Abstract
Background: Lipid biomarkers, such as HDL-cholesterol concentrations, have been shown to have positive, inverse, and null associations with total, breast, and colorectal cancer risks. Studies of novel lipid biomarkers, such as apolipoprotein A-I (apo A-I) and apolipoprotein B-100 (apo B-100), and cancer risk have been sparse, to our knowledge.
Objectives: We evaluated the prospective association of total, breast, colorectal, and lung cancers and cancer mortality with circulating lipid biomarkers in 15,602 female health professionals in the Women’s Health Study (aged ≥45 y, free of cardiovascular disease and cancer, and without hormone replacement therapy or lipid-lowering medications at baseline).
Design: Cox regression models estimated HRs of cancer endpoints (19 y median follow-up) across quartiles 1 (reference) to 4 of each lipid biomarker after adjustment for cancer risk factors.
Results: Confirmed cases included 2163 incident cancer cases (864 breast, 198 colorectal, and 190 lung cancers) and 647 cancer deaths. Total cancer risk was significantly lower in the highest quartile of apo A-I (adjusted HR: 0.79; 95% CI: 0.70, 0.90; P-trend = 0.0008) and HDL cholesterol (HR: 0.85; 95% CI: 0.75, 0.97; P-trend = 0.01). For site-specific cancers, significant associations included colorectal cancer risk with HDL cholesterol (HR: 0.63; 95% CI; 0.41, 0.98; P-trend = 0.03), triglycerides (HR: 1.86; 95% CI: 1.17, 2.97; P-trend = 0.02), and apo B-100 (HR: 1.60; 95% CI: 1.03, 2.49; P-trend = 0.006) and lung cancer risk with HDL cholesterol (HR: 0.59; 95% CI: 0.38, 0.93; P-trend = 0.01). LDL cholesterol was not significantly associated with risk of total cancer or any site-specific cancers. In time-dependent models that were adjusted for the use of a lipid-lowering medication after baseline, these associations remained.
Conclusions: Lipids were associated with total, lung, and colorectal cancer risks in women. Lifestyle interventions for heart-disease prevention, which reduce apo B-100 or raise HDL cholesterol, may be associated with reduced cancer risk. The Women’s Health Study was registered at clinicaltrials.gov as NCT00000479.
Keywords: cancer, lipids, metabolomics, triglycerides, women
INTRODUCTION
Cancer is the second leading cause of death in the United States, of which breast cancer, colorectal cancer (CRC),11 and lung cancer are frequently diagnosed cancers in women (1). Findings from extensive epidemiologic and experimental investigations have linked factors that alter blood lipid markers, such as diet, smoking, and obesity, with cancer risk. Blood lipids and lipoproteins, which are used for energy storage and membrane production (2), may influence carcinogenesis through insulin resistance, inflammation, and oxidative stress pathways (3).
As established risk factors for cardiovascular disease (CVD) that are routinely measured in the clinic, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides (4) have shown positive, inverse, and null associations with total cancer risk (5–9), breast cancer risk (7) and CRC risk (10). Studies of novel lipid biomarkers, such as apolipoprotein A-I (apo A-I) and apolipoprotein B-100 (apo B-100), and cancer risk have been sparse, to our knowledge. Apo A-I and apo B-100, which are the main protein components of HDL cholesterol and LDL cholesterol, respectively, are synthesized in the intestines and liver and are largely responsible for the antiatherogenic effect of HDL cholesterol and the proatherogenic effect of LDL cholesterol. Furthermore, apo B-100 is a requisite for the formation of triglyceride-rich VLDL.
Intervention strategies to reduce LDL cholesterol and triglycerides or raise HDL cholesterol for heart-disease prevention may have beneficial effects on cancer risk. Previous studies have shown that effective lifestyle interventions for cancer prevention such as dietary changes (11, 12), exercise (13), and smoking cessation (14) are effective in raising HDL cholesterol. However, the bidirectional relation between overall obesity and regional obesity with circulating lipids (15, 16) adds complexity to the association between lipids and cancer risk.
To further evaluate the association between plasma lipids and risks of cancers, we conducted a prospective analysis in a large cohort of women aged ≥45 y who were free of cancer and CVD at baseline. Although apo A-I is correlated with HDL cholesterol, and apo B-100 is correlated with total cholesterol and LDL cholesterol, we hypothesized that apo A-I and apo B-100 may provide a superior risk prediction for certain cancers than would standard lipid markers.
METHODS
Study design
The WHS (Women’s Health Study) (clinicaltrials.gov; NCT00000479) began in 1992 and is a completed randomized, double-blind, placebo-controlled trial that was originally designed to examine the role of aspirin (100 mg every other day), vitamin E (600 IU every other day), and β-carotene (50 mg on alternate days) in the prevention of cancer and CVD in 39,876 women in the United States. The β-carotene component was terminated early after a median treatment duration of 2.1 y. During follow-up, every 6 mo in the first year and every year thereafter, participants received questionnaires that assessed their compliance, potential side effects, updated risk factors, and outcomes of interest. When the trial ended in 2004, 33,682 women (88.6% of those alive) consented to continue with an observational follow-up whereby they reported their health habits and medical histories annually on questionnaires. A morbidity follow-up in the WHS was complete for 97.2% of women, and a mortality follow-up was complete for 99.4% of women. All subjects provided written informed consent. The project was approved by the Institutional Review Board of Brigham and Women’s Hospital. Because it is well known that the use of postmenopausal hormone replacement therapy (HRT) and lipid-lowering medications affect blood lipid concentrations, we excluded participants who were taking these medications at the WHS baseline. Thus, 15,602 women ≥45 y of age and without CVD, cancer, or use of HRT or lipid-lowering medications at baseline were included in this analysis (Supplemental Figure 1).
Cancer endpoint ascertainment
For cases of cancer that were reported during the study period, subjects provided written consent for a medical record review. Medical records were reviewed by a committee of physicians. We included confirmed invasive cancer cases through 2013. With a median follow-up of 19 y, confirmed cancer cases include 834 breast cancers, 198 CRCs, 190 lung cancers, and 647 cancer deaths. The endpoint review was complete for 95% of reported cancer cases. The confirmation rate in participants with records was 82%. Of all deaths, 60% had a cause that was confirmed by medical records, and 85% were confirmed with death certificates or National Death Index reports.
Baseline blood collection
Before random assignment in the WHS, blood was collected from participants by mail. Women returned the completed blood kit in a gel-filled freezer pack via overnight courier. Each Participants were instructed to record the time of venipuncture and the time of their last meal and to send their sample to our blood laboratory in the freezer packs ≤24 h of the blood draw. A fasting blood sample at the time of blood draw was defined as the elapse of ≥8 h since the most-recent meal. Of 39,876 randomly assigned women in the trial, 28,345 women (71%) provided a baseline blood sample. Women who did and did not donate blood were similar on a wide range of variables related to cancer (17).
Measurement of biomarkers and baseline covariates
A standard lipid profile (total cholesterol, HDL cholesterol, triglycerides, and LDL cholesterol) and apo A-I and apo B-100 were measured as described previously (18, 19). Anthropometric, lifestyle, and dietary data were derived from the baseline questionnaire. The validity and reproducibility of a semiquantitative food-frequency questionnaire have been described previously (20–22).
Statistical analysis
SAS version 9.2 software (SAS Institute) was used for all analyses. All P values were 2 sided, and P < 0.05 was considered statistically significant. Lipid biomarkers, including total cholesterol, HDL cholesterol, triglycerides, LDL cholesterol, apo A-I, and apo B-100, were divided into quartiles. The associations of biomarkers with cancer-related baseline covariates were assessed with the use of general linear models for continuous variables and chi-square tests for categorical variables. HRs and 95% CIs of incident cancer and cancer mortality were calculated with the use of a Cox proportional hazards regression model that compared the increasing quartiles to the lowest quartile. The linear trend for an association was tested with the use of the median of each quartile as an ordinal variable. We evaluated the association of the lipid biomarkers total cholesterol, HDL cholesterol, triglycerides, LDL cholesterol, apo A-I, and apo B-100 with total cancer and site-specific cancers (CRCs, breast cancers, and lung cancers). We assumed a P value <0.05 for significance for the primary endpoints total cancer and site-specific cancers (CRCs, breast cancers, and lung cancers).
Because of the large number of tests performed, we also interpreted associations with marginal significance with caution. In the crude models for total cancer, we adjusted for the trial treatment assignment (aspirin, vitamin E, and β-carotene) and age. In the multivariable model for total cancer, we further adjusted for established cancer risk factors including cigarette smoking (never, past, or current), exercise [total kilocalories from exercise and the number of stairs taken per week (4 categories as follows: <200, 200 to <600, 600 to <1500, and ≥1500)], alcohol consumption (continuous; grams per day), postmenopausal status (yes or no), postmenopausal hormone use (never or past), family history of cancer (yes or no), aspirin use (current use >1 time/wk compared with no use), and mammogram screening (yes or no). An additional model included adjustment for BMI (in kg/m2) in 5 categories (<18.5, 18.5 to <25, 25 to <30, 30 to <35, and ≥35; the reference group was BMI from 18.5 to <25). For lung cancer, we adjusted for the same covariates as for total cancer. For other site-specific cancers, we further adjusted for other known risk factors. Specifically, for breast cancer, we also adjusted for a family history of breast cancer, the reproductive history including the age of menarche (≤11, 12, 13, and ≥14 y), oral contraceptive use (no use or ever use for ≥2 mo), age at first birth lasting ≥6 mo (nulliparous, ≤29 y, and ≥30 y), and history of benign breast disease; for CRC, we also adjusted for intakes of red meat and total vegetables and fruit (servings/d).
We further stratified total cancer analyses by cancer risk factors of baseline BMI, menopausal status, physical activity, and alcohol use. We used a time-dependent variable for lipid-medication use to assess the impact of lipid-lowering medications on the observed associations. Overweight and overweight-associated risk factors (e.g., lipid concentrations) differ in premenopausal and postmenopausal breast cancer cases. Thus, we further stratified breast cancer analyses by age (<55 and ≥55 y) to evaluate for potential differences by premenopause compared with postmenopause because of the heterogeneity in the causes of premenopausal and postmenopausal breast cancers. We conducted secondary analyses with a lag time to evaluate the associations after the exclusion of cancers that occurred in the first 2–4 y of follow-up to address possible reverse causation that was due to preexisting cancers that might have influenced lipid concentrations.
RESULTS
Baseline characteristics
In 15,602 women who did not use HRT or a lipid-lowering medication at the WHS baseline, subjects in the highest quartile (quartile 4) of triglycerides and apo B-100 had significantly higher BMI than that of women in the lowest quartile (quartile 1) (Table 1). In contrast, women in quartile 4 of HDL cholesterol and apo A-I had significantly lower BMI than that of women in quartile 1. Women in quartile 4 of triglycerides and apo B-100 engaged in less exercise than did those in quartile 1. In contrast, women in quartile 4 of HDL cholesterol and apo A-I performed significantly more exercise than did those in quartile 1. During a median follow-up of 19 y (10 y-trial period and 9-y posttrial observation period), 2163 incident cancer cases (864 breast cancers, 198 CRCs, and 190 lung cancers) and 647 cancer deaths were confirmed. Concentrations of total cholesterol, HDL cholesterol, triglycerides, LDL cholesterol, apo A-I, and apo B-100 were correlated, with the strongest positive correlation (Spearman r ranging from 0.13 to 0.91) for total cholesterol and LDL cholesterol and the strongest negative correlation (Spearman r ranging from −0.13 to −0.51) for HDL cholesterol and triglycerides (Supplemental Table 1).
TABLE 1.
Baseline characteristics by extreme quartiles of blood lipids1
| Total cholesterol |
HDL cholesterol |
Triglycerides |
LDL cholesterol |
apo A-I |
apo B-100 |
|||||||
| Quartile 1 (n = 3902) | Quartile 4 (n = 3797) | Quartile 1 (n = 3935) | Quartile 4 (n = 3881) | Quartile 1 (n = 3942) | Quartile 4 (n = 3898) | Quartile 1 (n = 3903) | Quartile 4 (n = 3899) | Quartile 1 (n = 3890) | Quartile 4 (n = 3864) | Quartile 1 (n = 3890) | Quartile 4 (n = 3856) | |
| Range, mg/dL | 72–180 | 235–474 | 17–41 | 59–151 | 13–76 | 163–995 | 17–102 | 147–335 | 12–127 | 156–238 | 22–83 | 120–257 |
| Age, y | 51.3 ± 6.22 | 56.6 ± 8.0 | 54.2 ± 7.7 | 54.0 ± 7.63 | 52.2 ± 6.7 | 55.3 ± 7.9 | 51.5 ± 6.3 | 56.5 ± 8.0 | 53.1± 7.2 | 54.6 ± 7.8 | 51.2 ± 6.0 | 56.8 ± 8.0 |
| BMI, kg/m2 | 25.7 ± 5.5 | 26.8 ± 5.1 | 29.1 ± 5.8 | 23.8 ± 3.9 | 23.9 ± 3.9 | 28.7 ± 5.5 | 25.4 ± 5.3 | 27.0 ± 5.2 | 28.1 ± 5.8 | 24.4 ± 4.3 | 24.5 ± 4.7 | 27.8 ± 5.4 |
| Exercise, MET-h/wk | 15.2 ± 19.1 | 13.9 ± 18.0 | 11.9 ± 17.3 | 17.5 ± 21.1 | 17.4 ± 20.6 | 12.3 ± 16.7 | 16.1 ± 19.9 | 13.4 ± 17.7 | 12.6 ± 18.1 | 17.2 ± 21.1 | 16.9 ± 20.7 | 12.8 ± 17.2 |
| Alcohol intake, g/d | 3.5 ± 7.2 | 4.5 ± 9.8 | 2.1 ± 5.4 | 6.8 ± 10.9 | 4.9 ± 8.5 | 3.2 ± 8.1 | 4.4 ± 8.2 | 3.9 ± 8.64 | 2.1 ± 5.3 | 7.3 ± 11.7 | 4.4 ± 8.3 | 3.9 ± 9.03 |
| Current smoking,5 % | 9.8 | 15.3 | 17.3 | 9.1 | 9.0 | 15.7 | 10.1 | 15.3 | 16.4 | 9.4 | 9.4 | 16.9 |
| Ever use of OCP, % | 76.4 | 59.8 | 67.6 | 68.16 | 74.1 | 64.5 | 76.5 | 60.6 | 70.4 | 66.23 | 76.3 | 59.1 |
| Family history of cancer, % | 17.2 | 20.13 | 18.0 | 18.06 | 16.8 | 18.56 | 17.6 | 20.03 | 17.5 | 18.36 | 16.5 | 19.73 |
| Mammogram, % | 62.1 | 58.43 | 58.3 | 63.23 | 62.1 | 58.43 | 62.5 | 57.5 | 59.4 | 63.03 | 62.7 | 57.7 |
P-trend for continuous variables; P-χ2 for categorical variables. P ≤ 0.0001 for all markers unless otherwise noted. apo A-I, apolipoprotein A-I; apo B-100, apolipoprotein B-100; MET-h, metabolic equivalent hours, OCP, oral contraceptive pill.
Mean ± SD (all such values for continuous variables).
P > 0.0001, but P ≤ 0.01.
P > 0.01 but P < 0.05.
Percentages are given for all categorical variables.
P > 0.05.
Total cancer
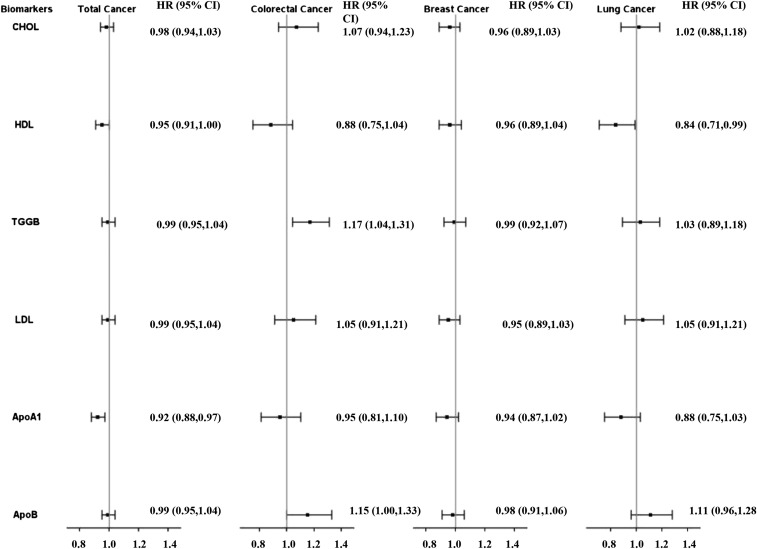
After multivariable adjustment, women in increasing quartiles of apo A-I and HDL cholesterol had significantly decreased risk of incident total cancer (Table 2). The association was slightly attenuated with adjustment for BMI (Table 2). Total cholesterol:HDL cholesterol, LDL cholesterol:HDL cholesterol, and apo B-100:apo A-I showed similar associations as for HDL cholesterol with the total cancer incidence (Supplemental Table 2). Other lipid biomarkers were not associated with the incidence of total cancer. Subgroup analyses by alcohol use, physical activity, menopausal status, and BMI revealed no significant interaction between these cancer risk factors and apo A-I (all P-interaction > 0.05). With the use of the multivariable model that included BMI, the HR per 1 SD of lipid biomarker was significant only for apo A-I (HR: 0.92; 95% CI: 0.88, 0.97; P = 0.0007) (Figure 1).
TABLE 2.
Total cancer incidence according to quartiles of lipid biomarkers1
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trend | |
| Total cholesterol, mg/dL | 72.0–180.02 | 181.0–205.0 | 206.0–234.0 | 235.0–474.0 | — |
| Cases/noncases, n | 480/3422 | 520/3402 | 585/3396 | 578/3219 | — |
| Age and treatment | 1.00 (—)3 | 1.03 (0.91, 1.16) | 1.07 (0.94, 1.21) | 1.06 (0.94, 1.20) | 0.30 |
| Model 2 | 1.00 (—) | 1.01 (0.89, 1.15) | 1.03 (0.91, 1.17) | 1.03 (0.91, 1.17) | 0.66 |
| Model 2 + BMI | 1.00 (—) | 1.01 (0.89, 1.14) | 1.02 (0.90, 1.16) | 1.04 (1.03, 1.05) | 0.79 |
| HDL cholesterol, mg/dL | 17.1–41.3 | 41.4–49.2 | 49.3–58.9 | 59.0–151.1 | — |
| Cases/noncases, n | 585/3350 | 566/3335 | 517/3367 | 495/3386 | — |
| Age and treatment | 1.00 (—) | 0.96 (0.86, 1.08) | 0.88 (0.78, 0.99) | 0.82 (0.73, 0.93) | 0.0005 |
| Model 2 | 1.00 (—) | 0.95 (0.84, 1.07) | 0.87 (0.77, 0.99) | 0.80 (0.71, 0.91) | 0.0003 |
| Model 2 + BMI | 1.00 (—) | 0.98 (0.87, 1.10) | 0.91 (0.81, 1.04) | 0.85 (0.75, 0.97) | 0.01 |
| Triglycerides, mg/dL | 13.0–76.0 | 77.0–110.0 | 111.0–162.0 | 163.0–995.0 | — |
| Cases/noncases, n | 480/3462 | 548/3376 | 584/3254 | 551/3347 | — |
| Age and treatment | 1.00 (—) | 1.10 (0.98, 1.25) | 1.18 (1.05, 1.33) | 1.09 (0.96, 1.23) | 0.35 |
| Model 2 | 1.00 (—) | 1.11 (0.98, 1.26) | 1.17 (1.03, 1.32) | 1.08 (0.95, 1.23) | 0.45 |
| Model 2 + BMI | 1.00 (—) | 1.08 (0.96, 1.23) | 1.12 (0.98, 1.27) | 1.01 (0.88, 1.15) | 0.69 |
| LDL cholesterol, mg/dL | 17.3–102.0 | 102.1–123.3 | 123.4–146.9 | 147.0–335.4 | — |
| Cases/noncases, n | 505/3398 | 491/3421 | 562/3326 | 605/3294 | — |
| Age and treatment | 1.00 (—) | 0.91 (0.81, 1.03) | 1.01 (0.89, 1.14) | 1.03 (0.91, 1.16) | 0.34 |
| Model 2 | 1.00 (—) | 0.90 (0.79, 1.02) | 0.99 (0.87, 1.12) | 1.01 (0.89, 1.15) | 0.47 |
| Model 2 + BMI | 1.00 (—) | 0.89 (0.78, 1.01) | 0.98 (0.86, 1.11) | 0.99 (0.88, 1.13) | 0.65 |
| apo A-I, mg/dL | 12.3–127.3 | 127.4–140.4 | 140.5–155.7 | 155.7–238.3 | — |
| Cases/noncases, n | 577/3313 | 531/3344 | 533/3334 | 498/3366 | — |
| Age and treatment | 1.00 (—) | 0.88 (0.78, 0.99) | 0.87 (0.77, 0.98) | 0.79 (0.70, 0.89) | 0.0002 |
| Model 2 | 1.00 (—) | 0.89 (0.79, 1.01) | 0.87 (0.77, 0.99) | 0.76 (0.67, 0.86) | <0.0001 |
| Model 2 + BMI | 1.00 (—) | 0.91 (0.80, 1.02) | 0.90 (0.80, 1.02) | 0.79 (0.70, 0.90) | 0.0008 |
| Apo B-100, mg/dL | 21.8–82.4 | 82.6–98.9 | 99.0–120.2 | 120.4–257.4 | — |
| Cases/noncases, n | 477/3413 | 500/3373 | 570/3305 | 593/3263 | — |
| Age and treatment | 1.00 (—) | 0.99 (0.87, 1.12) | 1.09 (0.96, 1.23) | 1.09 (0.96, 1.23) | 0.09 |
| Model 2 | 1.00 (—) | 0.98 (0.86, 1.11) | 1.07 (0.94, 1.22) | 1.07 (0.93, 1.21) | 0.18 |
| Model 2 + BMI | 1.00 (—) | 0.96 (0.84, 1.09) | 1.04 (0.91, 1.18) | 1.01 (0.89, 1.16) | 0.58 |
Treatment denotes the treatment random assignment (aspirin, vitamin E, or β-carotene). Model 2 was adjusted for age, race, treatment random assignment (aspirin, vitamin E, or β-carotene), hormone replacement therapy (past or none), cigarette smoking (never, past, or current), exercise [total kilocalories from exercise and the number of stairs taken per week (4 categories as follows: <200, 200 to <600, 600 to <1500, and ≥1500)], alcohol consumption (continuous; grams per day), postmenopausal status (yes or no), family history of cancer (yes or no), aspirin use (current use >1 time/wk compared with no use), history of colon polyps (yes or no), history of fibrocystic or benign breast disease (yes or no), total vegetable and fruit intake (servings/d), and history of mammogram (yes or no). Model 2 + BMI was adjusted as for model 2 as well as for BMI (in kg/m2) in 5 categories (<18.5, 18.5 to <25, 25 to <30, 30 to <35, and ≥35; the reference group was BMI from 18.5 to <25). apo A-I, apolipoprotein A-I; apo B-100, apolipoprotein B-100.
Range (all such values).
HR; 95% CI in parentheses (all such values).
FIGURE 1.
HRs per 1 SD for incident cancer events are shown on the y axis (log scale) for CHOL, HDL, TGGB, LDL, ApoA1, and ApoB. Incident cancer cases were as follows: total, n = 2163; lung, n = 190; colorectal, n = 198; and breast, n = 864. Values were evaluated with the use of Cox proportional hazard models. SD concentrations were as follows: CHOL, 209.2 mg/dL; HDL, 51.1 mg/dL; TGGBs, 133.8 mg/dL; LDL, 126.1 mg/dL; ApoA1, 142.3 mg/dL; and ApoB, 102.7 mg/dL. ApoA1, apolipoprotein A-I; ApoB, apolipoprotein B-100; CHOL, total cholesterol; HDL, HDL cholesterol; LDL, LDL cholesterol; TGGB, triglyceride.
CRC
In multivariable models with and without BMI, women in increasing quartiles of apo B-100 and triglycerides had significantly increased risk of CRC (Table 3). In contrast, HDL cholesterol showed an inverse association with risk of CRC in the multivariable model with and without BMI (Table 3). With the use of multivariable models that included BMI, the HRs of CRC per 1 SD of lipid biomarker for apo B-100, triglycerides, and HDL cholesterol were 1.15 (95% CI: 1.00, 1.33; P = 0.06), 1.17 (95% CI: 1.04, 1.31; P = 0.0008), and 0.88 (95% CI: 0.75, 1.04; P = 0.13), respectively (Figure 1). Total cholesterol:HDL cholesterol, LDL cholesterol:HDL cholesterol, and apo B-100:apo A-I showed associations of similar magnitudes but inverse directions with CRC incidence compared with those for HDL cholesterol (Supplemental Table 3). There was no significant interaction between cancer risk factors including alcohol use, physical activity, menopausal status, and BMI with the lipid biomarkers (P-interaction > 0.05).
TABLE 3.
Incidence of colorectal cancer according to quartiles of lipid biomarkers1
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trend | |
| Total cholesterol, mg/dL | 72.0–180.02 | 181.0–205.0 | 206.0–234.0 | 235.0–474.0 | — |
| Cases/noncases, n | 36/3866 | 46/3876 | 46/3935 | 70/3727 | — |
| Age and treatment | 1.00 (—)3 | 1.10 (0.71, 1.70) | 0.91 (0.59, 1.42) | 1.32 (0.87, 1.99) | 0.19 |
| Model 2 | 1.00 (—) | 1.02 (0.65, 1.58) | 0.84 (0.54, 1.32) | 1.23 (0.81, 1.86) | 0.30 |
| Model 2 + BMI | 1.00 (—) | 1.02 (0.65, 1.58) | 0.84 (0.54, 1.31) | 1.21 (0.80, 1.84) | 0.34 |
| HDL cholesterol, mg/dL | 17.1–41.3 | 41.4–49.2 | 49.3–58.9 | 59.0–151.1 | — |
| Cases/noncases, n | 60/3875 | 54/3847 | 42/3842 | 42/3839 | — |
| Age and treatment | 1.00 (—) | 0.92(0.64, 1.33) | 0.72 (0.49, 1.07) | 0.69 (0.47, 1.03) | 0.04 |
| Model 2 | 1.00 (—) | 0.82 (0.56, 1.19) | 0.67 (0.45, 1.00) | 0.59 (0.39, 0.89) | 0.008 |
| Model 2 + BMI | 1.00 (—) | 0.84 (0.57, 1.22) | 0.71 (0.47, 1.07) | 0.63 (0.41, 0.98) | 0.03 |
| Triglycerides, mg/dL | 13.0–76.0 | 77.0–110.0 | 111.0–162.0 | 163.0–995.0 | — |
| Cases/noncases, n | 29/3913 | 49/3875 | 53/3785 | 67/3831 | — |
| Age and treatment | 1.00 (—) | 1.51 (0.95, 2.39) | 1.56 (0.99, 2.46) | 1.86 (1.20, 2.89) | 0.01 |
| Model 2 | 1.00 (—) | 1.54 (0.97, 2.46) | 1.58 (0.99, 2.51) | 1.99 (1.27, 3.12) | 0.005 |
| Model 2 + BMI | 1.00 (—) | 1.50 (0.94, 2.41) | 1.51 (0.94, 2.42) | 1.86 (1.17, 2.97) | 0.02 |
| LDL cholesterol, mg/dL | 17.3–102.0 | 102.1–123.3 | 123.4–146.9 | 147.0–335.4 | — |
| Cases/noncases, n | 44/3859 | 35/3877 | 43/3845 | 76/3823 | — |
| Age and treatment | 1.00 (—) | 0.68 (0.44, 1.06) | 0.74 (0.48, 1.13) | 1.16 (0.79, 1.69) | 0.16 |
| Model 2 | 1.00 (—) | 0.71 (0.45, 1.12) | 0.71 (0.46, 1.10) | 1.17 (0.79, 1.73) | 0.16 |
| Model 2 + BMI | 1.00 (—) | 0.70 (0.45, 1.10) | 0.70 (0.45, 1.08) | 1.14 (0.77, 1.68) | 0.20 |
| apo A-I, mg/dL | 12.3–127.3 | 127.4–140.4 | 140.5–155.7 | 155.7–238.3 | — |
| Cases/noncases, n | 47/3843 | 55/3820 | 44/3823 | 50/3814 | — |
| Age and treatment | 1.00 (—) | 1.08 (0.73, 1.59) | 0.85 (0.56, 1.28) | 0.90 (0.61, 1.35) | 0.42 |
| Model 2 | 1.00 (—) | 1.04 (0.70, 1.53) | 0.79 (0.52, 1.19) | 0.77 (0.51, 1.17) | 0.12 |
| Model 2 + BMI | 1.00 (—) | 1.05 (0.71, 1.56) | 0.82 (0.54, 1.25) | 0.82 (0.53, 1.26) | 0.23 |
| apo B-100, mg/dL | 21.8–82.3 | 82.6–98.9 | 99.0–120.2 | 120.4–257.4 | — |
| Cases/noncases, n | 32/3858 | 37/3836 | 49/3826 | 79/3777 | — |
| Age and treatment | 1.00 (—) | 0.96 (0.60, 1.55) | 1.16 (0.74, 1.82) | 1.65 (1.08, 2.53) | 0.004 |
| Model 2 | 1.00 (—) | 0.94 (0.58, 1.53) | 1.15 (0.72, 1.82) | 1.69 (1.09, 2.60) | 0.003 |
| Model 2 + BMI | 1.00 (—) | 0.92 (0.57, 1.50) | 1.10 (0.69, 1.76) | 1.60 (1.03, 2.49) | 0.006 |
Treatment denotes the treatment random assignment (aspirin, vitamin E, or β-carotene). Model 2 was adjusted for age, race, treatment random assignment (aspirin, vitamin E, or β-carotene), hormone replacement therapy (past or none), cigarette smoking (never, past, or current), exercise [total kilocalories from exercise and the number of stairs taken per week (4 categories as follows: <200, 200 to <600, 600 to <1500, and ≥1500)], alcohol consumption (continuous; grams per day), postmenopausal status (yes or no), family history of cancer (yes or no), aspirin use (current use >1 time/wk compared with no use), history of colon polyps (yes or no), total vegetable and fruit intake (servings/d), history of mammogram (yes or no), and red meat intake (servings/d). Model 2 + BMI was adjusted as for model 2 as well as for BMI (in kg/m2) in 5 categories (<18.5, 18.5 to <25, 25 to <30, 30 to <35, and ≥35; the reference group was BMI from 18.5 to <25). apo A-I, apolipoprotein A-I; apo B-100, apolipoprotein B-100.
Range (all such values).
HR; 95% CI in parentheses (all such values).
Breast cancer
The median age at the time of diagnosis of breast cancer was 63.3 y (IQR: 57.6–69.8 y). In age- and treatment-adjusted models and multivariable-adjusted models that included reproductive history, history of benign breast disease, mammogram screening, and BMI, no lipid biomarker was associated with incident breast cancer (all P-trend > 0.10) (Table 4). Similarly, multivariable HRs per 1-SD increase were not significantly associated with breast cancer for any lipid marker (Figure 1). Total cholesterol:HDL cholesterol, LDL cholesterol:HDL cholesterol, and apo B-100:apo A-I were also not significantly associated with incident breast cancer (Supplemental Table 4). There was also no interaction between cancer risk factors and lipid markers with the exception of age and total cholesterol (P-interaction = 0.01), age and LDL (P-interaction = 0.01), and BMI and triglycerides (P-interaction = 0.04). In the stratified analyses by age <55 y (Supplemental Figure 2, Supplemental Table 5) and ≥55 y (Supplemental Figure 2, Supplemental Table 6), HRs in the highest quartile were 0.78 (95% CI: 0.59, 1.03; P-trend = 0.19) and 1.44 (95% CI: 0.97, 2.13; P-trend = 0.03) for total cholesterol and 0.82 (95% CI: 0.62, 1.07; P-trend = 0.11) and 1.44 (95% CI: 0.97, 2.13; P-trend = 0.14) for LDL cholesterol in the groups aged <55 and ≥ 55 y, respectively. In the stratified analyses by BMI, HRs in the highest quartile of total cholesterol were 0.79 (95% CI: 0.62, 1.01; P-trend = 0.04) and 1.18 (95% CI: 0.65, 2.14; P-trend = 0.49) in groups with BMI <30 compared with ≥30, respectively.
TABLE 4.
Incidence of breast cancer according to quartiles of lipid biomarkers1
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trend | |
| Total cholesterol, mg/dL | 72.0–180.02 | 181.0–205.0 | 206.0–234.0 | 235.0–474.0 | — |
| Cases/noncases, n | 202/3700 | 213/3709 | 245/3736 | 204/3593 | — |
| Age and treatment | 1.00 (—)3 | 1.03 (0.85, 1.25) | 1.13 (0.94, 1.37) | 0.98 (0.80, 1.19) | 0.96 |
| Model 2 | 1.00 (—) | 1.04 (0.85, 1.27) | 1.17 (0.96, 1.42) | 1.02 (0.83, 1.26) | 0.67 |
| Model 2 + BMI | 1.00 (—) | 1.03 (0.85, 1.26) | 1.15 (0.95, 1.40) | 1.01 (0.82, 1.24) | 0.80 |
| HDL cholesterol, mg/dL | 17.1–41.3 | 41.4–49.2 | 49.3–58.9 | 59.0–151.1 | — |
| Cases/noncases, n | 206/3729 | 223/3678 | 226/3658 | 209/3672 | — |
| Age and treatment | 1.00 (—) | 1.07 (0.88, 1.29) | 1.08 (0.89, 1.30) | 0.98 (0.81, 1.19) | 0.76 |
| Model 2 | 1.00 (—) | 1.02 (0.84, 1.24) | 1.05 (0.86, 1.28) | 0.92 (0.75, 1.13) | 0.42 |
| Model 2 + BMI | 1.00 (—) | 1.03 (0.85, 1.25) | 1.08 (0.88, 1.32) | 0.96 (0.78, 1.19) | 0.72 |
| Triglycerides, mg/dL | 13.0–76.0 | 77.0–110.0 | 111.0–162.0 | 163.0–995.0 | — |
| Cases/noncases, n | 227/3715 | 226/3698 | 209/3629 | 202/3696 | — |
| Age and treatment | 1.00 (—) | 0.99 (0.82, 1.19) | 0.93 (0.77, 1.13) | 0.89(0.73, 1.08) | 0.18 |
| Model 2 | 1.00 (—) | 1.00 (0.83, 1.21) | 0.94 (0.77, 1.14) | 0.93 (0.76, 1.13) | 0.37 |
| Model 2 + BMI | 1.00 (—) | 0.98 (0.81, 1.19) | 0.90 (0.74, 1.10) | 0.87 (0.71, 1.07) | 0.16 |
| LDL cholesterol, mg/dL | 17.3–102.0 | 102.1–123.3 | 123.4–146.9 | 147.0–335.4 | — |
| Cases/noncases, n | 206/3697 | 233/3679 | 214/3674 | 211/3688 | — |
| Age and treatment | 1.00 (—) | 1.09 (0.91, 1.32) | 0.99 (0.82, 1.21) | 0.96 (0.78, 1.16) | 0.44 |
| Model 2 | 1.00 (—) | 1.10 (0.91, 1.34) | 1.03 (0.84, 1.25) | 1.01 (0.83, 1.24) | 0.90 |
| Model 2 + BMI | 1.00 (—) | 1.09 (0.90, 1.33) | 1.01 (0.83, 1.24) | 0.99 (0.81, 1.22) | 0.74 |
| apo A-I, mg/dL | 12.3–127.3 | 127.4–140.4 | 140.5–155.7 | 155.7–238.3 | — |
| Cases/noncases, n | 214/3676 | 213/3662 | 231/3636 | 196/3668 | — |
| Age and treatment | 1.00 (—) | 0.97 (0.80, 1.17) | 1.03 (0.86, 1.24) | 0.86 (0.71, 1.05) | 0.19 |
| Model 2 | 1.00 (—) | 0.97 (0.80, 1.18) | 1.03 (0.85, 1.25) | 0.81 (0.66, 1.00) | 0.07 |
| Model 2 + BMI | 1.00 (—) | 0.97 (0.80, 1.18) | 1.04 (0.86, 1.26) | 0.83 (0.67, 1.03) | 0.14 |
| apo B-100, mg/dL | 21.8–82.4 | 82.6–98.9 | 99.0–120.2 | 120.4–257.4 | — |
| Cases/noncases, n | 200/3690 | 206/3667 | 243/3632 | 205/3651 | — |
| Age and treatment | 1.00 (—) | 1.00 (0.82, 1.22) | 1.18 (0.97, 1.42) | 0.99 (0.81, 1.21) | 0.80 |
| Model 2 | 1.00 (—) | 1.01 (0.83, 1.24) | 1.25 (1.03, 1.52) | 1.05 (0.85, 1.30) | 0.34 |
| Model 2 + BMI | 1.00 (—) | 1.00 (0.82, 1.22) | 1.22 (1.00, 1.49) | 1.02 (0.82, 1.26) | 0.55 |
Treatment denotes the treatment random assignment (aspirin, vitamin E, or β-carotene). Model 2 was adjusted for age, race, treatment random assignment (aspirin, vitamin E, or β-carotene), hormone replacement therapy (past or none), cigarette smoking (never, past, or current), exercise [total kilocalories from exercise and the number of stairs taken per week (4 categories as follows: <200, 200 to <600, 600 to <1500, and ≥1500)], alcohol consumption (continuous; grams per day), postmenopausal status (yes or no), family history of cancer (yes or no), aspirin use (current use >1 time/wk compared with no use), history of colon polyps (yes or no), history of fibrocystic or benign breast disease (yes or no), total vegetable and fruit intake (servings/d), history of mammogram (yes or no), reproductive history including the age of menarche (≤11, 12, 13, and ≥14 y), oral contraceptive use (yes or no), and age at first birth lasting ≥6 mo (nulliparous, ≤29 y, and ≥30 y). Model 2 + BMI was adjusted as for model 2 as well as for BMI (in kg/m2) in 5 categories (<18.5, 18.5 to <25, 25 to <30, 30 to <35, and ≥35; the reference group was BMI from 18.5 to <25). apo A-I, apolipoprotein A-I; apo B-100, apolipoprotein B-100.
Range (all such values).
HR; 95% CI in parentheses (all such values).
Lung cancer
In multivariable models with and without BMI, women in increasing quartiles of HDL cholesterol and apo A-I had significantly decreased risk of lung cancer (Table 5). The multivariable HR of lung cancer with adjustment for BMI per 1 SD in lipid markers were significant only for HDL cholesterol (HR: 0.84; 95% CI: 0.71, 0.99; P = 0.04) (Figure 1). Women in increasing quartiles of total cholesterol:HDL cholesterol, LDL cholesterol:HDL cholesterol, and apo B-100:apo A-I had increased risk of lung cancer incidence in multivariable models with and without BMI (Supplemental Table 7). Subgroup analyses revealed no significant interaction between physical activity, BMI, and lipid biomarkers (P-interaction > 0.05) but a significant interaction between alcohol use and HDL cholesterol and apo A-I (P-interaction < 0.01). The observed association between HDL cholesterol and apo A-I and cancer risk was dependent on the alcohol intake category; multivariable HRs of lung cancer per 1 SD of HDL were 0.79 (95% CI: 0.61, 1.01; P = 0.06) in abstainers, 0.96 (95% CI: 0.50, 1.82; P = 0.89) in subjects who had 1–3 drinks/mo, 0.87 (95% CI: 0.62, 1.22; P = 0.40) in subjects who had 1–6 drinks/wk, and 0.90 (95% CI: 0.62, 1.32; P = 0.60) in subjects who had ≥1 drink/d. The corresponding multivariable HRs of lung cancer per 1 SD of apo A-I were 0.83 (95% CI: 0.66, 1.05; P = 0.12), 1.04 (95% CI: 0.58, 1.87; P = 0.89), 0.90 (95% CI: 0.64, 1.25; P = 0.52), and 0.97 (95% CI: 0.67, 1.41; P = 0.88), respectively.
TABLE 5.
Incidence of lung cancer according to quartiles of lipid biomarkers1
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trend | |
| Total cholesterol, mg/dL | 72.0–180.02 | 181.0–205.0 | 206.0–234.0 | 235.0–474.0 | — |
| Cases/noncases, n | 25/3877 | 43/3879 | 64/3917 | 58/3739 | — |
| Age and treatment | 1.00 (—)3 | 1.55 (0.95, 2.54) | 2.01 (1.26, 3.21) | 1.77 (1.10, 2.86) | 0.03 |
| Model 2 | 1.00 (—) | 1.47 (0.89, 2.43) | 1.72 (1.07, 2.76) | 1.34 (0.82, 2.18) | 0.42 |
| Model 2 + BMI | 1.00 (—) | 1.49 (0.90, 2.47) | 1.74 (1.08, 2.80) | 1.37 (0.84, 2.24) | 0.36 |
| HDL cholesterol, mg/dL | 17.1–41.3 | 41.4–49.2 | 49.3–58.9 | 59.0–151.1 | — |
| Cases/noncases, n | 62/3873 | 51/3850 | 40/3844 | 37/3844 | — |
| Age and treatment | 1.00 (—) | 0.83 (0.57, 1.20) | 0.65 (0.44, 0.97) | 0.59 (0.39, 0.88) | 0.006 |
| Model 2 | 1.00 (—) | 0.95 (0.66, 1.39) | 0.75 (0.49, 1.14) | 0.74 (0.49, 1.14) | 0.11 |
| Model 2 + BMI | 1.00 (—) | 0.87 (0.60, 1.27) | 0.65 (0.42, 0.99) | 0.59 (0.38, 0.93) | 0.01 |
| Triglycerides, mg/dL | 13.0–76.0 | 77.0–110.0 | 111.0–162.0 | 163.0–995.0 | — |
| Cases/noncases, n | 29/3913 | 47/3877 | 57/3781 | 57/3841 | — |
| Age and treatment | 1.00 (—) | 1.49 (0.94, 2.37) | 1.76 (1.12, 2.76) | 1.69 (1.07, 2.65) | 0.06 |
| Model 2 | 1.00 (—) | 1.40 (0.87, 2.25) | 1.53 (0.96, 2.44) | 1.35 (0.85, 2.15) | 0.47 |
| Model 2 + BMI | 1.00 (—) | 1.46 (0.91, 2.35) | 1.67 (1.04, 2.66) | 1.58 (0.98, 2.56) | 0.15 |
| LDL cholesterol, mg/dL | 17.3–102.0 | 102.1–123.3 | 123.4–146.9 | 147.0–335.4 | — |
| Cases/noncases, n | 31/3872 | 36/3876 | 59/3829 | 64/3835 | — |
| Age and treatment | 1.00 (—) | 1.03 (0.64, 1.67) | 1.55 (1.00, 2.41) | 1.53 (0.99, 2.38) | 0.02 |
| Model 2 | 1.00 (—) | 0.93 (0.57, 1.52) | 1.39 (0.89, 2.17) | 1.21 (0.77, 1.88) | 0.23 |
| Model 2 + BMI | 1.00 (—) | 0.94 (0.57, 1.54) | 1.41 (0.90, 2.21) | 1.25 (0.80, 1.96) | 0.17 |
| apo A-I, mg/dL | 12.3–127.3 | 127.4–140.4 | 140.5–155.7 | 155.7–238.3 | — |
| Cases/noncases, n | 60/3830 | 44/3831 | 43/3824 | 39/3825 | — |
| Age and treatment | 1.00 (—) | 0.68 (0.46, 1.00) | 0.65 (0.44, 0.97) | 0.56 (0.38, 0.84) | 0.006 |
| Model 2 | 1.00 (—) | 0.80 (0.54, 1.19) | 0.80 (0.54, 1.21) | 0.68 (0.44, 1.06) | 0.09 |
| Model 2 + BMI | 1.00 (—) | 0.78 (0.52, 1.16) | 0.74 (0.49, 1.12) | 0.60 (0.38–0.94) | 0.03 |
| apo B-100, mg/dL | 21.8–82.4 | 82.6–98.9 | 99.0–120.2 | 120.4–257.4 | — |
| Cases/noncases, n | 31/3859 | 32/3841 | 55/3820 | 68/3788 | — |
| Age and treatment | 1.00 (—) | 0.90 (0.55, 1.49) | 1.45 (0.93, 2.27) | 1.63 (1.05, 2.52) | 0.004 |
| Model 2 | 1.00 (—) | 0.90 (0.54, 1.50) | 1.32 (0.84, 2.09) | 1.24 (0.79, 1.94) | 0.17 |
| Model 2 + BMI | 1.00 (—) | 0.94 (0.56, 1.57) | 1.41 (0.89, 2.22) | 1.38 (0.87, 2.18) | 0.07 |
Treatment denotes the treatment random assignment (aspirin, vitamin E, or β-carotene). Model 2 was adjusted for age, race, treatment random assignment (aspirin, vitamin E, or β-carotene), hormone replacement therapy (past or none), cigarette smoking (never, past, or current), exercise [total kilocalories from exercise and the number of stairs taken per week (4 categories as follows: <200, 200 to <600, 600 to <1500, and ≥1500)], alcohol consumption (continuous; grams per day), postmenopausal status (yes or no), family history of cancer (yes or no), aspirin use (current use >1 time/wk compared with no use), and history of mammogram (yes or no). Model 2 + BMI was adjusted as for model 2 as well as for BMI (in kg/m2) in 5 categories (<18.5, 18.5 to <25, 25 to <30, 30 to <35, and ≥35; the reference group was BMI from 18.5 to <25). apo A-I, apolipoprotein A-I; apo B-100, apolipoprotein B-100.
Range (all such values).
HR; 95% CI in parentheses (all such values).
Fasting status
With the exception of triglycerides, a previous study in this cohort revealed no substantial changes in the distributions of lipids and apolipoprotein concentrations as a function of time since the last meal (23). Of 15,602 participants, 11,300 subjects (72%) provided fasting blood samples. Subgroup analyses for fasting status (time since last meal <8 compared with ≥8 h) revealed no significant interaction between fasting status and triglycerides (P-interaction > 0.05) for total cancer, CRC, breast cancer, and lung cancer. The association between triglycerides and incident CRC was stronger but in the same direction with fasting compared with nonfasting [triglycerides per 1 SD: HR, 1.23 (95% CI: 1.08, 1.41; P = 0.002) and HR, 1.04 (95% CI: 0.79, 1.37; P = 0.77), respectively. In contrast, breast, lung, and total cancers displayed similar magnitudes of association for fasting and nonfasting triglycerides.
Cancer mortality
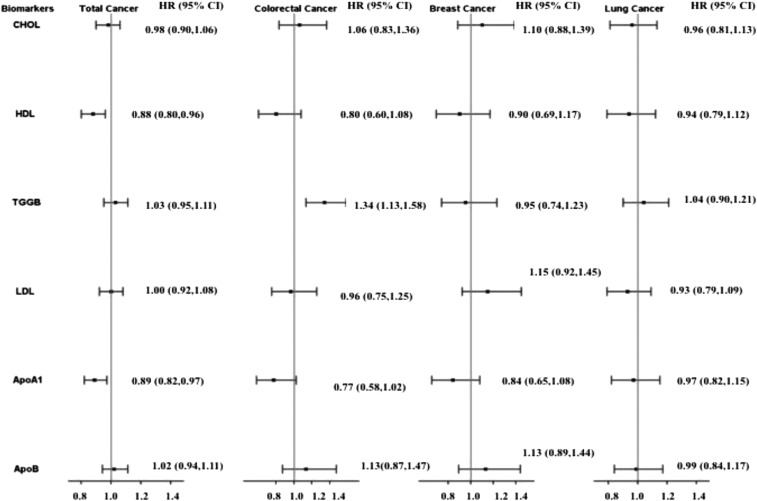
Higher apo A-I and HDL were associated with decreased risk of total cancer mortality in multivariable models with and without BMI (Figure 2, Supplemental Table 8). Higher apo A-I was also associated with decreased risk of CRC death, and higher triglycerides was associated with increased risk of CRC deaths, in multivariable models with and without BMI (Supplemental Table 9). Higher apo A-I tended to be associated with decreased risk of breast cancer (P = 0.06) (Supplemental Table 10). No lipid marker was associated with lung cancer deaths in multivariable models with and without BMI (all P-trend > 0.05) (Supplemental Table 11).
FIGURE 2.
HRs per 1 SD for cancer mortality events are shown on the y axis (log scale) for CHOL, HDL, TGGBs, LDL, ApoA1, and ApoB. Cancer death cases were as follows: total, n = 647; lung, n = 156; colorectal, n = 62; and breast. n = 80. Values were evaluated with the use of Cox proportional hazard models. SD concentrations were as follows: CHOL, 209.2 mg/dL; HDL, 51.1 mg/dL; TGGB, 133.8 mg/dL; LDL, 126.1 mg/dL; ApoA1, 142.3 mg/dL; and ApoB, 102.7 mg/dL. Categories for variables were as follows: treatment random assignment (aspirin, vitamin E, beta-carotene), hormone replacement therapy (past or none), cigarette smoking (never, past, or current), exercise [total kilocalories from exercise and the number of stairs taken per week (4 categories as follows: <200, 200 to <600, 600 to <1500, ≥ 1500)], alcohol consumption (continuous; grams per day), postmenopausal status (yes or no), family history of cancer (yes or no), aspirin use (current use >1 time/wk or no use), history of colon polyps (yes or no), history of fibrocystic or benign breast disease (yes or no), total vegetables and fruit intake (servings/d), history of mammogram (yes or no), age of menarche (≤11, 12, 13, or ≥14 y), gestational age in weeks at completion of first pregnancy lasting >6 mo (nulliparous, ≤29, or ≥30), and BMI (in kg/m2) in 5 categories (<18.5, 18.5 to <25, 25 to <30, 30 to <35, or ≥35; the reference group was 18.5 to <25). HRs were adjusted for the following variables—for total cancer: age, race, treatment random assignment, hormone replacement therapy, cigarette smoking, exercise, alcohol consumption, postmenopausal status, family history of cancer, aspirin use, history of colon polyps, history of fibrocystic or benign breast disease, total vegetable and fruit intake, history of mammogram, and BMI; for colorectal cancer: age, race, treatment random assignment, hormone replacement therapy, cigarette smoking, exercise, alcohol consumption, postmenopausal status, family history of cancer, aspirin use, history of colon polyps, total vegetable and fruit intake, history of mammogram, red meat intake, and BMI; for breast cancer: age, race, treatment random assignment, hormone replacement therapy, cigarette smoking, exercise, alcohol consumption, postmenopausal status, family history of cancer, aspirin use, history of colon polyps, history of fibrocystic or benign breast disease, total vegetable and fruit intake, history of mammogram, family history of breast cancer in mother or sister at <60 y of age, reproductive history, and BMI; and for lung cancer: age, race, treatment random assignment, hormone replacement therapy, cigarette smoking, exercise, alcohol consumption, postmenopausal status, family history of cancer, aspirin use, history of mammogram, and BMI. ApoA1, apolipoprotein A-I; ApoB, apolipoprotein B-100; CHOL, total cholesterol; HDL, HDL cholesterol; LDL, LDL cholesterol; TGGB, triglyceride.
Lipid-medication use
The use of time-dependent variables to assess the impact of lipid-lowering medications on the observed associations revealed no significant interaction between such medications and lipid and lipoprotein biomarkers with risk of incident total cancer, breast cancer, CRC, or lung cancer (P-interaction > 0.05).
Secondary analyses for lag time
In secondary analyses in which participants who were diagnosed with cancer ≤2 or 4 y of the baseline blood draw were excluded, the observed direction of an association between apo A-I and HDL for total cancer incidence and mortality; HDL, triglycerides, and apo B-100 for CRC incidence and mortality; and HDL for lung cancer incidence and mortality remained. The association between each lipid biomarker and total cancer and site-specific cancers are shown in the plots of cancer incidence [only adjusted for age and treatment random assignment (i.e., aspirin, vitamin E, or β-carotene] over time according to baseline lipid values (Supplemental Figures 3–14).
DISCUSSION
In this prospective study of middle-aged and older women, we showed that circulating apo A-I and HDL were inversely associated with risk of total cancer and lung cancer, whereas triglycerides and apo B-100 were positively associated, and HDL was inversely associated, with the risk of CRC. These findings support a possible role of lipid metabolism in the development of cancers. An attenuation of the associations with adjustment for BMI suggested that these serum lipids may be involved in carcinogenesis through pathophysiologic processes that are related to obesity.
The development and progression of cancer is a multistep process with complex interactions between behavioral and environmental factors, dysregulated lipid homeostasis (30, 31), and the cancer microenvironment. Fatty acids are required for energy storage, membrane production, and the generation of signaling molecules in cancer cells (32, 33). However, it is unclear if the cancer-related lipid-metabolism changes can be detected at the concentration of circulating lipid markers.
Our results on apo A-I and total cancer are in agreement with previously published work (24). The prospective European Prospective Investigation into Cancer and Nutrition study, with 1238 cancer cases, showed a significant inverse association between apo A-I concentrations and risk of colon cancer (RR for 1-SD increase: 0.82; 95% CI: 0.72, 0.94) (25). The association was not influenced by biomarkers of systemic inflammation, insulin resistance, and oxidative stress or by the exclusion of cancers that occurred in the first 2 y of follow-up. Two small case-control studies of apo A-I and breast cancer have mixed results (26, 27). Han et al. (27) showed increased risk of developing breast cancer with elevated serum apo A-I in Chinese women (multivariable models including BMI). In contrast, Chang et al. (26) showed reduced risk of breast cancer with higher apo A-I in Taiwanese women, and BMI was not measured and adjusted for in the study. A large nested case-control study, in which concentrations of lipid markers were measured at multiple times before the diagnosis of breast cancer, showed decreased risk in breast cancer with higher apo A-I (28). Prospective data on apo A-I and lung cancer risk are lacking.
A recent study in the prospective Metabolic Syndrome and Cancer Project reported RRs of 1.16 (95% CI: 1.06, 1.26) in men and 1.15 (95% CI: 1.05, 1.27) in women for total cancer with the top quintile compared with the bottom quintile of triglycerides, which suggested a potential role of triglycerides in cancer development. In our study, higher triglycerides were not associated with total cancer risk but was associated with increased risk of CRC. CRC risk is increased with obesity (29–33), hyperinsulinemia (34–36), type 2 diabetes (30), and lower plasma adiponectin (a marker of insulin resistance) (37). Triglyceride concentrations may reflect insulin status and glycemia status and may also represent the complex effects of hyperglycemia and hyperinsulinemia on carcinogenesis pathways (38). Insulin increases the production of free insulin-like growth factor-1, which is a mitogenic molecule (38), and adiopocyte-derived vascular endothelial growth factor, which is a critical angiogenic factor that influences cell survival and migration (39). In addition, hyperinsulinemia induces inflammation, which is an established risk factor that promotes carcinogenesis (40). In contrast, the positive association between triglycerides and CRC risk may be explained by the dysregulation of lipid metabolism that is caused by cancer development such as imbalances in the gut microbiota (41) and elevated concentrations of fecal bile acids (42).
Previous case-control studies have shown that HDL-cholesterol concentrations were lower in lung cancer cases than in controls (43, 44). Kucharska-Newton et al. (45) prospectively evaluated the association of baseline HDL cholesterol with the incidence of lung cancer and showed a weak inverse association that was dependent on smoking status. Our study showed an inverse association of HDL cholesterol with lung cancer and CRC. Because smoking is a major risk factor for cancer, particularly for lung cancer, and smoking is associated with lower HDL cholesterol, a potential confounder that underlies the observed association between HDL cholesterol and cancer risk may be smoking.
Apolipoprotein B (apoB) contains the binding site for the LDL receptor that is present on the cell surface (46) and plays a critical role in the uptake of cholesterol by the cell (47). The prospective Swedish Apolipoprotein Mortality Risk study observed no significant association of apoB with CRC but showed a significant positive association between triglycerides and colon cancer risk (48). This study had a larger sample size but a shorter duration of follow up (mean: 12 y) and included both men and women. No adjustment was made for menopausal status or HRT use. Information on smoking status and alcohol use was unavailable and could not be adjusted (48). Similarly, the European Prospective Investigation into Cancer and Nutrition nested case-control study reported no association between apoB and CRC but noted an inverse association between HDL cholesterol and CRC (25). A case-control study of human surgical specimens showed that glycated apoB was associated with colorectal adenomas and CRC (49). In a cross-sectional adenoma study, a nonsignificant association between higher concentrations of LDL cholesterol and apoB with a higher prevalence of advanced adenoma was observed along with a significant association between high triglycerides and an increased prevalence of adenomas.
Our study has numerous strengths, including a large number of cancer cases, high follow-up rate, prospective study design, standard measurement of lipid markers, and comprehensive covariate information including lifestyle, medical history, and medication use. Time-dependent variables were used to assess the impact of lipid-lowering medications on the observed associations. The long duration of follow-up reduced the likelihood of reverse causation as a possible explanation for the observed results.
The current study also has notable limitations. First, the lack of repeated measurements of lipid markers to reflect changes during follow-up may have biased the HRs toward the null. Second, residual confounding may explain our findings. However, multiple confounders were accounted for in multivariable models. We did not observe significant changes in risk estimates before or after controlling for these confounders. Third, WHS participants were predominantly white women, which limits the generalizability to other populations. To further evaluate the role of serum lipids in cancer development, more prospective cohort studies and experimental studies are needed to elucidate both systemic and regional effects of lipid constituents on carcinogenesis.
In conclusion, this large prospective cohort study of women showed that plasma apo A-I is inversely associated with risk of total cancer, whereas triglycerides and apo B-100 are associated with increased risk and HDL cholesterol with decreased risk of CRC. Evidence for a positive association of apo B-100 and an inverse association of HDL cholesterol with lung cancer risk exists after adjustment for BMI. Future studies are warranted to confirm or refute our findings.
Acknowledgments
We thank Kevin P McMahon for manuscript preparation assistance.
The authors’ responsibilities were as follows—PDC, YS, JL, SZ, HDS, SM, ELG, KER, PMR, I-ML, JEM, JEB, and LW: designed the research; PDC, MVM, CL, and LW: analyzed the data; PDC, MVM, and LW: performed the statistical analyses; PDC and LW: had primary responsibility for the final content of the manuscript; and all authors: wrote the manuscript and read and approved the final manuscript. SM has received research support from the National Heart, Lung, and Blood Institute, AstraZeneca, and Atherotech Diagnostics and has served as a consultant to Lilly, Pfizer, and Cerenis Therapeutics. PMR has received research support from AstraZeneca, Novartis, Roche, and Sanofi-Aventis. The other authors reported no conflicts of interest related to the study.
Footnotes
Abbreviations used: apo A-I, apolipoprotein A-I; apo B-100, apolipoprotein B-100; apoB, apolipoprotein B; CRC, colorectal cancer; CVD, cardiovascular disease; HRT, hormone replacement therapy; WHS, Women’s Health Study.
REFERENCES
- 1.Pathak K, Soares MJ, Calton EK, Zhao Y, Hallett J. Vitamin D supplementation and body weight status: a systematic review and meta-analysis of randomized controlled trials. Obes Rev 2014;15:528–37. [DOI] [PubMed] [Google Scholar]
- 2.Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr. Cellular fatty acid metabolism and cancer. Cell Metab 2013;18:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackerman D, Simon MC. Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment. Trends Cell Biol 2014;24:472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech 2013;6:1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichholzer M, Stahelin HB, Gutzwiller F, Ludin E, Bernasconi F. Association of low plasma cholesterol with mortality for cancer at various sites in men: 17-y follow-up of the prospective Basel study. Am J Clin Nutr 2000;71:569–74. [DOI] [PubMed] [Google Scholar]
- 6.Nago N, Ishikawa S, Goto T, Kayaba K. Low cholesterol is associated with mortality from stroke, heart disease, and cancer: the Jichi Medical School Cohort Study. J Epidemiol 2011;21:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strohmaier S, Edlinger M, Manjer J, Stocks T, Bjorge T, Borena W, Haggstrom C, Engeland A, Nagel G, Almquist M, et al. Total serum cholesterol and cancer incidence in the Metabolic syndrome and Cancer Project (Me-Can). PLoS One 2013;8:e54242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitahara CM, Berrington de Gonzalez A, Freedman ND, Huxley R, Mok Y, Jee SH, Samet JM. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol 2011;29:1592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iso H, Ikeda A, Inoue M, Sato S, Tsugane S. Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. Int J Cancer 2009;125:2679–86. [DOI] [PubMed] [Google Scholar]
- 10.Aleksandrova K, Jenab M, Bueno-de-Mesquita HB, Fedirko V, Kaaks R, Lukanova A, van Duijnhoven FJ, Jansen E, Rinaldi S, Romieu I, et al. Biomarker patterns of inflammatory and metabolic pathways are associated with risk of colorectal cancer: results from the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Epidemiol 2014;29:261–75. [DOI] [PubMed] [Google Scholar]
- 11.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 2003;348:2082–90. [DOI] [PubMed] [Google Scholar]
- 12.Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, Fiol M, Gomez-Gracia E, Lopez-Sabater MC, Vinyoles E, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med 2006;145:1–11. [DOI] [PubMed] [Google Scholar]
- 13.Kelley GA, Kelley KS, Tran ZV. Aerobic exercise and lipids and lipoproteins in women: a meta-analysis of randomized controlled trials. J Womens Health (Larchmt) 2004;13:1148–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Criqui MH, Wallace RB, Heiss G, Mishkel M, Schonfeld G, Jones GT. Cigarette smoking and plasma high-density lipoprotein cholesterol. The Lipid Research Clinics Program Prevalence Study. Circulation 1980;62:IV70–6. [PubMed] [Google Scholar]
- 15.Torriani M, Gill CM, Daley S, Oliveira AL, Azevedo DC, Bredella MA. Compartmental neck fat accumulation and its relation to cardiovascular risk and metabolic syndrome. Am J Clin Nutr 2014;100:1244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lessard J, Laforest S, Pelletier M, Leboeuf M, Blackburn L, Tchernof A. Low abdominal subcutaneous preadipocyte adipogenesis is associated with visceral obesity, visceral adipocyte hypertrophy, and a dysmetabolic state. Adipocyte 2014;3:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang SM, Buring JE, Lee IM, Cook NR, Ridker PM. C-reactive protein levels are not associated with increased risk for colorectal cancer in women. Ann Intern Med 2005;142:425–32. [DOI] [PubMed] [Google Scholar]
- 18.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009;119:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA 2006;295:1412–9. [DOI] [PubMed] [Google Scholar]
- 20.Willett WC, Reynolds RD, Cottrell-Hoehner S, Sampson L, Browne ML. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc 1987;87:43–7. [PubMed] [Google Scholar]
- 21.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 22.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 23.Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation 2008;118:993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.His M, Zelek L, Deschasaux M, Pouchieu C, Kesse-Guyot E, Hercberg S, Galan P, Latino-Martel P, Blacher J, Touvier M. Prospective associations between serum biomarkers of lipid metabolism and overall, breast and prostate cancer risk. Eur J Epidemiol 2014;29:119–32. [DOI] [PubMed] [Google Scholar]
- 25.van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M, Jenab M, Pischon T, Jansen EH, Frohlich J, Ayyobi A, Overvad K, Toft-Petersen AP, et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut 2011;60:1094–102. [DOI] [PubMed] [Google Scholar]
- 26.Chang SJ, Hou MF, Tsai SM, Wu SH, Hou LA, Ma H, Shann TY, Wu SH, Tsai LY. The association between lipid profiles and breast cancer among Taiwanese women. Clin Chem Lab Med 2007;45:1219–23. [DOI] [PubMed] [Google Scholar]
- 27.Han C, Zhang HT, Du L, Liu X, Jing J, Zhao X, Yang X, Tian B. Serum levels of leptin, insulin, and lipids in relation to breast cancer in china. Endocrine 2005;26:19–24. [DOI] [PubMed] [Google Scholar]
- 28.Martin LJ, Melnichouk O, Huszti E, Connelly PW, Greenberg CV, Minkin S, Boyd NF. Serum lipids, lipoproteins, and risk of breast cancer: a nested case-control study using multiple time points. J Natl Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slattery ML. Physical activity and colorectal cancer. Sports Med 2004;34:239–52. [DOI] [PubMed] [Google Scholar]
- 30.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology 2007;132:2208–25. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 1995;122:327–34. [DOI] [PubMed] [Google Scholar]
- 32.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 2007;86:s836–42. [DOI] [PubMed] [Google Scholar]
- 33.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr 2001;131(11 Suppl):3109S–20S. [DOI] [PubMed] [Google Scholar]
- 34.Giovannucci E. Insulin and colon cancer. Cancer causes & control. CCC 1995;6:164–79. [DOI] [PubMed] [Google Scholar]
- 35.Wei EK, Ma J, Pollak MN, Rifai N, Fuchs CS, Hankinson SE, Giovannucci E. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 2005;14:850–5. [DOI] [PubMed] [Google Scholar]
- 36.Wei EK, Ma J, Pollak MN, Rifai N, Fuchs CS, Hankinson SE, Giovannucci E. C-peptide, insulin-like growth factor binding protein-1, glycosylated hemoglobin, and the risk of distal colorectal adenoma in women. Cancer Epidemiol Biomarkers Prev 2006;15:750–5. [DOI] [PubMed] [Google Scholar]
- 37.Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst 2005;97:1688–94. [DOI] [PubMed] [Google Scholar]
- 38.Gialamas SP, Petridou ET, Tseleni-Balafouta S, Spyridopoulos TN, Matsoukis IL, Kondi-Pafiti A, Zografos G, Mantzoros CS. Serum adiponectin levels and tissue expression of adiponectin receptors are associated with risk, stage, and grade of colorectal cancer. Metabolism 2011;60:1530–8. [DOI] [PubMed] [Google Scholar]
- 39.Williams CJ, Mitsiades N, Sozopoulos E, Hsi A, Wolk A, Nifli AP, Tseleni-Balafouta S, Mantzoros CS. Adiponectin receptor expression is elevated in colorectal carcinomas but not in gastrointestinal stromal tumors. Endocr Relat Cancer 2008;15:289–99. [DOI] [PubMed] [Google Scholar]
- 40.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA 2005;294:47–55. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Y, Michelle Luo T, Jobin C, Young HA. Gut microbiota and probiotics in colon tumorigenesis. Cancer Lett 2011;309:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong JL, Ran ZH, Shen J, Fan GQ, Xiao SD. Association between fecal bile acids and colorectal cancer: a meta-analysis of observational studies. Yonsei Med J 2008;49:792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siemianowicz K, Gminski J, Stajszczyk M, Wojakowski W, Goss M, Machalski M, Telega A, Brulinski K, Magiera-Molendowska H. Serum HDL cholesterol concentration in patients with squamous cell and small cell lung cancer. Int J Mol Med 2000;6:307–11. [DOI] [PubMed] [Google Scholar]
- 44.Fiorenza AM, Branchi A, Sommariva D. Serum lipoprotein profile in patients with cancer. A comparison with non-cancer subjects. Int J Clin Lab Res 2000;30:141–5. [DOI] [PubMed] [Google Scholar]
- 45.Kucharska-Newton AM, Rosamond WD, Schroeder JC, McNeill AM, Coresh J, Folsom AR. Members of the Atherosclerosis Risk in Communities Study. HDL-cholesterol and the incidence of lung cancer in the Atherosclerosis Risk in Communities (ARIC) study. Lung Cancer 2008;61:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldstein JL, Brown MS. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem 1974;249:5153–62. [PubMed] [Google Scholar]
- 47.Goldstein JL, Brown MS. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem 1977;46:897–930. [DOI] [PubMed] [Google Scholar]
- 48.Wulaningsih W, Garmo H, Holmberg L, Hammar N, Jungner I, Walldius G, Van Hemelrijck M.. Serum lipids and the risk of gastrointestinal malignancies in the Swedish AMORIS study. J Cancer Epidemiol 2012;2012:792034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reddavide R, Misciagna G, Caruso MG, Notarnicola M, Armentano R, Caruso ML, Pirrelli M, Valentini AM. Tissue expression of glycated apolipoprotein B in colorectal adenoma and cancer. Anticancer Res 2011;31:555–9. [PubMed] [Google Scholar]