Abstract
Background: Chronic obstructive pulmonary disease (COPD) is a condition characterized by systemic low-grade inflammation that could increase the production of nitric oxide (NO), of which arginine is the sole precursor. Arginine is derived from the breakdown of protein and through the conversion of citrulline to arginine (de novo arginine production).
Objective: Our objective was to study whole-body arginine and citrulline and related metabolism in stable COPD patients.
Design: With the use of stable isotope methodology, we studied whole-body arginine and citrulline rates of appearance, de novo arginine (citrulline-to-arginine flux) and NO (arginine-to-citrulline flux) production, protein synthesis and breakdown rates, and plasma amino acid concentrations in a heterogeneous group of patients with moderate-to-severe COPD [n = 23, mean ± SE age: 65 ± 2 y, forced expiratory volume in 1 s (FEV1): 40% ± 2% of predicted], and a group of healthy older adults (n = 19, mean ± SE age: 64 ± 2 y, FEV1: 95% ± 4% of predicted).
Results: Although plasma arginine and citrulline concentrations were comparable between COPD patients and controls, whole-body arginine (P = 0.015) and citrulline (P = 0.026) rates of appearance were higher in COPD patients and related to a 57% greater de novo arginine production (P < 0.0001). Despite a higher whole-body arginine clearance in COPD patients (P < 0.0001), we found no difference in NO production.
Conclusion: In stable patients with moderate-to-severe COPD, endogenous arginine production is upregulated to support a higher arginine utilization that is unrelated to whole-body NO production. This trial was registered at clinicaltrials.gov as NCT01173354 and NCT01172314.
Keywords: COPD, arginine, citrulline, nitric oxide, protein breakdown
INTRODUCTION
Chronic obstructive pulmonary disease (COPD)7 patients are characterized by both airway (1) and low-grade systemic inflammation (2). The large heterogeneity in patients is attributed to the variation in disease severity, symptoms, and comorbidities (3). We and others found changes in plasma concentrations of Arg and/or related metabolites in COPD and other inflammatory airway diseases (4–7), suggestive of a modified Arg metabolism.
Through the arginase pathway, Arg is converted to ornithine and urea. In both acute and chronic inflammatory conditions, arginase has been linked to systemic Arg depletion, increased disposal, and suppressed immune function (8–10). Furthermore, airway expression of arginase in COPD has been linked to airway remodeling (11, 12).
Through the nitric oxide synthase (NOS) pathway, Arg is converted to nitric oxide (NO). Like arginase, the enzyme NOS has multiple isoforms and is expressed in various organs, and its activity can change rapidly in response to disease related stimuli. In the presence of proinflammatory mediators, NO production is stimulated as part of the immune response (13), but also plays an important role in smooth-muscle relaxation, thereby affecting airway function (14, 15).
Aside from the above, Arg is primarily used toward protein synthesis (PS), and it is mainly derived from protein breakdown (PB) (∼80%) (8). A small amount of Arg is produced via the conversion of citrulline to Arg in the kidneys (de novo Arg production) (16). The direct relation between protein turnover and Arg metabolism suggests that, aside from the upregulated whole-body protein turnover (17, 18), COPD patients are also characterized by an elevated whole-body Arg metabolism. No data are available currently on Arg de novo production and Arg utilization in COPD.
We recently described an upregulated whole-body Arg and citrulline metabolism, NO production, and protein turnover in cystic fibrosis (CF) (4), another inflammatory airway disease characterized by low-grade systemic inflammation and reduced pulmonary function (19, 20). We therefore hypothesized that COPD patients have an altered Arg metabolism that is characterized by an upregulated whole-body (de novo) Arg and citrulline metabolism in support of a higher Arg utilization for the production of NO.
On that account, with the use of advanced stable isotope methodology, we measured in vivo 1) whole-body Arg and citrulline rates of appearance, 2) de novo Arg production (conversion of citrulline to Arg), 3) NO production (conversion of Arg to citrulline), 4) Arg clearance, and 5) PS and PB in a heterogeneous group of patients with stable moderate-to-severe COPD and healthy adults of similar age.
METHODS
Subject inclusion
We included 23 patients with a clinical diagnosis of moderate-to-severe airflow obstruction (grades II–IV), according to the established Global Initiative for Chronic Obstructive Lung Disease guidelines (3) (NCT01173354), and 19 healthy subjects of similar age (NCT01172314) (Supplemental Figure 1). Recruitment took place through pulmonologist referral and local advertising. Medical history and medication use were assessed as part of the screening process. All COPD patients were in clinically stable condition and not suffering from a respiratory tract infection or exacerbation of their disease ≤4 wk before the study. Except for 2 patients who did not receive any maintenance therapy, all received bronchodilator treatment. Eighteen patients also received inhalation corticosteroids and 9 were on long-term oxygen therapy. Use of systemic corticosteroids ≤1 mo before the study was an exclusion criteria, as were malignancy; recent surgery; and severe endocrine, hepatic, or renal disorders. Written informed consent was obtained from all subjects, and the Institutional Review Board of the University of Arkansas for Medical Sciences approved the study (nos. 105558 and 112254).
Lung function and anthropometric data
Height and weight were measured with the use of standard procedures, whereas body composition was assessed by dual-energy X-ray absorptiometry (Hologic QDR 4500). Values were standardized for height. Forced expiratory volume in 1 s (FEV1) was assessed with the highest value from ≥3 technically acceptable maneuvers being used (21).
Study design
All participants were studied 2 times within 1 wk (≥1 d apart) (Figure 1). Test days started in the early morning after an overnight fast and lasted 3 h each, during which subjects were in a supine position. A catheter was inserted into an antecubital vein to measure natural isotope enrichment, after which a primed (enterally or intravenously), constant intravenous infusion of stable amino acid isotopes was started to measure Arg and citrulline metabolism and protein turnover (Table 1). A second catheter for arterialized venous blood sampling was placed in a superficial dorsal vein of the contralateral hand or lower arm. The hand was placed in a thermostatically controlled heated box, a technique to mimic direct arterial sampling (22) for the measurement of isotope enrichment values. Blood was processed and analyzed in batch by liquid chromatography–electrospray ionization–tandem mass spectrometry with the use of routine laboratory procedures (4).
FIGURE 1.
Study design. All participants were studied 2 times within 1 wk (≥1 d apart) during a 3-h infusion protocol. t, time.
TABLE 1.
Infusion of stable isotopes1
| Isotope | Prime, μmol/kg BW | Infusion rate, μmol · kg BW−1 · h−1 |
| L-[guanidine-15N2]-arginine | 3.75 | 3.75 |
| L-[13C,2H4]-citrulline or L-[13C,2H2]-citrulline | 0.88 | 0.30 |
| L-[ring-2H5]-phenylalanine | 3.60 | 3.60 |
| L-[ring-2H2]-tyrosine | 1.14 | 1.14 |
| L-[ring-2H4]-tyrosine | 0.31 | — |
Stable isotopes were purchased from Cambridge Isotopic Laboratories. BW, body weight.
Calculation of metabolic markers
Whole-body Arg, citrulline, Phe, and Tyr appearance rates, de novo Arg production (citrulline-to-Arg flux), and NO production (Arg-to-citrulline flux), were calculated from isotope enrichment values as described previously (23). Calculations for whole-body protein metabolism included PS, PB, and net PS (PS − PB) (24). Calculations were based on metabolic data obtained under steady-state conditions.
Statistical analyses
Results are expressed as means ± SEs. Population characteristics and baseline measurements were compared with the use of the unpaired Student’s t test, Mann-Whitney test, or Fisher’s exact test, depending on the type of variable and distribution of the data. Calculations for Arg, citrulline, and protein metabolism were done with the use of the median value for measurements taken at 150, 165, and 180 min after the start of infusion. Two-factor repeated-measures ANOVA with “group” and “day” was used to compare differences between healthy controls and COPD patients, as well as between test days with the level of significance set at P < 0.05. The statistical package within Graphpad Prism (version 6.04) was used for data analysis.
RESULTS
COPD patients in this study formed a heterogeneous group that varied in lung function characteristics, exacerbation frequency, BMI and body composition, and comorbidities (Table 2). Of all patients, 35% were considered to be nutritionally depleted [fat-free mass index (in kg/m2) ≤15 (women) or ≤16 (men)] (25), 39% had ≥2 exacerbations/y or ≥1 hospitalization/y for an exacerbation, and 60% (based on n = 15) had a diffusing capacity of the lung for carbon monoxide (DLCO) <60 (percentage of predicted), indicative of a moderate-to-severe reduction in diffusing capacity (26). COPD patients were significantly different from the control subjects for lung function (FEV1 percentage of predicted) (P < 0.0001), smoking history and pack-years (P < 0.0001), time since smoking cessation (P = 0.006), and prevalence of depression and/or anxiety (P = 0.0478). Body weight and composition measures were comparable between the groups.
TABLE 2.
Population characteristics and baseline measurements1
| Healthy (n = 19) | COPD (n = 23) | |
| General characteristics | ||
| Sex, M/F | 11/8 | 13/10 |
| Age, y | 64 ± 2 | 65 ± 2 |
| Smoking history, yes/no | 7/12 | 23/0**** |
| Pack-years | 10 ± 5 | 55 ± 7**** |
| Smoking status, smoker/nonsmoker | 1/18 | 7/16 |
| Time since smoking cessation, y | 31 ± 8 | 10 ± 3** |
| Weight, kg | 81 ± 3 | 75 ± 3 |
| BMI, kg/m2 | 27.8 ± 1.1 | 26.3 ± 1.0 |
| hsCRP, mg/L | 2.3 ± 0.7 | 3.7 ± 0.9 |
| Pulmonary function– and COPD-related measures | ||
| FEV1, % of predicted | 95 ± 4 | 40 ± 2**** |
| FVC,2 % of predicted | 70 ± 4 | |
| FEV1:FVC ratio2 | 0.42 ± 0.02 | |
| DLCO,3 % of predicted | 52 ± 5 | |
| Time since initial diagnosis, y | 11 ± 2 | |
| Self-reported amount of time of COPD-related symptoms, y | 15 ± 3 | |
| 0–1 exacerbation/y and no hospitalization for exacerbation, yes/no | 14/9 | |
| ≥2 exacerbations/y or ≥1 hospitalization for exacerbation, yes/no | 9/14 | |
| Comorbidities, yes/no | ||
| Cardiovascular disease | 9/10 | 15/8 |
| Depression and/or anxiety | 3/16 | 11/12* |
| Gastroesophageal reflux disease | 3/16 | 9/14 |
| Hyperlipidemia | 6/13 | 8/15 |
| Body composition | ||
| Fat-free mass index,4 kg/m2 | 17.7 ± 0.6 | 17.0 ± 0.5 |
| Skeletal muscle index,5 kg/m2 | 7.1 ± 0.3 | 6.6 ± 0.2 |
| Total fat mass, % | 34 ± 2 | 33 ± 2 |
| Total fat mass index,6 kg/m2 | 9.5 ± 0.8 | 8.9 ± 0.8 |
| Trunk fat mass index,6 kg/m2 | 5.1 ± 0.5 | 4.6 ± 0.4 |
Values are means ± SEs or n/n. For comparison of numerical values, statistics were obtained with the use of either the unpaired Student’s t test (if data were distributed normally) or the Mann-Whitney test. Alternatively, categorical values were compared with the use of Fisher’s exact test. *,**,****Different from controls, *P < 0.05, **P < 0.01, and ****P < 0.0001. COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; hsCRP, high-sensitivity C-reactive protein.
Data from most recent pulmonary function test were used, n = 17.
Data from most recent pulmonary function test were used, n = 15.
Calculated as (muscle mass + bone mineral content)/height squared.
Appendicular skeletal muscle mass/height squared.
(Trunk) fat mass index = (trunk) fat mass/height squared.
Plasma amino acid concentrations
We found a lower plasma Gln concentration in COPD patients (P = 0.0001), but no difference in plasma Arg or citrulline (Table 3). In addition, for all plasma amino acid concentrations listed in Table 3, no differences were found between COPD patients with 0–1 compared with ≥2 exacerbations/y, a DLCO >60 compared with <60 (percentage of predicted), or nutritional depletion compared with no nutritional depletion. An overall reduction in plasma essential (831 ± 29 compared with 622 ± 17 μM, P < 0.0001) and nonessential amino acids was present in COPD patients (1796 ± 62 compared with 1556 ± 35 μM, P = 0.0011). The plasma concentration ratio between Arg and ornithine was not significantly different between the groups; neither was the ratio between Arg and the sum of Pro and Lys.
TABLE 3.
Postabsorptive plasma amino acid concentrations1
| Concentration, μM |
||
| Amino acid | Healthy (n = 19) | COPD (n = 23) |
| Arg | 87 ± 3 | 79 ± 4 |
| Citrulline | 34 ± 1 | 35 ± 2 |
| Gln | 677 ± 21 | 569 ± 20*** |
| Glutamate | 41 ± 3 | 37 ± 2 |
| Ornithine | 51 ± 2 | 50 ± 2 |
Values are means ± SEs. Statistics were obtained with the use of 2-factor repeated-measures ANOVA with “group” and “day” used to compare differences between groups and test days. Besides a significant group effect for Gln, no significant group or day effect or interaction was observed for any of the other measures; therefore, data are shown as the mean value of both days. ***Different from controls, P < 0.001. COPD, chronic obstructive pulmonary disease.
Whole-body amino acid fluxes and protein kinetics
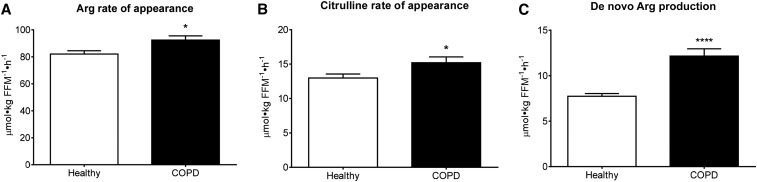
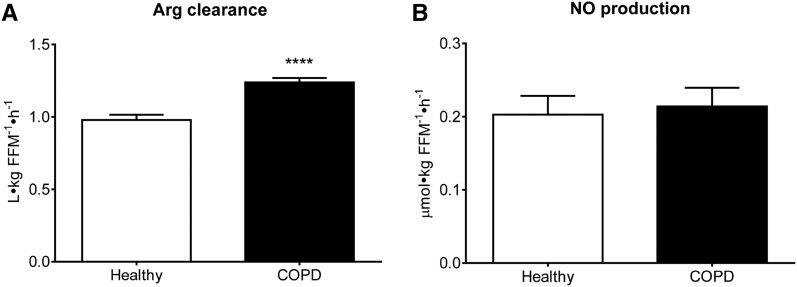
Isotope enrichment data are shown in Supplemental Figures 2–4. We found significantly higher Arg (P = 0.015) and citrulline (P = 0.026) rates of appearance, and a 57% greater de novo Arg production (P < 0.0001) in COPD patients (Figure 2). The extent to which citrulline was used toward de novo Arg production (citrulline rate of appearance divided by de novo Arg production) was also greater (81% ± 2% compared with 60% ± 2%; P < 0.0001). PS and PB rates and net PS were not significantly different (Table 4). The 13% greater Arg rate of appearance was accounted for by a 6% greater de novo Arg production, whereas the remaining 7% only could have come from whole-body PB (8). Calculating the contribution of PB from the appearance rate of Phe, based on a 1:1.7 ratio between Phe and Arg in body protein (27, 28), shows an 8% contribution, supporting this notion. The increase in Arg rate of appearance was associated with a higher Arg clearance (P < 0.0001) that was not related to NO production (Figure 3). No day effect was found for any of the measures, but an interaction between group and day was observed for NO production (P = 0.0325; Supplemental Figure 5). In addition, no differences were found for any of the above measures between COPD patients with 0–1 compared with ≥2 exacerbations/y, a DLCO >60 compared with <60 (percentage of predicted), or nutritional depletion compared with no nutritional depletion, except for a higher Arg rate of appearance (P = 0.0328) in those who were nutritionally depleted.
FIGURE 2.
Mean ± SE postabsorptive whole-body Arg rate of appearance (A), citrulline rate of appearance (B), and de novo Arg production (conversion of citrulline to Arg) (C) in healthy controls (n = 19) and COPD patients (n = 23). Statistics were obtained by using 2-factor repeated-measures ANOVA with “group” and “day” used to compare differences between groups and test days. A significant group effect was found, but no significant day effect or interaction. Data are shown as the mean value of both days. *,****Different from controls, *P < 0.05, ****P < 0.0001. COPD, chronic obstructive pulmonary disease; FFM, fat-free mass.
TABLE 4.
Postabsorptive protein kinetics1
| Values, μmol · kg FFM−1 · h−1 |
||
| Healthy (n = 19) | COPD (n = 23) | |
| PB | 54.9 ± 2.1 | 58.8 ± 1.7 |
| PS | 50.2 ± 1.9 | 54.7 ± 1.7 |
| Net PS2 | −4.7 ± 0.3 | −4.1 ± 0.2 |
| Hydroxylation | 4.7 ± 0.3 | 4.1 ± 0.2 |
Values are means ± SEs. Statistics were obtained with the use of 2-factor repeated-measures ANOVA with “group” and “day” used to compare differences between groups and test days. No significant group or day effect or interaction was observed for any of the measures; therefore, data are shown as the mean value of both days. COPD, chronic obstructive pulmonary disease; FFM, fat-free mass; PB, protein breakdown; PS, protein synthesis.
Calculated as PS − PB.
FIGURE 3.
Mean ± SE postabsorptive whole-body Arg clearance (A) and NO production (conversion of Arg to citrulline) (B) in healthy controls (n = 19) and COPD patients (n = 23). Statistics were obtained by using 2-factor repeated-measures ANOVA with “group” and “day” used to compare differences between groups and test days. A significant group effect was found for Arg clearance, but no significant day effect or interaction. For NO production, only a significant interaction between group and day was observed (P = 0.0325; Supplemental Figure 5). Data are shown as the mean value of both days. ****Different from controls, P < 0.0001. COPD, chronic obstructive pulmonary disease; FFM, fat-free mass; NO, nitric oxide.
DISCUSSION
We found that stable patients with moderate-to-severe COPD were characterized by an upregulated whole-body Arg rate of appearance, mainly because of an increase in de novo Arg production. The higher Arg clearance in COPD, however, was not associated with alterations in whole-body NO production.
Whole-body Arg and citrulline rates of appearance
The whole-body Arg rate of appearance was higher in COPD patients than in healthy controls, which is in line with our findings in patients with CF (4), another chronic airway disease that is characterized by local and systemic inflammation and reduced pulmonary function (19, 20). Because participants were studied while fasting, the Arg rate of appearance was determined primarily by the rate of whole-body PB (∼80%) (8), and, to a lesser extent by the conversion of citrulline to Arg, i.e., de novo Arg production (16). In our COPD group, the higher whole-body Arg rate of appearance mainly was due to an increase in de novo Arg production. We do not know yet whether the higher Arg rate of appearance was a consequence of the higher Arg clearance (29).
We found that the whole-body citrulline rate of appearance was also higher in COPD patients, similar again to what we observed in CF patients (4). Citrulline primarily is synthesized from Gln in the gut (16, 30). The gut therefore plays a crucial role in citrulline homeostasis. Although there is evidence of enterocyte dysfunction in COPD (31), the citrulline rate of appearance was higher and not lower in the studied COPD group. To what extent this indicates that stable COPD patients have adequate gut Gln metabolism (23, 32) remains unclear. We previously found a direct relation between gut Gln delivery and disposal, which suggests that when more Gln is presented to the gut, consumption is higher as well (32). In that regard, a higher citrulline rate of appearance in COPD is likely a reflection of higher Gln delivery and possibly higher Gln turnover. Because plasma Gln is depleted in critically ill patients (23), and pulmonary Gln production and utilization is increased in the presence of pulmonary inflammation in these patients (33), the lower observed plasma Gln concentration in COPD patients may be indicative of an enhanced Gln clearance via this route. Evidently, more research is required to examine whole-body Gln turnover and clearance rates in COPD with the use of stable isotope methodology.
Arg clearance
A third similarity between CF and COPD patients was the greater whole-body Arg clearance (4). The metabolic fate of Arg is diverse, but a higher clearance rate indicates potential changes in 3 main pathways (8). First, as a constituent of body protein, Arg is used toward PS, and most of our previous studies in COPD patients show an upregulation of whole-body PS (17, 18). However, in the present study, the upregulation was not significant and could account for only 37% of the increase in Arg clearance.
The second and third uses of Arg are highly dependent on the interplay between 2 enzymes, i.e., arginase and NOS (8). Constitutively expressed neuronal and endothelial NOS catalyze the conversion of Arg to NO to facilitate vasodilation and smooth-muscle relaxation (13). NO produced via inducible NOS is highly involved in immune function, as reflected by the increased inducible NOS expression in the presence of (pro)inflammatory mediators (13). COPD patients in a stable condition are characterized by low-grade systemic inflammation (2), which in our study was not reflected by an increase in high-sensitivity C-reactive protein. However, increases in other respective biomarkers of systemic inflammation could still be present (2) but did not lead to higher whole-body NO production. On a pulmonary level, COPD patients are characterized by higher neuronal NOS expression in peripheral lung tissue (34) as well as inducible NOS expression in bronchial tissue and smooth-muscle cells (35), both of which are negatively related to disease severity. Because 87% of the studied patients had COPD Global Initiative for Chronic Obstructive Lung Disease grade III or IV, the increase in Arg clearance potentially could be related to higher Arg utilization in the lung for NO production. Some studies support that notion, because they have found increases in fractional exhaled nitric oxide (FeNO) in stable COPD patients (36, 37). However, our pilot findings in another group of patients comparable to the current one (FEV1: 36% ± 3% of predicted) did not show an increase in FeNO (COPD (n = 12): 13 parts per billion; healthy (n = 14): 19 parts per billion) (38). Current smoking (37) and use of inhaled corticosteroids (39) could have downregulated FeNO in our study to some extent.
Alternatively, Arg can be hydrolyzed by arginase to ornithine and urea, which in healthy young men accounts for ∼15% of plasma Arg consumption (40). Inflammation stimulates arginase activity, leads to higher Arg utilization, and subsequently causes Arg deficiency (9, 10, 41). Recently, higher serum arginase activity and myeloid-derived suppressor cell (MDSC) expression were described in COPD (42–44). MDSCs stimulate arginase I activity and have been linked to Arg depletion and/or immunosuppression in several inflammatory conditions (45), including COPD (42–44). The exact contribution of MDSCs to Arg clearance in COPD still needs to be established. The previously described increased airway arginase activity in COPD (35), however, could indicate greater conversion of Arg to ornithine in the lung for the production of polyamines and Pro, components of tissue repair processes that are suggested to contribute to airway remodeling in COPD (11, 12).
Future research and current study limitations
Further exploration is needed to determine the clinical relevance of the upregulated endogenous Arg production and Arg clearance in COPD. However, if these changes are part of a compensatory mechanism for higher Arg demand, then most likely the magnitude is increased during acute pulmonary exacerbations, which may then lead to Arg depletion and immune suppression. In support, reduced plasma Arg status and increased serum arginase activity have been found in both asthma and CF at times of an exacerbation (5, 6), and FeNO is increased in unstable COPD (37, 46, 47).
We concluded the study with a group of COPD patients who were rather uniform in terms of pulmonary function (FEV1 percentage of predicted), with 20 of the 23 patients classified as having severe to very severe COPD. Because of this small variability in lung function, we were not able to assess its direct relation to Arg metabolism. Also, the measurement of exhaled NO or bioactive NO metabolites in sputum would have allowed us to determine the relation between pulmonary and whole-body NO production, albeit whole-body NO production was unchanged in this group of COPD patients. The determination of whole-body NO production required the analysis of enriched citrulline derived from the continuous infusion of isotopically labeled Arg, which posed a challenge because the enrichment values were at the detection limit of our equipment.
In conclusion, alterations in whole-body Arg metabolism are present in stable COPD and may be indicative of a compensatory mechanism for higher Arg demand.
Acknowledgments
We thank chief analytical chemist John Thaden for the sample analysis.
The authors’ responsibilities were as follows—NEPD and MPKJE: designed the research; MLE and PJA: recruited study participants; RJ, NEPD, and MPKJE: conducted the research and data analysis, wrote the manuscript, had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity of the lung for carbon monoxide; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; MDSC, myeloid-derived suppressor cell; NO, nitric oxide; NOS, nitric oxide synthase; PB, protein breakdown; PS, protein synthesis.
REFERENCES
- 1.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–53. [DOI] [PubMed] [Google Scholar]
- 2.Gan WQ, Man SFP, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004;59:574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016 [Internet] [cited 2016 Mar 30]. Available from: http://www.goldcopd.org/.
- 4.Engelen MP, Com G, Luiking YC, Deutz NE. Stimulated nitric oxide production and arginine deficiency in children with cystic fibrosis with nutritional failure. J Pediatr 2013;163:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasemann H, Schwiertz R, Grasemann C, Vester U, Racke K, Ratjen F. Decreased systemic bioavailability of L-arginine in patients with cystic fibrosis. Respir Res 2006;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM. Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med 2004;170:148–53. [DOI] [PubMed] [Google Scholar]
- 7.Ruzsics I, Nagy L, Keki S, Sarosi V, Illes B, Illes Z, Horvath I, Bogar L, Molnar T. L-arginine pathway in COPD patients with acute exacerbation: a new potential biomarker. COPD 2016;13:139–45. [DOI] [PubMed] [Google Scholar]
- 8.Luiking YC, Ten Have GA, Wolfe RR, Deutz NE. Arginine de novo and nitric oxide production in disease states. Am J Physiol Endocrinol Metab 2012;303:E1177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raber P, Ochoa AC, Rodriguez PC. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol Invest 2012;41:614–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris SM., Jr Arginases and arginine deficiency syndromes. Curr Opin Clin Nutr Metab Care 2012;15:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pera T, Zuidhof AB, Smit M, Menzen MH, Klein T, Flik G, Zaagsma J, Meurs H, Maarsingh H. Arginase inhibition prevents inflammation and remodeling in a guinea pig model of chronic obstructive pulmonary disease. J Pharmacol Exp Ther 2014;349:229–38. [DOI] [PubMed] [Google Scholar]
- 12.Maarsingh H, Pera T, Meurs H. Arginase and pulmonary diseases. Naunyn Schmiedebergs Arch Pharmacol 2008;378:171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 1993;329:2002–12. [DOI] [PubMed] [Google Scholar]
- 14.Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev 2004;84:731–65. [DOI] [PubMed] [Google Scholar]
- 15.Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax 2003;58:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ligthart-Melis GC, van de Poll MCG, Boelens PG, Dejong CHC, Deutz NEP, van Leeuwen PAM. Glutamine is an important precursor for de novo synthesis of arginine in humans. Am J Clin Nutr 2008;87:1282–9. [DOI] [PubMed] [Google Scholar]
- 17.Engelen MP, De Castro CL, Rutten EP, Wouters EF, Schols AM, Deutz NE. Enhanced anabolic response to milk protein sip feeding in elderly subjects with COPD is associated with a reduced splanchnic extraction of multiple amino acids. Clin Nutr 2012;31:616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Supplementation of soy protein with branched-chain amino acids alters protein metabolism in healthy elderly and even more in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 2007;85:431–9. [DOI] [PubMed] [Google Scholar]
- 19.Elizur A, Cannon CL, Ferkol TW. Airway inflammation in cystic fibrosis. Chest 2008;133:489–95. [DOI] [PubMed] [Google Scholar]
- 20.Ngan DA, Wilcox PG, Aldaabil M, Li Y, Leipsic JA, Sin DD, Man SF. The relationship of systemic inflammation to prior hospitalization in adult patients with cystic fibrosis. BMC Pulm Med 2012;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–87. [DOI] [PubMed] [Google Scholar]
- 22.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism 1981;30:936–40. [DOI] [PubMed] [Google Scholar]
- 23.Luiking YC, Poeze M, Ramsay G, Deutz NE. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr 2009;89:142–52. [DOI] [PubMed] [Google Scholar]
- 24.Jonker R, Deutz NE, Erbland ML, Anderson PJ, Engelen MP. Hydrolyzed casein and whey protein meals comparably stimulate net whole-body protein synthesis in COPD patients with nutritional depletion without an additional effect of leucine co-ingestion. Clin Nutr 2014;33:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis 1993;147:1151–6. [DOI] [PubMed] [Google Scholar]
- 26.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–68. [DOI] [PubMed] [Google Scholar]
- 27.Reeds PJ, Fjeld CR, Jahoor F. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J Nutr 1994;124:906–10. [DOI] [PubMed] [Google Scholar]
- 28.Wu G, Ott TL, Knabe DA, Bazer FW. Amino acid composition of the fetal pig. J Nutr 1999;129:1031–8. [DOI] [PubMed] [Google Scholar]
- 29.Luiking YC, Poeze M, Deutz NE. Arginine infusion in patients with septic shock increases nitric oxide production without haemodynamic instability. Clin Sci (Lond) 2015;128:57–67. [DOI] [PubMed] [Google Scholar]
- 30.Deutz NE. The 2007 ESPEN Sir David Cuthbertson Lecture: amino acids between and within organs. The glutamate-glutamine-citrulline-arginine pathway. Clin Nutr 2008;27:321–7. [DOI] [PubMed] [Google Scholar]
- 31.Rutten EPA, Lenaerts K, Buurman WA, Wouters EFM. Disturbed intestinal integrity in patients with COPD: effects of activities of daily living. Chest 2014;145:245–52. [DOI] [PubMed] [Google Scholar]
- 32.van de Poll MC, Ligthart-Melis GC, Boelens PG, Deutz NE, van Leeuwen PA, Dejong CH. Intestinal and hepatic metabolism of glutamine and citrulline in humans. J Physiol 2007;581:819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hulsewé KW, van der Hulst RR, Ramsay G, van Berlo CL, Deutz NE, Soeters PB. Pulmonary glutamine production: effects of sepsis and pulmonary infiltrates. Intensive Care Med 2003;29:1833–6. [DOI] [PubMed] [Google Scholar]
- 34.Brindicci C, Kharitonov SA, Ito M, Elliott MW, Hogg JC, Barnes PJ, Ito K. Nitric oxide synthase isoenzyme expression and activity in peripheral lung tissue of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;181:21–30. [DOI] [PubMed] [Google Scholar]
- 35.Tadié JM, Henno P, Leroy I, Danel C, Naline E, Faisy C, Riquet M, Levy M, Israel-Biet D, Delclaux C. Role of nitric oxide synthase/arginase balance in bronchial reactivity in patients with chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 2008;294:L489–97. [DOI] [PubMed] [Google Scholar]
- 36.Corradi M, Majori M, Cacciani GC, Consigli GF, de’Munari E, Pesci A. Increased exhaled nitric oxide in patients with stable chronic obstructive pulmonary disease. Thorax 1999;54:572–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maziak W, Loukides S, Culpitt S, Sullivan P, Kharitonov SA, Barnes PJ. Exhaled nitric oxide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:998–1002. [DOI] [PubMed] [Google Scholar]
- 38.Engelen M, Jonker R, Veley E, Harrykissoon R, Thaden J, Deutz N. MON-PP282: Exhaled concentration of nitric oxide (feno) does not reflect the upregulated whole body nitric oxide synthesis in patients with chronic obstructive pulmonary disease. Clinical Nutrition 2015;34:S232. [Google Scholar]
- 39.Ferreira IM, Hazari MS, Gutierrez C, Zamel N, Chapman KR. Exhaled nitric oxide and hydrogen peroxide in patients with chronic obstructive pulmonary disease: effects of inhaled beclomethasone. Am J Respir Crit Care Med 2001;164:1012–5. [DOI] [PubMed] [Google Scholar]
- 40.Castillo L, Beaumier L, Ajami AM, Young VR. Whole body nitric oxide synthesis in healthy men determined from [15N] arginine-to-[15N]citrulline labeling. Proc Natl Acad Sci USA 1996;93:11460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Waardenburg DA, de Betue CT, Luiking YC, Engel M, Deutz NE. Plasma arginine and citrulline concentrations in critically ill children: strong relation with inflammation. Am J Clin Nutr 2007;86:1438–44. [DOI] [PubMed] [Google Scholar]
- 42.Kalathil SG, Lugade AA, Pradhan V, Miller A, Parameswaran GI, Sethi S, Thanavala Y. T-regulatory cells and programmed death 1+ T cells contribute to effector T-cell dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014;190:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scrimini S, Pons J, Agusti A, Clemente A, Sallan MC, Bauca JM, Soriano JB, Cosio BG, Lopez M, Crespi C, et al. Expansion of myeloid-derived suppressor cells in chronic obstructive pulmonary disease and lung cancer: potential link between inflammation and cancer. Cancer Immunol Immunother 2015;64:1261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scrimini S, Pons J, Agusti A, Soriano JB, Cosio BG, Torrecilla JA, Nunez B, Cordova R, Iglesias A, Jahn A, et al. Differential effects of smoking and COPD upon circulating myeloid derived suppressor cells. Respir Med 2013;107:1895–903. [DOI] [PubMed] [Google Scholar]
- 45.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9:162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agustí AG, Villaverde JM, Togores B, Bosch M. Serial measurements of exhaled nitric oxide during exacerbations of chronic obstructive pulmonary disease. Eur Respir J 1999;14:523–8. [DOI] [PubMed] [Google Scholar]
- 47.Bhowmik A, Seemungal TA, Donaldson GC, Wedzicha JA. Effects of exacerbations and seasonality on exhaled nitric oxide in COPD. Eur Respir J 2005;26:1009–15. [DOI] [PubMed] [Google Scholar]