Abstract
Phytoliths can occlude some organic carbon during their deposition in plants. This carbon fraction is recognised as an ideal dating material because of its high resistance to decomposition and post-deposition contamination at the time of phytolith formation. However, the reliability of phytolith radiocarbon dating has recently been questioned. The development of a new extraction protocol for phytoliths, with paired dating between phytoliths and other materials from the same sediment, may provide further evidence for the reliability of phytolith dating. We present an improved method for extracting phytoliths from soils. We compared the dating of phytoliths and other materials (e.g., charcoal and plant seeds) recovered at the same depth from seven pits at six archaeological sites in China. The estimated ages of the phytoliths and other materials were generally consistent, except for one outlier. We attribute this inconsistency to the post-depositional processes of phytoliths in soil, rather than to the uptake of old carbon from the soil. Our results clearly show the potential for phytolith carbon dating at archaeological sites in the absence of other dating materials.
Radiocarbon dating has proven to be a powerful tool for reliably obtaining the ages of past events recorded in sediments and archaeological sites during the late Quaternary period. However, the selection of materials has a profound effect on the quality of radiocarbon dating1. Wood, plant residue, and charcoal are generally accepted as robust dating materials because of their homogeneity and relatively good preservation2. However, these remains are often absent from many sedimentary archives and archaeological sites. Consequently, it is necessary to identify alternative materials that might enable reliable and effective dating.
Phytoliths(SiO2·nH2O) are non-crystalline minerals that are deposited within the cells and cell walls in various parts of plants3. Some organic carbon of plant origin is occluded by phytoliths during their deposition4,5. When the plant dies and decays, this carbon fraction, encapsulated by silica, can survive for long periods due to the phytolith’s high resistance to decomposition. Phytolith-occluded carbon (PhytOC) has been demonstrated to be an important form of carbon sequestration6,7,8,9. Because PhytOC is usually taken to be a product of photosynthesis, it has been used to reconstruct C3/C4 plants of the past10,11,12, for paleo-CO2 concentration13, and in radiocarbon dating tests4,14,15,16,17,18.
The earliest radiocarbon dating studies using phytoliths were carried out by Jones and Beavers19 and Wilding et al.14. They investigated the potential of PhytOC for radiocarbon analysis, and found that the measurements obtained using phytoliths were older than those expected sediments developed from the soil. Studies on phytolith dating of lake, terrestrial soil sediments, and archaeological sites showed good agreement between phytolith dating and methods utilizing other dating materials4,16,17,20,21,22,23. However, in a small number of studies, phytolith dating was attempted but was unsuccessful because no expected phytoliths ages were retrieved1,24. A few studies attributed this distortion of phytolith dating to old carbon absorbed from soils25,26,27. Further testing of phytolith dating at archaeological sites is required to confirm whether or not phytolith dating can be influenced by the carbon content of old soils.
In this study, we collected palaeosoil samples from pits dug at archaeological sites in China. A new, improved method was developed to extract phytoliths from soils. Scanning electron microscopy–energy-dispersive X-ray spectroscopy (SEM-EDS) analysis was performed to check the purity of concentrated phytoliths. Then, the pure phytolith and other dating materials were dated by accelerator mass spectrometry. Finally, phytolith dating was compared with dating results obtained using other materials (charcoal, plant residue) recovered from the same pit depth or cultural layer.
Materials and Method
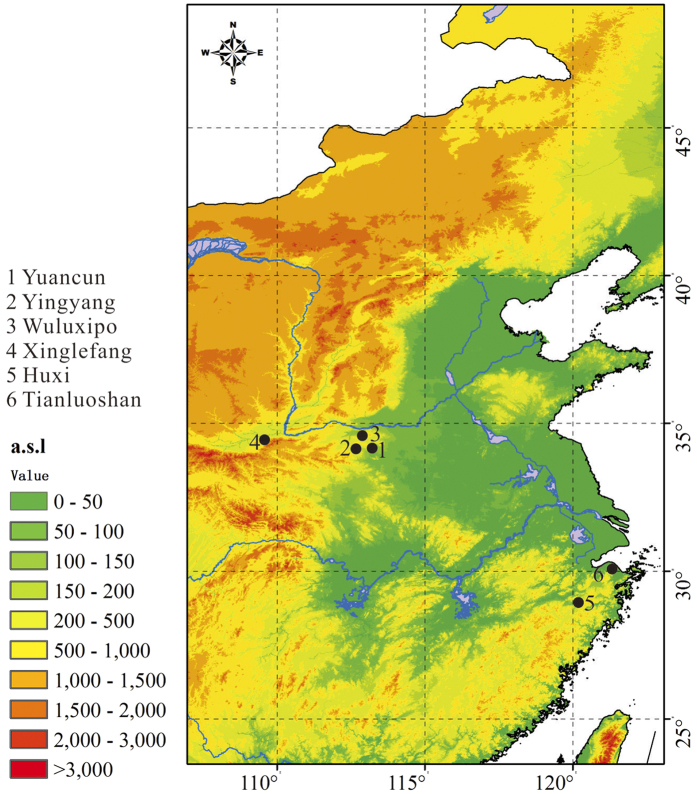
Fourteen samples were collected from six archaeological sites. Soil and charcoals or seeds were simultaneously selected at the same depth from the pits and cultural layers. The Tianluoshan and Huxi sites are located in Zhejiang province, southeastern China. The Yingyang, Yuancun, and Wuluoxipo sites are located in Henan province, central China. The Xinglefang site is located in Shanxi province, western China (Fig. 1). Wuluoxipo is attributed to the Peiligang culture (7000–5000 BCE). Yingyang and Yuancun are attributed to the Yangshao culture (5000–3000 BCE). Xinglefang is attributed to the Miaodigou culture (3900–3600 BCE). Huxi and Tianluoshan are attributed to the Shangshan (8000–5500 BCE) and Hemudu (5000–4000 BCE) cultures, respectively (Table 1)28.
Figure 1. Locations of archaeological sites.
The figure was generated using GRASS GIS 7.0.3: https://grass.osgeo.org/.
Table 1. Sites, locations, and weights of selected samples.
| Archaeological sites | Sample code | Location | Cultural period | Other dating materials | Soil dry weight (g) |
|---|---|---|---|---|---|
| Yuancun | YC | Henan province | Yangshao | Charcoal | 25.024 |
| Yingyang | YY | Henan province | Yangshao | Charcoal | 135.645 |
| Wuluoxipo | WLXP | Henan province | Peiligang | Charcoal | 137.222 |
| Xinglefang | XLF | Shanxi province | Miaodigou | Charcoal | 80.16 |
| Huxi | HX | Zhejiang province | Shangshan | Plant residue | 102.4 |
| Tianluoshan | TLS-2 | Zhejiang province | Hemudu | Seeds | 70.513 |
| Tianluoshan | TLS-3 | Zhejiang province | Hemudu | Seeds | 56.75 |
A modified wet oxidation method was used for extracting phytoliths from soil3,13,29,30. The detailed steps are as follows: (a) Dry soil was crushed and sieved at 500 μm; (b) The sample was deflocculated with 5% sodium polyphosphates, and then washed three to four times with distilled water; (c) Organic matter was first oxidised by 250 ml of H2O2 (30%) for 12 h at room temperature and then heated in a water bath until the reaction stopped; (d) Carbonates were eliminated using 200 ml of HCl (10%) with heating for 30 min; (e) The >250 μm fraction was separated by wet sieving, and the remaining sample was disaggregated from the organic material and clay by ultrasonic treatment for 20 min; (f) Clays (<5 μm) were removed by gravity sedimentation until the sample was clear; (g) The remaining higher-resistance materials were oxidised by 200 ml of HNO3 and pinches of KClO3 with heating for 1 h, and were then centrifuged and decanted; (h) Phytoliths were extracted three times by 200 ml of heavy liquid (ZnBr2) with a specific density of 2.35 g/cm3 and then washed three times with distilled water; (i) Extracted phytoliths were further sieved at 7 μm to remove clay. Then, the recovered part of remains in the sieve were treated by 20 ml of H2O2 (30%) in the tube for 20 min; (j) Finally, the recovered phytoliths were dried at 60 °C for 24 h prior to testing.
The phytolith and most of the other materials were dated by Beta Analytic Lab, except for two plant samples from the Tianluoshan site, which were sent to the Peking University accelerator mass spectrometry (AMS) laboratory. The phytolith dating processes can generally be described by the following three steps: First, the sample is placed into a combustion vessel (quartz glass) and combusted at 1500 °C to generate CO2. The high temperature is necessary to melt the phytolith and ensure that all the carbon is combusted. Secondly, the CO2 is collected and converted to graphite. Finally, the graphite is measured by the accelerator mass spectrometer.
The purity of the phytoliths was checked by SEM-EDS analysis. This is recognised as a robust method for checking phytolith purity and has also been applied to evaluating routine extraction processes26,31. The steps were previously described by Corbineau et al.31. In this study, the extracted phytoliths were analysed using an SEM (LEO1450VP) in association with an EDS system (INCA ENERGY 300).
Results
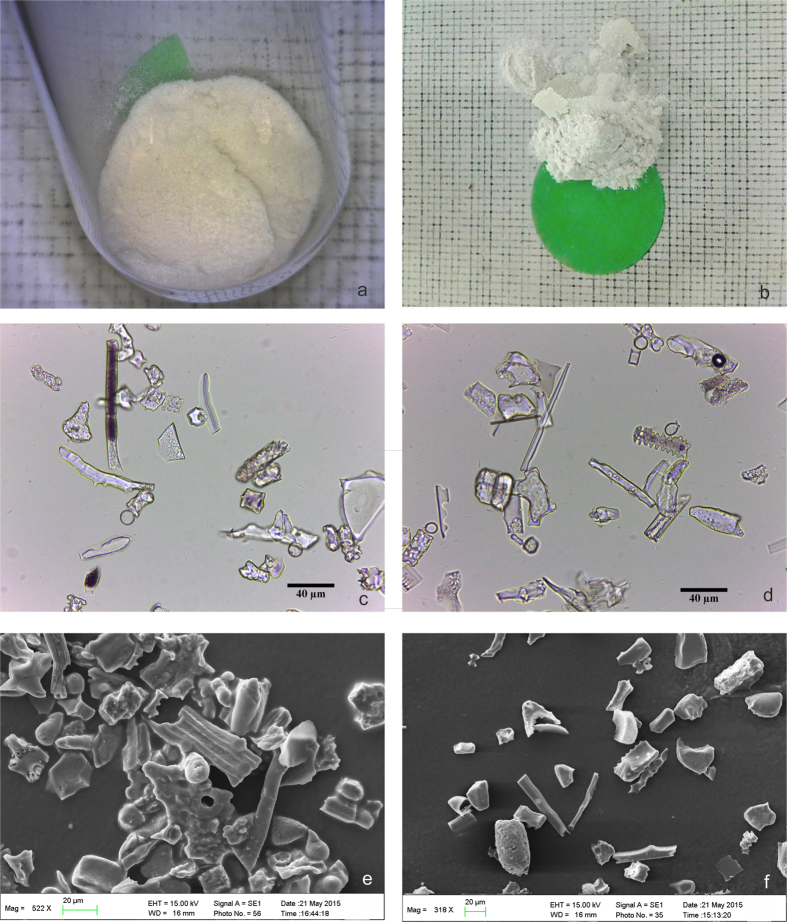
As shown in Fig. 2a,b, the extracted phytoliths appeared as white or grey-white. None of the charcoal or clays were observed with an optical microscope (Fig. 2c,d). The absence of extraneous organic materials was further checked by SEM-EDS analysis (Fig. 2e,f). Four micro-areas on the phytoliths were randomly selected for EDS analysis. The EDS spectrum showed two peaks caused by X-rays that were given off as electrons returning to the Si and O electron shells. The Si and O comprised more than 90% of the total mass, and the atomic ratio was nearly 2:1. Note that a few K atoms were detected in the EDS analysis of a micro-area; however, no C was found in the EDS spectra of the phytoliths.
Figure 2. Images of phytoliths extracted from soils:
(a,b) images of phytoliths; (c,d) optical microscopy images of phytoliths; (e,f) SEM images of phytoliths.
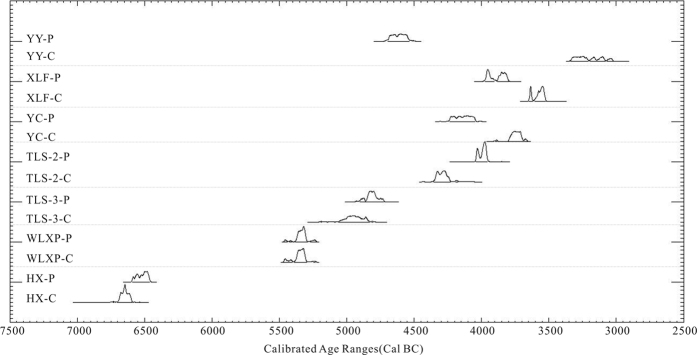
All conventional ages were calibrated to calendar years using Calib Rev 7.0.4 and the IntCal13 calibration curve32. The ages of the phytoliths were consistent with the cultural periods. Thus, all of the dating results were generally acceptable, regardless of which materials were being dated. In general, the phytolith dating results were concordant or similar to those of other materials, except for one sample from the YY site, which indicated an age 1000 years older than the charcoal date. The results listed in Table 2 can be categorised into three groups: (1) phytolith dating substantially consistent with the other materials within an error bar of 2σ (HX, WLXP, and TLS-3); (2) phytolith dating within ±200–300 years of dating with the other materials (XLF, YC, and TLS-2); and (3) phytolith dating was an outlier, and thousands of years older than the dating with other materials (YY). The detailed extracted phytoliths from soils, analysed phytoliths for combustion, graphite, and carbon yield rates are shown in Table S1.
Table 2. AMS radiocarbon dating results with uncertainty ±2σ.
| Lab ID | Archaeological sites | Sample code | Dating materials | 13/12C Ratio | Conventional age (BP) | 2σ Calibration (Cal BC) |
|---|---|---|---|---|---|---|
| Beta-407469 | Huxi | HX-C | Plant remains | −25.9 0/00 | 7820 ± 30 | 6690–6595 |
| Beta-406654 | Huxi | HX-P | Phytolith | −25.7 0/00 | 7680 ± 30 | 6590–6460 |
| Beta-404827 | Wuluoxipo | WLXP-C | Char | −25.5 0/00 | 6360 ± 30 | 5460–5450 |
| Beta-404848 | Wuluoxipo | WLXP-P | Phytolith | −26.0 0/00 | 6350 ± 30 | 5370–5300 |
| BA07763 | Tianluoshan | TLS-3-C | Flatstalk bulrush | NA | 6045 ± 45 | 5060–4800 |
| Beta-409348 | Tianluoshan | TLS-3-P | Phytolith | −32.1 0/00 | 5940 ± 30 | 4895–4865 |
| BA08204 | Tianluoshan | TLS-2-C | Yagara bulrush seed | NA | 5430 ± 40 | 4200–4170 |
| Beta-409347 | Tianluoshan | TLS-2-P | Phytolith | −31.2 0/00 | 5180 ± 30 | 4040–3955 |
| Beta-392838 | Xinglefang | XLF-C | Char | −24.9 0/00 | 4800 ± 30 | 3645–3625 |
| Beta-409349 | Xinglefang | XLF-P | Phytolith | NA* | 5110 ± 30 | 3970–3910 |
| Beta-404835 | Yuancun | YC-C | Char | −25.2 0/00 | 4970 ± 30 | 3890–3885 |
| Beta-404844 | Yuancun | YC-P | Phytolith | −24.6 0/00 | 5310 ± 30 | 4240–4040 |
| Beta-404837 | Yingyang | YY-C | Char | −26.0 0/00 | 4470 ± 30 | 3340–3080 |
| Beta-404846 | Yingyang | YY-P | Phytolith | −24.40/00 | 5760 ± 40 | 4710–4500 |
*The original sample was too small to provide a δ13C on the original material. However, a ratio including both natural and laboratory effects was measured during 14C detection in order to calculate the true Conventional Radiocarbon Age.
Discussion
The extraction of pure phytolith content is of fundamental importance to radiocarbon dating. In our previous experiments, the conventional extraction methods that only involve H2O2 and HCl pretreatment were usually unable to exclude all exogenous organic materials and clays33. Thus, the ages of phytoliths were likely distorted when employing the conventional extraction method27. In this study, we developed three stages of sieving for our extraction protocol. Firstly, plant residues and roots are sieved at 500 μm. Secondly, macro-charcoal and micro-plant residues are sieved at 250 μm. Finally, extracted phytoliths are sieved at 7 μm to remove clay. Exogenous organic materials are excluded by H2O2 and acid. Rapid digestion (H2SO4 and H2O2) has previously been used for the extraction of phytoliths30,34. A recent study argued that rapid digestion was so harsh that it led to the consumption of carbon occluded in phytoliths35. Hence, we used HNO3/KClO3 rather than rapid digestion. This improved method is widely employed to extract soil phytoliths for isotopic analysis3, and has proven to be efficient for the removal of organic materials.
Based on Fig. 2, we conclude that extracted phytoliths vary in colour from white to grey-white. Exogenous organic materials and clays were not detected in the microscopic examination. EDS analysis indicated that Si and O were the main elements of the phytoliths. No carbon was found in the EDS results (Fig. 3). The analysis results verified the purity of the phytoliths extracted using the improved method.
Figure 3. EDS analysis of phytolith surface.
1 and 4 are EDS spectra of elongate phytoliths; 2 is EDS spectrum of acicular phytolith; 3 is EDS spectrum of square phytolith.
Table 2 and Fig. 4 show AMS radiocarbon dating of phytoliths and other materials. Three phytoliths dates partly or completely overlapped with the other materials within an uncertainty of 2σ, which confirmed their concordance. Three other phytolith dating results were slightly older or younger (<300 years) than those for other materials. A portion of the soil phytoliths was probably inherited from previous grasses, demonstrating a long deposition history of the soil phytoliths29,36. In this case, the soil phytolith dating results could only represent the mean yielded time of phytoliths. Due to their differing depositional processes in soils, phytoliths may have different ages from that of charcoal at the same soils profile depth18. When sampling a thick soil layer of 5–10 cm, a difference of hundreds of years between the dating results of soil phytoliths and other materials is generally acceptable. However, that does not account for the discrepancy of thousands of years, between the dates for one phytolith/non-phytolith pair obtained from one pit. The post-dispositional processes of phytoliths in archaeological pits might be considered for a possible explanation.
Figure 4. Calibrated two-sigma probability distributions for radiocarbon assays of phytoliths and other materials.
Post-depositional movements of phytoliths after depositing in pits have a fundamental effect on either the chronology or composition of phytoliths37,38. Phytoliths are subjected to translocation, bioturbation, and stratigraphic mixing processes after being incorporated into a soil18,39. Heavy translocation and extreme bioturbation may produce a phytolith pool that differs in chronology and composition even given the same soil profiles38. Based on the depositional processes described above, we believe that the discrepancy between the charcoal and phytolith dating results at the YY site is likely due to vertical translocation of the phytolith composition within the sequence.
In this study, the dating results of six phytolith samples were generally consistent with those of other dating materials, except for one sample from the pits of the YY site. Our results pose questions concerning the presence in phytoliths of old carbon taken up by plants from soils. Although discussion on this issue is ongoing25,40,41,42,43, it is probably not the most important factor for consideration, at least in prehistoric phytolith dating. Further data are required for deeper discussion on the issue of old carbon within phytoliths.
Conclusions
In this study, we present an improved method for extracting phytoliths from pits and cultural layers. The proposed method was employed to compare dating results obtained from phytoliths with those of other common materials at the same depth, for materials recovered from seven pits or cultural layers at six archaeological sites in China. We found that phytolith carbon dating could provide a reliable and accurate chronometer. The ages of soil phytoliths were generally consistent with those of other dating materials sampled at the same depths within the pits and cultural layers. We speculate that the observed inconsistencies can be attributed to the post-depositional processes of phytoliths. Our results do not support that phytolith dating could be distorted by the presence of old carbon, absorbed by plants from soils. However, we emphasise the importance of extracting pure phytolith from soils for dating tests.
Additional Information
How to cite this article: Zuo, X. et al. Radiocarbon dating of prehistoric phytoliths: a preliminary study of archaeological sites in China. Sci. Rep. 6, 26769; doi: 10.1038/srep26769 (2016).
Supplementary Material
Acknowledgments
We are grateful to Jeffrey F. Parr, and Zhaoyan Gu for their useful discussions that considerably improved the manuscript. This work was funded jointly by the National Natural Science Foundation of China (41230104, 41401230, and 41271226), the 973 Program (2015CB953801), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA05130602), and the China Postdoctoral Science Foundation-funded project (2014M561050).
Footnotes
Author Contributions Z.X. and L.H. co-wrote the main manuscript text. Z.X. conducted the experiments. Z.X. and Z.J. put forward the ideas of the study. W.C., S.G. and Z.Y. collected samples. All authors reviewed the manuscript.
References
- Boaretto E. Dating materials in good archaeological contexts: the next challenge for radiocarbon analysis. Radiocarbon 51, 275–281 (2009). [Google Scholar]
- Yates A. B., Smith A. M. & Bertuch F. Residue radiocarbon AMS dating review and preliminary sampling protocol suggestions. J. Archaeol. Sci. 61, 223–234, 10.1016/j.jas.2015.06.011 (2015). [DOI] [Google Scholar]
- Piperno D. Phytoliths: a comprehensive guide for archaeologists and paleoecologists. Ch.1, 5–21(AltaMira Press, Lanham MD, 2006).
- Parr J. F. & Sullivan L. A. Soil carbon sequestration in phytoliths. Soil Biol. Biochem. 37, 117–124, 10.1016/j.soilbio.2004.06.013 (2005). [DOI] [Google Scholar]
- Smith F. A. & Anderson K. B. Characterization of organic compounds in phytoliths: improving the resolving power of phytolith δ 13 C as a tool for palaeoecological reconstruction of C3 and C4 grasses, in Phytoliths: applications in earth sciences and human history (eds Meunier J. D. & Colin F), Ch. 4, 317–327 (A.A. Balkema Publishers, Lisse, 2001). [Google Scholar]
- Parr J. & Sullivan L. Phytolith occluded carbon and silica variability in wheat cultivars. Plant Soil 342, 165–171, 10.1007/s11104-010-0680-z (2011). [DOI] [Google Scholar]
- Parr J., Sullivan L., Chen B., Ye G. & Zheng W. Carbon bio-sequestration within the phytoliths of economic bamboo species. Global Change Biol. 16, 2661–2667 10.1111/j.1365-2486.2009.02118.x (2010). [DOI] [Google Scholar]
- Song Z., Müller K. & Wang H. Biogeochemical silicon cycle and carbon sequestration in agricultural ecosystems. Earth-Sci. Rev. 139, 268–278, 10.1016/j.earscirev.2014.09.009 (2014). [DOI] [Google Scholar]
- Song Z., Wang H., Strong P. J. & Guo F. Phytolith carbon sequestration in China’s croplands. Eur. J. Agron. 53, 10–15, 10.1016/j.eja.2013.11.004 (2014). [DOI] [Google Scholar]
- Stromberg C. A. E. & McInerney F. A. The Neogene transition from C3 to C4 grasslands in North America: assemblage analysis of fossil phytoliths. Paleobiology 37, 50–71, 10.1666/09067.1 (2011). [DOI] [Google Scholar]
- Hodson M. J., Parker A. G., Leng M. J. & Sloane H. J. Silicon, oxygen and carbon isotope composition of wheat (Triticum aestivum L.) phytoliths: implications for palaeoecology and archaeology. J. Quat. Sci. 23, 331–339, 10.1002/jqs.1176 (2008). [DOI] [Google Scholar]
- Krull E. S. et al. 13C-depleted charcoal from C4 grasses and the role of occluded carbon in phytoliths. Org. Geochem. 34, 1337–1352, 10.1016/S0146-6380(03)00100-1 (2003). [DOI] [Google Scholar]
- Carter J. A. Atmospheric carbon isotope signatures in phytolith-occluded carbon. Quat. Int. 193, 20–29, 10.1016/j.quaint.2007.11.013 (2009). [DOI] [Google Scholar]
- Wilding L. P., Brown R. E. & Holowaychuk N. Accessibility and Properties of Occluded Carbon in Biogenetic Opal. Soil Sci. 103, 56–61 (1967). [Google Scholar]
- Mulholland S. C. & Prior C. AMS Radiocarbon Dating of Phytoliths, in Current Research in Phytolith Analysis: Applications in Archaeology and Paleoecology (eds Pearsall D. M. & Piperno D. R.), Ch. 2, 21–23 (The University Museum of Archaeology and Anthropology, University of Pennsylvania Press, Philadelphia, 1993). [Google Scholar]
- Piperno D. R. & Jones J. G. Paleoecological and archaeological implications of a late Pleistocene/Early holocene record of vegetation and climate from the pacific coastal plain of panama. Quat. Res. 59, 79–87, 10.1016/S0033-5894(02)00021-2 (2003). [DOI] [Google Scholar]
- Piperno D. R. & Stothert K. E. Phytolith Evidence for Early Holocene Cucurbita Domestication in Southwest Ecuador. Science 299, 1054–1057, 10.1126/science.1080365 (2003). [DOI] [PubMed] [Google Scholar]
- Piperno D. R. & Becker P. Vegetational History of a Site in the Central Amazon Basin Derived from Phytolith and Charcoal Records from Natural Soils. Quat. Res. 45, 202–209, 10.1006/qres.1996.0020 (1996). [DOI] [Google Scholar]
- Jones R. L. & Beavers A. H. Aspects of Catenary and Depth Distribution of Opal Phytoliths in Illinois Soils. Soil Sci. Soc. Am. J. 28, 413–416 (1964). [Google Scholar]
- Piperno D. R., Ranere A. J., Holst I. & Hansell P. Starch grains reveal early root crop horticulture in the Panamanian tropical forest. Nature 407, 894–897, 10.1038/35038055 (2000). [DOI] [PubMed] [Google Scholar]
- Piperno D. R., Andres T. C. & Stothert K. E. Phytoliths in Cucurbita and other Neotropical Cucurbitaceae and their Occurrence in Early Archaeological Sites from the Lowland American Tropics. J. Archaeol. Sci. 27, 193–208, 10.1006/jasc.1999.0443 (2000). [DOI] [Google Scholar]
- Kelly E. F., Amundson R. G., Marino B. D. & Deniro M. J. Stable Isotope Ratios of Carbon in Phytoliths as a Quantitative Method of Monitoring Vegetation and Climate Change. Quat. Res. 35, 222–233, 10.1016/0033-5894(91)90069-H (1991). [DOI] [Google Scholar]
- McMichael C. H. et al. Spatial and temporal scales of pre-Columbian disturbance associated with western Amazonian lakes. The Holocene 22, 131–141, 10.1177/0959683611414932 (2012). [DOI] [Google Scholar]
- Prior C. A., Carter J. & Rieser U. Are phytolith radiocarbon dates reliable? Abstract, P1592, Poster Session II. The 10th International Conference on Accelerator Mass Spectrometry, Berkeley, USA. (2005, September 5th–10th).
- Reyerson P. E. et al. Unambiguous evidence of old soil carbon in grass biosilica particles. Biogeosciences 13, 1269–1286, 10.5194/bg-13-1269-2016 (2016). [DOI] [Google Scholar]
- Santos G. M. et al. Possible source of ancient carbon in phytolith concentrates from harvested grasses. Biogeosciences 9, 1873–1884, 10.5194/bg-9-1873-2012 (2012). [DOI] [Google Scholar]
- Yin J., Yang X. & Zheng Y. Influence of increasing combustion temperature on the AMS 14C dating of modern crop phytoliths. Sci. Rep. 4, 10.1038/srep06511 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. & Chen X. The archaeology of China: from the late Paleolithic to the early Bronze Age. 123–250 (Cambridge University Press, 2012). [Google Scholar]
- Zuo X., Lu H. & Gu Z. Distribution of soil phytolith-occluded carbon in the Chinese Loess Plateau and its implications for silica–carbon cycles. Plant Soil 374, 223–232, 10.1007/s11104-013-1850-6 (2014). [DOI] [Google Scholar]
- Santos G. M. et al. The phytolith 14C puzzle: a tale of background determinations and accuracy tests. Radiocarbon 52, 113–128 (2010). [Google Scholar]
- Corbineau R., Reyerson P. E., Alexandre A. & Santos G. M. Towards producing pure phytolith concentrates from plants that are suitable for carbon isotopic analysis. Rev. Palaeobot. Palynol. 197, 179–185, 10.1016/j.revpalbo.2013.06.001 (2013). [DOI] [Google Scholar]
- Reimer P. J. et al. IntCal13 and Marine13 Radiocarbon Age Calibration Curves 0–50,000 Years cal BP. Radiocarbon 55, 1869–1887 (2013). [Google Scholar]
- Zhang J. P. et al. Early Mixed Farming of Millet and Rice 7800 Years Ago in the Middle Yellow River Region, China. Plos ONE 7(12), e52146, 10.1371/journal.pone.0052146 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X. X. & Lü H. Y. Carbon sequestration within millet phytoliths from dry-farming of crops in China. Chin. Sci. Bull. 56, 3451–3456, 10.1007/s11434-011-4674-x (2011). [DOI] [Google Scholar]
- Parr J. F. & Sullivan L. A. Comparison of two methods for the isolation of phytolith occluded carbon from plant material. Plant Soil 374, 45–53, 10.1007/s11104-013-1847-1 (2014). [DOI] [Google Scholar]
- Borrelli N., Osterrieth M. & Marcovecchio J. Interrelations of vegetal cover, silicophytolith content and pedogenesis of Typical Argiudolls of the Pampean Plain, Argentina. Catena 75, 146–153, 10.1016/j.catena.2008.05.001 (2008). [DOI] [Google Scholar]
- Wallis L. A. Environmental history of northwest Australia based on phytolith analysis at Carpenter’s Gap 1. Quat. Int. 83–85, 103–117, 10.1016/S1040-6182(01)00033-7 (2001). [DOI] [Google Scholar]
- Madella M. & Lancelotti C. Taphonomy and phytoliths: A user manual. Quat. Int. 275, 76–83, 10.1016/j.quaint.2011.09.008 (2012). [DOI] [Google Scholar]
- Shillito L.-M. Grains of truth or transparent blindfolds? A review of current debates in archaeological phytolith analysis. Veget. Hist. Archaeobot. 22, 71–82, 10.1007/s00334-011-0341-z (2013). [DOI] [Google Scholar]
- Santos G. M., Alexandre A. & Prior C. A. From radiocarbon analysis to interpretation: A comment on “Phytolith radiocarbon dating in archaeological and paleoecological research: A case study of phytoliths from modern neotropical plants and a review of the previous dating evidence”. J. Archaeol. Sci. 66, 36–43, 10.1016/j.jas.2015.11.012 (2016). [DOI] [Google Scholar]
- Piperno D. R. Phytolith radiocarbon dating in archaeological and paleoecological research: a case study of phytoliths from modern Neotropical plants and a review of the previous dating evidence. J. Archaeol. Sci. 68, 54–61, 10.1016/j.jas.2015.06.002 (2016). [DOI] [Google Scholar]
- Gallagher K. L., Alfonso-Garcia A., Sanchez J., Potma E. O. & Santos G. M. Plant growth conditions alter phytolith carbon. Front. Plant Sci. 6, 9, 10.3389/fpls.2015.00753 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno D. R. “Standard Evaluations of Bomb Curves and Age Calibrations Along with Consideration of Environmental and Biological Variability Show the Rigor of Phytolith Dates on Modern Neotropical Plants: Review of Comment by Santos, Alexandre, and Prior” J. Archaeol. Sci. in press, 10.1016/j.jas.2016.01.013 (2016). [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.