put forth specific forms, for example, for village inventory data, these were not universally followed. SCORE had planned to use mobile data collection and centralised data storage to ensure consistency; however, this was more difficult than anticipated. The expected adaptation of the software and use of the same mobile data collection tools used in lymphatic filariasis studies was not possible due to the extensive reprogramming required to adapt the software and to the evolving and rapid changes in the tools used for mobile data collection. SCORE partnered with Imperial College London to develop an application of their EpiCollect® software, but this took longer than expected and was not ready to be implemented by all sites in time for year 1 data collection. Since then, use of mobile devices for NTD programme data collection has expanded and advanced, and the ease of developing applications has increased markedly. Mobile systems (either EpiCollect or the LINKS system) are currently being used by four of the five study countries. Systems for data cleaning and submission to SCORE have been standardised, with data cleaned in-country, then submitted to SCORE, where they are reviewed and put into a standardised format (i.e. SCORE uniform data set (SUDS)). The uniform data sets are then returned to the originating country PIs, and can also be combined for multi-country analyses, as in the current paper.
We anticipate that the optimal PCT strategy for a given region may, in part, depend on the starting prevalence and intensity of infection and contextual local factors that must be carefully considered in the adoption for schistosomiasis control. The results of these gaining and sustaining studies will provide data-driven decision frameworks for national NTD control programme managers, as well as what should be an invaluable source for researchers and mathematical modellers. The infrastructure from this research laid by the SCORE programme has already contributed to important spin-off efforts, including schistosome infection modelling [33–36]. Of note, SCORE-related efforts or support have already resulted in more than 40 peer-reviewed publications and one non peer-reviewed white paper (all publications and the white paper can be accessed via the SCORE website, available on: http://www.score.uga.edu).
Conclusion
World Health Assembly (WHA) resolution number 65.21 of March 2012 encourages the world to move towards elimination of schistosomiasis. Although the gaining and sustaining studies described here mainly focus on morbidity control by carefully examining different PCT schemes with PZQ, it is widely agreed that to achieve the reductions in prevalence and intensity needed to approach the goal of breaking transmission (i.e. elimination), enhanced PCT such as more frequent dosing than once a year, additional interventions, such as snail control, improvement in water, sanitation and hygiene (WASH), setting-specific information, education and communication (IEC), paediatric PZQ formulations and, possibly, drugs that more effectively kill adult worms and treat juvenile worms, are necessary [8, 37–44]. The results of these gaining and sustaining studies, however, will provide strategic information about how best to implement PCT and use treatment-related resources in countries with moderate and high prevalence of infection at the onset of multi-year treatment interventions.
Abbreviations
CDC, centers for disease control and prevention; CWT, community-wide treatment; DALY, disability-adjusted life year; IEC, information, education and communication; IRB, institutional review board; KEMRI, Kenyan Medical Research Institute; NIMR, National Institute for medical research; NTD, neglected tropical disease; PCT, preventive chemotherapy; PI, principal investigator; PZQ, Praziquantel; RAP, rapid answers project; SAC, school-aged children; SBT, school-based treatment; SCI, schistosomiasis control initiative; SCORE, Schistosomiasis Consortium for Operational Research and Evaluation; Sh1, Schistosoma haematobium sustaining control study; Sh2, Schistosoma haematobium gaining control study; Sm1, Schistosoma mansoni sustaining control study; Sm2, Schistosoma mansoni gaining control study; SOP, standard operating procedure; SUDS, SCORE uniform data set; UGA, University of Georgia; WASH, water, sanitation and hygiene; WHA, World Health Assembly; WHO, World Health Organization
Funding
These studies received financial support from University of Georgia Research Foundation, Inc., which was funded by the Bill & Melinda Gates Foundation for the SCORE project. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
All data supporting our findings are already contained within this manuscript.
Authors’ contributions
Each author contributed to data interpretation and manuscript development, critique and revision for important intellectual content by providing comment, reading, editing and approving the final version of the manuscript. AEE, CLH, YS, XPY, SCB, CHC, SR, CCW, EKN, JU, AO, PM, SK, AF, AP, JF, DMSK, PNMM, SM, WES, AH, AG, CHK and DGC.
Competing interests
The authors declare that they have no competing interests.
Consent to publish
Not applicable.
Ethics and consent to participate
Written informed consent was obtained from adults (including parents/legal guardians of children in the study) and assent was obtained from children less than 18 years old, except in places where village-level consent is the standard, in which case local requirements were met. Ethical review of research protocol was implemented by human subjects committee in each African country and by the institutional review board (IRB) of their respective northern partners. Sm1 and Sm2 studies in Kenya were reviewed and approved by the National Ethics Review Committee of the Kenyan Medical Research Institute (KEMRI; approval numbers SCC 1800 and SCC 1820, respectively) and by the IRB of the Centers for Disease Control and Prevention (CDC; approval #: 1661). For the Sm1 study in Côte d’Ivoire, ethical approval was obtained from the ethics committees in Côte d’Ivoire (reference no. 1994MSHP/CNER) and Basel (reference no. EKBB 279/10). In Niger, ethical approval was obtained from the Niger Republic National Consulate for ethical review (reference no. 012/2010/CCNE) and from the Imperial College Research Ethic Committee (ICREC_8_2_2). In Mozambique, ethical approval was received from the Ministry of Health (reference no. 235/CNBS/10) and the Imperial College Research Ethic Committee (ICREC_10_8_2). In Tanzania, ethical approval was obtained from the National Institute for Medical Research (NIMR; reference no. NIMR/HQ/R.8a/Vol. IX/1022). In addition to these, the UGA IRB implemented an administrative human subjects review and issued additional approval per country’s protocol as follows: 10021–0, 10221–0, 10267–0, 10353–0, 10431–0 and 10533–0 for Côte d’Ivoire, Kenya Sm1, Kenya Sm2, Tanzania, Niger and Mozambique, respectively.
The trials have been registered with the International Standard Randomised Controlled Trial registry under ISRCT numbers 99401114 (Côte d’Ivoire), 14849830 (Kenya Sm1), 16755535 (Kenya Sm2), 95819193 (Tanzania), 32045736 (Niger), and 14117624 (Mozambique).
Data analysis
CLH implemented data analysis, in collaboration with AEE, YS and SR who contributed to analysis.
Study design/conceptualization
The SCORE project was designed and conceptualised by a team of United States and international collaborators: DGC, SB, CHC, EKN, JU, AO, PM, SK, AF, AP, JF, DMSK, PNMM, SM, WES, AH, AG and CHK.
Disclaimers
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
References
- 1.Colley DG. Morbidity control of schistosomiasis by mass drug administration: How can we do it best and what will it take to move on to elimination? Trop Med Health. 2014;42(2 Suppl):25–32. doi: 10.2149/tmh.2014-S04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coulibaly JT, Knopp S, N’Guessan NA, Silué KD, Fürst T, Lohourignon LK, et al. Accuracy of urine circulating cathodic antigen (CCA) test for Schistosoma mansoni diagnosis in different settings of Côte d’Ivoire. PLoS Negl Trop Dis. 2011;5(11):e1384. doi: 10.1371/journal.pntd.0001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colley DG, Binder S, Campbell C, King CH, Tchuem Tchuenté LA, N’Goran EK, et al. A five-country evaluation of a point-of-care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. Am J Trop Med Hyg. 2013;88(3):426–32. doi: 10.4269/ajtmh.12-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stothard JR, Stanton MC, Bustinduy AL, Sousa-Figueiredo JC, Van Dam GJ, Betson M, et al. Diagnostics for schistosomiasis in Africa and Arabia: a review of present options in control and future needs for elimination. Parasitology. 2014;141(14):1947–61. doi: 10.1017/S0031182014001152. [DOI] [PubMed] [Google Scholar]
- 5.Knopp S, Corstjens PL, Koukounari A, Cercamondi CI, Ame SM, Ali SM, et al. Sensitivity and specificity of a urine circulating anodic antigen test for the diagnosis of Schistosoma haematobium in low endemic settings. PLoS Negl Trop Dis. 2015;9(5):e0003752. doi: 10.1371/journal.pntd.0003752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glenn TC, Lance SL, McKee AM, Webster BL, Emery AM, Zerlotini A, et al. Significant variance in genetic diversity among populations of Schistosoma haematobium detected using microsatellite DNA loci from a genome-wide database. Parasit Vectors. 2013;6(1):300. doi: 10.1186/1756-3305-6-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King CH, Bertsch D. Historical perspective: snail control to prevent schistosomiasis. PLoS Negl Trop Dis. 2015;9(4):e0003657. doi: 10.1371/journal.pntd.0003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knopp S, Mohammed KA, Ali SM, Khamis IS, Ame SM, Albonico M, et al. Study and implementation of urogenital schistosomiasis elimination in Zanzibar (Unguja and Pemba islands) using an integrated multidisciplinary approach. BMC Public Health. 2012;12:930. doi: 10.1186/1471-2458-12-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knopp S, Becker SL, Ingram KJ, Keiser J, Utzinger J. Diagnosis and treatment of schistosomiasis in children in the era of intensified control. Expert Rev Anti Infect Ther. 2013;11(11):1237–58. doi: 10.1586/14787210.2013.844066. [DOI] [PubMed] [Google Scholar]
- 10.Assaré RK, Knopp S, N’Guessan NA, Yapi A, Tian-Bi YN, Yao PK, et al. Sustaining control of schistosomiasis mansoni in moderate endemicity areas in western Côte d’Ivoire: a SCORE study protocol. BMC Public Health. 2014;14:1290. doi: 10.1186/1471-2458-14-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagan P, Chandiwana S, Ndhlovu P, Woolhouse MEJ, Dessein A. The epidemiology, immunology and morbidity of Schistosoma haematobium infections in diverse communities in Zimbabwe. I. The study design. Trop Geogr Med. 1994;46(4 Spec No):227–32. [PubMed] [Google Scholar]
- 12.Vennervald BJ, Ouma JH, Butterworth AE. Morbidity in schistosomiasis: assessment, mechanisms and control. Parasitol Today. 1998;14(10):385–90. doi: 10.1016/S0169-4758(98)01311-8. [DOI] [PubMed] [Google Scholar]
- 13.Woolhouse MEJ, Mutapi F, Ndhlovu PD, Chandiwana SK, Hagan P. Exposure, infection and immune responses to Schistosoma haematobium in young children. Parasitology. 2000;120(Pt 1):37–44. doi: 10.1017/S0031182099005156. [DOI] [PubMed] [Google Scholar]
- 14.Kabatereine NB, Kemijumbi J, Ouma JH, Kariuki HC, Richter J, Kadzo H, et al. Epidemiology and morbidity of Schistosoma mansoni infection in a fishing community along Lake Albert in Uganda. Trans R Soc Trop Med Hyg. 2004;98(12):711–8. doi: 10.1016/j.trstmh.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Salam RA, Maredia H, Das JK, Lassi ZS, Bhutta ZA. Community-based interventions for the prevention and control of helmintic neglected tropical diseases. Infect Dis Poverty. 2014;3:23. doi: 10.1186/2049-9957-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365(9470):1561–9. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 17.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6(7):411–25. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 18.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383(9936):2253–64. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 20.WHO. Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva: World Health Organization; 2006. In. World Health Organization; 2006.
- 21.Brady MA, Hooper PJ, Ottesen EA. Projected benefits from integrating NTD programs in sub-Saharan Africa. Trends Parasitol. 2006;22(7):285–91. doi: 10.1016/j.pt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Toure S, Zhang Y, Bosque-Oliva E, Ky C, Ouedraogo A, Koukounari A, et al. Two-year impact of single praziquantel treatment on infection in the national control programme on schistosomiasis in Burkina Faso. Bull World Health Organ. 2008;86(10):780–7. doi: 10.2471/BLT.07.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabatereine NB, Malecela M, Lado M, Zaramba S, Amiel O, Kolaczinski JH. How to (or not to) integrate vertical programmes for the control of major neglected tropical diseases in sub-Saharan Africa. PLoS Negl Trop Dis. 2010;4(6):e755. doi: 10.1371/journal.pntd.0000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King CH, Olbrych SK, Soon M, Singer ME, Carter J, Colley DG. Utility of repeated praziquantel dosing in the treatment of schistosomiasis in high-risk communities in Africa: a systematic review. PLoS Negl Trop Dis. 2011;5(9):e1321. doi: 10.1371/journal.pntd.0001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanson C, Weaver A, Zoerhoff KL, Kabore A, Linehan M, Doherty A, et al. Integrated implementation of programs targeting neglected tropical diseases through preventive chemotherapy: identifying best practices to roll out programs at national scale. Am J Trop Med Hyg. 2012;86(3):508–13. doi: 10.4269/ajtmh.2012.11-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assaré RK, Hürlimann E, Ouattara M, N’Guessan NA, Tian-Bi YN, Yapi A, et al. Sustaining the control of schistosoma mansoni in Western Côte d’Ivoire: baseline findings before the implementation of a randomized trial. Am J Trop Med Hyg. 2015. [DOI] [PMC free article] [PubMed]
- 27.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14(6):397–400. [PubMed] [Google Scholar]
- 28.Peters PAS, Kazura JW. Update on diagnostic methods for schistosomiasis. In: Mahmoud AA, editor. Balliere’s clinical tropical medicine and communicable diseases, schistosomiasis. Volume. London: Bailliere Tindall; 1987: 419–433.
- 29.WHO. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level: a guide for managers of control programmes. Geneva: World Health Organization; 1998. p. 45.
- 30.King CH, Bertsch D. Meta-analysis of urine heme dipstick diagnosis of Schistosoma haematobium infection, including low-prevalence and previously-treated populations. PLoS Negl Trop Dis. 2013;7(9):e2431. doi: 10.1371/journal.pntd.0002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen A, Kinung’hi S, Magnussen P. Schistosoma mansoni infection along the coast of Lake Victoria in Mwanza region, Tanzania. Am J Trop Med Hyg. 2015;92(6):1240–4. doi: 10.4269/ajtmh.14-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Organization WH. Schistosomiasis: number of people treated worldwide in 2013. Wkly Epidemiol Rec. 2015;90(5):25–32. [PubMed] [Google Scholar]
- 33.Wang X, Gurarie D, Mungai PL, Muchiri EM, Kitron U, King CH. Projecting the long-term impact of school- or community-based mass-treatment interventions for control of Schistosoma infection. PLoS Negl Trop Dis. 2012;6(11):e1903. doi: 10.1371/journal.pntd.0001903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurarie D, King CH. Population biology of Schistosoma mating, aggregation, and transmission breakpoints: more reliable model analysis for the end-game in communities at risk. PLoS One. 2014;9(12):e115875. doi: 10.1371/journal.pone.0115875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King CH. Health metrics for helminth infections. Acta Trop. 2015;141(Pt B):150–60. doi: 10.1016/j.actatropica.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olliaro PL, Vaillant M, Diawara A, Coulibaly JT, Garba A, Keiser J, et al. Toward measuring schistosoma response to praziquantel treatment with appropriate descriptors of egg excretion. PLoS Negl Trop Dis. 2015;9(6):e0003821. doi: 10.1371/journal.pntd.0003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aagaard-Hansen J, Mwanga JR, Bruun B. Social science perspectives on schistosomiasis control in Africa: past trends and future directions. Parasitology. 2009;136(13):1747–58. doi: 10.1017/S0031182009006404. [DOI] [PubMed] [Google Scholar]
- 38.Liese B, Rosenberg M, Schratz A. Programmes, partnerships, and governance for elimination and control of neglected tropical diseases. Lancet. 2010;375(9708):67–76. doi: 10.1016/S0140-6736(09)61749-9. [DOI] [PubMed] [Google Scholar]
- 39.Utzinger J, N’Goran EK, Caffrey CR, Keiser J. From innovation to application: social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 2011;120(Suppl 1):S121–37. doi: 10.1016/j.actatropica.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 40.Freeman MC, Ogden S, Jacobson J, Abbott D, Addiss DG, Amnie AG, et al. Integration of water, sanitation, and hygiene for the prevention and control of neglected tropical diseases: a rationale for inter-sectoral collaboration. PLoS Negl Trop Dis. 2013;7(9):e2439. doi: 10.1371/journal.pntd.0002439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuenté LA, Garba A, et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128(2):423–40. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Spear RC. Commentary by Spear, R. on “Integration of water, sanitation, and hygiene for the prevention and control of neglected tropical diseases: a rationale for inter-sectoral collaboration:” can the control of NTDs profit from a good WASH? PLoS Negl Trop Dis. 2013;7(9):e2473. doi: 10.1371/journal.pntd.0002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grimes JE, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The relationship between water, sanitation and schistosomiasis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8(12):e3296. doi: 10.1371/journal.pntd.0003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grimes JE, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The roles of water, sanitation and hygiene in reducing schistosomiasis: a review. Parasit Vectors. 2015;8:156. doi: 10.1186/s13071-015-0766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting our findings are already contained within this manuscript.


