Abstract
Background: There is a paucity of studies that have prospectively tested the energy surfeit theory of obesity with the use of objectively estimated energy intake and energy expenditure in humans. An alternative theory is that homeostatic regulation of body weight is more effective when energy intake and expenditure are both high (high energy flux), implying that low energy flux should predict weight gain.
Objective: We aimed to examine the predictive relations of energy balance and energy flux to future weight gain and tested whether results were replicable in 2 independent samples.
Design: Adolescents (n = 154) and college-aged women (n = 75) underwent 2-wk objective doubly labeled water, resting metabolic rate, and percentage of body fat measures at baseline. Percentage of body fat was measured annually for 3 y of follow-up for the adolescent sample and for 2 y of follow-up for the young adult sample.
Results: Low energy flux, but not energy surfeit, predicted future increases in body fat in both studies. Furthermore, high energy flux appeared to prevent fat gain in part because it was associated with a higher resting metabolic rate.
Conclusion: Counter to the energy surfeit model of obesity, results suggest that increasing energy expenditure may be more effective for reducing body fat than caloric restriction, which is currently the treatment of choice for obesity. This trial was registered at clinicaltrials.gov as NCT02084836.
Keywords: energy balance, energy flux, physical activity, doubly labeled water, weight gain
INTRODUCTION
Because obesity is credited with 2.8 million deaths annually (1), it is vital to identify factors that predict weight gain. On the basis of the first law of thermodynamics (2), it is widely accepted that a chronic positive energy balance is the core factor that drives excessive weight gain (3). Highly controlled metabolic ward experiments have found that humans assigned to a positive energy balance condition show greater weight gain than do humans assigned to a balanced energy condition (4, 5). Yet, to our knowledge, no study with free-living humans has tested whether objectively measured energy balance, operationalized by habitual energy intake minus habitual energy expenditure, predicts weight gain over a multiyear follow-up, despite the fact that reduced-calorie diets are the frontline treatment of obesity. Thus, we tested whether objectively measured energy surfeit predicted future body fat gain in 2 prospective studies.
Moreover, reduced-calorie diets have not produced the rate and magnitude of weight loss predicted by the law of thermodynamics (6), and most people who complete reduced-calorie weight loss treatments regain the lost weight (7). According to the metabolic adaptation hypothesis, physiologic compensatory responses to a negative energy balance produce energy-sparing changes in metabolism that attenuate weight loss and promote weight regain (8). Substrate conserving responses include reduced resting metabolic rate (RMR)6 and thermic effects of feeding (9, 10), as well as increased metabolic efficiency (11) and fractional energy absorption (12). Low patient adherence also limits the efficacy of reduced-calorie treatment (8), apparently because caloric deprivation increases the reward value of food (13).
One explanation for these findings is that homeostatic regulation of body weight is most effective when energy balance is sustained at a high level of energy flux, operationalized as the total sum of energy moving through a system with similar energy intake and expenditure (14, 15). Maintaining high energy flux (high energy intake and expenditure) induces metabolic changes that are more protective against weight gain than a prolonged negative energy balance from dietary restriction. Among endurance athletes who train regularly, fat-free mass–adjusted RMR is elevated substantially during high energy flux periods compared with low energy flux periods induced by reductions in energy intake and volitional exercise (16). Among adults who regularly engage in aerobic endurance exercise, proportional reductions of energy intake and expenditure reduce RMR and skeletal muscle sympathetic nervous system activity (15). Because RMR is the largest constituent of daily total energy expenditure (TEE) (17) and some (but not all) studies show that low RMR predicts future weight gain (18), high physical activity coupled with high energy intake may protect against weight gain. Thus, we also tested whether objectively measured energy flux (habitual energy intake plus habitual energy expenditure) showed a negative relation to future body fat gain, because this too has not yet been tested with prospective data.
All together, the aim of this study was to determine how best to combine the predictive power of habitual energy intake and energy expenditure to forecast future weight change reliably, because the results should guide the development of more effective obesity treatment strategies (NCT02084836).
METHODS
Research participants
To address the dual aims of our article, we conducted 2 independent prospective studies with humans wherein we collected objective measures of total energy intake (TEI) and TEE over a 2-wk period and assessed body fat percentage over a 3-y follow-up for study 1 and over a 2-y follow-up for study 2.
Data in text, tables, and figures are presented as means ± SDs. Participants in study 1 included 162 adolescents (82 female, 80 male; age = 15.3 ± 1.1 y; BMI (in kg/m2) = 20.8 ± 1.9; 4% Hispanic, 1% Native American, 1% Asian/Pacific Islander, 76% European American, and 18% mixed racial heritage) recruited in Eugene, Oregon, via advertisements. Exclusion criteria were a BMI <18 or >25, current use of psychoactive medications or drugs more than weekly, pregnancy, head injury with a loss of consciousness, substantial cognitive impairment, major medical problems, or current Axis I psychiatric disorder (Supplemental Table 1).
Participants in study 2 included 91 late-adolescent women aged 18–20 y (age = 18.4 ± 0.57 y; BMI = 23.72 ± 4.07; 90% White, 2% American Indian or Alaska Native, 3% Asian, and 5% unreported) who were randomly selected from a large obesity prevention trial in Eugene, Oregon targeting young women with weight concerns. Exclusion criteria included those who had diabetes, conditions requiring supplemental oxygen, or pregnancy (Supplemental Table 1).
Participants and their parents (for minors) provided written informed consent, and all research was conducted according to the ethical standards required by these institutional review board–approved studies.
Experimental design
Participants reported to the laboratory after a 10- to 12-h overnight fast, at which time RMR was assessed (19). Air-displacement plethysmography (ADP) was used to assess the percentage of body fat of participants at baseline and at 1-, 2-, and 3-y follow-ups in study 1 and at baseline and at 1- and 2-y follow-ups in study 2 with the Bod Pod S/T (COSMED, Rome, Italy). After baseline RMR and ADP measures, daily TEI and TEE were quantified for each participant. Given that self-reported energy intake and energy expenditure are very inaccurate (20), we used doubly labeled water (DLW) to provide an objective measure of TEI and TEE over a 2-wk period (21).
RMR
RMR was assessed via the measurement of oxygen uptake and carbon dioxide production for 20 min with the use of a ventilated hood, as described elsewhere (19). Participants arrived at the laboratory after a 10- to 12-h overnight fast, and then assumed supine rest (≥10 min) before the start of the test. For each RMR trial, the first 5 min were discarded to ensure that participants had reached steady state (CV <10%) (Quark RMR; Cosmed). All trials were performed in a sound- and light-controlled room. Before the start of testing each day, the gas analyzer was calibrated with a 3-L syringe and standard gas mixtures of oxygen (26% O2, with the balance nitrogen) and carbon dioxide (4% CO2, 16% O2, and the balance nitrogen).
Percentage of body fat
ADP was used to assess the percentage of body fat of participants. This was accomplished with the Bod Pod S/T by using recommended procedures and age- and sex-appropriate equations (22). Body density was calculated as body mass (assessed by direct weighing) divided by body volume. Body fat percentage estimates showed test–retest reliability (r = 0.92–0.99) and correlated with dual-energy X-ray absorptiometry and hydrostatic weighing estimates (r = 0.98–0.99) (23). In study 1, body composition data were complete at baseline, 7% were missing at the 1-y follow-up, 11% were missing at the 2-y follow-up, and 15% were missing at the 3-y follow-up (Supplemental Figure 1). In study 2, body composition data were complete at baseline, 7% were missing at the 1-y follow-up, and 12% were missing at the 2-y follow-up (Supplemental Figure 2).
Energy balance and energy flux
We used DLW to provide an objective measure of TEI and TEE over a 2-wk period because this is the gold standard measure of these 2 constructs (21). The DLW method was validated previously against continuous indirect calorimetry and weighed food intake and has 1–2% accuracy (CV = 2–12%; error of <3%) (24). Furthermore, the 2-wk DLW protocol yields energy intake data that are ∼25% more accurate than measures collected via survey instruments (25), and it is a reliable measure of habitual energy expenditure (test–retest measures reflecting a CV of 5.1%) (26). DLW also has shown 18-mo test–retest reliability (r = 0.80) (27).
At baseline, DLW was administered immediately after participants tested negatively for pregnancy (if applicable). Doses were 1.6–2.0 g H218O (10 atom %)/kg estimated total body water. Spot urine samples were collected immediately before DLW was administered and 1, 3, and 4 h after dosing. Two weeks later, 2 additional spot urine samples were collected at the same time of day as 3- and 4-h postdosing samples. No samples were the first void of the day. Participants were required to avoid traveling >322 km from the study site in the 2 wk between the second and third visits to the laboratory because regional variation in the amount of 18O and D218O in water would have introduced error variance.
TEE was calculated with the use of equation A6 (1986), dilution space ratios (28), and the modified Weir’s equation (1949), as previously described (29). TEE per day was divided by the number of days between baseline and 2 wk after testing to calculate mean TEE. Mean energy intake per day was calculated from the sum of TEE from DLW and the estimated change in body energy stores from serial body fat measurements, assessed via ADP, performed at baseline and 2 wk after dosing. This figure was divided by the number of days between baseline and 2 wk after testing to calculate the daily source of energy substrates from weight loss or storage of excess energy intake as weight gain (30). The equation used for each participant was as follows: TEI = TEE + [(2-wk weight – baseline weight) × 7800)]/(2-wk date – baseline date). The 7800 kcal/kg value is an estimate of the energy density of adipose tissue (31).
For both samples, we calculated a continuous variable representing energy balance (TEI − TEE) and a second continuous variable representing energy flux (TEI + TEE) for each participant. In both samples, TEE and energy flux were normally distributed (P > 0.05). It is important to note that, by definition, energy flux refers to the absolute level of energy balance (15). Accordingly, any participants with a TEI value that was >33% greater or less than their TEE value were considered to be markedly out of energy balance and omitted from the analyses involving energy flux. This is necessary because otherwise it would be possible for participants to have the same energy flux value, but for completely opposing reasons (e.g., one participant could be consuming 3000 kcal/d but expending only 2000 kcal/d, whereas another could be consuming 2000 kcal/d, but expending 3000 kcal/d, but both would have the same energy flux value). In total, 60 participants in study 1 (39%) and 20 participants in study 2 (27%) were excluded from analyses involving the energy flux variable because they were out of energy balance.
Statistical analyses
The Stata 12 software package was used for the statistical analyses. Shapiro-Wilk W tests were conducted to examine data normality. Pearson product moment or Spearman rank-order correlations, as appropriate, were used to determine relations between RMR and DLW measures of energy flux. Multiple linear regression analyses, which support multilevel longitudinal data arrangements, were used to determine whether DLW-measured energy balance and energy flux at baseline predicted change in percentage of body fat over follow-ups (both samples), with final models controlling for baseline energy balance, age, and percentage of body fat. Results were very similar when controlling for fat-free mass and BMI instead of percentage of body fat. The assumptions of statistical tests regarding the homogeneity of variances were tested with Bartlett’s test for parametric data and Levene’s test for nonparametric data. The statistical threshold was set to an α of P < 0.05.
RESULTS
Sample characteristics
For 8 adolescents, DLW data were invalid, reducing this sample (study 1) to n = 154. For study 2, records for ADP-measured percentage of body fat and/or DLW data were missing or void for 16 participants, reducing this sample to n = 75.
Multiple linear regression analyses
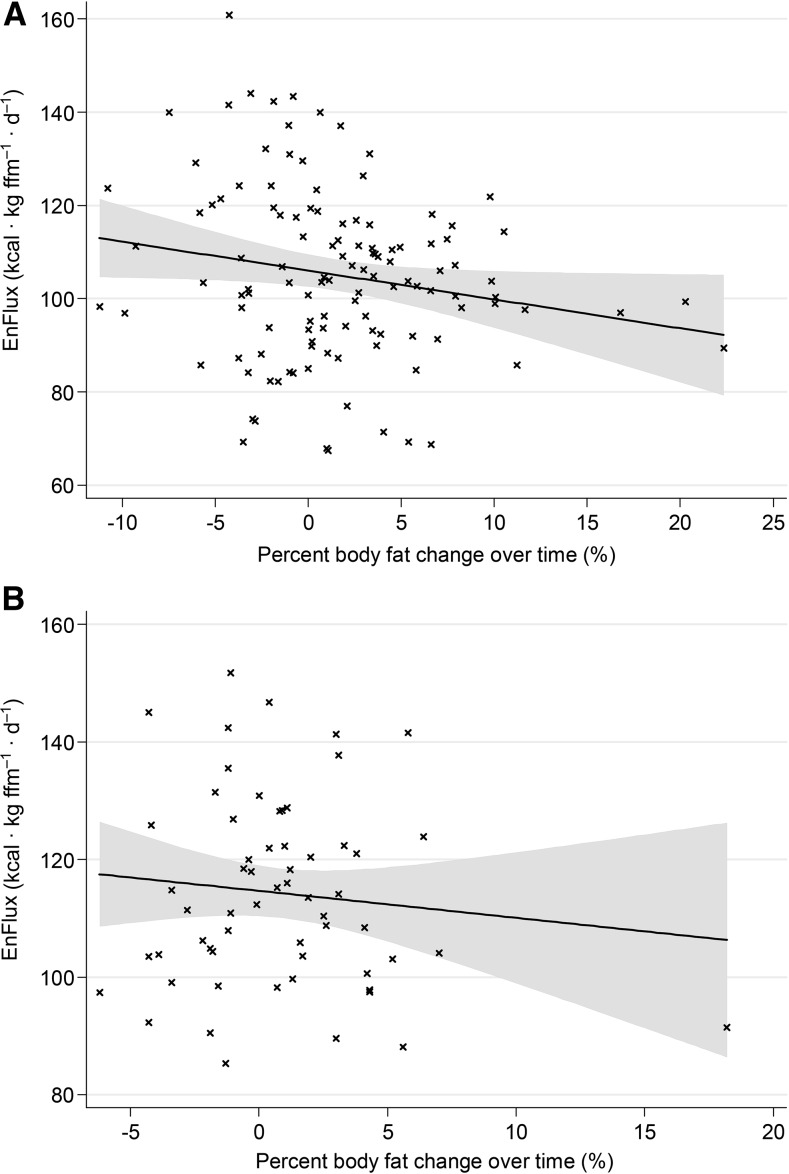
The negative association we observed upon initial inspection of the data between energy flux and percentage of body fat change is depicted for sample 1 in Figure 1A and sample 2 in Figure 1B. Change in percentage of body fat was modeled as 3- and 4-level models in studies 1 and 2, respectively, in which participants were nested within groups. We undertook several analyses to determine the relative contributions of energy balance and energy flux to future gains in body fat. Specifically, a 5-tiered hierarchical regression analysis was conducted with percentage of body fat and age at baseline included as fixed regressor variables in all models. First, energy balance was entered as the sole predictor of percentage of body fat change over follow-up for all participants in study 1 (n = 154) and study 2 (n = 75). Second, energy balance was entered as the sole predictor of percentage of body fat change for participants who were not markedly out of energy balance in study 1 (n = 94) and study 2 (n = 55). Third, energy flux was entered as the sole predictor of percentage of body fat change with the same participants that were included in the last step for studies 1 and 2. Fourth, both energy balance and energy flux were entered as predictors of percentage of body fat change to determine the unique predictive effects of these 2 factors in these same samples. Last, RMR was entered with energy flux to determine whether this diminishes the predictive effects of energy flux, which would be consistent with the notion that RMR at least partially explains the effect of energy flux on percentage of body fat change, again in the same samples.
FIGURE 1.
The relation of EnFlux, or energy intake plus energy expenditure, to percentage of body fat change over time (body fat percentage at final follow-up minus body fat percentage at baseline) for both study 1 (n = 91) (A) and study 2 (n = 54) (B). EnFlux, energy flux; ffm, fat-free mass.
The results of the regression analyses for study 1 and study 2 are shown in Tables 1 and 2, respectively. Energy balance did not significantly predict future body fat change in study 1 or study 2, irrespective of which participants were included (all participants or with markedly out-of-balance participants removed) or which covariates were incorporated. In contrast, energy flux showed a significant inverse relation to future change in body fat in both study 1 (step 3: r = −0.24, P = 0.019; Table 1) and study 2 (step 3: r = −0.28, P = 0.045; Table 2). That is, higher energy flux predicted less future body fat gain, and lower energy flux predicted greater future body fat gain. Furthermore, the predictive effects of energy flux remained significant even when covarying for energy balance in both studies in step 4 (study 1: r = −0.24, P = 0.021; study 2: r = −0.33, P = 0.017). The inclusion of RMR in the model rendered energy flux a nonsignificant predictor of body fat gain in both studies, consistent with the notion that individual differences in RMR mediated the effects of energy flux on body fat change. Notably, the predictive effects of energy balance and energy flux were similar between studies, despite the fact that the participants in the sample for study 1 were all in a healthy BMI range at baseline, whereas the participants in the sample for study 2 had higher body fat and variability in body fat at baseline.
TABLE 1.
Effects for percentage of body fat change (final-year follow-up and baseline Δ) over time for sample 11
| Measures added to model | Coefficient | SE | df | t | P | Partial r |
| Step 1: DLW EnBal (whole-sample analysis) | ||||||
| Intercept | −12.55 | 7.48 | 118 | −1.68 | 0.096 | |
| Baseline %BF | −0.04 | 0.07 | 153 | 0.56 | 0.168 | 0.04 |
| Age, y | 0.97 | 0.48 | 153 | 2.04 | 0.043 | 0.16 |
| DLW EnBal | −0.05 | 0.04 | 153 | −1.06 | 0.290 | −0.09 |
| Step 2: DLW EnBal (out-of-balance group excluded) | ||||||
| Intercept | −12.38 | 8.51 | 75 | −1.45 | 0.150 | |
| Baseline %BF | 0.00 | 0.08 | 93 | 0.00 | 0.977 | 0.00 |
| Age, y | 0.88 | 0.54 | 93 | 1.62 | 0.109 | 0.17 |
| DLW EnBal | −0.07 | 0.05 | 93 | −1.47 | 0.147 | −0.15 |
| Step 3: DLW EnFlux (out-of-balance group excluded) | ||||||
| Intercept | −4.07 | 9.14 | 75 | −0.44 | 0.658 | |
| Baseline %BF | −0.01 | 0.08 | 93 | −0.10 | 0.921 | −0.01 |
| Age, y | 0.79 | 0.53 | 93 | 1.49 | 0.142 | 0.15 |
| DLW EnFlux | −0.06 | 0.03 | 93 | −2.41 | 0.019 | −0.24 |
| Step 4: DLW EnBal + DLW EnFlux (out-of-balance group excluded) | ||||||
| Intercept | 4.36 | 10.88 | 75 | 0.40 | 0.690 | |
| Baseline %BF | −0.01 | 0.08 | 93 | −0.08 | 0.938 | −0.01 |
| Age, y | 0.73 | 0.53 | 93 | 1.39 | 0.170 | 0.14 |
| DLW EnBal | 0.14 | 0.10 | 93 | 1.41 | 0.164 | 0.14 |
| DLW EnFlux | −0.14 | 0.06 | 93 | −2.36 | 0.021 | −0.24 |
| Step 5: DLW EnFlux + RMR at baseline (out-of-balance group excluded) | ||||||
| Intercept | 22.94 | 12.6 | 75 | 1.82 | 0.073 | |
| Baseline %BF | 0.03 | 0.07 | 93 | 0.35 | 0.728 | 0.04 |
| Age, y | 0.22 | 0.56 | 93 | 0.39 | 0.696 | 0.04 |
| DLW EnFlux | −0.03 | 0.03 | 93 | −0.96 | 0.339 | −0.10 |
| Baseline RMR | −0.79 | 0.24 | 93 | −3.32 | 0.001 | −0.43 |
DLW, doubly labeled water; EnBal, energy balance; EnFlux, energy flux; RMR, resting metabolic rate; %BF, percentage of body fat.
TABLE 2.
Effects for percentage of body fat change (final-year follow-up and baseline Δ) over time for sample 21
| Measures added to model | Coefficient | SE | df | t | P | Partial r |
| Step 1: DLW EnBal (whole-sample analysis) | ||||||
| Intercept | −8.30 | 4.31 | 61 | −1.93 | 0.064 | |
| Baseline %BF | −0.01 | 0.03 | 74 | −0.53 | 0.601 | −0.06 |
| Age, y | 0.51 | 0.24 | 74 | 2.14 | 0.041 | 0.24 |
| DLW EnBal | 0.01 | 0.02 | 74 | 0.40 | 0.692 | 0.04 |
| Step 2: DLW EnBal (out-of-balance group excluded) | ||||||
| Intercept | −5.08 | 5.07 | 45 | −1.00 | 0.327 | |
| Baseline %BF | −0.03 | 0.03 | 54 | −1.03 | 0.315 | −0.14 |
| Age, y | 0.37 | 0.27 | 54 | 1.38 | 0.182 | 0.18 |
| DLW EnBal | −0.02 | 0.02 | 54 | −0.78 | 0.444 | −0.11 |
| Step 3: DLW EnFlux (out-of-balance group excluded) | ||||||
| Intercept | −0.80 | 5.13 | 45 | −0.16 | 0.877 | |
| Baseline %BF | −0.02 | 0.03 | 54 | −0.81 | 0.426 | −0.11 |
| Age, y | 0.25 | 0.25 | 54 | 1.00 | 0.326 | 0.13 |
| DLW EnFlux | −0.02 | 0.01 | 54 | −2.13 | 0.045 | −0.28 |
| Step 4: DLW EnBal + DLW EnFlux (out-of-balance group excluded) | ||||||
| Intercept | 0.68 | 5.04 | 45 | 0.14 | 0.894 | |
| Baseline %BF | −0.01 | 0.03 | 54 | −0.54 | 0.598 | −0.07 |
| Age, y | 0.28 | 0.25 | 54 | 1.15 | 0.264 | 0.15 |
| DLW EnBal | 0.05 | 0.03 | 54 | 1.60 | 0.124 | 0.21 |
| DLW EnFlux | −0.04 | 0.02 | 54 | −2.58 | 0.017 | −0.33 |
| Step 5: DLW EnFlux + RMR at baseline (out-of-balance group excluded) | ||||||
| Intercept | −0.35 | 5.19 | 45 | −0.07 | 0.946 | |
| Baseline %BF | −0.02 | 0.03 | 54 | −0.78 | 0.446 | −0.11 |
| Age, y | 0.33 | 0.27 | 54 | 1.21 | 0.239 | 0.16 |
| DLW EnFlux | −0.02 | 0.01 | 54 | −1.63 | 0.117 | −0.22 |
| Baseline RMR | −0.08 | 0.10 | 54 | −0.83 | 0.417 | −0.43 |
Data are log-transformed. DLW, doubly labeled water; EnBal, energy balance; EnFlux, energy flux; RMR, resting metabolic rate; %BF, percentage of body fat.
RMR
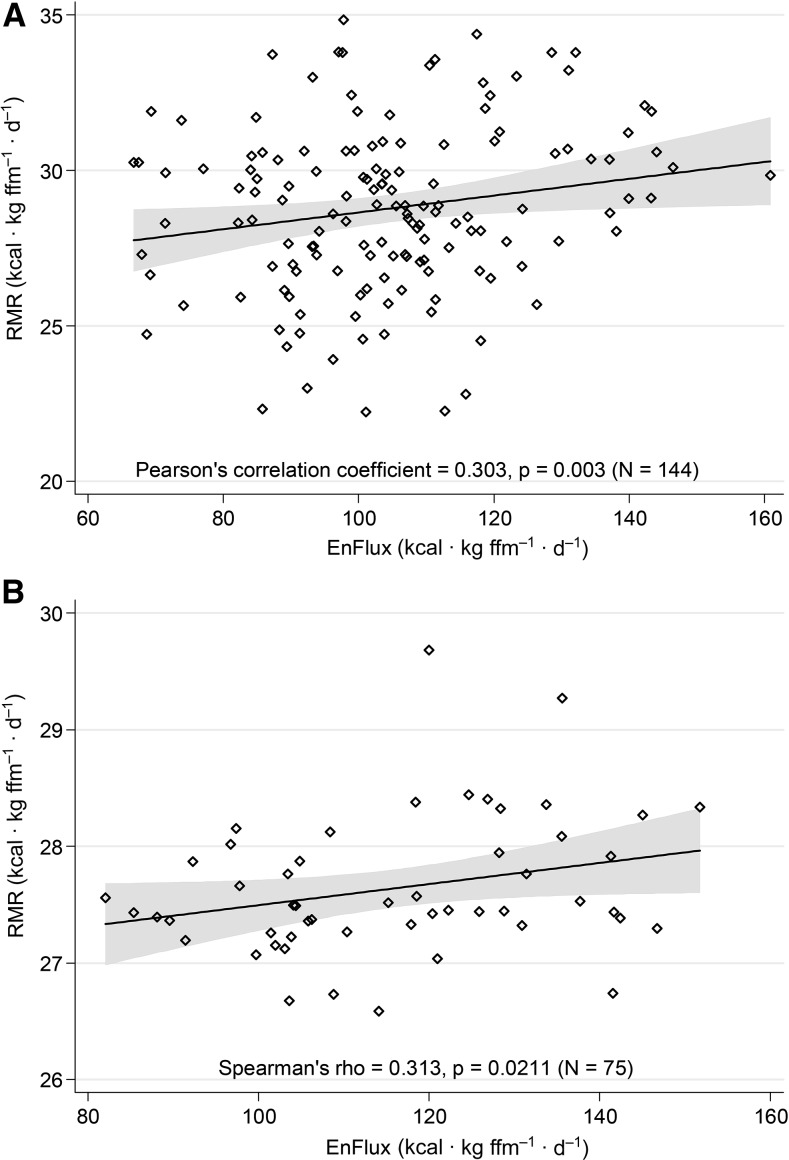
Analyses indicated that baseline RMR values for the adolescent sample (study 1) were normally distributed (permitting the use of Pearson correlational analysis) and that data for the young adult sample (study 2) were skewed negatively (necessitating Spearman correlational analysis). RMR (kcal · kg fat-free mass–1 · d–1) correlated positively with energy flux (kcal · kg fat-free mass–1 · d–1) in study 1 (r = 0.303, P = 0.003; Figure 2A) as well as in study 2 (r = 0.313, P = 0.021; Figure 2B) (out-of-balance groups excluded from analyses).
FIGURE 2.
Significant associations between EnFlux and RMR for both study 1 (A) and study 2 (B). EnFlux, energy flux; ffm, fat-free mass; RMR, resting metabolic rate.
RMR was positively correlated with change in percentage of body fat in study 1 (r = 0.26), but not in study 2 (r = 0.01). In both studies, change in percentage of body fat over follow-up was not significantly related to baseline fat-free mass (study 1, r = 0.05; Study 2, r = 0.01), TEI (study 1, r = −0.021; study 2, r = 0.03), or TEE (study 1, r = −0.09; study 2, r = −0.03).
Percentage of body fat change over time
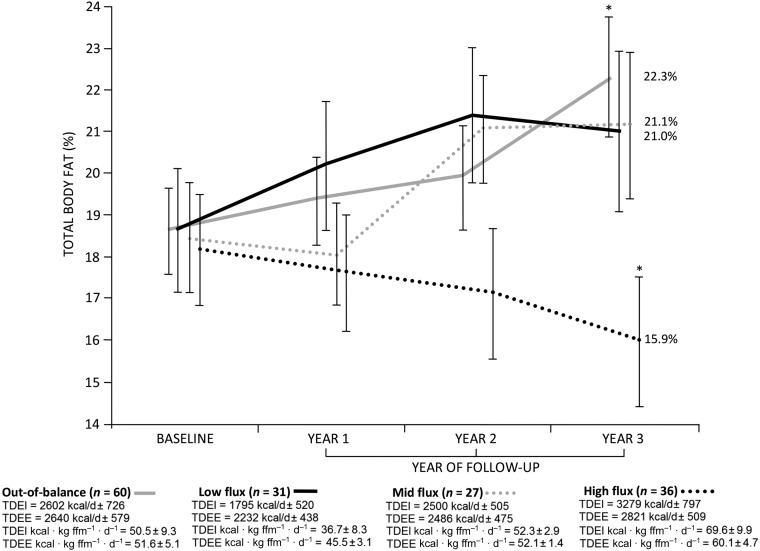
To provide a clear indication of the amounts of energy intake and energy expenditure that were associated with body fat gain compared with loss, participants in study 1 were divided into 4 groups as follows: 1) TEI and TEE were arranged into tertiles; 2) low energy flux participants were identified as those who occupied the lower tertile for both TEI and TEE; 3) midenergy flux participants were classified as those who occupied the middle tertile for both TEI and TEE; 4) high energy flux participants were those who occupied the upper tertile for both TEI and TEE; and 5) the remainder of the sample (those who showed mismatched TEI − TEE tertile gradations) was classified as “out-of-balance.” In study 1, 39.0% of adolescents (n = 60) were classified as out-of-balance (unmatched TEI − TEE tertile gradations), 20.1% (n = 31) were classified as low flux (energy flux = 82.1 ± 9.1 kcal · kg fat-free mass−1 · d−1), 17.5% (n = 27) were midflux (energy flux = 104.3 ± 3.4 kcal · kg fat-free mass−1 · d−1), and 23.4% (n = 36) were high flux (energy flux = 129.7 ± 11.1 kcal · kg fat-free mass−1 · d−1) (Figure 3).
FIGURE 3.
Total fat mass (percentage) at baseline and years 1–3 of follow-up (study 1). Data are means ± SEs. Labels refer to body fat (percentage) at final follow-up. Whereas no group differences were seen for percentage of body fat at baseline or follow-up at years 1 and 2, out-of-balance participants exhibited greater overall body adiposity at year 3 of follow-up than did their high-flux counterparts. Per group is reported. *P = 0.005; 1-factor ANOVA with Bonferroni adjustment. ffm, fat-free mass; TDEE, total daily energy expenditure; TDEI, total daily energy intake.
Total percentage of fat mass at baseline and at years 1–3 of follow-up for these 4 groups is depicted in Figure 3. Notably, percentage of body fat increased for the low energy flux, midenergy flux, and out-of-balance groups (increases of 2.3%, 2.7%, and 3.7%, respectively), whereas percentage of body fat decreased for the high flux group (reduction of 2.2%). Furthermore, even though adolescents who were out-of-balance showed a similar level of energy surfeit than did their high flux peers (out-of-balance = 8.4 ± 2.4 kcal · kg fat-free mass–1 · d–1 compared with high flux = 9.5 ± 10.8 kcal · kg fat-free mass–1 · d–1, P = 0.79), the former group showed a greater percentage of body fat gain from baseline through 3-y follow-up (out-of-balance = 3.7% compared with high flux = −2.2%; z = 2.93, P = 0.003).
Self-reported habitual dietary macronutrient (carbohydrate, fat, and protein) and total daily sugar intake measured with the Block food-frequency questionnaire (32) were analyzed to test for confounding due to between-group differences in dietary intake. However, groups were similar when these data were expressed in absolute (g/d) and relative (% TEI) terms (data collected at baseline).
DISCUSSION
A noteworthy finding to emerge from our data is that energy balance did not prospectively predict weight gain in either sample, despite the fact that we used gold-standard objective estimates of TEI and TEE, objectively measured body fat, and had power to detect clinically meaningful effects. To our knowledge, these null findings are novel, because no prospective study with free-living humans has tested whether energy balance, defined as habitual energy intake minus habitual energy expenditure, predicts body fat change over a multiyear follow-up. One can argue that the 2-wk interval during which TEI and TEE were assessed was too brief to provide accurate estimates, but TEI and TEE did have predictive validity when combined via addition (energy flux) rather than subtraction (energy balance). These null findings from these 2 studies do not provide support for the predictive validity of the energy surfeit theory of obesity.
In contrast, low energy flux predicted future body fat gain in both samples; these predictive effects were medium (r = −0.24 and r = −0.33 in studies 1 and 2, respectively), whereas those for energy balance were trivial (r = −0.09 and r = 0.04 in studies 1 and 2, respectively). Moreover, the predictive effects for energy flux are larger than those for other established obesity risk factors, such as parental obesity (mean r = 0.20) (33, 34) and impulsivity (mean r = 0.13) (35, 36). To our knowledge, this represents another novel finding, because no study has tested whether energy flux predicts future body fat gain in free-living humans over a multiyear follow-up with the use of objective measures of energy intake and expenditure. Collectively, the results suggest that relatively low energy flux, rather than a positive energy balance, predicts future weight gain.
Even more remarkable, given the energy surfeit model of obesity, the low energy flux group that showed increases in body fat was in a negative energy balance at baseline (−437 kcal/d), whereas the high energy flux group that showed decreases in body fat was in a positive energy balance at baseline (458 kcal/d). Indeed, TEI for the high energy flux group was greater than for all of the other groups, yet this was the only group that lost body fat over time.
These results imply that high energy flux predicted future body fat loss because it was associated with a higher RMR: the correlations between energy flux and RMR were r = 0.30 and r = 0.31 in studies 1 and 2, respectively, and the predictive relations between energy flux and future body fat change became nonsignificant when RMR was entered as a covariate. The results are consistent with the thesis that homeostatic regulation of body weight is more effective when energy balance is sustained at high levels of energy intake and energy expenditure (14, 15), supporting the premise that energy-restrictive diets, particularly when coupled with low exercise, may not be optimal for weight loss. Results converge with evidence that 1) assignment to a 7-d high-intensity exercise condition increases RMR relative to a no-exercise condition (d = 1.8) (37), 2) mean daily RMR is higher in obese persons (mean BMI = 35) during periods of high than during low energy flux (1816 compared with 1747 kcal/d, respectively) (38), 3) caloric restriction diets reduce oxygen requirements and RMR (39, 40), and 4) restrictions in energy intake induce less metabolic compensation when accompanied by exercise than when not accompanied by exercise (41).
High energy flux may also reduce body fat gain because exercise reduces brain reward region response to food cues. Acute high-intensity exercise decreased reward (putamen, orbitofrontal cortex) and gustatory (insula, rolandic operculum) region response to high- and low-calorie food images compared with responsivity after a sedentary condition (42, 43). Furthermore, exercise reduces the preference for high-fat foods compared with a nonexercise control condition (44). Pretest-to-posttest reductions in anterior insula response to food cues after a 6-mo supervised exercise program correlated positively with changes in fat mass (r = 0.61) (45). Exercise-induced fat mass loss, even as modest as 2.1%, was associated with reduced activation in intrinsic default mode network activity (46).
Although we did not assess physical activity in research participants, the findings imply that regular physical activity may be key for effective long-term weight management, extending previous evidence that exercise is the best predictor of successful weight loss maintenance (47), and that high objectively measured exercise correlates with weight loss maintenance (48). Daily exercise can attenuate and even prevent increases in visceral adipose tissue in the presence of overeating (49).
Low-calorie diets theoretically reduce volitional physical activity and nonexercise-associated thermogenesis, which promote weight regain because they foster energy conservation (50). Critically, DLW-assessed physical activity and total daily kilocalorie expenditure decreased in overweight participants when placed on a low-calorie diet (±890 kcal/d) (41). These data suggest that low physical activity is both a cause and a consequence of the preservation of a eucaloric state at low levels of energy flux.
The present study has several limitations. First, we did not have sufficient power to detect small effects. Second, although energy flux, but not energy balance, predicted future body fat gain in healthy-weight adolescents and slightly heavier young adults, results may not generalize to other populations (e.g., children and older adults). Third, because we did not assess TEI and TEE repeatedly during follow-up, we could not confirm the temporal stability of energy flux and energy balance or test whether chronic low energy flux is associated with future body fat gain and chronic high energy flux with future body fat loss. Fourth, we did not include an objective measure of macronutrient intake, which would be useful for determining whether individuals at a high compared with a low energy flux habitually consume low-energy–density foods with a more favorable macronutrient profile than low energy flux. Fifth, we did not include an objective measure of physical activity so that we could investigate the effect of chronic physical activity on body fat change over time.
In conclusion, the results indicated that low energy flux (i.e., low levels of habitual caloric intake coupled with low levels of energy expenditure) predicted future body fat gain. The fact that the null predictive relations between energy balance and future body fat gain, the significant predictive inverse relation between energy flux and future body fat gain, and the association between energy flux and RMR each replicated in 2 samples increases the confidence that can be placed in these findings. Results also implied that a low RMR may be a key physiologic mechanism underlying this relation. These findings do not provide support for the widely accepted theory that energy surfeit drives weight gain, and imply that weight loss might be more attainable via high physical activity that is coupled with high energy intake, rather than subscription to commonly prescribed low calorie diets.
Acknowledgments
We thank Dale Schoeller for conducting the doubly labeled water assessments of total daily caloric intake and expenditure, as well as for providing comments on the manuscript.
The authors’ responsibilities were as follows—SY and ES: designed and conducted the research; DJH and SY: performed the statistical analyses; and all authors: wrote the manuscript and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: ADP, air-displacement plethysmography; DLW, doubly labeled water; RMR, resting metabolic rate; TEE, total energy expenditure; TEI, total energy intake.
REFERENCES
- 1.World Health Organization. Obesity and overweight [Internet]. Fact Sheet 311. Version current 2016 [cited 2016 Jan 21]. Available from: http://amro.who.int/common/Display.asp?Lang=E&RecID=10203.
- 2.Guggenheim EA. Thermodynamics: an advanced treatment for chemists and physicists. 1st ed Amsterdam: North-Holland Publishing Company; 1949. [Google Scholar]
- 3.Chow CC, Hall KD. Short and long-term energy intake patterns and their implications for human body weight regulation. Physiol Behav 2014;134:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forbes GB, Brown MR, Welle SL, Lipinski BA. Deliberate overfeeding in women and men: energy cost and composition of the weight gain. Br J Nutr 1986;56:1–9. [DOI] [PubMed] [Google Scholar]
- 5.Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO. Fat and carbohydrate overfeeding in humans: different effects on energy storage. Am J Clin Nutr 1995;62:19–29. [DOI] [PubMed] [Google Scholar]
- 6.Avenell A, Broom JI, Brown TJ, Poobalan A, Aucott LS, Stearns SC, Smith WC, Jung RT, Campbell MK, Grant AM. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004;8:iii–iv. [DOI] [PubMed] [Google Scholar]
- 7.Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and meta-analysis. Obesity (Silver Spring) 2006;14:1283–93. [DOI] [PubMed] [Google Scholar]
- 8.Heymsfield SB, Harp JB, Reitman ML, Beetsch JW, Schoeller DA, Erondu N, Pietrobelli A. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. Am J Clin Nutr 2007;85:346–54. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr 2008;88:906–12. [DOI] [PubMed] [Google Scholar]
- 10.Chaput JP, Pelletier C, Després J, Lemieux S, Tremblay A. Metabolic and behavioral vulnerability related to weight regain in reduced-obese men might be prevented by an adequate diet-exercise intervention. Appetite 2007;49:691–5. [DOI] [PubMed] [Google Scholar]
- 11.Blackburn GL, Wilson GT, Kanders BS, Stein LJ, Lavin PT, Adler J, Brownell KD. Weight cycling: the experience of human dieters. Am J Clin Nutr 1989;49:1105–9. [DOI] [PubMed] [Google Scholar]
- 12.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–5. [DOI] [PubMed] [Google Scholar]
- 13.Stice E, Yokum S, Burger KS. Elevated reward region responsivity predicts future substance use onset but not overweight/obesity onset. Biol Psychiatry 2013;73:869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev 2006;27:750–61. [DOI] [PubMed] [Google Scholar]
- 15.Bell C, Day DS, Jones PP, Christou DD, Petitt DS, Osterberg K, Melby CL, Seals DR. High energy flux mediates the tonically augmented beta-adrenergic support of resting metabolic rate in habitually exercising older adults. J Clin Endocrinol Metab 2004;89:3573–8. [DOI] [PubMed] [Google Scholar]
- 16.Bullough RC, Gillette CA, Harris MA, Melby CL. Interaction of acute changes in exercise energy expenditure and energy intake on resting metabolic rate. Am J Clin Nutr 1995;61:473–81. [DOI] [PubMed] [Google Scholar]
- 17.Speakman JR, Selman C. Physical activity and resting metabolic rate. Proc Nutr Soc 2003;62:621–34. [DOI] [PubMed] [Google Scholar]
- 18.Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, Boyce V, Howard BV, Bogardus C. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med 1988;318:467–72. [DOI] [PubMed] [Google Scholar]
- 19.Hume DJ, Kroff J, Clamp LD, Lambert EV. Compensations for weight loss in successful and unsuccessful dieters. Am J Health Behav 2015;39:589–600. [DOI] [PubMed] [Google Scholar]
- 20.Lichtman SW, Pisarska K, Berman ER, Pestone M, Dowling H, Offenbacher E, Weisel H, Heshka S, Matthews DE, Heymsfield SB. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med 1992;327:1893–8. [DOI] [PubMed] [Google Scholar]
- 21.Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jequier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol 1986;250:R823–30. [DOI] [PubMed] [Google Scholar]
- 22.Lohman TG. Assessment of body composition in children. Pediatr Exerc Sci 1989;1:19–30. [DOI] [PubMed] [Google Scholar]
- 23.Fields DA, Goran MI, McCrory MA. Body-composition assessment via air-displacement plethysmography in adults and children: a review. Am J Clin Nutr 2002;75:453–67. [DOI] [PubMed] [Google Scholar]
- 24.Schoeller DA. Measurement of energy expenditure in free-living humans by using doubly labeled water. J Nutr 1988;118:1278–89. [DOI] [PubMed] [Google Scholar]
- 25.Schoeller DA. Validation of habitual energy intake. Public Health Nutr 2002;5(6A):883–8. [DOI] [PubMed] [Google Scholar]
- 26.Trabulsi J, Troiano RP, Subar AF, Sharbaugh C, Kipnis V, Schatzkin A, Schoeller DA. Precision of the doubly labeled water method in a large-scale application: evaluation of a streamlined-dosing protocol in the Observing Protein and Energy Nutrition (OPEN) study. Eur J Clin Nutr 2003;57:1370–7. [DOI] [PubMed] [Google Scholar]
- 27.Luke A, Dugas LR, Ebersole K, Durazo-Arvizu RA, Cao G, Schoeller DA, Adeyemo A, Brieger WR, Cooper RS. Energy expenditure does not predict weight change in either Nigerian or African American women. Am J Clin Nutr 2009;89:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of h-2-labeled and o-18-labeled water in humans. Am J Physiol 1994;267:E585–90. [DOI] [PubMed] [Google Scholar]
- 29.Black AE. The use of recommended daily allowances to assess dietary adequacy. Proc Nutr Soc 1986;45:369–81. [DOI] [PubMed] [Google Scholar]
- 30.Forbes GB. Body fat content influences the body composition response to nutrition and exercise. Ann N Y Acad Sci 2000;904:359–65. [DOI] [PubMed] [Google Scholar]
- 31.Poehlman ET. A review: exercise and its influence on resting energy metabolism in man. Med Sci Sports Exerc 1989;21:515–25. [PubMed] [Google Scholar]
- 32.Stice E, Durant S. Elevated objectively measured but not self-reported energy intake predicts future weight gain in adolescents. Appetite 2014;81:84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salbe AD, Weyer C, Lindsay RS, Ravussin E, Tataranni PA. Assessing risk factors for obesity between childhood and adolescence: I. Birth weight, childhood adiposity, parental obesity, insulin, and leptin. Pediatrics 2002;110:299–306. [DOI] [PubMed] [Google Scholar]
- 34.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 1997;337:869–73. [DOI] [PubMed] [Google Scholar]
- 35.Schlam TR, Wilson NL, Shoda Y, Mischel W, Ayduk O. Preschoolers’ delay of gratification predicts their body mass 30 years later. J Pediatr 2013;162:90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seeyave DM, Coleman S, Appugliese D, Corwyn RF, Bradley RH, Davidson NS, Kaciroti N, Lumeng JC. Ability to delay gratification at age 4 years and risk of overweight at age 11 years. Arch Pediatr Adolesc Med 2009;163:303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stubbs RJ, Hughes DA, Johnstone AM, Whybrow S, Horgan GW, King N, Blundell J. Rate and extent of compensatory changes in energy intake and expenditure in response to altered exercise and diet composition in humans. Am J Physiol Regul Integr Comp Physiol 2004;286:R350–8. [DOI] [PubMed] [Google Scholar]
- 38.Foright R, Paris H, Werth K, Larson L, Beals J, Wilburn J, Davis J, Bell C, Melby C. Decreasing the biological drive toward weight gain by increasing energy flux. FASEB J 2014;28(1 Suppl):LB431. [Google Scholar]
- 39.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, et al. . Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress. JAMA 2006;295:1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keys A, Brozek J, Henschel A, Mickelson O, Taylor HL. The biology of human starvation. Minneapolis (MN): University of Minnesota Press; 1950. [Google Scholar]
- 41.Redman LM, Heilbronn LK, Martin CK, De Jonge L, Williamson DA, Delany JP, Ravussin E, Pennington CALERIE Team. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One 2009;4:e4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crabtree DR, Chambers ES, Hardwick RM, Blannin AK. The effects of high-intensity exercise on neural responses to images of food. Am J Clin Nutr 2014;99:258–67. [DOI] [PubMed] [Google Scholar]
- 43.Evero N, Hackett LC, Clark RD, Phelan S, Hagobian TA. Aerobic exercise reduces neuronal responses in food reward brain regions. J Appl Physiol 2012;112:1612–9. [DOI] [PubMed] [Google Scholar]
- 44.McNeil J, Cadieux S, Finlayson G, Blundell JE, Doucet É. The effects of a single bout of aerobic or resistance exercise on food reward. Appetite 2015;84:264–70. [DOI] [PubMed] [Google Scholar]
- 45.Cornier MA, Melanson EL, Salzberg AK, Bechtell JL, Tregellas JR. The effects of exercise on the neuronal response to food cues. Physiol Behav 2012;105:1028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McFadden KL, Cornier M, Melanson EL, Bechtell JL, Tregellas JR. Effects of exercise on resting-state default mode and salience network activity in overweight/obese adults. Neuroreport 2013;24:866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pronk NP, Wing RR. Physical activity and long-term maintenance of weight loss. Obes Res 1994;2:587–99. [DOI] [PubMed] [Google Scholar]
- 48.Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev 2005;6:67–85. [DOI] [PubMed] [Google Scholar]
- 49.Krogh-Madsen R, Pedersen M, Solomon TP, Knudsen SH, Hansen LS, Karstoft K, Lehrskov-Schmidt L, Pedersen KK, Thomsen C, Holst JJ, et al. . Normal physical activity obliterates the deleterious effects of a high-caloric intake. J Appl Physiol 2014;116:231–9. [DOI] [PubMed] [Google Scholar]
- 50.King NA, Caudwell P, Hopkins M, Byrne NM, Colley R, Hills AP, Stubbs JR, Blundell JE. Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity (Silver Spring) 2007;15:1373–83. [DOI] [PubMed] [Google Scholar]