Abstract
Background: Previous studies have suggested that metabolite profiles of elevated acylcarnitines were associated with increased risk of cardiovascular disease (CVD) in populations with established coronary disease. However, to our knowledge, this association has not been evaluated in the context of primary cardiovascular prevention.
Objectives: We evaluated the association between 28 plasma acylcarnitine species and risk of incident CVD and the potential modifying effect of Mediterranean diet (MedDiet) interventions.
Design: We measured plasma acylcarnitines with the use of high-throughput liquid chromatography–tandem mass spectrometry at baseline and after 1 y of follow-up, both individually and classified into short-, medium-, or long-chain scores, in a case-cohort study within the Prevención con Dieta Mediterránea (PREDIMED) study, which is a randomized Mediterranean dietary intervention for primary cardiovascular prevention. A randomly selected subcohort (n = 751) and all available incident CVD cases (n = 229) after 4.8 y of follow-up were included in the current study.
Results: After adjustment for age, sex, body mass index, and other CVD risk factors, participants in the highest quartile of baseline short- and medium-chain acylcarnitines had a higher risk of CVD than did participants in the lowest quartile [HRs: 1.80 (95% CI: 1.11, 2.91; P-trend 0.01) and 1.55 (95% CI: 1.01, 2.48; P-trend = 0.04), respectively]. Increased short-chain acylcarnitines after 1 y were associated with higher risks of total CVD and stroke. Participants with higher baseline concentrations of short-, medium-, and long-chain acylcarnitines who were randomly assigned to the control group had a higher risk of CVD than did subjects with lower concentrations of acylcarnitines who were assigned to the MedDiet group.
Conclusions: Our data support the conclusion that metabolite profiles characterized by elevated concentrations of acylcarnitines are independently associated with risks of total CVD and stroke alone in participants at high risk of CVD. MedDiet interventions may mitigate the adverse associations shown between higher concentrations of acylcarnitines and CVD. This trial was registered at www.controlled-trials.com as ISRCTN35739639.
Keywords: acylcarnitines, cardiovascular disease, Mediterranean diet, metabolomics, PREDIMED
INTRODUCTION
Cardiovascular disease (CVD)20 is the leading cause of death and disability in industrialized countries (1). Although many CVD risk factors, such as smoking, obesity, diabetes, and poor dietary habits, have been identified (2), the understanding of CVD etiology and its mechanisms is still incomplete. More comprehensive approaches may help to deepen our knowledge of CVD physiopathology and also identify patients at high risk, potentially years before diagnosis, who will benefit most from preventive strategies. Thus, emerging high-throughput metabolomics has been proposed as one of the novel and useful tools for the early diagnosis of metabolic diseases (3, 4).
Of several metabolites identified as potential keys to metabolic diseases, acylcarnitines, which are intermediates of fatty acid oxidation, have been associated with CVD risk (5–8). Elevated concentrations of these metabolites may be indicative of impaired β-oxidation and mitochondrial dysfunction (5) and have been associated with higher risks of obesity, insulin resistance, and type 2 diabetes (9, 10), all of which are recognized risk factors of CVD. To date, only a few studies have examined the associations between acylcarnitines and CVD, and these studies have had small sample sizes and were conducted only in patients with established coronary artery disease. Also, they included no or scarce information on diet and lifestyles (5–8). The association between acylcarnitines and CVD, and in particular changes in their concentrations, in the context of primary cardiovascular prevention remains therefore to be elucidated.
Importantly, a Western dietary pattern was associated with a specific metabolite signature characterized by increased concentrations of short-chain acylcarnitines (11), and some dietary components have been shown to change the acylcarnitine metabolite profile in plasma or other fluids and tissues (12–15). However, whether the potential association between acylcarnitines and CVD risk is modified by an intervention with an overall healthy dietary pattern such as the Mediterranean diet (MedDiet) has not yet been investigated.
In the current prospective nested case-cohort study, we performed quantitative profiling of 28 acylcarnitine species in plasma samples of elderly participants at high CVD risk from the Prevención con Dieta Mediterránea (PREDIMED) trial. We hypothesized that higher concentrations at baseline and 1-y changes in acylcarnitine concentrations would be associated with risk of CVD and that these associations might be modified by a MedDiet intervention. Our aim was to determine the association of baseline acylcarnitine profiles and 1-y changes in acylcarnitine profiles with risk of incident CVD and stroke, and to examine whether these associations may be mitigated by MedDiet interventions.
METHODS
Study population
The design and protocol of the PREDIMED study (http://www.predimed.es) have been described in detail elsewhere (16, 17) (ISRCTN35739639). In brief, the PREDIMED study was a large, multicenter, parallel-group, randomized controlled trial, evaluating the effect of MedDiet on the primary prevention of CVD, conducted in Spain from 2003 until 2010. Participants were men (aged 55–80 y) and women (aged 60–80 y) who were free of CVD at baseline but at high CVD risk because they had either type 2 diabetes mellitus or ≥3 of the following CVD risk factors: current smoking, hypertension, hypercholesterolemia, low HDL cholesterol, overweight/obesity, or family history of premature coronary heart disease (myocardial infarction or sudden death). Exclusion criteria were the presence of any severe chronic illness, alcohol or drug abuse, BMI (in kg/m2) ≥40, and an allergy or intolerance to olive oil or nuts (16). Participants were randomly assigned to receive one of the following 3 interventions: a MedDiet supplemented with extra-virgin olive oil, a MedDiet supplemented with mixed nuts, or advice about a low-fat diet (control group). The primary endpoint of the PREDIMED trial was a composite of cardiovascular events (myocardial infarction, stroke, or death from cardiovascular causes); 288 incident CVD cases occurred during 4.8 y of follow-up.
We designed a case-cohort study in the framework of the PREDIMED trial (18). A case-cohort study design dictates that all cases and a randomly selected percentage of the original cohort (referred to as the subcohort, which, by its random selection, may include some cases) are selected as participants. In the context of a trial, the case-cohort study design allowed us to 1) maintain the trial’s original random-assignment scheme, 2) limit the randomly selected subcohort to fewer participants than in the entire cohort for cost savings, and 3) extrapolate the results to all participants in the study. In keeping with the study design, from the eligible cohort of participants with available plasma samples at baseline, we selected a random, nonstratified subsample of 10% of all PREDIMED participants at baseline plus all incident CVD cases with available blood samples taken during follow-up. Of 980 participants included in the analyses, 751 subjects were noncases, and 229 subjects were cases (there were 37 overlapping cases between the subcohort and total cases). Of these subjects, 923 of 980 participants had available samples after 1 y of follow-up and were included in the 1-y change analyses (Supplemental Figure 1). We defined cases as participants who developed a cardiovascular event (stroke, myocardial infarction, or cardiovascular death) during follow-up. We defined the subcohort as the random sample selected from the full roster of the PREDIMED study (including some incident CVD cases). We defined internal cases as cases that were randomly included in this random subcohort. We defined external cases as cases that were not randomly included in the subcohort. All participants provided written informed consent according to a protocol approved by the institutional review boards before inclusion in the study.
Metabolite profiling
At baseline and at yearly follow-up visits, trained nurses collected fasting blood samples from the PREDIMED participants. After an overnight fast, tubes for EDTA plasma were collected, and aliquots were coded and kept refrigerated until they were stored at −80°C in freezers. Pairs of samples (baseline and first-year visit) were randomly ordered and shipped on dry ice to the Broad Institute for the metabolomics analysis.
Amino acids, acylcarnitines, and other polar plasma metabolites were profiled with the use of liquid chromatography–tandem mass spectrometry (MS) on a system comprised of a Shimadzu Nexera ×2 U-HPLC (Shimadzu Corp.) coupled to a Q Exactive hybrid quadrupole orbitrap mass spectrometer (Thermo Fisher Scientific) (19). Liquid chromatography, through the application of a number of distinct stationary phase chemistries, affords the reproducible separation of metabolites in complex mixtures on the basis of their physical properties. MS enables the additional resolution of metabolites on the basis of the m/z and quantification over a wide linear dynamic range. Metabolite extracts were prepared from plasma samples (10 μL) via protein precipitation with the addition of 9 volumes of 74.9:24.9:0.2 (volume:volume:volume) of acetonitrile:methanol:formic acid–containing stable isotope-labeled internal standards [valine-d8 (Sigma-Aldrich) and phenylalanine-d8 (Cambridge Isotope Laboratories)]. The samples were centrifuged (10 min; 9000 × g; 4°C), and the supernatant fluid was injected directly onto a 150 × 2-mm, 3-μm Atlantis HILIC column (Waters). The column was eluted isocratically at a flow rate of 250 μL/min with 5% mobile phase A (10 mmol ammonium formate/L and 0.1% formic acid in H2O) for 0.5 min followed by a linear gradient to 40% mobile phase B (acetonitrile with 0.1% formic acid) over 10 min. MS analyses were carried out with the use of electrospray ionization in the positive-ion mode with the use of a full-scan analysis over 70–800 m/z at 70,000 resolution and a 3-Hz data-acquisition rate. Other MS settings were as follows: sheath gas, 40; sweep gas, 2; spray voltage, 3.5 kV; capillary temperature, 350°C; S-lens radio frequency, 40; heater temperature 300°C microscans, 1; automatic gain control target, 1e6; and maximum ion time, 250 ms. Metabolite identities were confirmed with the use of authentic reference standards. Free carnitine and 27 acylcarnitine subtypes were analyzed with the use of this approach. Raw data were processed with the use of TraceFinder 3.1 (Thermo Fisher Scientific) and Progenesis CoMet v2.0 (Nonlinear Dynamics)
Case ascertainment
For the current analysis, the primary endpoint was a composite of cardiovascular events (myocardial infarction, stroke, or death from cardiovascular causes), and as a secondary endpoint, we separately analyzed incident stroke because this was the most-common element included in the definition of the composite primary endpoint in the PREDIMED trial. The endpoint adjudication committee, whose members were blinded to the treatment allocation and dietary information, updated information on these endpoints once a year. The committee used the following different sources of information: 1) yearly questionnaires and examinations for all participants, 2) primary care physicians, 3) a comprehensive yearly review of medical records of all participants, and 4) yearly consultation of the National Death Index. Medical records of participants were requested, and the endpoint committee adjudicated major events and determined the cause of death.
Covariate assessment
At baseline and yearly follow-up visits, a questionnaire was administered about lifestyle variables, educational achievement, history of illnesses, medication use, and family history of disease. Physical activity was assessed with the use of the validated Spanish version of the Minnesota Leisure-Time Physical Activity questionnaire (20). Participants were considered to have diabetes, hypercholesterolemia, or hypertension if they had previously been diagnosed or if they were being treated with antidiabetic, cholesterol-lowering, or antihypertensive agents, respectively. Trained dietitians completed a 137-item validated semiquantitative food-frequency questionnaire in face-to-face interviews with participants (21). We used Spanish food-composition tables to estimate energy and nutrient intakes (22). Trained personnel took anthropometric and blood pressure measurements.
Statistics
We applied an inverse normal transformation to approximate a normal distribution of metabolite concentrations (23). Baseline characteristics of participants are presented according to case status as means ± SDs for quantitative traits and as n (%) for categorical variables. Baseline characteristics were compared between cases and noncases with the use of t tests for continuous variables and chi-square tests for categorical variables.
We used Cox proportional hazard models with Barlow weights [to account for oversampling of cases in the study design (18)] to estimate HRs and their 95% CIs for the primary combined endpoint of CVD and, separately, for nonfatal stroke. The follow-up time was calculated as the interval between the date of random assignment and the date of a cardiovascular event, death, or end of follow-up, whichever came first. Cox models were adjusted for age (y), sex (men or women), family history of premature heart disease (yes or no), smoking (never, former, or current), and BMI and were stratified by intervention group (both MedDiet interventions and a low-fat control group) (model 1). Model 2 was further adjusted for leisure-time physical activity (metabolic equivalent tasks in min/d), baseline hypertension (yes or no), dyslipidemia (yes or no), and diabetes (yes or no). At baseline, 28 individual acylcarnitine species were analyzed as both continuous variables and quartiles (with the use of cutoffs defined in noncases). To account for multiple testing in the individual analysis of the 28 acylcarnitines, we used a corrected P value according to the Benjamini-Hochberg method. We calculated 3 acylcarnitine scores. An inverse normal transformation was applied to raw values of baseline acylcarnitines, and a weighted sum of these values was computed to calculate scores for 1) short-chain acylcarnitines (10 acylcarnitines species): from C2carnitine to C7carnitine; 2) medium-chain acylcarnitines (9 species): from C8carnitine to C14:2carnitine; and 3) long-chain acylcarnitines (8 species): from C16carnitine to C26carnitine. Weights corresponded to the respective coefficients from the multivariable Cox regression model that was fitted with each individual metabolite (19). We conducted additional models that were adjusted for total energy intake (kcal/d) and branched-chain amino acid (valine, isoleucine, and leucine) scores calculated with the use of the same method as used for carnitine scores (24). To test for the linear trend across quartiles, the median of each quartile was assigned and analyzed as a continuous variable. To compare the predictive ability of a classical model (multivariable model 2 without acylcarnitines) and the same model with the inclusion of acylcarnitine scores, we further calculated the AUC.
We also examined the associations between 1-y changes in the 28 individual acylcarnitines species and 1-y changes in acylcarnitine scores (short-, medium-, and long-chain acylcarnitines) and CVD risk. We used the same models as in the baseline analyses but we further adjusted for baseline concentrations and an interaction term between acylcarnitines at baseline and the 1-y change of acylcarnitines (both as continuous variables).
We repeated the analysis with the use of incident stroke as the outcome both including and excluding nonstroke CVD cases (i.e., treating the 113 nonstroke CVD cases as noncases or removing them from the analyses) and showed similar results. Therefore, we present stroke results only after the removal of nonstroke CVD cases.
To assess the effect of the MedDiet interventions, we introduced a multiplicative interaction term with 1 df between each of the 3 baseline acylcarnitine scores (continuous) and the MedDiet-intervention groups (the 2 MedDiet group as a single group compared with the control group). We stratified the analysis by intervention group (both MedDiet interventions compared with the control). We conducted joint analyses for both the baseline and 1-y changes in acylcarnitine scores and the intervention group with the use of, as the reference group, participants assigned to the MedDiet group and with lower baseline concentrations of acylcarnitines (quartile 1 of the 3 scores). Finally, we compared the adjusted mean changes in the individual metabolites from baseline to 1 y by intervention group with the use of the same models as previously described.
All statistical analyses were performed with the use of SAS software (v9.4; SAS Institute) and R software (v2.13.0; R Foundation). P < 0.05 was considered statistically significant for the analysis of acylcarnitine scores.
RESULTS
Baseline characteristics of the 980 individuals (229 cases and 751 noncases) included in the current case-cohort study are described in Table 1. The mean age of participants at baseline was 68 y, and the mean ± SD BMI was 29.7 ± 3.7. Compared with noncases, participants who developed CVD were more likely to be older, men, and current smokers; have diabetes; and be less likely to have dyslipidemia and a family history of premature coronary heart disease (myocardial infarction or sudden death) (Table 1).
TABLE 1.
Baseline characteristics of the PREDIMED nested case-cohort study population1
| Total | Cases | Noncases | P | |
| n | 980 | 229 | 751 | |
| Age, y | 67.6 ± 6 | 69.4 ± 6.5 | 67.0 ± 6 | <0.01 |
| Women | 528 (53.9) | 91 (39.7) | 437 (58.1) | <0.01 |
| BMI, kg/m2 | 29.7 ± 3.7 | 29.6 ± 3.7 | 29.7 ± 3.6 | 0.67 |
| Intervention group | 0.15 | |||
| MedDiet + EVOO | 363 (37.0) | 82 (35.8) | 281 (37.4) | |
| MedDiet + nuts | 314 (32.0) | 65 (28.4) | 249 (33.1) | |
| Control group | 303 (30.9) | 82 (35.8) | 221 (29.4) | |
| Family history of CHD | 237 (24.2) | 44 (19.2) | 193 (25.7) | 0.04 |
| Hypertension | 817 (83.3) | 189 (82.5) | 628 (83.6) | 0.69 |
| Dyslipidemia | 692 (70.6) | 134 (58.5) | 558 (74.3) | <0.01 |
| Diabetes | 494 (50.4) | 147 (64.2) | 347 (46.2) | <0.01 |
| Smoking | <0.01 | |||
| Never | 578 (58.9) | 104 (45.4) | 474 (63.1) | |
| Former | 263 (26.8) | 79 (34.5) | 184 (24.5) | |
| Current | 139 (14.2) | 46 (20.1) | 93 (12.4) |
Baseline characteristics of participants are presented according to case status as means ± SDs for quantitative traits and as n (%) for categorical variables. P values are for comparisons between cases and controls (Pearson’s chi-square test was used for categorical variables, and a 1-factor ANOVA was used for continuous variables). CHD, family history of premature coronary heart disease (myocardial infarction or sudden death); EVOO, extra-virgin olive oil; MedDiet, Mediterranean diet; PREDIMED, Prevención con Dieta Mediterránea.
Baseline acylcarnitines and the effect of dietary interventions on risks of CVD and stroke
Associations between baseline acylcarnitine scores and risk of CVD are presented in Table 2. Across all models in the overall study population, higher risk of CVD was observed per SD increase in short-, medium-, and long-chain acylcarnitine scores. After adjustment for age, sex, BMI, family history of premature heart disease, and smoking (model 1), participants in the top quartile of short-and medium-chain acylcarnitine scores had significantly higher CVD risk. In the second model (further adjusted for physical activity, baseline hypertension, diabetes, and dyslipidemia), HRs for CVD in the top compared with bottom quartiles were 1.80 (95% CI: 1.11, 2.91; P-trend = 0.01) for the short-chain acylcarnitine score and 1.55 (95% CI: 1.01, 2.48; P-trend = 0.04) for the medium-chain acylcarnitine score. In analyses stratified by intervention group, participants who were assigned to the control group and were in the top baseline quartile of short- and medium-chain acylcarnitines had significantly higher risk of CVD than did those in the bottom baseline quartile [HRs: 3.19 (95% CI: 1.34, 7.56; P-trend = 0.02) and 2.17 (95% CI: 1.02, 4.63; P-trend = 0.03), respectively]. In contrast, no significant associations between acylcarnitine scores and CVD were shown for participants who were assigned to the MedDiet interventions. When baseline quartiles of long-chain acylcarnitine scores were analyzed, the results were nonsignificant in both the overall and stratified analyses. The associations between baseline concentrations of each of the 28 acylcarnitine species and risk of CVD are presented in Supplemental Table 1. In the second model, and after the application of a multiple testing correction, no significant associations were shown between individual acylcarnitines at baseline and CVD risk.
TABLE 2.
Associations between baseline acylcarnitine scores and risk of incident cardiovascular disease1
| Composite cardiovascular disease |
||||||
| Overall (751 noncases and 229 cases) |
Both MedDiet2 groups (530 noncases and 147 cases) |
Control group (221 noncases and 82 cases) |
||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Short-chain acylcarnitines (C2–C7) | ||||||
| Crude per SD | 1.70 (1.14, 2.52) | <0.01 | 1.33 (0.85, 2.10) | 0.21 | 3.00 (1.33, 6.78) | <0.01 |
| Model 1 | ||||||
| Per SD | 2.09 (1.36, 3.21) | <0.01 | 1.57 (0.96, 2.57) | 0.07 | 4.00 (1.68, 9.53) | <0.01 |
| Across quartiles | ||||||
| 1 | 1 (reference) | — | 1 (reference) | — | 1 (reference) | — |
| 2 | 1.11 (0.69, 1.78) | — | 1.18 (0.67, 2.08) | — | 1.08 (0.45, 2.60) | — |
| 3 | 1.33 (0.86, 2.08) | — | 1.53 (0.89, 2.64) | — | 1.06 (0.48, 2.31) | — |
| 4 | 1.77 (1.12, 2.78) | — | 1.43 (0.81, 2.51) | — | 2.67 (1.22, 5.87) | — |
| P-trend | — | <0.01 | — | 0.13 | — | 0.02 |
| Model 2 | ||||||
| Per SD | 2.08 (1.33, 3.25) | <0.01 | 1.50 (0.90, 2.49) | 0.11 | 5.22 (1.87, 14.50) | <0.01 |
| Across quartiles | ||||||
| 1 | 1 (reference) | — | 1 (reference) | — | 1 (reference) | — |
| 2 | 1.18 (0.72, 1.95) | — | 1.31 (0.70, 2.43) | — | 1.08 (0.42, 2.74) | — |
| 3 | 1.35 (0.85, 2.14) | — | 1.65 (0.92, 2.95) | — | 1.07 (0.47, 2.44) | — |
| 4 | 1.80 (1.11, 2.91) | — | 1.39 (0.76, 2.54) | — | 3.19 (1.34, 7.56) | — |
| P-trend | — | 0.01 | — | 0.19 | — | 0.02 |
| Medium-chain acylcarnitines (C8–C14) | ||||||
| Crude per SD | 1.67 (1.17, 2.40) | <0.01 | 1.41 (0.89, 2.21) | 0.13 | 2.79 (1.49, 5.24) | <0.01 |
| Model 1 | ||||||
| Per SD | 1.65 (1.12, 2.43) | 0.01 | 1.32 (0.83, 2.10) | 0.23 | 2.75 (1.36, 5.57) | <0.01 |
| Across quartiles | ||||||
| 1 | 1 (reference) | — | 1 (reference) | — | 1 (reference) | — |
| 2 | 0.88 (0.54, 1.44) | — | 1.01 (0.54, 1.86) | — | 0.70 (0.31, 1.59) | — |
| 3 | 1.25 (0.78, 1.98) | — | 1.24 (0.68, 2.25) | — | 1.27 (0.60, 2.68) | — |
| 4 | 1.60 (1.02, 2.53) | — | 1.36 (0.76, 2.43) | — | 2.35 (1.12, 4.93) | — |
| P-trend | — | 0.02 | — | 0.21 | — | 0.01 |
| Model 2 | ||||||
| Per SD | 1.53 (1.04, 2.26) | 0.03 | 1.23 (0.76, 2.00) | 0.38 | 2.36 (1.17, 4.76) | 0.02 |
| Across quartiles | ||||||
| 1 | 1 (reference) | — | 1 (reference) | — | 1 (reference) | — |
| 2 | 0.92 (0.55, 1.51) | — | 0.90 (0.48, 1.68) | — | 0.74 (0.31, 1.78) | — |
| 3 | 1.19 (0.74, 1.92) | — | 1.10 (0.59, 2.07) | — | 1.39 (0.63, 3.02) | — |
| 4 | 1.55 (1.01, 2.48) | — | 1.30 (0.70, 2.39) | — | 2.17 (1.02, 4.63) | — |
| P-trend | — | 0.04 | — | 0.28 | — | 0.03 |
| Long-chain acylcarnitines (C16–C26) | ||||||
| Crude per SD | 1.54 (1.19, 1.99) | <0.01 | 1.42 (1.04, 1.94) | 0.02 | 1.94 (1.20, 3.13) | <0.01 |
| Model 1 | ||||||
| Per SD | 1.33 (1.00, 1.76) | 0.04 | 1.19 (0.86, 1.67) | 0.28 | 1.66 (0.95, 2.90) | 0.07 |
| Across quartiles | ||||||
| 1 | 1 (reference) | — | 1 (reference) | — | 1 (reference) | — |
| 2 | 1.01 (0.61, 1.65) | — | 1.33 (0.71, 2.47) | — | 0.55 (0.21, 1.44) | — |
| 3 | 1.11 (0.68, 1.78) | — | 1.38 (0.74, 2.57) | — | 0.81 (0.36, 1.81) | — |
| 4 | 1.44 (0.90, 2.29) | — | 1.56 (0.85, 2.87) | — | 1.26 (0.56, 2.84) | — |
| P-trend | — | 0.09 | — | 0.16 | — | 0.38 |
| Model 2 | ||||||
| Per SD | 1.31 (0.98, 1.75) | 0.07 | 1.20 (0.85, 1.69) | 0.30 | 1.59 (0.87, 2.91) | 0.12 |
| Across quartiles | ||||||
| 1 | 1 (reference) | — | 1 (reference) | — | 1 (reference) | — |
| 2 | 1.02 (0.61, 1.71) | — | 1.29 (0.68, 2.47) | — | 0.59 (0.21, 1.63) | — |
| 3 | 1.14 (0.69, 1.88) | — | 1.45 (0.75, 2.80) | — | 0.87 (0.37, 2.02) | — |
| 4 | 1.47 (0.91, 2.40) | — | 1.61 (0.85, 3.03) | — | 1.29 (0.54, 3.10) | — |
| P-trend | — | 0.08 | — | 0.14 | — | 0.43 |
Inverse normal transformation was applied to raw values of acylcarnitines, and a weighted sum of these values was computed to calculate the scores (short-chain acylcarnitines were as follows: C2carnitine, C3DCCH3carnitine, C3carnitine, C4carnitine, C4OHcarnitine, C5carnitine, C5:1carnitine, C5:DCcarnitine, C6carnitine, and C7carnitine; medium-chain acylcarnitines were as follows: C8carnitine, C9carnitine, C10carnitine, C10:2carnitine, C12carnitine, C12:1carnitine, C14carnitine, C14:1carnitine, and C14:2carnitine; long-chain acylcarnitines were as follows: C16carnitine, C18carnitine, C18:1carnitine, C18:1OHcarnitine, C18:2carnitine, C20carnitine, C20:4carnitine, and C26carnitine). Model 1 was adjusted for age, sex, BMI, family history of premature heart disease, and smoking and was stratified by intervention group (only in the overall analyses). Model 2 was adjusted as for model 1 and for physical activity (metabolic equivalent tasks in min/d), hypertension, dyslipidemia, and diabetes.
MedDiet, Mediterranean diet.
Table 3 shows the associations between baseline acylcarnitine scores and risk of stroke. A total of 869 individuals (118 stroke cases and 751 noncases) were included in these analyses (participants who experienced myocardial infarction or cardiovascular death but not stroke were removed from these analyses). Short-chain acylcarnitines were strongly associated with higher stroke risk [HR for quartile 4 compared with quartile 1: 2.53; 95% CI: 1.24, 5.18; P-trend < 0.01). Medium- and long-chain acylcarnitines were not significantly associated with stroke in the adjusted models. Similar to CVD results, participants in the control group and in the highest quartile of baseline short-chain acylcarnitines had higher risk of stroke than that of subjects in the lowest quartile (HR: 4.37; 95% CI: 1.24, 15.38; P-trend = 0.01). No significant associations between acylcarnitine scores and stroke were shown for individuals assigned to MedDiet interventions.
TABLE 3.
Associations between baseline acylcarnitine scores and risk of incident stroke1
| Stroke |
||||||
| Overall (751 noncases and 118 cases) |
Both MedDiet groups (530 noncases and 72 cases) |
Control group (221 noncases and 46 cases) |
||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Short-chain acylcarnitines (C2–C7) | ||||||
| Crude per SD | 1.64 (1.18, 2.28) | <0.01 | 1.43 (0.97, 2.11) | 0.06 | 2.45 (1.24, 4.83) | <0.01 |
| Model 1 | ||||||
| Per SD | 1.84 (1.30, 2.59) | <0.01 | 1.46 (1.00, 2.13) | 0.05 | 2.68 (1.35, 5.33) | <0.01 |
| Across quartiles | ||||||
| 1 | 1 (reference) | — | 1 (reference) | — | 1 (reference) | — |
| 2 | 2.49 (1.27, 4.90) | — | 2.93 (1.28, 6.71) | — | 1.89 (0.56, 6.38) | — |
| 3 | 2.91 (1.47, 5.74) | — | 2.58 (1.12, 5.97) | — | 3.52 (1.04, 11.87) | — |
| 4 | 2.52 (1.26, 5.02) | — | 1.88 (0.78, 4.51) | — | 3.36 (1.05, 10.77) | — |
| P-trend | — | 0.01 | — | 0.35 | — | 0.02 |
| Model 2 | ||||||
| Per SD | 1.82 (1.27, 2.61) | <0.01 | 1.41 (0.96, 2.07) | 0.07 | 3.38 (1.49, 7.66) | <0.01 |
| Across quartiles | ||||||
| 1 | 1 (reference) | — | 1 (reference) | — | 1 (reference) | — |
| 2 | 2.46 (1.23, 4.91) | — | 3.10 (1.31, 7.32) | — | 1.60 (0.46, 5.54) | — |
| 3 | 2.86 (1.42, 5.74) | — | 2.73 (1.13, 6.59) | — | 3.40 (0.99, 11.70) | — |
| 4 | 2.53 (1.24, 5.18) | — | 1.83 (0.71, 4.54) | — | 4.37 (1.24, 15.38) | — |
| P-trend | — | 0.01 | — | 0.43 | — | 0.01 |
| Medium-chain acylcarnitines (C8–C14) | ||||||
| Crude per SD | 1.88 (1.04, 3.42) | 0.03 | 1.55 (0.71, 3.37) | 0.26 | 3.28 (1.26, 8.55) | 0.01 |
| Model 1 | ||||||
| Per SD | 1.76 (0.95, 3.28) | 0.07 | 1.34 (0.63, 2.85) | 0.44 | 3.45 (1.14, 10.38) | 0.02 |
| Across quartiles | ||||||
| 1 | 1 (reference) | — | 1 (reference) | — | 1 (reference) | — |
| 2 | 1.09 (0.59, 2.00) | — | 1.41 (0.62, 3.19) | — | 0.85 (0.33, 2.22) | — |
| 3 | 0.98 (0.52, 1.84) | — | 1.27 (0.57, 2.80) | — | 0.58 (0.18, 1.84) | — |
| 4 | 1.64 (0.93, 2.91) | — | 1.42 (0.66, 3.06) | — | 2.37 (1.01, 5.67) | — |
| P-trend | — | 0.09 | — | 0.44 | — | 0.07 |
| Model 2 | ||||||
| Per SD | 1.59 (0.85, 2.98) | 0.14 | 1.23 (0.56, 2.72) | 0.60 | 2.92 (1.01, 8.62) | 0.05 |
| Across quartiles | ||||||
| 1 | 1 (reference) | — | 1 (reference) | — | 1 (reference) | — |
| 2 | 1.16 (0.62, 2.17) | — | 1.26 (0.55, 2.89) | — | 1.05 (0.34, 3.18) | — |
| 3 | 1.01 (0.52, 1.95) | — | 1.21 (0.52, 2.75) | — | 0.70 (0.20, 2.44) | — |
| 4 | 1.55 (0.86, 2.79) | — | 1.30 (0.59, 2.87) | — | 2.37 (0.95, 5.87) | — |
| P-trend | — | 0.16 | — | 0.56 | — | 0.09 |
| Long-chain acylcarnitines (C16–C26) | ||||||
| Crude per SD | 1.66 (1.06, 2.59) | 0.03 | 1.32 (0.77, 2.26) | 0.31 | 2.69 (1.12, 6.42) | 0.03 |
| Model 1 | ||||||
| Per SD | 1.41 (0.85, 2.32) | 0.17 | 1.15 (0.65, 2.05) | 0.62 | 2.09 (0.76, 5.82) | 0.15 |
| Across quartiles | ||||||
| 1 | 1 (reference) | — | 1 (reference) | — | 1 (reference) | — |
| 2 | 0.90 (0.48, 1.69) | — | 1.00 (0.45, 2.19) | — | 0.77 (0.27, 2.20) | — |
| 3 | 0.93 (0.49, 1.74) | — | 1.43 (0.65, 3.12) | — | 0.42 (0.13, 1.34) | — |
| 4 | 1.15 (0.62, 2.16) | — | 1.09 (0.48, 2.49) | — | 1.14 (0.40, 3.23) | — |
| P-trend | — | 0.60 | — | 0.67 | — | 0.73 |
| Model 2 | ||||||
| Per SD | 1.37 (0.82, 2.29) | 0.22 | 1.15 (0.64, 2.08) | 0.63 | 2.09 (0.70, 6.18) | 0.18 |
| Across quartiles | ||||||
| 1 | 1 (reference) | — | 1 (reference) | — | 1 (reference) | — |
| 2 | 0.95 (0.49, 1.82) | — | 1.00 (0.44, 2.26) | — | 0.84 (0.27, 2.57) | — |
| 3 | 0.96 (0.51, 1.81) | — | 1.53 (0.69, 3.39) | — | 0.46 (0.14, 1.49) | — |
| 4 | 1.18 (0.62, 2.24) | — | 1.11 (0.48, 2.56) | — | 1.13 (0.43, 4.15) | — |
| P-trend | — | 0.59 | — | 0.62 | — | 0.68 |
Inverse normal transformation was applied to raw values of acylcarnitines, and a weighted sum of these values was computed to calculate the scores (short-chain acylcarnitines were as follows: C2carnitine, C3DCCH3carnitine, C3carnitine, C4carnitine, C4OHcarnitine, C5carnitine, C5:1carnitine, C5:DCcarnitine, C6carnitine, and C7carnitine; medium-chain acylcarnitines were as follows: C8carnitine, C9carnitine, C10carnitine, C10:2carnitine, C12carnitine, C12:1carnitine, C14carnitine, C14:1carnitine, and C14:2carnitine; long-chain acylcarnitines were as follows: C16carnitine, C18carnitine, C18:1carnitine, C18:1OHcarnitine, C18:2carnitine, C20carnitine, C20:4carnitine, and C26carnitine). Nonstroke CVD cases were excluded from the analysis. Model 1 was adjusted for age, sex, BMI, family history of premature heart disease, and smoking and was stratified by intervention group (only in the overall analyses). Model 2 was adjusted as for model 1 and for physical activity (metabolic equivalent tasks in min/d), hypertension, dyslipidemia, and diabetes. CVD, cardiovascular disease; MedDiet, Mediterranean diet.
Results for additional adjustment of multivariable model 2 for total energy intake and a branched-chain amino acid score were consistent with the primary analysis and resulted in higher risks of CVD for short-, medium, and long-chain acylcarnitine scores. Per SD increase in short-chain acylcarnitines, the HR was 2.43 (95% CI: 1.49, 3.97), for medium-chain acylcarnitines, the HR was 1.51 (95% CI: 1.02, 2.24), and for long-chain acylcarnitine, the HR was 1.34 (95% CI: 1.01, 1.81). The AUC for the model without the inclusion of acylcarnitine scores was 0.70, and with the inclusion of acylcarnitine scores, the AUC increased to 0.72, but there were no significant differences between both AUCs.
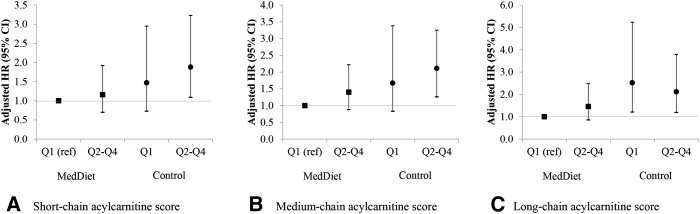
Figure 1 shows risk of incident CVD with the use of a joint classification of baseline acylcarnitine scores and intervention groups in which the reference category was composed of participants in the bottom quartile of scores who were assigned to MedDiet interventions (both MedDiet groups merged together). Participants in the control group and in the top quartiles of short-, medium-, and long-chain acylcarnitines had higher risk of CVD than did the reference group [HRs: 2.11 (95% CI: 1.26, 3.25), 1.88 (95% CI: 1.09, 3.23), and 2.12 (95% CI: 1.19, 3.79), respectively].
FIGURE 1.
Multivariate adjusted HRs (95% CIs) of incident CVD and Qs of baseline acylcarnitine scores stratified by intervention group (Mediterranean interventions compared with a control group). An inverse normal transformation was applied to raw baseline values, and a weighted sum of these values was computed to calculate the scores. (A) Short-chain acylcarnitines (C2carnitine, C3DCCH3carnitine, C3carnitine, C4carnitine, C4OHcarnitine, C5carnitine, C5:1carnitine, C5:DCcarnitine, C6carnitine, and C7carnitine). (B) Medium-chain acylcarnitines (C8carnitine, C9carnitine, C10carnitine, C10:2carnitine, C12carnitine, C12:1carnitine, C14carnitine, C14:1carnitine, and C14:2carnitine). (C) Long-chain acylcarnitines (C16carnitine, C18carnitine, C18:1carnitine, C18:1OHcarnitine, C18:2carnitine, C20carnitine, C20:4carnitine, and C26carnitine). n = 980 for the 3 carnitine scores. Analyses were adjusted for age, sex, BMI, physical activity (metabolic equivalent tasks in min/d), family history of premature heart disease, and smoking. The P values for the interactions between each MedDiet intervention group (extra-virgin olive oil and nuts) (as binary; yes or no) and acylcarnitine scores (continuous) with 2 product terms (extra-virgin olive oil × acylcarnitine score and nuts × acylcarnitine score) and 2 df were 0.04, 0.09, and 0.48 for short-, medium-, and long-chain acylcarnitine scores, respectively. CVD, cardiovascular disease; MedDiet, Mediterranean diet; Q, quartile; ref, reference.
One-year change in acylcarnitines and the effect of dietary interventions on risks of CVD and stroke
Associations between 1-y changes in acylcarnitine scores and risk of CVD are presented in Supplemental Table 2. Per each SD increase in the 1-y change in the short-chain acylcarnitine score, risk of CVD was 36% higher (HR: 1.36; 95% CI: 1.01, 1.83) and corresponding risk per each additional SD of the 1-y change in the long-chain acylcarnitine score was 71% higher (HR: 1.71; 95% CI: 1.04, 2.81). For the score of medium-chain acylcarnitines, an increase of 1 SD after 1 y was associated with increased risk of CVD in model 1 (HR: 1.47; 95% CI: 1.04, 1.09), but results were attenuated and no longer significant after additional adjustment. The associations between 1-y changes in acylcarnitine scores and risk of stroke were similar to those of CVD (Supplemental Table 3). One-year changes in short- and long-chain acylcarnitine scores were associated with increased risk of stroke in both analyses. Consistent with the CVD analyses, 1-y changes in medium-chain acylcarnitine scores were associated with increased stroke risk in the first model but were attenuated in the second model. A joint classification of 1-y changes in acylcarnitine scores and intervention groups and risk of CVD are presented in Supplemental Figure 2. Although the P-interaction was NS, participants in the control group who had higher increases in long-chain acylcarnitines had higher risk of CVD than did the reference group (subjects in the MedDiet group in the lower quartile of scores) (HR: 1.93; 95% CI: 1.05, 3.53). Supplemental Figure 3 shows adjusted means for 1-y changes in acylcarnitine scores by intervention group. No significant differences between control and intervention groups were observed.
DISCUSSION
In this prospective case-cohort study of individuals at high CVD risk, we showed that a baseline profile of increased short- and medium-chain plasma acylcarnitines was associated with higher risk of CVD independent of established CVD risk factors. We also identified elevated concentrations of short-chain acylcarnitines as potential biomarkers of future stroke risk. These metabolites were altered up to 3–4 y before the onset of CVD. We also observed that 1-y changes in short- and long-chain acylcarnitine scores were directly associated with increased risks of both total CVD and stroke alone independent of baseline concentrations of these metabolites and recognized risk factors of CVD. Participants with higher concentrations of short- and medium-chain acylcarnitines at baseline who were assigned to the control group had higher risks of both total CVD and stroke alone, which suggested that MedDiet interventions can mitigate, at least in part, the harmful effects of elevated acylcarnitines on CVD risk. These alterations in acylcarnitine concentrations provide pathway hypotheses about their implications in the development of CVD. However, no effect of the MedDiet was observed for 1-y changes in acylcarnitine scores.
Our observations, which revealed a metabolomic signature of increased baseline acylcarnitines that were related to higher CVD risk, are consistent with those of previous studies, but to our knowledge, our findings are novel and go beyond those of previous studies because we also assessed 1-y changes in the context of a dietary intervention for primary cardiovascular prevention (5–8). In a prospective cohort study of 2023 patients who were undergoing cardiac catheterization, a metabolic panel composed of short-, medium-, and long-chain acylcarnitines was associated with 14–24% higher risks of myocardial infarction and death after 3.1 y of follow-up (6). A previous case-control study conducted by the same authors revealed that a factor composed of dicarboxylacylcarnitines was predictive of cardiovascular or death events in individuals with coronary artery disease (5). Similarly, a report of 2 independent nested case-control studies showed that increased plasma concentrations of long-chain acylcarnitines were associated with 1-y cardiovascular mortality in dialysis patients (7). Finally, results from a principal component analysis conducted in Italian elderly patients with a high rate of previous CVD showed that medium- and long-chain acylcarnitines were associated with early cardiovascular events independent of recognized CVD risk predictors (8). With the addition of this evidence, our study is the first study to our knowledge to describe the association between plasma acylcarnitines and CVD risk in individuals at high CVD risk after controlling for well-established CVD risk factors and with repeated longitudinal measurements of acylcarnitines. Our findings are noteworthy in the context of the primary prevention of CVD because it is important to identify individuals who are more likely to progress in their disease to target preventive strategies, thereby reducing future clinical and public health impacts. Consequently, it is expected that this type of research will lead towards a more personalized and precise medicine, which is widely considered to be the medical future.
Additional novel findings, to our knowledge, of the current study included the apparent mitigating effect of the MedDiet intervention on the association between baseline acylcarnitine scores and incident CVD as well as the direct associations between 1-y changes in acylcarnitine scores and risks of stroke and CVD. Participants with relatively high baseline acylcarnitine concentrations who were assigned to the control group showed higher risk of CVD than did individuals with lower concentrations of acylcarnitines who were assigned to the MedDiet interventions. These results were more pronounced in short- and medium-chain acylcarnitines, which suggested that these metabolites could have a role in the pathophysiology of CVD (6). These findings indicate that pathways related to acylcarnitine metabolism may be influenced by the diet (12–14), and the MedDiet may potentially counteract the harmful effects of elevated acylcarnitines in blood. Compared with the prudent diet, the Western dietary pattern was associated with a specific metabolite signature characterized by increased concentrations of short-chain acylcarnitines (11); in addition, meat eaters tended to have increased concentrations of acylcarnitines and other metabolites (15), which indicated that the acylcarnitine metabolite profile can be affected by dietary components (12–14). In the context of previous cross-sectional studies, our study provided novel and useful longitudinal information with repeated measurements of acylcarnitines after a 1-y intervention. However, contrary to our expectations, no significant differences in 1-y changes in acylcarnitines between control and intervention groups were observed in our study, perhaps because only 1 y might be a short period to observe these effects or because other mechanisms and pathways instead of the changes in acylcarnitines underlie the observed beneficial effect of the MedDiet on CVD. Gut microbiota that can be modified by diet may also play a role in the relation between acylcarnitines and CVD (25). Various short- and long-chain acylcarnitines possess a trimethylamine moiety, and consequently, they are likely to be involved in gut microbe–dependent pathways that contribute to the formation of trimethylamine and trimethylamine-N-oxide, which may increase risk of atherosclerosis and consequently of CVD (26).
Because acylcarnitine concentrations have been associated with increased risks of insulin resistance and type 2 diabetes (27), our results were biologically plausible and consistent with previous cross-sectional assessments of acylcarnitines. Our study also showed, for the first time to our knowledge, direct associations between 1-y changes in short- and long-chain acylcarnitines and risk of CVD, thereby reinforcing the hypothesis that pathways related to acylcarnitines may play an important role in CVD development. However, medium-chain acylcarnitines were not significantly related to total CVD or stroke alone in the current study. In addition, we showed strong associations between acylcarnitines and CVD, and the AUC from the classical risk-factor model showed a reasonable predictive ability, but the addition of acylcarnitine scores to the classical risk-factor model did not add much to the C statistic from the classical model. Although acylcarnitines did not add the predictive power of CVD beyond traditional risk factors, our findings improve our understanding of the metabolic pathways related to the MedDiet and CVD.
Acylcarnitines are derived from both fatty acid and amino acid β oxidation (27). Acylcarnitines can be formed from almost any CoA ester. Acylcarnitines can also be derived from other intermediates such as ketone bodies, degradation products of lysine, tryptophan, valine, leucine, and isoleucine, and carbon atoms from glucose (28). The main function of l-carnitine is to transport fatty acids from the cytosol to the mitochondrial matrix where β oxidation takes place; this process results in the esterification of l-carnitine to form acylcarnitine derivatives (29). Acylcarnitines can be measured in plasma after being transported across cell membranes (27). The accumulation of acylcarnitines may be indicative of inefficient β oxidation and altered mitochondrial metabolism (8). These processes are enhanced in the aging process, in particular, advanced age leads to an impaired flux of carnitines through the mitochondrial pathway, thereby reflecting mitochondrial dysfunction (8). Elderly individuals also have an increased mitochondrial production of reactive oxygen species that enhances vascular inflammation and contributes to alterations in the composition of plaque and to its rupture (8). In addition, increased acylcarnitines have been associated with higher risks of insulin resistance and type 2 diabetes (9, 30), both of which are strong risk factors for CVD.
The main limitation of the current study was that our results may not be generalized to other populations. However, the case-cohort design maximized the efficiency of the high-throughput metabolomics profiling, which allowed us to extend the results to all PREDIMED participants. Other limitations included that the liquid chromatography–tandem MS–based metabolite measurements may not have a direct clinical translation for each metabolite trait. Several strengths, such as the prospective design, the ability to control for potential confounders because of the recording of comprehensive data, and the accurate and blind assessment of incident cases, also deserve mention.
In conclusion, our data provide strong support for the association between metabolite profiles composed of elevated acylcarnitines with risks of stroke and CVD in individuals at high CVD risk. MedDiet interventions may partially mitigate the deleterious effects of increased acylcarnitines on CVD risk.
Acknowledgments
The authors’ responsibilities were as follows—MG-F: drafted the manuscript; M-GF and YZ: analyzed the data; MG-F, YZ, MR-C, AH, MAM-G, FBH, and JS-S: supervised the study; MG-F, MR-C, MAM-G, DC, RE, ER, MF, FA, MF, JL, LS-M, and JS-S: conducted the research; MG-F, YZ, MAM-G, FBH, and JS-S: interpreted the statistical analysis and data; MG-F, FBH, and JS-S: had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis; MAM-G, DC, RE, ER, MF, JL, LS-M, and JS-S: coordinated the subject recruitment at outpatient clinics; MAM-G and FBH: designed the research; CBC: analyzed the metabolomics data; and all authors: revised the manuscript for important intellectual content and read and approved the final manuscript. ER and FBH have received grants from the California Walnut Commission, and MAM-G and JS-S have received grants from the International Nut Council. The remaining authors reported no conflicts of interest related to the study.
Footnotes
Abbreviations used: CVD, cardiovascular disease; MedDiet, Mediterranean diet; MS, mass spectrometry; PREDIMED, Prevención con Dieta Mediterránea.
REFERENCES
- 1.World Health Organization Report. Global atlas on cardiovascular disease prevention and control. In: Mendis S, Puska P, Norrving B, editors. Geneva (Switzerland): World Health Organization; 2011.
- 2.World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks; Geneva (Switzerland): World Health Organization; 2009.
- 3.Rhee EP, Gerszten RE. Metabolomics and cardiovascular biomarker discovery. Clin Chem 2012;58:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kordalewska M, Markuszewski MJ. Metabolomics in cardiovascular diseases. J Pharm Biomed Anal 2015;113:121–36. [DOI] [PubMed] [Google Scholar]
- 5.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, Dungan J, Newby LK, Hauser ER, Ginsburg GS, et al. . Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet 2010;3:207–14. [DOI] [PubMed] [Google Scholar]
- 6.Shah SH, Sun J-L, Stevens RD, Bain JR, Muehlbauer MJ, Pieper KS, Haynes C, Hauser ER, Kraus WE, Granger CB, et al. . Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J 2012;163:844–50.e1. [DOI] [PubMed] [Google Scholar]
- 7.Kalim S, Clish CB, Wenger J, Elmariah S, Yeh RW, Deferio JJ, Pierce K, Deik A, Gerszten RE, Thadhani R, et al. . A plasma long-chain acylcarnitine predicts cardiovascular mortality in incident dialysis patients. J Am Heart Assoc 2013;2:e000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizza S, Copetti M, Rossi C, Cianfarani MA, Zucchelli M, Luzi A, Pecchioli C, Porzio O, Di Cola G, Urbani A, et al. . Metabolomics signature improves the prediction of cardiovascular events in elderly subjects. Atherosclerosis 2014;232:260–4. [DOI] [PubMed] [Google Scholar]
- 9.Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, DeLany JP. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 2010;18:1695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts LD, Koulman A, Griffin JL. Towards metabolic biomarkers of insulin resistance and type 2 diabetes: progress from the metabolome. Lancet Diabetes Endocrinol 2014;2:65–75. [DOI] [PubMed] [Google Scholar]
- 11.Bouchard-Mercier A, Rudkowska I, Lemieux S, Couture P, Vohl M-C. The metabolic signature associated with the Western dietary pattern: a cross-sectional study. Nutr J 2013;12:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kien CL, Everingham KID, Stevens R, Fukagawa NK, Muoio DM. Short-term effects of dietary fatty acids on muscle lipid composition and serum acylcarnitine profile in human subjects. Obesity (Silver Spring) 2011;19:305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross AB, Pere-Trépat E, Montoliu I, Martin F-PJ, Collino S, Moco S, Godin J-P, Cléroux M, Guy PA, Breton I, et al. . A whole-grain-rich diet reduces urinary excretion of markers of protein catabolism and gut microbiota metabolism in healthy men after one week. J Nutr 2013;143:766–73. [DOI] [PubMed] [Google Scholar]
- 14.Kien CL, Bunn JY, Stevens R, Bain J, Ikayeva O, Crain K, Koves TR, Muoio DM. Dietary intake of palmitate and oleate has broad impact on systemic and tissue lipid profiles in humans. Am J Clin Nutr 2014;99:436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt JA, Rinaldi S, Ferrari P, Carayol M, Achaintre D, Scalbert A, Cross AJ, Gunter MJ, Fensom GK, Appleby PN, et al. . Metabolic profiles of male meat eaters, fish eaters, vegetarians, and vegans from the EPIC-Oxford cohort. Am J Clin Nutr 2015;102:1518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, et al. . Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-González MÁ, Corella D, Salas-Salvado J, Ros E, Covas MI, Fiol M, Warnberg J, Aros F, Ruiz-Gutierrez V, Lamuela-Raventos RM, et al. . Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol 2012;41:377–85. [DOI] [PubMed] [Google Scholar]
- 18.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol 1999;52:1165–72. [DOI] [PubMed] [Google Scholar]
- 19.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, et al. . Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elosua R, Marrugat J, Molina L, Pons S, Pujol E. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish men. The MARATHOM Investigators. Am J Epidemiol 1994;139:1197–209. [DOI] [PubMed] [Google Scholar]
- 21.Fernández-Ballart JD, Pinol JL, Zazpe I, Corella D, Carrasco P, Toledo E, Perez-Bauer M, Martinez-Gonzalez MA, Salas-Salvado J, Martin-Moreno JM. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr 2010;103:1808–16. [DOI] [PubMed] [Google Scholar]
- 22.Mataix J. Tablas de composición de alimentos. [Food composition tables.] 4th ed. Granada (Spain): Universidad de Granada; 2003. (in Spanish). [Google Scholar]
- 23.Beasley TM, Erickson S, Allison DB. Rank-based inverse normal transformations are increasingly used, but are they merited? Behav Genet 2009;39:580–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz-Canela M, Toledo E, Clish CB, Hruby A, Liang L, Salas-Salvado J, Razquin C, Corella D, Estruch R, Ros E, et al. . Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED trial. Clin Chem 2016;62:582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang WHW, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest 2014;124:4204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ussher JR, Lopaschuk GD, Arduini A. Gut microbiota metabolism of L-carnitine and cardiovascular risk. Atherosclerosis 2013;231:456–61. [DOI] [PubMed] [Google Scholar]
- 27.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 2013;62:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinaldo P, Cowan TM, Matern D. Acylcarnitine profile analysis. Genet Med 2008;10:151–6. [DOI] [PubMed] [Google Scholar]
- 29.Reuter SE, Evans AM. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clin Pharmacokinet 2012;51:553–72. [DOI] [PubMed] [Google Scholar]
- 30.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 2009;139:1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]