Abstract
Post-weaning diarrhoea (PWD) in piglets is associated with colonization of the intestine with bacterial pathogens. In this study, we evaluated the use of recombinant porcine β-defensin 2 (rpBD2) as a medicated feed additive for weaned piglets. The crude extract from the culture supernatant of rpBD2-expressing Pichia pastoris was used as a medicated feed additive for weaned piglets. Dietary treatments included a positive control (basal diet + antibiotics, designated PC) and three different rpBD2 treatments without antibiotics (basal diet supplemented with 1, 5, or 15 g of crude rpBD2/kg basal diet, designated 1PD, 5PD, and 15PD, respectively). Of all the treatments, 5PD had the greatest impact on the weaned piglets. It increased their body weight, average daily weight gain, average daily feed intake, and intestinal villus height in the duodenum and jejunum, and reduced the incidence of PWD. The diversity of the cecal digesta and mucosa microflora was compared between the weaned piglets in the PC and 5PD groups. Piglets treated with 5PD had lower diversity indices and fewer bacterial pathogens in their cecal digesta and mucosa than the PC group. Our results demonstrate that crude rpBD2 could provide an alternative to the traditional antibiotic feed additives given to weaned piglets.
Post-weaning diarrhoea (PWD) is caused by a number of bacterial pathogens, including Escherichia coli, Clostridium spp., and Lawsonia spp., and is a significant gastrointestinal disease in pigs, entailing high economic losses in pig herds1,2,3. For decades, antibiotic growth promoters have been widely used in piglets to reduce enteric infections and improve the composition of the intestinal microflora, thereby reducing the risk of PWD4,5. The limitations of dietary antibiotics include the presence of drug residues in edible animal products and the reduction of the innate immune defences of young animals6,7. Therefore, natural products are urgently required for use as alternatives to antibiotic feed additives8,9,10,11,12.
Defensins are a family of endogenous cationic antimicrobial peptides that play an important role in the innate and adaptive immune systems of mammals, and provide protection against intestinal bacterial infections by modulating the composition of the intestinal microbiota13,14. Bacteria are less able to develop resistance to defensins than to traditional antibiotics, because defensins disrupt the bacterial membrane by forming non-specific electrostatic interactions with the membrane lipid components15. Therefore, the administration of defensins is a potentially novel therapeutic strategy for inflammatory and infectious diseases of the gastrointestinal tract16, and may present a promising alternative to the traditional antibiotic feed additives used in the livestock industry.
Mature porcine β-defensin 2 (pBD2), whose expression in the pig intestine is induced by infection with intestinal pathogens14, exerts strong antimicrobial activity against a broad range of pathogenic intestinal bacteria, with very limited haemolytic activity against porcine red blood cells17. With a triple-stranded β-sheet fold and a framework of six disulfide-linked cysteines, the arginine-rich cationic peptide exerts its antimicrobial and cytotoxic effects by permeabilizing the target membrane when it inserts into it in response to the electrical forces that act on the positively charged defensin molecule17,18. As shown in our previous study19, recombinant pBD2 overexpressed in Pichia pastoris can pass through the stomach and intestine and tolerate feed pellet processing with no loss of its antimicrobial activity because its thermal and pH stability and proteolytic resistance are high. Furthermore, a crude extract of the culture supernatant of rpBD2-expressing P. pastoris (designated ‘crude rpBD2’) can be added directly to feed without rpBD2 purification. This advantage reduces the production costs and allows its application to be scaled up. The objective of this follow-up study was to rigorously evaluate the effects of dietary supplementation with crude rpBD2 as a medicated feed additive on the growth performance, intestinal morphology, and intestinal microflora of weaned piglets on a commercial farm.
Results
Experimental design
A clear rpBD2 band was observed following tricine–sodium dodecyl sulfate–polyacrylamide gel electrophoresis (tricine–SDS–PAGE) of the crude rpBD2 powder (concentration 474.8 ± 34.2 mg/g). The application of crude rpBD2 caused an obvious inhibition zone on a bacterial lawn of Staphylococcus aureus ATCC 6538. The minimal inhibitory concentrations of crude rpBD2 against a broad range of pig pathogenic bacteria were previously shown to range from 32 to 128 μg/mL19. Three different rpBD2-supplemented feeds without antibiotics (basal diet supplemented with 1, 5, or 15 g crude rpBD2/kg, designated 1PD, 5PD, and 15PD, respectively) and the positive control (PC; basal diet + 1.5% crude P. pastoris X-33 + 200 mg/kg 10% colistin sulfate + 1,000 mg/kg 10% zinc bacitracin) were given to weaned piglets on a commercial farm to determine their effects on the growth performance, incidence of PWD, small intestinal morphology, and intestinal microflora of the piglets. Our aim was to identify the appropriate dose of the crude rpBD2 additive and confirm its utility as an alternative to traditional antibiotic feed additives.
Growth performance and incidence of PWD
The growth performance and incidence of PWD in all the weaned piglet groups tested are listed in Table 1. In phase I, the average daily weight gain (ADG) and average daily feed intake (ADFI) values of the 5PD group were significantly higher than those of the 1PD and 15PD groups, but were not significantly different from those of the PC group. In phase II, the ADG and ADFI values of the 5PD group were superior to those of the 1PD, 15PD, and PC groups. Throughout the experimental period, the overall values for body weight (BW), ADG, and ADFI were significantly higher and the incidence of PWD was significantly lower in the 5PD group than in the 1PD, 15PD, and PC groups, whereas the 5PD and PC groups had similar feed conversion (G/F) values (1.80 vs 1.81, respectively). Therefore, in general, 5PD was more effective than 1PD, 15PD, or PC in improving the growth performance of weaned piglets and reducing the incidence of PWD.
Table 1. Growth performance and incidence of diarrhoea in weaned piglets.
| Item | PC | PD |
SEM | P Value | ||
|---|---|---|---|---|---|---|
| 1 | 5 | 15 | ||||
| Body weight, kg | ||||||
| Initial weight (1 d) | 9.38a | 9.35a | 9.38a | 9.39a | 0.033 | 0.984 |
| Final weight (28 d) | 16.79b | 17.04b | 17.62a | 16.98b | 0.106 | 0.007 |
| Average daily weight gain, g/d | ||||||
| 1–14 d | 159.5a | 167.9a | 173.8a | 164.2a | 3.020 | 0.442 |
| 14–28 d | 369.5b | 381.7b | 414.3a | 378.1b | 5.806 | 0.006 |
| 1–28 d | 264.5b | 274.8b | 294.0a | 271.1b | 3.754 | 0.005 |
| Average daily feed intake, g | ||||||
| 1–14 d | 333.6ab | 317.1bc | 344.0a | 309.3c | 4.977 | 0.022 |
| 14–28 d | 625.0b | 634.5b | 717.9a | 640.3b | 12.469 | 0.004 |
| 1–28 d | 479.3b | 475.8b | 531.0a | 474.8b | 7.575 | 0.001 |
| Feed conversion (g feed/g weight gain) | ||||||
| 1–14 d | 2.10a | 1.89a | 1.98a | 1.89a | 0.047 | 0.409 |
| 14–28 d | 1.69a | 1.66a | 1.73a | 1.69a | 0.012 | 0.211 |
| 1–28 d | 1.81a | 1.73b | 1.80a | 1.75b | 0.012 | 0.008 |
| Diarrhoea incidence (%) | 4.4a | 3.9ab | 2.4c | 2.9bc | 0.277 | 0.009 |
PC: positive control; PD: rpBD2 treatment groups; SEM: standard error of the mean. a,b,cMean values within a row with unlike superscript letters are significantly different (P < 0.05).
Small intestinal morphology
As shown in Table 2, the villus height values in the duodenum and jejunum were significantly greater in the 5PD group than in the 1PD, 15PD, or PC group. Dietary treatment did not appear to affect the crypt depth in the duodenum in any of the PD treatment groups, but the duodenal crypt depth was much shallower in the 5PD group than in the PC group (317.6 μm vs 351.7 μm, respectively). The crypt depth in the ileum was significantly shallower in the 5PD group than in the 1PD, 15PD, or PC group. No significant differences in the villus height/crypt depth ratio were observed among the treatment groups. Overall, 5PD was more effective than 1PD, 15PD, or PC in improving the small intestinal morphology of the weaned piglets. Representative micrographs of small intestinal morphology of the PC and 5PD groups are shown in Fig. S1.
Table 2. Characteristics of the duodenum, jejunum, and ileum in weaned piglets (28 days).
| Item | PC | PD |
PValue | ||
|---|---|---|---|---|---|
| 1 | 5 | 15 | |||
| Duodenum | |||||
| Villus height, μm | 541.1 ± 66.3b | 500.8 ± 65.7b | 702.3 ± 55.9a | 656.2 ± 61.1a | 0.025 |
| Crypt depth, μm | 351.7 ± 54.4a | 296.6 ± 65.2a | 317.6 ± 32.5a | 361.1 ± 50.7a | 0.443 |
| Villus height/crypt depth | 1.5 ± 0.5a | 1.8 ± 0.6a | 2.2 ± 0.3a | 1.8 ± 0.4a | 0.362 |
| Jejunum | |||||
| Villus height, μm | 507.7 ± 60.4b | 540.7 ± 36.9b | 651.4 ± 20.5a | 623.3 ± 22.2a | <0.010 |
| Crypt depth, μm | 211.8 ± 37.0b | 297.7 ± 23.4a | 252.0 ± 19.0ab | 303.6 ± 61.7a | 0.063 |
| Villus height/crypt depth | 2.5 ± 0.8a | 1.8 ± 0.3a | 2.6 ± 0.3a | 2.1 ± 0.5a | 0.307 |
| Ileum | |||||
| Villus height, μm | 369.0 ± 66.1ab | 454.1 ± 68.9a | 327.6 ± 44.2b | 384.3 ± 34.6ab | 0.110 |
| Crypt depth, μm | 243.3 ± 29.4ab | 272.7 ± 55.2a | 200.7 ± 21.5b | 312.0 ± 32.0a | 0.031 |
| Villus height/crypt depth | 1.5 ± 0.5a | 1.7 ± 0.6a | 1.7 ± 0.2a | 1.2 ± 0.02a | 0.461 |
PC: positive control; PD: rpBD2 treatment groups; SEM: standard error of the mean. a,b,cMean values within a row with unlike superscript letters are significantly different (P < 0.05).
Intestinal bacterial community
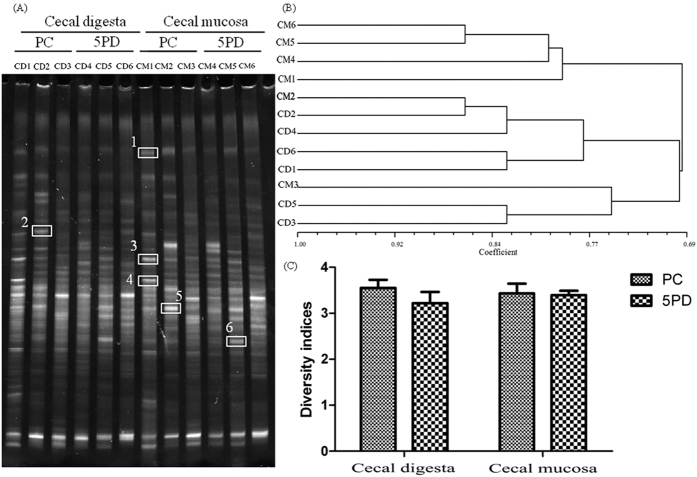
In total, 12 denaturing gradient gel electrophoresis (DGGE) profiles were generated when DGGE was used to analyse the cecal digesta and mucosal samples of three piglets randomly selected from the PC and 5PD pens on day 28 (Fig. 1A). The relative intensities of bands 1, 2, 3, 4, and 5 were considerably lower in the 5PD group than in the PC group and, moreover, the bands 1, 2, and 4 in the 5PD group were below the limit of detection. By contrast, the relative intensity of band 6 was higher in the 5PD group than in the PC group. Band 2 was present in the cecal digesta but not found in the mucosal samples (CD2 vs CM2 profile in Fig. 1A, respectively), whereas band 5 was constantly visible in the cecal mucosal samples but almost invisible in the digesta samples.
Figure 1. Denaturing gradient gel electrophoresis (DGGE) results.
(A) DGGE profiles. 12 DGGE profiles of the PCR products from the V3 region of the 16S rRNA gene from the PC and 5PD cecal digesta and mucosal samples on day 28. Bands identified from the 16S rRNA gene clone libraries are numbered and indicated with square frames. The bands are also described in Table 3. (B) Clustered DGGE profiles. UPGMA clustering diagram of Dice similarity indices of the 12 DGGE profiles. (C) Calculated diversity indices. Diversity indices were used to compare the PC and 5PD cecal digesta and mucosa samples on day 28. Triplicate observations from three individual piglets were made and data are means ± standard errors (SE).
The 16S rRNA-DGGE bands had sequence similarities to those of organisms closely related to Solobacterium moorei NR_113039.1 (band 1), Helicobacter canadensis NR_115104.1 (band 2), Eubacterium eligens NR_074613.1 (band 3), Coprococcus comes NR_044048.1 (band 4), Clostridium polysaccharolyticum NR_119085.1 (band 5), and Fusicatenibacter saccharivorans NR_114326.1 (band 6), with similarities of 87%, 100%, 100%, 100%, 99%, and 100%, respectively (Table 3).
Table 3. Alignment of sequenced clones to the most similar GenBank sequences.
| Sequenced clones | Corresponding sample | Length of 16S rDNA V3 fragments (bp) | GenBank accession number | Most similar GenBank sequence |
|||
|---|---|---|---|---|---|---|---|
| Aligned site | % similarity | Bacterium | Accession number | ||||
| 1 | CM1 | 196 | KM220765 | 334–524 | 87 | Solobacterium moorei | NR_113039.1 |
| 2 | CD2 | 171 | KM220766 | 296–466 | 100 | Helicobacter canadensis | NR_115104.1 |
| 3 | CM1 | 171 | KM220767 | 349–519 | 100 | Eubacterium eligens | NR_074613.1 |
| 4 | CM1 | 171 | KM220768 | 327–497 | 100 | Coprococcus comes | NR_044048.1 |
| 5 | CM2 | 173 | KM220769 | 346–516 | 99 | Clostridium polysaccharolyticum | NR_119085.1 |
| 6 | CM5 | 171 | KM220770 | 333–503 | 100 | Fusicatenibacter saccharivorans | NR_114326.1 |
The 12 DGGE profiles formed a coherent cluster, with similarity indices above 69% (Fig. 1B). The cecal mucosal profiles of the 5PD group (CM4/CM5/CM6) had the highest intra-group similarity (>80%) when compared with the other three groups (~69% each): cecal digesta PC group (CD1/CD2/CD3), cecal digesta 5PD group (CD4/CD5/CD6), and cecal mucosa PC group (CM1/CM2/CM3). The two DGGE profiles CM2 and CD2 had the highest pairwise similarity (>86%) compared with all the other pairwise similarity values, which ranged from 69% to 75%. These results indicate that rpBD2 increased the coherence of the mucosal microbiota in the piglet caecum, thereby reducing the diversity of microflora.
The Shannon index of diversity (H′) for each cecal digesta and mucosa sample was lower in the 5PD group than in the PC group, but no statistical significant difference was observed among the four groups. Furthermore, in the PC and 5PD groups, the Shannon indices were more similar among the cecal mucosal samples than among the digesta samples (Fig. 1C). These results indicate that rpBD2 reduces the intestinal pathogenic microbiota in the piglet cecal digesta more than that in the mucosa.
Discussion
Defensin is an important component of the nonspecific immune system and acts as a gene-encoded antibiotic to repel the assault of diverse infectious agents, including bacteria, viruses, fungi, and parasites18,20,21,22. In this study, we have shown that dietary supplementation with crude rpBD2 improved the growth performance of weaned piglets and reduced the incidence of PWD more effectively than antibiotic supplementation. These results suggest that rpBD2 can be used as a growth promoter and medicinal agent instead of antibiotics to improve the growth performance of piglets and prevent PWD. Importantly, 5 g/kg crude rpBD2 most effectively improved the growth performance of the weaned piglets and reduced the incidence of PWD. Lower (1 g/kg) or higher (15 g/kg) doses of rpBD2 were less effective. Why higher dose of rpBD2 did not acquire better growth performance is unclear. Probably, higher dose of rpBD2 would inhibit the survival of probiotic in porcine intestines as shown in our previous study19. Notably, significant improvement in piglet growth performance was observed in phase II but not in phase I, probably because piglet growth in response to rpBD2 occurred at least 2 weeks after weaning.
The intestinal morphology is indicative of pig gut health. Villus height and the villus height/crypt depth ratio correlate with epithelial turnover, and longer villi are associated with active cell mitosis and greater nutrient absorption23,24. After weaning, the villus height is reduced and the crypt depth increases, primarily because feed intake decreases immediately after weaning23. In swine and broiler models, dietary supplementation with probiotics, antimicrobial peptides (cecropin AD, antimicrobial peptide A3, or antimicrobial peptide P5), or glucose has a beneficial effect on mucosal development, increasing the villus height and causing weight gain25,26,27,28,29,30. Shorter villi and deeper crypts are associated with the presence of toxins or pathogens31. In this study, greater villus heights and shallower crypt depths were observed in the weaned piglets treated with 5PD compared with the PC group, and this may have contributed to their improved growth performance.
The pig large intestine is densely colonized with bacteria, but little is known about the microfloral composition and its variation after weaning, which significantly affect piglet growth performance and health3,32. During weaning, major quantitative and qualitative changes occur in the composition of the piglet intestinal microbiota, providing an opportunity for pathogenic coliforms and other bacteria to invade, contributing to gastric disorders and hence reduced performance33. Dietary supplementation with an antimicrobial peptide, such as a fusion peptide of lactoferricin and lactoferrampin, potato protein, antimicrobial peptide A3, cecropin AD, or antimicrobial peptide P5, reduces the total numbers of aerobes while simultaneously enhancing the total amount of anaerobes and beneficial lactobacilli in the intestines of weaned piglets24,25,27,28,33.
The intestinal microbial community of pigs has been investigated in several studies with traditional microbial culture techniques. However, many strictly anaerobic intestinal bacteria are difficult to culture and remain undetectable with conventional techniques. The combined use of 16S rRNA gene-targeted PCR, DGGE, cloning, and sequencing is a powerful approach to identifying the gastrointestinal microbiota, including bacteria that are difficult to culture34,35. As shown with PCR–DGGE, rpBD2 potentially suppresses harmful intestinal microflora in weaning piglets. The five DGGE bands attenuated in the 5PD-treated group relative to the PC group were from organisms most similar to S. moorei, H. Canadensis, E. eligens, C. comes, and C. polysaccharolyticum. Another band, representing F. saccharivorans, was more intense in the 5PD-treated group than in the PC group. S. moorei is a non-spore-forming, strictly anaerobic Gram-positive bacillus that has been identified in specimens from human patients with dental diseases and wound infections36. Because the partial 16S rRNA sequences showed a similarity to S. moorei much lower than 97% in this study, the corresponding bacterium probably represents a new Solobacterium species. H. Canadensis has been isolated from swine faeces in Europe and has been associated with diarrhoea37. The administration of clindamycin increases the bacterial load of E. eligens in standardized human faecal microbiota38. C. comes is an anaerobic Gram-positive coccoid rod isolated from the faecal flora and sera of patients with Crohn’s disease39. C. polysaccharolyticum is a common bacterium in animal intestines40, and Clostridium species are common anaerobic pathogens in the pig intestines28,33. F. saccharivorans is a Gram-positive, obligately anaerobic, non-motile, non-spore-forming, spindle-shaped bacterium isolated from human faeces41. All the bacteria sequenced in this study, except H. canadensis, have not previously been reported in pigs.
The microbiota in post-weaning piglets formed a more coherent cluster with greater similarity indices than that in the pre-weaning piglets. Weaning causes significant changes in the mucosa-associated bacterial communities and digesta samples from the jejuna of piglets34. The clustering analyses performed in this study indicated that post-weaning piglets formed a coherent cluster, with similarity indices above 69%. Interestingly, the rpBD2-supplemented mucosal microbiota showed high sequence similarities (>80%), whereas the rpBD2-supplemented digesta microbiota showed the lowest coherence. However, the Shannon index of diversity for the mucosal samples from the PC group was almost the same as that for the 5PD-treated group. These phenomena suggest that rpBD2 increased the coherence of the mucosal microbiota among different pens of piglets but did not affect the microbial diversity on the intestinal mucosa.
Before the establishment of a developed immune system, which generally takes 4–5 weeks, newly weaned piglets are very susceptible to diseases and stressors. At this stage, dietary supplementation with innate immunity factors is important for the growth performance and health of the weaning piglets28. In this study, we have demonstrated that dietary supplementation with crude rpBD2 has beneficial effects on the growth performance and intestinal morphology of weaned piglets, reducing the incidence of PWD and the numbers of potential pathogens in the caecum. Therefore, rpBD2 can potentially be used as an alternative to traditional antibiotic feed additives for weaned piglets on commercial farms. The detailed mechanism(s) by which rpBD2 promotes the growth performance of weaned piglets and improves their intestinal health requires further clarification.
Methods
Preparation of crude rpBD2
The method for preparing crude rpBD2 has been described by Peng et al.19. Briefly, the rpBD2 supernatant was harvested from the culture medium of rpBD2-experssing P. pastoris X-33, dialyzed with a 1-kDa nanofiltration membrane (Laungy Membrane Filtration Technology, Shanghai, China), and spray-dried. The resulting crude rpBD2 powder was confirmed with an inhibition zone assay42 against S. aureus ATCC 6538 and with tricine–SDS–PAGE43. The concentration of rpBD2 in the crude powder was quantified with a Porcine β Defensin 2 Enzyme-linked Immunosorbent Assay (ELISA) Kit (Cloud-Clone, Houston, TX, USA). The crude powdered P. pastoris X-33 culture supernatant was used as the blank control.
Weaned piglets and dietary treatments
All animal experiments were performed in accordance with protocols approved by the Ethics Committee of the State Key Laboratory of Direct-Fed Microbial Engineering, China National Center for Food Safety Risk Assessment and the Beijing Institute of Microbiology and Epidemiology. In total, 120 weaned piglets (Landrace × Yorkshire × Duroc; 9.375 ± 0.017 kg in body weight [BW]; 21 ± 2 days old; 60 males and 60 females) with the same ancestry were used in a 28-day growth study on a commercial farm in Henan Province in July 2014. The piglets were randomly assigned to four groups for dietary treatment: PC, 1PD, 5PD, and 15PD. The ingredients and chemical composition of the basal diet are shown in Table 4. The groups were composed of three replicate pens with 10 piglets each, and all groups were supplied with either antibiotics or rpBD2. The experiment was divided into phases I (0–14 days post-weaning) and II (14–28 days post-weaning).
Table 4. Ingredients and chemical composition of the basal diet.
| Ingredients | % | Nutrient composition | % |
|---|---|---|---|
| Maize | 59.37 | Crude protein | 19.19 |
| Soybean meal | 25.00 | Calcium | 0.583 |
| Fishmeal | 4.00 | P | 0.464 |
| Dried whey power | 4.00 | Lysine | 1.198 |
| Cream from bovine milk | 5.00 | Methionine | 0.397 |
| Limestone | 0.30 | Threonine | 0.850 |
| Monocalcium phosphate | 1.10 | Digestible energy†/kg feed | 14.3 MJ |
| Anti-mould agent | 0.10 | NA | NA |
| Antioxidant | 0.02 | NA | NA |
| Vitamin premix* | 0.04 | NA | NA |
| Trace mineral premix# | 0.30 | NA | NA |
| Salt | 0.30 | NA | NA |
| Flavour | 0.06 | NA | NA |
| L-lysine-HCl | 0.23 | NA | NA |
| L-methionine | 0.05 | NA | NA |
| L-threonine | 0.05 | NA | NA |
*Vitamin premix consisted of the following ingredients per kilogram of complete diet: 11,000 IU (3,300 μg) vitamin A, 1,100 IU (27.5 μg) vitamin D3, 22 IU (14.67 μg) vitamin E, 4 mg menadione as dimethylpyrimidinol bisulfite, 0.03 mg vitamin B12, 28 mg d-pantothenic acid, 33 mg niacin, and 0.08% choline chloride.
#Trace mineral premix consisted of the following ingredients per kilogram of complete diet: 165 mg Zn (ZnSO4), 165 mg Fe (FeSO4), 33 mg Mn (MnSO4), 16.5 mg Cu (CuSO4), 297 μg I (CaI2), and 297 μg Se (Na2SeO3);
†Digestible energy was based on calculated values. NA: not applicable.
The diets were formulated to meet or exceed the National Research Council guidelines44 for 10–20-kg pigs. The pigs were housed in temperature-controlled (29 °C) nursery rooms and grouped in elevated pens with wire flooring. Feed and water were available to the pigs ad libitum. The pigs were individually weighed on an empty stomach on days 0, 14, and 28. Feed consumption was recorded on days 14 and 28. All feed remaining in the feed trough at the next feeding period was weighed and subtracted from the daily allowance to determine the actual daily feed intake. Growth performance indicators, such as BW, ADG, ADFI, and G/F, were determined for each pen at the end of every phase.
Faecal consistency and the incidence of PWD
The occurrence of PWD in each piglet was assessed visually each afternoon using the method of Hart and Dobb45. The scores were: 0 = normal, firm faeces; 1 = possible slight diarrhoea; 2 = definitely unformed/moderately fluid faeces; and 3 = very watery and frothy diarrhoea. A cumulative diarrhoea score per diet and day was then calculated25. The occurrence of diarrhoea was defined as the maintenance of faecal scores of 2 or 3 for 2 consecutive days and was determined with the formula: diarrhoea incidence (%) = ([number of piglets with diarrhoea within a treatment]/[number of piglets × total experimental days]) × 100, where ‘number of piglets with diarrhoea’ was the total number of piglets with diarrhoea observed each day46.
Small intestinal morphology
To study the effects of rpBD2 on the small intestinal morphology, six pigs were randomly selected from each treatment group (two piglets per pen, one male and one female, reflecting the average BW of the pen) and killed by electrocution on day 28. The samples of intestinal segments from the middle regions of the duodenum, jejunum, and ileum, after removal of their contents, were aseptically isolated, flushed with physiological saline, and submerged in a fixative solution (0.1 M collidine buffer, pH 7.3) containing 3% glutaraldehyde, 2% paraformaldehyde, and 1.5% acrolein, for further analysis. Three cross-sections were prepared from each intestinal sample after staining with haematoxylin and eosin, using standard paraffin-embedding and staining procedures33. In total, 10 intact, well-oriented crypt–villus units were selected in triplicate for each intestinal cross-section (30 measurements of each sample; a total of 180 measurements per dietary treatment). The villus heights and crypt depths were determined with an image processing and analysis system (Leica Imaging Systems, version 1, Cambridge, UK)23.
Intestinal microfloral analysis
Fresh digesta from cecal samples (approximate middle segments) from piglets in the PC and 5PD treatment groups were collected aseptically, immediately immersed in liquid nitrogen, and stored at −20 °C. To analyse the microflora intimately attached to the cecal mucosa, the luminal fluid was drained, and the middle segments of the caecum (3–4 cm) were excised, washed with sterile phosphate-buffered saline (pH 7.0), immediately snap frozen in liquid nitrogen, and stored at −20 °C.
For the DGGE analysis, total DNA was extracted from the digesta and mucosal samples with a TIANamp Stool DNA Kit (Tiangen, Beijing, China). The variable V3 region of 16S rRNA was amplified with PCR using the universal primers 341F (5′-CCTACGGGAGGCAGCAG-3′) and 534R (5′-ATTACCGCGGCTGCTGG-3′), and a GC clamp (5′-CGCCCGCCGCGCGCGGCGGGCGGGGCGGGGGCACGGGGGG-3′) was attached to the 5′-terminus of 341F47,48. The PCR products were loaded onto 8% polyacrylamide gels containing 37.5:1 acrylamide:bisacrylamide and a denaturing gradient of 38–51% (100% denaturant was equivalent to 7 M urea and 40% deionised formamide) using the DCode Universal Mutation Detection System (Bio-Rad Laboratories, Hercules, CA, USA). Electrophoresis was initiated by pre-running the samples for 10 min at 200 V and then continued at a fixed voltage of 85 V for 12 h at 60 °C49. The gels were stained with Gene Green I (Tiangen) after electrophoresis and scanned with the Quantity One software (version 4.6.3, Bio-Rad, USA). The DGGE profiles were clustered using the unweighted pair group method with arithmetic averages (UPGMA) in the MEGA 4.0 software. Preliminary diversity data were collected from the DGGE bands with the Quantity One analysis software, and Shannon’s diversity index was calculated to measure microfloral diversity49.
Six 16S rRNA PCR products from prominent bands that differed between the cecal digesta and mucosal samples from the PC and 5PD groups were selected, purified with the Gel Recovery Purification Kit (Tiangen), cloned into the pMD18-T vector (Takara, Dalian, China), and used to transform E. coli DH5α cells. Ten clones were randomly selected for each band and the cloned inserts amplified with PCR. Sequencing was performed by Invitrogen (Beijing, China) using a universal primer pair for the pMD18-T vector: M13F (−40) 5′-GTTTTCCCAGTCACGAC-3′ and M13R (−26) 5′-CAGGAAACAGCTATGAC-3′. The 16S rRNA gene sequences are available in GenBank under accession numbers KM220765, KM220766, KM220767, KM220768, KM220769, and KM220770.
Statistical analysis
The data were analysed according to a randomized complete block design using the general linear model procedure of the SAS Statistical Software (SAS Institute, Cary, NC, USA). One-way analysis of variance (ANOVA) was applied to all parameters. When significant differences were observed among treatment means, they were separated with Tukey’s honest significant difference test. ‘Pen’ was the experimental unit for the analysis of all parameters. Differences with P < 0.01, <0.05, and <0.10 were considered extremely significant, significant, and a trend, respectively.
Additional Information
How to cite this article: Peng, Z. et al. Use of recombinant porcine β-defensin 2 as a medicated feed additive for weaned piglets. Sci. Rep. 6, 26790; doi: 10.1038/srep26790 (2016).
Supplementary Material
Acknowledgments
This study was financially supported by the Beijing Talents Fund of the Beijing Municipal Organization Department (grant 2014000021223ZK46), and the Beijing Nova Program Interdisciplinary Cooperation Project (grant xxjc201601).
Footnotes
Author Contributions Z.P. performed the animal experiments, interpreted the results, and participated in the preparation of the manuscript. A.W. prepared the crude rpBD2 and the dietary treatments for the weaned piglet. L.X. and W.S. performed the DGGE experiment and contributed to the analysis of the results. J.W. and Z.Y. were responsible for the analysis of the small intestinal morphology. D.Z. and F.L. were responsible for the study design, supervision of the whole study, interpretation of the results, and the preparation and submission of the manuscript. All authors read and approved the final manuscript.
References
- Stensland I. et al. A Comparison of Diets Supplemented with a Feed Additive Containing Organic Acids, Cinnamaldehyde and a Permeabilizing Complex, or Zinc Oxide, on Post-Weaning Diarrhoea, Selected Bacterial Populations, Blood Measures and Performance in Weaned Pigs Experimentally Infected with Enterotoxigenic E. coli. Anim 5, 1147–1168, 10.3390/ani5040403 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J. M. et al. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol An N 97, 207–237, 10.1111/j.1439-0396.2012.01284.x (2013). [DOI] [PubMed] [Google Scholar]
- Britton R. A. & Young V. B. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology 146, 1547–1553, 10.1053/j.gastro.2014.01.059 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vondruskova H., Slamova R., Trckova M., Zraly Z. & Pavlik I. Alternatives to antibiotic growth promoters in prevention of diarrhoea in weaned piglets: a review. Vet Med-Czech 55, 199–224 (2010). [Google Scholar]
- Lin J. Effect of Antibiotic Growth Promoters on Intestinal Microbiota in Food Animals: A Novel Model for Studying the Relationship between Gut Microbiota and Human Obesity? Front Microbiol 2, 53, 10.3389/fmicb.2011.00053 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee S. E., Cunningham R. & Elliott C. T. Simultaneous immunochemical detection of four banned antibiotic growth promoters in raw and cooked poultry tissue. Food Addit Contam A 30, 1270–1278, 10.1080/19440049.2013.801087 (2013). [DOI] [PubMed] [Google Scholar]
- Millet S. & Maertens L. The European ban on antibiotic growth promoters in animal feed: from challenges to opportunities. Vet J 187, 143–144, 10.1016/j.tvjl.2010.05.001 (2011). [DOI] [PubMed] [Google Scholar]
- Zhao X., Guo Y., Guo S. & Tan J. Effects of Clostridium butyricum and Enterococcus faecium on growth performance, lipid metabolism, and cecal microbiota of broiler chickens. Appl Microbiol Biotechnol 97, 6477–6488, 10.1007/s00253-013-4970-2 (2013). [DOI] [PubMed] [Google Scholar]
- Huyghebaert G., Ducatelle R. & Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet J 187, 182–188, 10.1016/j.tvjl.2010.03.003 (2011). [DOI] [PubMed] [Google Scholar]
- De Schryver P. et al. Poly-beta-hydroxybutyrate (PHB) increases growth performance and intestinal bacterial range-weighted richness in juvenile European sea bass, Dicentrarchus labrax. Appl Microbiol Biotechnol 86, 1535–1541, 10.1007/s00253-009-2414-9 (2010). [DOI] [PubMed] [Google Scholar]
- Sang Y. & Blecha F. Porcine host defense peptides: expanding repertoire and functions. Dev Comp Immunol 33, 334–343, 10.1016/j.dci.2008.05.006 (2009). [DOI] [PubMed] [Google Scholar]
- Thacker P. A. Alternatives to antibiotics as growth promoters for use in swine production: a review. J Anim Sci Biotechno 4, 35–46 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen E. J. et al. Expression of beta-defensins pBD-1 and pBD-2 along the small intestinal tract of the pig: lack of upregulation in vivo upon Salmonella typhimurium infection. Mol Immunol 44, 276–283, 10.1016/j.molimm.2006.03.005 (2007). [DOI] [PubMed] [Google Scholar]
- Veldhuizen E. J., Koomen I., Ultee T., van Dijk A. & Haagsman H. P. Salmonella serovar specific upregulation of porcine defensins 1 and 2 in a jejunal epithelial cell line. Vet microbiol 136, 69–75, 10.1016/j.vetmic.2008.09.072 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. High expression of a plectasin-derived peptide NZ2114 in Pichia pastoris and its pharmacodynamics, postantibiotic and synergy against Staphylococcus aureus. Appl Microbiol Biotechnol 98, 681–694 (2014). [DOI] [PubMed] [Google Scholar]
- Salzman N. H. et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 11, 76–82 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen E. J., Rijnders M., Claassen E. A., van Dijk A. & Haagsman H. P. Porcine beta-defensin 2 displays broad antimicrobial activity against pathogenic intestinal bacteria. Mol Immunol 45, 386–394, 10.1016/j.molimm.2007.06.001 (2008). [DOI] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3, 710–720, 10.1038/nri1180 (2003). [DOI] [PubMed] [Google Scholar]
- Peng Z. et al. High-level expression, purification and characterisation of porcine β-defensin 2 in Pichia pastoris and its potential as a cost-efficient growth promoter in porcine feed. Appl Microbiol Biotechnol 98, 5487–5497 (2014). [DOI] [PubMed] [Google Scholar]
- Klotman M. E. & Chang T. L. Defensins in innate antiviral immunity. Nat Rev Immunol 6, 447–456, 10.1038/nri1860 (2006). [DOI] [PubMed] [Google Scholar]
- Dorin J. R. & Jackson I. J. Genetics. Beta-defensin repertoire expands. Science 318, 1395, 10.1126/science.1151370 (2007). [DOI] [PubMed] [Google Scholar]
- Lai Y. & Gallo R. L. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol 30, 131–141, 10.1016/j.it.2008.12.003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Shan T., Xu Z., Liu J. & Feng J. Effect of lactoferrin on the growth performance, intestinal morphology, and expression of PR-39 and protegrin-1 genes in weaned piglets. J Anim Sci 84, 2636–2641, 10.2527/jas.2005-544 (2006). [DOI] [PubMed] [Google Scholar]
- Yoon J. H. et al. Effects of dietary supplementation of antimicrobial peptide-A3 on growth performance, nutrient digestibility, intestinal and fecal microflora and intestinal morphology in weanling pigs. Anim Feed Sci Tech 177, 98–107, 10.1016/j.anifeedsci.2012.06.009 (2012). [DOI] [Google Scholar]
- Wu S. et al. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides 35, 225–230, 10.1016/j.peptides.2012.03.030 (2012). [DOI] [PubMed] [Google Scholar]
- Bao H. et al. Effects of pig antibacterial peptides on growth performance and intestine mucosal immune of broiler chickens. Poultry Sci 88, 291–297, 10.3382/ps.2008-00330 (2009). [DOI] [PubMed] [Google Scholar]
- Tang Z. et al. Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin-lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Brit J Nutr 101, 998–1005, 10.1017/S0007114508055633 (2009). [DOI] [PubMed] [Google Scholar]
- Yoon J. H. et al. Effects of dietary supplementation with antimicrobial peptide-P5 on growth performance, apparent total tract digestibility, faecal and intestinal microflora and intestinal morphology of weanling pigs. J Sci Food Agr 93, 587–592, 10.1002/jsfa.5840 (2013). [DOI] [PubMed] [Google Scholar]
- Wang H. T. et al. Effects of albusin B (a bacteriocin) of Ruminococcus albus 7 expressed by yeast on growth performance and intestinal absorption of broiler chickens–its potential role as an alternative to feed antibiotics. J Sci Food Agr 91, 2338–2343, 10.1002/jsfa.4463 (2011). [DOI] [PubMed] [Google Scholar]
- Vente-Spreeuwenberg M. A. M., Verdonk J. M. A. J., Verstegen M. W. A. & Beynen A. C. Villus height and gut development in weaned piglets receiving diets containing either glucose, lactose or starch. Brit J Nutr 90, 907, 10.1079/bjn2003981 (2007). [DOI] [PubMed] [Google Scholar]
- Wan M. L., Woo C. S., Allen K. J., Turner P. C. & El-Nezami H. Modulation of porcine beta-defensins 1 and 2 upon individual and combined Fusarium toxin exposure in a swine jejunal epithelial cell line. Appl Environ Microb 79, 2225–2232, 10.1128/AEM.03277-12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao L. et al. Cello-oligosaccharide influences intestinal microflora, mucosal architecture and nutrient transport in weaned pigs. Anim Feed Sci Tech 20, 478–486 (2014). [Google Scholar]
- Jin Z. et al. Potato (Solanum tuberosum L. cv. Gogu valley) protein as a novel antimicrobial agent in weanling pigs. J Anim Sci 86, 1562–1572, 10.2527/jas.2007-0414 (2008). [DOI] [PubMed] [Google Scholar]
- Su Y., Yao W., Perez-Gutierrez O. N., Smidt H. & Zhu W. Y. Changes in abundance of Lactobacillus spp. and Streptococcus suis in the stomach, jejunum and ileum of piglets after weaning. FEMS Microbiol Ecol 66, 546–555, 10.1111/j.1574-6941.2008.00529.x (2008). [DOI] [PubMed] [Google Scholar]
- Konstantinov S. R. et al. Microbial diversity studies of the porcine gastrointestinal ecosystem during weaning transition. Anim Res 53, 317–324 (2004). [Google Scholar]
- Zheng G. et al. Phenotypic and molecular characterization of Solobacterium moorei isolates from patients with wound infection. J Clin Microbiol 48, 873–876, 10.1128/JCM.01381-09 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis G. D., McConville M. & de Jong A. Atypical Helicobacter canadensis strains associated with swine. Appl Environ Microb 72, 4464–4471, 10.1128/AEM.02843-05 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman A. et al. Effects of probiotics and antibiotics on the intestinal homeostasis in a computer controlled model of the large intestine. BMC Microbiol 12, 47, 10.1186/1471-2180-12-47 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazenberg M. P., van de Merwe J. P., Pena A. S., Pennock-Schroder A. M. & van Lieshout L. M. Antibodies to Coprococcus comes in sera of patients with Crohn’s disease. Isolation and purification of the agglutinating antigen tested with an ELISA technique. J Clin & Lab Immunol 23, 143–148 (1987). [PubMed] [Google Scholar]
- Varel V. H., Tanner R. S. & Woese C. R. Clostridium herbivorans sp. nov., a cellulolytic anaerobe from the pig intestine. Int J Syst Bacteriol 45, 490–494 (1995). [DOI] [PubMed] [Google Scholar]
- Takada T., Kurakawa T., Tsuji H. & Nomoto K. Fusicatenibacter saccharivorans gen. nov., sp. nov., isolated from human faeces. Int J Syst Evol Micr 63, 3691–3696, 10.1099/ijs.0.045823-0 (2013). [DOI] [PubMed] [Google Scholar]
- Tian Z.-g., Dong T.-t., Yang Y.-l., Teng D. & Wang J.-h. Expression of antimicrobial peptide LH multimers in Escherichia coli C43 (DE3). Appl Microbiol Biotechnol 83, 143–149 (2009). [DOI] [PubMed] [Google Scholar]
- Schagger H. Tricine-SDS-PAGE. Nat Protoc 1, 16–22, 10.1038/nprot.2006.4 (2006). [DOI] [PubMed] [Google Scholar]
- NRC. Nutrient Requirements of Swine. 10th edn, (Natl. Acad. Press, 1998). [Google Scholar]
- Hart G. K. & Dobb G. J. Effect of a fecal bulking agent on diarrhea during enteral feeding in the critically ill. JPEN-Parenter Enteral 12, 465–468 (1988). [DOI] [PubMed] [Google Scholar]
- Sun P., Li D., Li Z., Dong B. & Wang F. Effects of glycinin on IgE-mediated increase of mast cell numbers and histamine release in the small intestine. J Nutr Biochem 19, 627–633, 10.1016/j.jnutbio.2007.08.007 (2008). [DOI] [PubMed] [Google Scholar]
- Ramond J. B., Welz P. J., Tuffin M. I., Burton S. G. & Cowan D. A. Selection of diazotrophic bacterial communities in biological sand filter mesocosms used for the treatment of phenolic-laden wastewater. Microbial Ecol 66, 563–570, 10.1007/s00248-013-0258-4 (2013). [DOI] [PubMed] [Google Scholar]
- Oguntoyinbo F. A., Tourlomousis P., Gasson M. J. & Narbad A. Analysis of bacterial communities of traditional fermented West African cereal foods using culture independent methods. Int J Food Microbiol 145, 205–210, 10.1016/j.ijfoodmicro.2010.12.025 (2011). [DOI] [PubMed] [Google Scholar]
- Su Y., Yao W., Perez-Gutierrez O. N., Smidt H. & Zhu W. Y. 16S ribosomal RNA-based methods to monitor changes in the hindgut bacterial community of piglets after oral administration of Lactobacillus sobrius S1. Anaerobe 14, 78–86, 10.1016/j.anaerobe.2007.12.004 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.