Abstract
Background: Antibodies to LPS and flagellin have been described as indirect measures of increased gastrointestinal permeability and may be markers of environmental enteric dysfunction (EED), which is a condition associated with poor child growth.
Objective: We assessed whether LPS- and flagellin-specific immunoglobulin (Ig) concentrations were associated with poor growth in young Tanzanian children at risk of EED.
Design: Blood samples were obtained from 590 children at 6 wk, 6 mo, and 12 mo of age. Serum LPS- and flagellin-specific Ig concentrations (IgA and IgG) were measured with the use of an ELISA. Growth was measured on a monthly basis for 18 mo.
Results: Anti-LPS and anti-flagellin IgA and IgG concentrations increased over the first year of life and were higher than concentrations (measured at 9 mo of age) in healthy controls. Children with anti-flagellin IgA, anti-LPS IgA, anti-flagellin IgG, and anti-LPS IgG concentrations in the highest quartile at 6 wk of age were 2.02 (95% CI: 1.11, 3.67), 1.84 (95% CI: 1.03, 3.27), 1.94 (95% CI: 1.04, 3.62), and 2.31 (95% CI: 1.25, 4.27) times, respectively, more likely to become underweight (weight-for-age z score <−2) after adjustment for covariates (P-trend < 0.05) than were children with Ig concentrations in the lowest quartile. Children with increased concentrations of anti-flagellin IgA were also more likely to become wasted; however, there was no association between any of the markers and subsequent stunting.
Conclusion: Serologic measures of increased intestinal permeability to bacterial components are associated with subsequent poor growth and could help identify children who may benefit most from preventive interventions. This trial was registered at clinicaltrials.gov as NCT00421668.
Keywords: child growth, environmental enteric dysfunction, intestinal biomarkers, anti-flagellin antibodies, anti-LPS antibodies, intestinal permeability, environmental enteropathy
INTRODUCTION
Environmental enteric dysfunction (EED)10 is a subclinical condition of the small intestine that is characterized by villous atrophy, crypt hyperplasia, increased intestinal permeability, inflammatory cell infiltrate, and malabsorption (1–3). Although the exact cause is unknown, EED is thought to result from a chronic fecal-oral exposure to enteropathogens that are common in poor sanitary and hygiene conditions. The subclinical characteristics of EED can ultimately lead to serious functional consequences including impaired child growth and development (4). Because of the global burden of these problems, there is a critical need to identify sensitive and early biomarkers of EED to target vulnerable infants who may benefit most from appropriate interventions.
The diagnosis of EED has remained challenging. Small bowel biopsies have been considered the gold standard for the assessment of the mucosal structure; however, such procedures are invasive, expensive, and impractical for widespread use in community-based settings. Carbohydrate-absorption tests, such as the d-xylose and lactulose:mannitol ratio tests, have also been used, but these tests are typically time-consuming procedures, and questions have been raised regarding the correlation of these tests results with actual enteropathy, clinical symptoms, and impaired growth (5, 6).
The bacterial protein flagellin, which mediates bacteria motility, and LPS, a major structural component of bacteria, are normally present in the gut lumen but are usually excluded from absorption by the gut epithelium. However, in conditions of gut-barrier dysfunction and perhaps alterations in the amount or composition of the gut microbiota, the translocation of LPS and flagellin across the intestinal mucosa may activate the adaptive immune response resulting in the generation of anti-flagellin and anti-LPS antibodies. Although anti-flagellin and anti-LPS Igs are present at low but detectable concentrations in the serum of healthy children, in certain disease states such as short bowel syndrome (7) and Crohn disease (8), the concentrations of these antibodies are considerably higher than in healthy controls, which suggests that they may be markers of impaired gastrointestinal barrier function. To our knowledge, these biomarkers have not been previously evaluated in resource-limited settings as indicators of EED. Therefore, we aimed to determine whether serum LPS- and flagellin-specific Ig concentrations were associated with poor growth in young Tanzanian children at risk of EED.
METHODS
Subjects included in this analysis were part of a randomized, double-blind, placebo-controlled trial that was designed to investigate whether the daily administration of zinc or multivitamins to Tanzanian infants reduced risk of infectious morbidity compared with that shown with the administration of a placebo. Results of this trial have been published previously (9). In brief, HIV-negative mothers of potentially eligible infants were recruited into the study, and their infants were randomly assigned to one of 4 study arms between 5 and 7 wk of age. Infants of multiple births and those with congenital anomalies or other conditions that would interfere with the study procedures were excluded from the trial.
Birth characteristics were obtained immediately after delivery. At the time of random assignment, a study physician performed a clinical examination, and a study nurse performed a history of morbidity and infant-feeding practices, anthropometric measurements, and a blood draw. Mothers were asked to return to the study clinic with their infants every 4 wk for data collection and standard clinical care including growth monitoring, immunizations, routine medical treatment of illnesses, and periodic vitamin A supplementation (100,000 IU at 9 mo of age and 200,000 IU at 15 mo of age). At these visits, study nurses performed a morbidity history, measured the child’s weight with the use of a digital infant balance with a 10-g precision (Tanita), and measured the child’s length with a 1-mm precision with the use of a rigid length board with a movable foot piece.
Blood samples were obtained from children at baseline and at 6, 12, and 18 mo of age. To be eligible for inclusion in this subanalysis of anti-flagellin and anti-LPS Igs and child growth, children were required to have a blood sample available at 6 wk and 6 mo of age and have a length-for-age z score (LAZ) of ≥−2 at 6 wk of age. We anticipated that 590 children would meet both eligibility criteria, which would have provided 99% power to detect a stunting HR of 1.5 for every 0.4-U increase in anti-LPS IgG and 91% power to detect a stunting HR of 1.5 for every 0.2-U increase in anti-flagellin IgG. These calculations assumed SDs of 0.48 and 0.19 for anti-LPS IgG and anti-flagellin IgG (10), respectively, an estimated stunting incidence of 30% by 2 y of age (11), and a 15% rate of loss to follow-up.
Flagellin- and LPS-specific IgA and IgG concentrations were measured with the use of an ELISA as previously reported (7). Microtiter plates were coated with purified Escherichia coli flagellin (100 ng/well) or purified E. coli LPS (2 μg/well). Serum samples from study participants were diluted 1:200 and applied to wells coated with flagellin or LPS. After incubation and washing, the wells were incubated with anti-human IgA (KPL) or IgG (GE Healthcare) coupled to a horseradish peroxidase. The quantification of total Igs was performed with the use of the colorimetric peroxidase substrate tetramethylbenzidine, and absorbance (optical density) was read at 450 nm with the use of an ELISA plate reader. Data are reported as optical density–corrected data by subtracting background concentrations, which were determined from the readings in samples that lacked serum.
For purposes of comparison, anti-flagellin and anti-LPS Igs were also measured in a cohort of 36 healthy infants seen at Boston Children’s Hospital. After institutional review board approval was obtained, any infant from birth to 12 mo of age with an excess blood sample in the Boston Children’s Hospital central laboratory was eligible for inclusion. Subjects were excluded from participation in the research study if 1) their LAZ was <−2 or >2; 2) their WAZ was <−2 or >2; 3) no growth data were available for the review and analysis; 4) there was evidence of a recent fever, which was defined as any temperature >38.5°C in the past 48 h; and 5) there was any evidence of a systemic illness (e.g., malignancy). A total of 67 infants were screened for enrollment, and 31 infants were excluded. The mean age of the remaining 36 infants at the time of the blood draw was 9.5 mo (range: 5–12 mo).
Data were double entered with the use of Microsoft Access software (version 12.0; Microsoft Corp.), converted to SAS (version 9.2; SAS Institute) data sets, and uploaded to a secured UNIX-based server (Oracle Solaris 10 x64) for analysis. Descriptive statistics were used to summarize baseline characteristics of the study population. Frequencies were reported for categorical variables and means ± SDs for continuous variables.
The possibility of nonlinear growth in children’s LAZ and weight-for-age z score (WAZ) over the course of follow-up was examined nonparametrically with restricted cubic splines (12). An automated stepwise selection with entry and retain criteria of P < 0.05 was used for the placement of 21 knots in each curve.
The concentrations of each of the 4 biomarkers (i.e., anti-flagellin IgA, anti-flagellin IgG, anti-LPS IgA, and anti-LPS IgG) at 6 wk and 6 mo of age were categorized into quartiles to examine their associations with child growth. Separate Cox proportional hazards models were constructed to estimate HRs and corresponding 95% CIs with the time to the first episode of stunting, wasting, and underweight as the outcome and the child’s age (in mo) as the metameter. Each outcome was modeled separately, and the first time the child reached a score of <−2 SDs was classified as an event. The follow-up time was censored at the time of the event or the time of the last anthropometric assessment. To assess the association between each biomarker and the anthropometric outcomes, we first ran a series of univariate models. Second, we ran multivariate models that adjusted for the following covariates: child sex, preterm birth, maternal age, maternal education, maternal midupper arm circumference, number of household assets, study regimen, and anthropometric z score at 6 wk of age.
All analyses were performed with the use of SAS version 9.2 software (SAS Institute). Institutional approval was granted by the Harvard T.H. Chan School of Public Health Human Subjects Committee; the Muhimbili University of Health and Allied Sciences Research and Publications Committee; the National Institute of Medical Research, Tanzania; the Tanzanian Food and Drugs Authority; and the Boston Children's Hospital Committee on Clinical Investigation. This trial was registered at clinicaltrials.gov as NCT00421668.
RESULTS
Of 2400 children who were enrolled in the parent trial for multivitamin, zinc, or multivitamin and zinc supplementation between August 2007 and December 2009, 590 children met the eligibility criteria and were included in the current substudy. As shown in Table 1, mothers of enrolled children had a mean age of 27 y, and only 25% of mothers had ≥8 y of formal education. Most mothers were married or cohabitating with a partner, and ∼70% of them had ≥1 previous pregnancy. One-half of all children were boys, and the prevalence of low birth weight was 2.1%.
TABLE 1.
Background characteristics of the study population (n = 590)
| Value | |
| Maternal characteristics | |
| Age, y | 26.6 ± 5.11 |
| Formal education, y, n (%) | |
| 0 | 14 (2.4) |
| 1–7 | 429 (73.2) |
| ≥8 | 143 (24.4) |
| Employment, n (%) | |
| Housewife without income | 346 (59.2) |
| Housewife with income | 193 (33.0) |
| Other | 46 (7.9) |
| Married or cohabitating with partner, n (%) | 537 (92.0) |
| Previous pregnancy, n (%) | |
| 0 | 172 (29.3) |
| 1–4 | 394 (67.1) |
| ≥5 | 21 (3.6) |
| Midupper arm circumference, cm | 26.8 ± 3.2 |
| Socioeconomic characteristics, n (%) | |
| Daily food expenditure per person in household is <1000 Tanzanian shillings2 | 140 (25.1) |
| Household possessions,3 n | |
| 0 | 176 (30.0) |
| 1–3 | 329 (56.1) |
| ≥3 | 81 (13.8) |
| Child characteristics | |
| Boys, n (%) | 293 (49.7) |
| Low birth weight (<2500g), n (%) | 12 (2.1) |
| Born preterm (<37 wk), n (%) | 56 (10.7) |
| Apgar score ≤7 at 5 min after birth, n (%) | 8 (1.4) |
| Length-for-age z score at 6 wk of age | −0.14 ± 1.01 |
| Weight-for-length z score at 6 wk of age | −0.01 ± 1.22 |
| Weight-for-age z score at 6 wk of age | −0.17 ± 0.87 |
Mean ± SD (all such values).
Approximately US$0.80 at the time of the study.
From a list that included a sofa, television, radio, refrigerator, and fan.
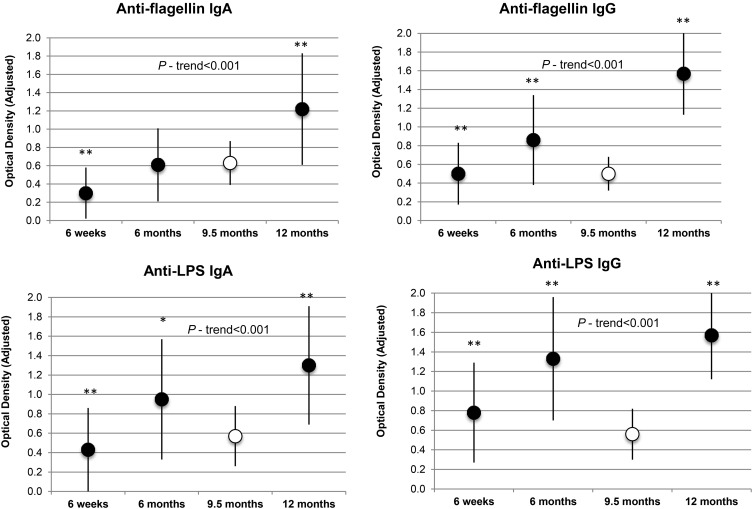
Figure 1 illustrates the progressive increase in the concentrations of all 4 biomarkers over the follow-up period. Figure 1 also shows that, in the case of anti-flagellin IgG, anti-LPS IgA, and anti-LPS IgG, mean concentrations were considerably lower in the healthy Boston children than in the Tanzanian children at 6 and 12 mo of age. The mean concentration of anti-flagellin IgA was also lower in the group of Boston children than in the Tanzanian infants at 12 mo of age.
FIGURE 1.
Anti-flagellin and anti-LPS Ig concentrations in Tanzanian infants compared with concentrations in healthy Boston infants. Black circles indicate means ± SDs in Tanzanian infants over the follow-up period. White circles indicate mean ± SD values in 36 healthy Boston infants with a mean age of 9.5 mo. n = 590 for all biomarkers in Tanzanian infants at 6 wk and 6 mo of age; n = 214 for all biomarkers in Tanzanian infants at 12 mo of age; n = 36 for all biomarkers in Boston infants at 9.5 mo of age. *,**For comparisons of mean biomarker concentrations in Tanzanian infants with those of healthy Boston infants (t tests): *P < 0.05, **P < 0.001. All P-trend values were calculated in Tanzania infants only.
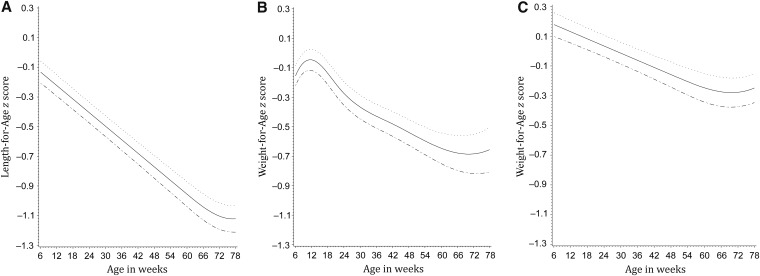
With the exclusion from the analysis of children with an LAZ <−2 at enrollment, mean ± SD LAZ, weight-for-length z score, and WAZ scores at 6 wk of age were −0.14 ± 1.01, −0.01 ± 1.22, and −0.17 ± 0.87, respectively. The restricted cubic spline models highlighted a marked decline in all 3 z scores over the course of the follow-up period (Figure 2). The mean LAZ declined in a linear fashion and reached −1.12 by 78 wk of age. The mean WAZ increased slightly from 6 to 15 wk of age but fell steadily to a low of ∼−0.68 by 72 wk of age. The drop in the weight-for-length z score was the least extreme and reached a low of ∼−0.28 by 68 wk of age.
FIGURE 2.
Mean (95% CI) length-for-age, weight-for-age, and weight-for-length z scores in Tanzanian infants over the follow-up period. Solid lines represent means; dotted lines represent 95% CIs.
The median duration of follow-up in the stunting analysis was 57 wk, over which time 27.6% of children became stunted. None of the biomarkers were significantly associated with risk of stunting in either the unadjusted or adjusted models (Table 2). The median duration of follow-up in the underweight analysis was 73 wk, over which time 18.7% of children became underweight. Contrary to the stunting models, an increased concentration of each biomarker was associated with a significant increase in risk of underweight. In adjusted analyses, children with anti-flagellin IgA, anti-LPS IgA, anti-flagellin IgG, and anti-LPS IgG concentrations in the highest quartile at 6 wk of age were 2.02 (95% CI: 1.11, 3.67; P-trend = 0.007), 1.84 (95% CI: 1.03, 3.27; P-trend = 0.02), 1.94 (95% CI: 1.04, 3.62; P-trend = 0.009), and 2.31 (95% CI: 1.25, 4.27; P-trend = 0.01) times, respectively, more likely to become underweight than were children with concentrations in the lowest quartile. At 15.8%, wasting was the least common outcome to occur in children with a median follow-up time of 61 wk. Although there was a borderline significant association between increased concentrations of anti-flagellin IgA at 6 wk of age and subsequent risk of wasting in the adjusted model (P-trend = 0.05), the other 3 biomarkers were not significantly related to risk of wasting. In parallel models that included the quartile of concentration of each biomarker at 6 mo of age as the exposure, there were no significant associations with any of the 3 outcomes, most likely because many cases of stunting, underweight, and wasting had already occurred by 6 mo of age.
TABLE 2.
Anti-flagellin and anti-LPS Ig concentrations at 6 wk of age as predictors of subsequent stunting, wasting, and underweight1
| Stunting |
Underweight |
Wasting |
|||||||||||||
| Events/infants, n | Unadjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | Events/infants, n | Unadjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | Events/infants, n | Unadjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | |
| Anti-flagellin IgA quartile2 | 0.93 | 0.75 | 0.03 | 0.007 | 0.04 | 0.05 | |||||||||
| 1 (0.00–0.14) | 40/147 | 1.00 | 1.00 | 19/141 | 1.00 | 1.00 | 11/137 | 1.00 | 1.00 | ||||||
| 2 (0.15–0.22) | 40/147 | 0.91 (0.59, 1.42) | 0.99 (0.62, 1.57) | 25/147 | 1.29 (0.71, 2.35) | 1.39 (0.75, 2.58) | 24/141 | 2.07 (1.02, 4.23) | 2.30 (1.12, 4.72) | ||||||
| 3 (0.23–0.37) | 39/147 | 0.86 (0.55, 1.34) | 0.97 (0.61, 1.54) | 33/146 | 1.78 (1.01, 3.13) | 2.22 (1.24, 3.99) | 30/137 | 2.79 (1.40, 5.57) | 2.79 (1.38, 5.62) | ||||||
| 4 (0.38–2.01) | 43/147 | 1.04 (0.67, 1.60) | 1.09 (0.69, 1.71) | 30/139 | 1.78 (1.00, 3.17) | 2.02 (1.11, 3.67) | 22/136 | 2.09 (1.01, 4.31) | 2.14 (1.02, 4.47) | ||||||
| Anti-LPS IgA quartile | 0.24 | 0.45 | 0.01 | 0.02 | 0.12 | 0.16 | |||||||||
| 1 (0.00–0.16) | 38/146 | 1.00 | 1.00 | 20/142 | 1.00 | 1.00 | 11/141 | 1.00 | 1.00 | ||||||
| 2 (0.17–0.30) | 36/148 | 0.88 (0.56, 1.39) | 0.91 (0.57, 1.45) | 25/146 | 1.17 (0.64, 2.11) | 1.16 (0.63, 2.15) | 27/137 | 2.69 (1.33, 5.41) | 2.87 (1.40, 5.87) | ||||||
| 3 (0.31–0.53) | 39/147 | 1.04 (0.66, 1.62) | 0.97 (0.62, 1.54) | 28/144 | 1.48 (0.84, 2.63) | 1.57 (0.87, 2.85) | 30/139 | 3.12 (1.56, 6.23) | 3.15 (1.56, 6.38) | ||||||
| 4 (0.54–2.82) | 49/147 | 1.24 (0.81, 1.89) | 1.16 (0.75, 1.79) | 34/141 | 1.92 (1.10, 3.34) | 1.84 (1.03, 3.27) | 19/134 | 1.86 (0.88, 3.90) | 1.81 (0.86, 3.82) | ||||||
| Anti-flagellin IgG quartile | 0.44 | 0.08 | 0.007 | 0.009 | 0.16 | 0.17 | |||||||||
| 1 (0.00–0.30) | 32/146 | 1.00 | 1.00 | 16/142 | 1.00 | 1.00 | 20/140 | 1.00 | 1.00 | ||||||
| 2 (0.31–0.42) | 48/148 | 1.65 (1.05, 2.58) | 1.54 (0.97, 1.45) | 28/146 | 1.85 (1.00, 3.43) | 1.11 (0.58, 2.13) | 18/141 | 0.89 (0.47, 1.68) | 0.85 (0.45, 1.63) | ||||||
| 3 (0.43–0.61) | 42/147 | 1.41 (0.89, 2.23) | 1.72 (1.07, 2.77) | 32/145 | 2.10 (1.15, 3.84) | 1.98 (1.06, 3.70) | 23/135 | 1.23 (0.67, 2.23) | 1.21 (0.66, 2.21) | ||||||
| 4 (0.62–2.56) | 40/147 | 1.30 (0.82, 2.07) | 1.52 (0.94, 2.45) | 31/140 | 2.31 (1.26, 4.24) | 1.94 (1.04, 3.62) | 26/135 | 1.39 (0.78, 2.50) | 1.38 (0.77, 2.49) | ||||||
| Anti-LPS IgG quartile | 0.63 | 0.50 | 0.03 | 0.01 | 0.62 | 0.75 | |||||||||
| 1 (0.00–0.42) | 38/147 | 1.00 | 1.00 | 18/142 | 1.00 | 1.00 | 20/141 | 1.00 | 1.00 | ||||||
| 2 (0.43–0.64) | 48/147 | 1.28 (0.84, 1.96) | 1.19 (0.77, 1.85) | 32/146 | 1.93 (1.07, 3.48) | 1.66 (0.90, 3.04) | 24/135 | 1.28 (0.71, 2.31) | 1.32 (0.73, 2.40) | ||||||
| 3 (0.65–0.99) | 39/148 | 1.02 (0.65, 1.59) | 1.15 (0.73, 1.83) | 23/144 | 1.41 (0.75, 2.64) | 1.59 (0.83, 3.03) | 19/140 | 0.95 (0.51, 1.79) | 0.92 (0.49, 1.74) | ||||||
| 4 (1.00–3.03) | 37/146 | 0.97 (0.63, 1.52) | 1.20 (0.75, 1.92) | 34/141 | 2.25 (1.26, 4.03) | 2.31 (1.25, 4.27) | 24/135 | 1.29 (0.71, 2.33) | 1.25 (0.68, 2.29) | ||||||
Adjusted HRs (95% CIs) were adjusted for child sex, preterm birth, maternal age, maternal education, maternal midupper arm circumference, number of household assets, treatment arm, and baseline z score. Cox proportional hazards models were constructed to estimate HRs and corresponding 95% CIs with the time to the first episode of stunting, wasting, and underweight as the outcome and the child’s age (in mo) as the metameter.
In optical density units.
In multivariate analyses that investigated the relation between the concentration of each biomarker at 6 wk of age and subsequent risks of severe stunting, severe wasting, and severe underweight (i.e., z scores <−3), no significant associations were detected between any of the biomarkers and severe stunting or severe underweight (Supplemental Table 1). There was a nonsignificant trend (P = 0.07) for children with anti-LPS IgA concentrations in the highest quartile to become severely wasted than was shown for children in the lowest quartile. There was also a nonsignificant trend between elevated anti-flagellin IgA concentrations and risk of severe wasting (P-trend = 0.07).
DISCUSSION
In this longitudinal study of 590 Tanzanian infants at risk of EED, we observed a clear trend of increasing anti-LPS and anti-flagellin IgA and IgG concentrations over the first year of life. Elevated concentrations of all 4 biomarkers at 6 wk of age were predictive of future underweight but not of stunting. There was also a borderline significant association between concentrations of anti-flagellin IgA at 6 wk of age and subsequent wasting. To our knowledge, our study is the first to explore the use of these Igs as potential indicators of EED in a resource-limited setting.
To date, most investigations of anti-flagellin and anti-LPS Ig concentrations have been conducted in clinical settings and have involved patients with various gastrointestinal conditions. Ziegler et al. (7) observed elevated anti-flagellin IgA, IgM, and IgG as well as anti-LPS IgA concentrations in adult patients with short bowel syndrome who were dependent on parenteral nutrition compared with the concentrations in healthy controls and concluded that this patient population was systemically exposed to flagellin and LPS from the gut lumen. Similarly, Galloway et al. (13) observed higher plasma concentrations of anti-flagellin IgA and anti-LPS IgA in children with intestinal failure than in healthy controls. These same authors also noted that anti-flagellin IgA concentrations increased during febrile episodes in all patients with intestinal failure. In contrast, anti-LPS and anti-flagellin IgA and IgG concentrations were lower in a small cohort of infants with short bowel syndrome than in healthy children; however, anti-flagellin IgA and IgG concentrations increased over the follow-up period (14). Diarrhea-predominant irritable bowel syndrome has also been characterized by elevated concentrations of LPS and flagellin antibodies (15).
Although no other studies have specifically explored anti-flagellin and anti-LPS Ig concentrations as biomarkers of EED in community-based settings in developing countries, our findings can be compared with previous investigations that examined other markers of impaired intestinal barrier function in relation to child growth. The lactulose:mannitol ratio test has been most commonly used to assess intestinal permeability, although its association with child growth outcomes has been inconsistent. Approximately 25 y ago, Lunn et al. (16) reported that the lactulose:mannitol ratio could predict 43% of the observed variation in linear growth and 39% of the weight gain in a cohort of Gambian infants. In a longitudinal study that was also conducted in the Gambia, Campbell et al. (5) observed that intestinal permeability more than doubled between 12 and 52 wk of age and was inversely related to age-corrected weight and length gains over the follow-up period. A study that involved rural Bangladeshi infants similarly showed that elevated lactulose:mannitol ratios were associated with lower HAZs and WAZs (17). In contrast, intestinal permeability was not related to the HAZ or WAZ in a study of mildly stunted Nepali children <5 y of age (18), and in a cross-sectional analysis that involved Gambian residents 2–60 y of age, the lactulose:mannitol ratio was only associated with the HAZ but not with the WAZ (19).
A smaller number of studies have also investigated whether increased intestinal permeability caused the translocation of bacteria and microbial-associated products from the lumen of the gut into the systemic circulation and resulted in immunostimulation. In the aforementioned study by Campbell et al. (5), plasma endotoxin concentrations were elevated and increased further with age. Plasma IgG endotoxin-core antibody concentrations increased even more dramatically with age and were inversely associated with HAZs and WAZs. Although this same study illustrated that plasma IgA, IgM, and IgG concentrations were similarly associated with child growth, a significant association was not detected between IgG concentrations and either HAZs or WAZs in Bangladeshi infants (17). A recent analysis that involved 700 Bangladeshi infants identified fecal reg1B, myeloperoxidase, and α-1 antitrypsin, among others, as biomarkers of environmental enteric dysfunction. More than 80% of infants had abnormal values of these biomarkers at 12 wk of age, and fecal reg1B and myeloperoxidase concentrations were negatively associated with changes in WAZs and HAZs from the time of enrollment to 1 y of age (20). Kosek et al. (21) similarly reported an association between neopterin, myeloperoxidase, and α-antitrypsin and linear growth in a cohort of 537 children from 8 countries. Unfortunately, because our study used stored serum samples, and stool sampling was not comprehensively performed, we were not able to compare these alternative measures of EED with our Ig concentrations.
Overall, our data are consistent with the notion that increased gut permeability in the first months of life results in an increased translocation of microbial products that drives proinflammatory gene expression that results in reduced growth. An important limitation of our study is that we could not assess whether such an increase in the bacteria product translocation may have occurred because of alterations in the microbial composition, including the presence of flagellated bacterial pathogens such as mildly pathogenic E. coli species. Furthermore, the detection of circulating Igs to bacterial components does not distinguish small bowel from colonic exposure. Another limitation is our inability to quantify the amount of IgG at 6 wk of age that crossed the placenta from the mother compared with that which was produced by the infant (22). The fact that we observed similar associations between IgA concentrations and underweight is encouraging, although breast milk may have contributed a portion of the IgA that was detected in the infants’ serum at 6 wk of age. Finally, because child stunting was initially the primary outcome of interest, we excluded infants who had this outcome at baseline, which limited the generalizability of our findings.
Some strengths of our study also deserve comment. We were able to obtain frequent, repeated measures of a large number of children over a follow-up period of 18 mo. Our antibody data were obtained in early infancy, which was well before the occurrence of growth faltering. Our analysis also involved multivariate modeling, which was a considerable advancement from many of the early studies of EED and child growth.
In conclusion, anti-flagellin and anti-LPS Ig concentrations increased substantially over the first year of life in young Tanzanian children. Elevated concentrations of all Igs at 6 wk of age independently predicted risk of subsequent underweight but not of stunting. The ability of these biomarkers to independently predict future nutritional deficits is encouraging because they may help identify children who are most vulnerable to undernutrition and who may benefit most from preventive interventions. An evaluation of these biomarkers as they relate to other measures of EED, including small bowel histology and other markers of gastrointestinal inflammation, is warranted.
Acknowledgments
The authors’ responsibilities were as follows—CMM and EL: analyzed the data; CMM and CPD: designed the research and wrote the manuscript; KPM, KG, HT, RK, SA, and ATG: conducted the research; WWF and ATG: provided input to the analysis and contributed to the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: EED, environmental enteric dysfunction; LAZ, length-for-age z score; WAZ, weight-for-age z score.
REFERENCES
- 1.Petri WA, Naylor C, Haque R. Environmental enteropathy and malnutrition: do we know enough to intervene? BMC Med 2014;12:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crane RJ, Jones K, Berkeley J. Environmental enteric dysfunction: an overview. Food Nutr Bull 2015;36:S76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prendergast A, Kelly P. Enteropathies in the developing world: neglected effects on global health. Am J Trop Med Hyg 2012;86:756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keusch GT, Rosenberg IH, Denno DM, Duggan C, Guerrant RL, Lavery JV, Tarr PI, Ward HD, Black RE, Nataro JP, et al. Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low- and middle-income countries. Food Nutr Bull 2013;34:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr 2003;133:1332–8. [DOI] [PubMed] [Google Scholar]
- 6.Denno DM, VanBuskirk K, Nelson ZC, Musser CA, Hay Burgess DC, Tarr PI. Use of the lactulose to mannitol ratio to evaluate childhood environmental enteric dysfunction: a systematic review. Clin Infect Dis 2014;59(Suppl 4):S213–9. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler TR, Luo M, Estivariz CF, Moore DA 3rd, Sitaraman SV, Hao L, Bazargan N, Klapproth JM, Tian J, Galloway JR, et al. Detectable serum flagellin and lipopolysaccharide and upregulated anti-flagellin and lipopolysaccharide immunoglobulins in human short bowel syndrome. Am J Physiol Regul Integr Comp Physiol 2008;294:R402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Targan SR, Landers CJ, Yang H, Lodes MJ, Cong Y, Papadakis KA, Vasiliauskas E, Elson CO, Hershberg RM. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology 2005;128:2020–8. [DOI] [PubMed] [Google Scholar]
- 9.McDonald CM, Manji KP, Kisenge R, Aboud S, Spiegelman D, Fawzi WW, Duggan CP. Daily zinc but not multivitamin supplementation reduces diarrhea and upper respiratory infections in Tanzanian infants: a randomized, double-blind, placebo-controlled clinical trial. J Nutr 2015;145:2153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole CR, Hansen NI, Higgins RD, Ziegler TR, Stoll BJ. Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics 2008;122:e573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald CM, Kupka R, Manji KP, Okuma J, Bosch RJ, Aboud S, Kisenge R, Spiegelman D, Fawzi WW, Duggan CP. Predictors of stunting, wasting and underweight among Tanzanian children born to HIV-infected women. Eur J Clin Nutr 2012;66:1265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 13.Galloway DP, Troutt ML, Kocoshis SA, Gewirtz AT, Ziegler TR, Cole CR. Increased anti-flagellin and anti-lipopolysaccharide immunoglobulins in pediatric intestinal failure: associations with fever and central line-associated bloodstream infections. JPEN J Parenter Enteral Nutr 2015;39:562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole CR, Frem JC, Schmotzer B, Gewirtz AT, Meddings JB, Gold BD, Ziegler TR. The rate of bloodstream infection is high in infants with short bowel syndrome: relationship with small bowel bacterial overgrowth, enteral feeding, and inflammatory and immune responses. J Pediatr 2010;156:941–7, 947.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dlugosz A, Nowak P, D’Amato M, Mohammadian Kermani G, Nystrom J, Abdurahman S, Lindberg G. Increased serum levels of lipopolysaccharide and antiflagellin antibodies in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 2015;27:1747–54. [DOI] [PubMed] [Google Scholar]
- 16.Lunn PG, Northrop-Clewes CA, Downes RM. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet 1991;338:907–10. [DOI] [PubMed] [Google Scholar]
- 17.Goto R, Mascie-Taylor CG, Lunn PG. Impact of anti-Giardia and anthelminthic treatment on infant growth and intestinal permeability in rural Bangladesh: a randomised double-blind controlled study. Trans R Soc Trop Med Hyg 2009;103:520–9. [DOI] [PubMed] [Google Scholar]
- 18.Goto R, Panter-Brick C, Northrop-Clewes CA, Manahdhar R, Tuladhar NR. Poor intestinal permeability in mildly stunted Nepali children: associations with weaning practices and Giardia lamblia infection. Br J Nutr 2002;88:141–9. [DOI] [PubMed] [Google Scholar]
- 19.Campbell DI, Lunn PG, Elia M. Age-related association of small intestinal mucosal enteropathy with nutritional status in rural Gambian children. Br J Nutr 2002;88:499–505. [DOI] [PubMed] [Google Scholar]
- 20.Naylor C, Lu M, Haque R, Mondal D, Buonomo E, Nayak U, Mychaleckyj JC, Kirkpatrick B, Colgate R, Carmolli M, et al. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine 2015;2:1759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosek M, Haque R, Lima A, Babji S, Shrestha S, Qureshi S, Amidou S, Mduma E, Lee G, Yori PP, et al. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg 2013;88:390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012;2012:985646. [DOI] [PMC free article] [PubMed] [Google Scholar]