Abstract
Endometrial cancer is the only gynecologic malignancy with a rising incidence and mortality. While cure is routinely achieved with surgery alone or in combination with adjuvant pelvic radiotherapy when disease is confined to the uterus, patients with metastatic or recurrent disease exhibit limited response rates to cytotoxic chemotherapy, targeted agents, or hormonal therapy. Given the unmet clinical need in this patient population, exploration of novel therapeutic approaches is warranted, and attention is turning to immunomodulation of the tumor microenvironment. Existing evidence suggests that endometrial cancer is sufficiently immunogenic to be a reasonable candidate for active and/or passive immunotherapy. In this review, we critically examine what is known about the microenvironment in endometrial cancer and what has been learned from preliminary immunotherapy trials that enrolled endometrial cancer patients, encouraging further attempts at immunomodulation in the treatment of aggressive forms of this disease.
Keywords: Adoptive cellular therapy, Bispecific T-cell engager antibodies, Endometrial cancer, Immune checkpoint inhibitors, Therapeutic vaccination, Tumor microenvironment
Background
As the most common cancer of the female genital tract, it is estimated that in 2015 endometrial cancer will be diagnosed in over 54,000 women and will be responsible for over 10,000 deaths in the United States [1]. At the time of diagnosis, 67 % of women have disease confined to the uterus and an associated 5-year survival rate of 95 % [1]. In contrast, the 8 % of patients with distant metastases at the time of diagnosis have a 5-year survival rate of 17 % [1] and face the prospect of cytotoxic chemotherapy (primarily with taxanes, anthracyclines, and platinum drugs) with limited response.
Since the completion of Gynecologic Oncology Group (GOG) protocol 177, which explored the triplet regimen of paclitaxel, doxorubicin and cisplatin (TAP) in patients with advanced stage and recurrent endometrial cancer, demonstrating an overall response rate of 57 % and median overall survival of 15.3 months, results have been clinically disappointing. Furthermore, the toxicity associated with the 3-drug regimen has limited its clinical utility [2]. The GOG, in the 229 queue, has evaluated a series of targeted agents [3–9] including bevacizumab (229E), aflibercept (229 F), bevacizumab/temsirolimus (229G), AZD6244 (229H), brivanib (229I), cediranib (229 J), AMG386 (229 L) and BIBF 1120 (229 K) with modest overall response rates, ranging from 0 % - 24.5 % (Table 1). Hormonal therapy is better tolerated but results in response rates between 18 % and 34 % [10]. With taxanes alone showing response rates of greater than 20 % in select patients (taxane-naïve) with recurrent disease [11, 12], effective second-line chemotherapeutic options are limited. Given the above unmet clinical need, exploration of novel therapeutic approaches is warranted in this patient population.
Table 1.
Clinical end points in the GOG 229 queue
| GOG Trial | N | ORR | PFS > 6mo | Median PFS (mo.) | Median OS (mo.) |
|---|---|---|---|---|---|
| 229 N [7] | 28 | 0 % | 11 % | 2.1 | 9.4 |
| 229 K [6] | 37 | 9.4 % | 22 % | 3.3 | 10.1 |
| 229 I [8] | 45 | 18.6 % | 30 % | 3.3 | 10.7 |
| 229 G* [5] | 53 | 24.5 % | 47 % | 5.6 | 16.9 |
| 229 F* [4] | 49 | 8.9 % | 40 % | 2.9 | 14.6 |
| 229 E [3] | 56 | 13.5 % | 40.4 % | 4.2 | 10.5 |
| 229 J [9] | 53 | 12.5 % | 29 % | 3.5 | 12.5 |
*Significant Grade 3/4 adverse events were encountered on these studies preventing subsequent development of a phase 3 trial; GOG = gynecologic oncology group; ORR = overall response rate; PFS = progression free survival; OS = overall survival
Within cancer drug development, a shift in focus from the tumor cell itself to the tumor microenvironment (TME) has been gradually gaining momentum. This shift has come with the recognition of the limitations of targeted therapy, which act by blocking essential biochemical pathways or mutant proteins that are required for tumor cell growth and survival. The ideal use of targeted therapies is in cancers with a single dominant driver mutation and a small mutational load, the classic example being chronic myeloid leukemia (CML) bearing the Philadelphia chromosome (bcr-abl gene translation) [13]. Most cancers, however, exhibit genetic heterogeneity. Fortunately, this same genetic heterogeneity that translates into limited therapeutic responses with targeted agents, may result in enhanced tumor immunogenicity, provoking an adaptive immune response. This concept of tumor immunogenicity is well appreciated for its role in determining the efficacy of immunotherapy [14]. Currently, our understanding of the somatic mutational load in endometrial cancer is evolving, and work is being done to identify the correlation between mutations and immunogenicity [15].
In this review, we critically examine what is known about the microenvironment in endometrial cancer and what has been learned from preliminary immunotherapy trials that enrolled endometrial cancer patients, encouraging further attempts at immunomodulation in the treatment of aggressive forms of this disease.
Characterizing the Tumor Microenvironment
Exploiting the immune system in cancer therapeutics relies on characterizing its components within the TME. This has proven to be a major endeavor, given the differences in the immune cell composition between different types of cancer, as well as between cancers of the same type. This diversity results from the phenotypic and functional plasticity of immune cells, which are responsible for a diverse set of tasks within the immune system’s overarching objective of host protection and tissue homeostasis. In both innate and adaptive immunity, immune cells are simultaneously responsible for promoting host defense while limiting collateral tissue damage. It is well established that the microenvironment has the capacity to regulate the phenotype and function of differentiated myeloid or lymphoid cells at the level of their progenitors, during their lineage-specific differentiation, and after they have matured into the fully differentiated cell types [16]. This plasticity lends support to the idea that differentiated hematopoietic cells should be viewed on a dynamic continuum rather than in distinct subcategories.
The lack of success in developing a cohesive picture of endometrial cancer on the molecular level may be explained, in part, by the fluctuations in immune cell composition of the endometrium that result from hormonal influences. As described in two recent reviews [17, 18], the immune system within the endometrium faces a unique challenge; it must be competent enough to provide protection against sexually transmitted pathogens while being permissive enough to allow the development of an allogeneic fetus. As such, this site within the female reproductive tract has evolved in such a way that sex hormones precisely regulate immune function to accomplish both tasks. The number of macrophages, neutrophils, and natural killer (NK) cells steadily increase throughout the menstrual cycle and are most abundant before menstruation, perhaps reflecting their role in the breakdown of the endometrium and in host defense during disruption of the mucosal barrier. Similarly, adaptive immune cells, which are present in the endometrium as unique aggregates consisting of a B-cell core surrounded by T cells and an outer halo of macrophages, increase in number throughout the proliferative phase and temporarily lose cytotoxic capabilities during the secretory phase, when conception may occur. These findings highlight the exceptional responsiveness of the immune system to hormonal fluctuations in this particular microenvironment.
In regards to the composition of the TME and corresponding associations with prognosis, endometrial cancer has been relatively understudied in comparison to other malignancies. In ovarian cancer, for example, it is well established that the presence of intraepithelial tumor infiltrating lymphocytes (TILs) is a robust predictor of a more favorable outcome, as demonstrated in a recent meta-analysis including 10 studies [19]. Early clinicopathologic studies were conflicting in regards to whether TILs were more common in low-grade [20] vs. high-grade endometrial cancers [21] and whether the location of TILs has prognostic implications. A perivascular lymphocytic infiltrate has been shown to correlate with poor overall survival (OS) on univariate analysis [22] while an intraepithelial lymphocytic infiltrate at the invasive border [23] has been shown to correlate with improved OS on multivariate analysis. On the whole, given the limited data set, and study heterogeneity, comparing studies to draw meaningful conclusions has been problematic.
Contemporary studies have not only examined the presence of intra-tumoral, cytotoxic T cells but also the ratio of CD8+ TILs to regulatory T cells (Tregs: CD4+ CD25+ FOXP3+), which are well known for their physiologic role in peripheral tolerance and their pathological role in antitumor immunity. After adjusting for well known prognostic factors in multivariate analysis, de Jong and colleagues [24] found that the presence of high numbers of CD8+ TILs was an independent predictor of increased OS (whole cohort and type II) and that the presence of a high CD8+/FoxP3+ ratio was an independent predictor of increased disease-free survival (DFS) in type I, though not type II, endometrial cancer patients. The importance of the ratio of CD8+ to FoxP3+ T cells to DFS was confirmed in an additional study that did not stratify by tumor type [25]. The amount of intra-tumoral Tregs alone has not been shown to impact recurrence and survival curves [26, 27], though a statistically significant correlation has been shown between the presence of Tregs and tumor stage, grade, and presence of myometrial invasion [28].
There appears to be a better understanding of the role of myeloid cells, in comparison to lymphoid cells, in endometrial pathology. While myeloid-derived suppressor cells (MDSCs) have been detected in tumor specimens [29], tumor associated macrophages (TAMs) have been consistently identified as the dominant contributor to a pro-tumorigenic environment [30]. TAM density, particularly within the stromal compartment, has been shown to steadily increase with disease progression from precancerous endometrial lesions (various forms of hyperplasia) to endometrial cancer [31, 32]. The presence of TAMs has been repeatedly correlated to aggressive features within the primary tumor, specifically higher stage and grade and the presence of lymphovascular and myometrial invasion [27, 33–36]. The results of only one study, containing a small and heterogeneous cohort of type I and II carcinomas, has differed from these consistent findings [31]. Additionally, the presence of TAMs has been strongly associated with pelvic lymph node metastases [27, 32, 35, 37] and an angiogenic profile [34–36, 38, 39].
Recently, Kubler and colleagues [27] were the first to demonstrate that TAM density is an independent prognostic factor for recurrence-free survival, finding that a high density compared to a low density of TAMs increased the risk of recurrence by a factor of 8.3. In their study, a significant relationship between the presence of TAMs and overall survival was found on univariate analysis but not on multivariate analysis. They hypothesize that detecting significance may not have been possible in this cohort of patients of mostly early stage disease due to the length of follow-up and the treatment of relapsed cases with curative intent. These same factors may explain why previous studies were only able to document a trend towards significance [32, 33, 35].
Immunotherapy in Endometrial Cancer
Therapeutic Vaccination
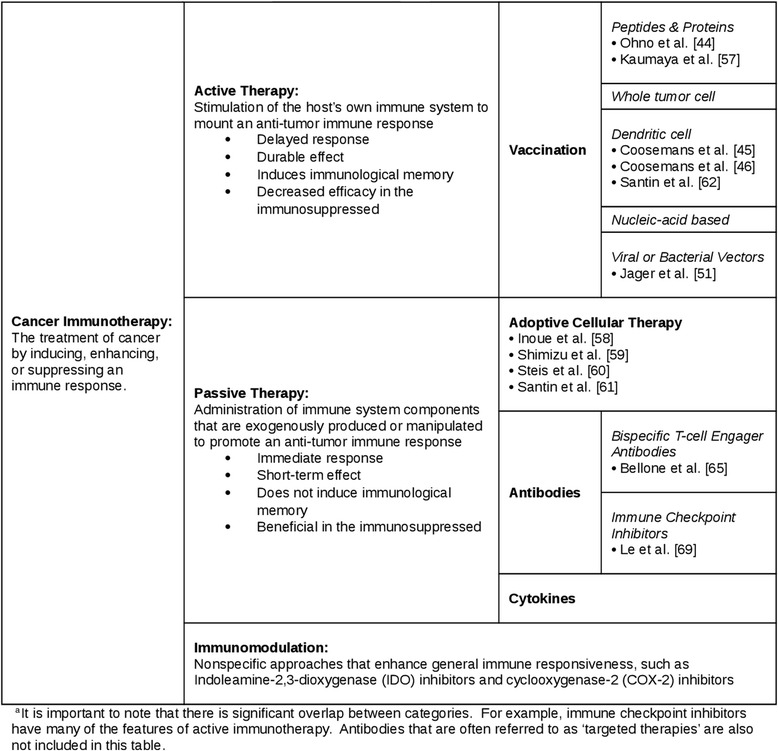
Therapeutic cancer vaccination is a form of active immunotherapy (Table 2). Active immunotherapies stimulate the host’s own immune system to mount an anti-tumor immune response and induce immunological memory, theoretically producing a durable effect after treatment is stopped. Cancer vaccines exploit the cellular arm of the immune system, inciting a cytotoxic T-lymphocyte response against tumor-associated antigens (TAAs). In concept, cancer vaccines offer the prospect of high specificity, low toxicity, and prolonged activity, though these properties have yet to be reliably translated in clinical practice [40].
Table 2.
Immunotherapeutic approaches and their application to endometrial cancera
The product of Wilms tumor gene 1 (WT1) has been identified as a TAA with therapeutic potential in endometrial cancer. WT1 is located on chromosome 11p13 and encodes a transcription factor that plays an essential role in the normal development of the urogenital system. It has been detected in 0 % to 79 % of endometrial cancer, depending on the immunohistochemical staining technique [41]. The safety and tolerability of a weekly WT1 peptide vaccine (HLA-A2402-restricted, modified 9-mer WT1 peptide emulsified with Montanide ISA51 adjuvant) in 12 patients with recurrent or progressive gynecologic malignancies was demonstrated in a recent phase I trial [42]. Adverse events were limited to erythema at the injection site, and the disease control rate in the initial 3 months was 25 % (stable disease [SD] in 3 patients, progressive disease [PD] in 9 patients). Unfortunately, the only subject with recurrent uterine carcinosarcoma failed to respond to treatment, with disease progression after 11 vaccine injections (3 months on therapy). Utilizing an alternative approach, Cooseman and colleagues have reported the vaccination of 4 patients with advanced serous endometrial cancer with autologous dendritic cells loaded with WT1 mRNA [43, 44]. After 4 weekly injections, 3 of 4 patients demonstrated an immunological response (defined as an increase in the percentage of WT1-specific T-cells or NK cells among peripheral blood mononuclear cells), 2 of 4 patients demonstrated a molecular response (defined as a decrease in CA-125), but no patients were found to have a decrease in tumor size on repeat CT scan. Similar to the experience in the WT1 peptide trial, adverse events were limited to erythema at the injection site and 1 local allergic reaction. The investigators hypothesized that the limited clinical response may be partially attributable to the advanced stage of the patients and the early termination of therapy once radiological progression was demonstrated.
Cancer testis (CT) antigens, expressed exclusively in male germ cells and placental tissue in healthy adults but ectopically in tumor cells of multiple types of human cancer, have emerged as excellent candidates for therapeutic manipulation. The restricted nature of their expression lends to high tumor-specificity and immunogenicity [45]. To date, several CT antigens have been identified in endometrial cancer. NY-ESO-1 and MAGE-A4 have been reported in 19 % and 12 % of endometrioid adenocarcinomas, respectively [46]. These numbers increase to 32 % and 63 % of USC, respectively. Additionally, KU-CT-1 has been identified in 64 % of cases of endometrial cancer [47] and SSX-4 in 24 % of cases [48]. In a two-part, open-label cohort study designed to test the safety and immunogenicity of recombinant vaccinia-NY-ESO-1 and recombinant fowlpox-NY-ESO-1, 36 patients with a wide range of tumor types experienced a similar, minor reaction to the vaccine (erythema and pruritis at the injection site) but differed significantly in their immunologic response [49]. The sole patient with endometrial cancer was one of three patients to demonstrate NY-ESO-1 sero-conversion and both a CD8+ and CD4+ T-cell response.
Additionally, human epidermal growth factor receptor 2 (HER-2/neu), the transmembrane receptor encoded by the ERBB2 gene, has exciting potential in the treatment of endometrial cancer. In USC, specifically, overexpression of HER-2/neu ranges from 16 % to 80 % and is associated with worse overall survival [50–52]. The success of targeted therapy with trastuzumab, a recombinant humanized monoclonal antibody against HER2, in producing impressive response rates and prolonged disease-free survival in patients with metastatic breast cancer [53, 54] has encouraged the development of active immunotherapies that may produce a more durable anti-tumor immune response. In a phase I, dose-escalating, safety trial in patients with various metastatic, heavily pretreated cancers, Kaumaya and colleagues [55] tested a novel peptide combination vaccine consisting of 2 B-cell epitopes derived from the HER2 extracellular domain. In utilizing B-cell epitopes rather than T-cell epitopes, they were able to overcome the requirement for specific HLA restrictions in their patient population and engage the humoral arm of the immune system. Between the 2 endometrial cancer patients enrolled in the study, 1 patient has a partial response, experiencing extended clinical benefit at 4 years after the initial vaccination. In functional studies, the vaccine elicited antibodies in this patient that disrupted 2 different HER2 signaling methods, ultimately suppressing HER2 phosphorylation and inhibiting cell proliferation. Vaccines such as this one offer hope that we may overcome the limitations of antibody therapy, namely the short half-life of IgG, requiring frequent treatments and accruing high costs.
Adoptive Cellular Therapy
Adoptive cellular therapy is a form of passive immunotherapy (Table 2). Passive immunotherapies involve the administration of immune system components (i.e. antibodies, cytokines, lymphocytes) that are exogenously produced or manipulated to promote an anti-tumor immune response. Unable to induce immunological memory, they offer immediate but short-term protection. In adoptive cellular therapy, cells from the blood or bone marrow are isolated, activated and expanded in vitro, and re-infused into the same patient (autologous) or a different patient (allogeneic). The technology has evolved substantially and now includes the generation of tumor-reactive T cells that are genetically engineered to express recombinant or chimeric T-cell receptors directed against common TAAs (CAR T cells).
Adoptive cellular therapy in the treatment of endometrial cancer has not yet exploited these most recent technological advances. The earliest animal studies involved the infusion of lymphokine-activated killer (LAK) cells with and without additional immuno-stimulatory components (Il-2, lentinan) [56, 57]. This therapy produced growth retardation of tumor in nude mice. Intraperitoneal adoptive transfer of LAK cells with IL-2 has also been tested in a phase I trial that enrolled 12 colorectal cancer patients, 10 ovarian cancer patients, and 1 endometrial cancer patient with abdominal metastases [58]. Thirty percent of patients had a laparoscopy- or laparotomy-documented PR, though this did not include the patient with endometrial cancer. While the majority of adverse events (minor to moderate hypotension, fever, chills, rash, nausea, vomiting, abdominal pain and distension, diarrhea, oliguria, fluid retention, thrombocytopenia, and minor elevations of liver function tests) were attributable to Il-2, intraperitoneal fibrosis (14 patients) was a notable toxic side effect of the therapy that led to treatment discontinuation in 5 patients. Adding to the question of safety, one patient had a grand mal seizure and another had colonic perforation.
The infusion of peripheral blood T cells stimulated with tumor lysate-pulsed autologous dendritic cells has been reported by Santin and colleagues [59] in a 65-year-old patient with advanced, chemoresistant endometrial cancer. Prior to the treatment, which consisted of 3 infusions administered every 3 to 4 weeks, the patient’s liver metastasis had substantially increased in size (9.5 X 8 cm to 14 X 10 cm in 3 weeks). During treatment, stabilization of the liver metastasis was achieved as a result of a tumor-specific, cytotoxic T-cell response. A more dramatic response was likely limited by the inability of the activated T cells to deeply infiltrate the large tumor mass, as evaluated in 3 dimensions by single photon emission computerized tomography (SPECT) imaging. Since publication of this report, these investigators have also demonstrated the ability to induce a tumor-specific, cytotoxic T-cell response in vitro through vaccination with tumor lysate-pulsed autologous dendritic cells in 3 patients with USC [60].
Bispecific T-cell Engager (BiTE) Antibodies
The diverse array of molecules employed within passive immunotherapeutic approaches now includes bispecific T-cell engager (BiTE) antibodies [61]. These novel molecules induce a transient cytolytic synapse between a cytotoxic T cell and the cancer target cell. This interaction results in discharge of cytotoxic T-cell contents following perforin fusion with the T-cell membrane resulting in direct tumor cell lysis. Currently, the only drug within this class with United States FDA approval is blinatumomab (BiTE for CD 19 and CD3) for patients with acute lymphoblastic leukemia (ALL), based on an impressive complete remission rate in a phase 2 clinical trial [62].
Solitomab, which targets epithelial-cell-adhesion-molecule (EpCAM) on tumor cells while also containing a CD3 binding region, is being pursued as treatment for metastatic, recurrent, or persistent USC overexpressing EpCAM (86 % of USC cell lines tested by flow cytometry) [63]. After exposure to peripheral blood lymphocytes in vitro, EpCAM positive USC cells were found to be resistant to NK or T-cell-mediated killing. This resistance was overcome by incubating the cell lines with solitomab. Additionally, ex vivo incubation of autologous tumor associated lymphocytes (TAL) with EpCAM expressing malignant cells in ascites with solitomab resulted in a significant increase in both CD4+ and CD8+ T-cell proliferation, an increase in T-cell activation markers, and a reduction in number of viable USC cells in ascites.
Immune Checkpoint Inhibitors
The therapies discussed thus far involve activating the immune system to achieve tumor cell death. However, recognition that the effectiveness of both active and passive immunotherapies are reduced by tumor immune evasion [40] has led to a recent paradigm shift within immunotherapeutics away from a focus on stimulating the immune system to a focus on inhibiting the inhibitors of an adequate immune response. Among the emerging strategies of tackling immune tolerance, immune checkpoint inhibitors are the most promising.
Immune checkpoints refer to a variety of inhibitory pathways employed by the immune system to maintain self-tolerance and minimize collateral damage during physiologic responses to pathogens. Many of these pathways are initiated by ligand-receptor interactions on the surface of immune cells and, thus, are logical targets for monoclonal antibodies. Cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) and programmed cell death protein-1 (PD-1) were the first, and remain the most relevant, immune-checkpoint receptors to be clinically targeted [64]. Although PD-1 and CTLA-4 belong to the same CD28 family of T-cell receptors, they assume very different roles in the down regulation of an inflammatory response. While CTLA-4 predominately regulates T cell activation within secondary lymphoid organs, PD-1 predominately regulates T cell effector function within peripheral tissues.
Importantly, immunohistochemical studies on endometrial cancer specimens have detailed PD-1 and PD-L1 expression levels surpassing those seen in ovarian and cervical carcinoma. Specifically, Vanderstraeten et al. described PD-L1 expression levels of 67-100 % in primary, recurrent and metastatic endometrial cancer specimens [29]. At the 2015 annual meeting of the Society of Gynecologic Oncology, Herzog et al. reported PD-1 expression levels of 75 %, and PD-L1 expression levels ranging from 25-47 %, once again surpassing all examined cervical and ovarian cancer specimens [65] (Table 3). Given the above, investigation of immune checkpoint inhibitors in patients with metastatic and recurrent endometrial cancer may represent a promising alternative to traditional cytotoxic therapies.
Table 3.
PD-1 and PD-L1 expression levels in uterine cancer (450 specimens) [67]
| Histology | PD-1 | PD-L1 |
|---|---|---|
| % Expression based on IHC staining* | ||
| Endometrioid | 77.9 | 39.7 |
| Serous Carcinoma | 68.2 | 10.2 |
| Carcinosarcoma | 80.0 | 22.2 |
| Leiomyosarcoma | 46.9 | 36.0 |
| Stromal Sarcoma | 64.3 | 64.3 |
| Clear Cell Carcinoma | 69.2 | 23.1 |
* IHC antibody = Spring Bioscience (Rabbit anti-Human IgG)
While preliminary evidence exists that tumor cell surface PD-L1 expression correlates with the likelihood of response to PD-1 pathway inhibition [66], the best argument for the use of checkpoint inhibitors in select endometrial cancer cases was recently put forth by a phase 2 trial of pembrolizumab, a humanized monoclonal antibody to the PD-1 receptor, in patients with mismatch repair- (MMR-) deficient tumors [67]. This trial was designed to test the hypothesis that MMR-deficient tumors are more responsive to PD-1 blockade than MMR-proficient tumors, due to the high somatic mutational load, resulting in neoantigen formation and a more prominent lymphocytic infiltrate. As predicted, the two cohorts with MMR-deficient cancers (one with colorectal cancer patients and the other with non-colorectal cancer patients, including 2 patients with endometrial cancer) had significantly higher objective response rates by immune-related response criteria and by Response Evaluation Criteria in Solid Tumors (RECIST). They also had a significantly better immune-related PFS at 20 weeks and disease control rate by RECIST. Interestingly, patients with sporadic MMR-deficient tumors responded more frequently to treatment than those with Lynch syndrome (100 % vs 27 %). This study provides preliminary clinical evidence that immune checkpoint inhibitors may be used effectively in the treatment of MMR-deficient endometrial cancers, and trials exploring this hypothesis are currently in development.
More recently, Howitt et al. specifically examined the hypothesis that microsatellite unstable endometrial cancers would exhibit more tumor specific neoantigens, resulting in increased tumor infiltrating lymphocytes and a compensatory up-regulation of immune checkpoints (9). Microsatellite unstable tumors exhibited higher numbers of CD3+ and CD8+ tumor infiltrating lymphocytes. Furthermore, PD-1 was overexpressed in tumor infiltrating lymphocytes, and peri-tumoral lymphocytes of microsatellite unstable tumors.
Conclusion
Despite existing evidence that endometrial cancer, particularly the most aggressive forms of the disease, is sufficiently immunogenic to be a reasonable candidate for immunomodulation, attempts to expand the role of active and/or passive immunotherapy in the treatment of this condition have been limited. At a time when the U.S. FDA-approved indications for immune checkpoint inhibitors is steadily amassing, progress in the endometrial cancer arena has been slow. Uniquely, endometrial cancer is the only gynecologic cancer with a rising incidence and mortality, and identifying effective therapies for patient with metastatic or recurrent disease is critical.
As reviewed here, these patients have been enrolled in small preclinical and phase I trials assessing the utility of immunotherapy. These studies have demonstrated encouraging immunologic responses but few clinical responses, provoking questions regarding the ability to establish therapeutic efficacy in a small number of heavily pretreated patients with advanced disease and short follow-up. The most urgent questions in identifying the utility of immunotherapy for the treatment of endometrial cancer are: how do we identify the subset of individuals most likely to respond to immunotherapy, the biomarkers most likely to predict successful treatment, and the therapy combinations most likely to enhance drug performance while limiting toxicity.
Acknowledgments
Financial Support: This research was supported by an institutional NIH-T32 training grant (Ruth L. Kirschenstein NRSA Institutional Research Grant, 2 T32 CA06039611).
Abbreviations
- ALL
Acute lymphoblastic leukemia
- BiTE antibody
Bispecific T-cell engager antibody
- CML
Chronic myeloid leukemia
- CR
Complete responses
- CT antigen
Cancer testis antigen
- CTLA-4
Cytotoxic T-lymphocyte-associated protein-4
- DFS
Disease-free survival
- EpCAM
Epithelial-cell-adhesion-molecule
- GOG
Gynecologic Oncology Group
- HER-2/neu
Human epidermal growth factor receptor 2
- LAK cells
Lymphokine-activated killer cells
- MDSCs
Myeloid-derived suppressor cells
- MMR
Mismatch repair
- NK cells
Natural killer cells
- OS
Overall survival
- PD
Progressive disease
- PD-1
Programmed cell death protein-1
- RECIST
Response Evaluation Criteria in Solid Tumors
- PR
Partial responses
- SD
Stable disease
- SPECT
Single photon emission computerized tomography
- TAAs
Tumor-associated antigens
- TALs
Tumor associated lymphocytes
- TAMs
Tumor associated macrophages
- TAP
Paclitaxel, doxorubicin and cisplatin
- TILs
Tumor infiltrating lymphocytes
- TME
Tumor microenvironment
- Tregs
Regulatory T cells
- WT1
Wilms tumor gene 1
Footnotes
Competing interests
The authors declare that they have no competing interest.
Authors’ contributions
All authors contributed substantially to the concept of this review article, evaluation of the existing literature, preparation and editing of the manuscript. All authors have given final approval for publication, and agree to be accountable for all aspects of the work.
Contributor Information
Teresa C. Longoria, Email: longoria@uci.edu
Ramez N. Eskander, Phone: 714.456.7971, Email: eskander@uci.edu
References
- 1.National Cancer Institute: SEER Stat Fact Sheets: Endometrial Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed August 23 2015.
- 2.Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22(11):2159–2166. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- 3.Aghajanian C, Sill MW, Darcy KM, Greer B, McMeekin DS, Rose PG, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011;29(16):2259–2265. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman RL, Sill MW, Lankes HA, Fader AN, Finkler NJ, Hoffman JS, et al. A phase II evaluation of aflibercept in the treatment of recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;127(3):538–543. doi: 10.1016/j.ygyno.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez EA, Brady WE, Walker JL, Rotmensch J, Zhou XC, Kendrick JE, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;129(1):22–27. doi: 10.1016/j.ygyno.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Dizon DS, Sill MW, Schilder JM, McGonigle KF, Rahman Z, Miller DS, et al. A phase II evaluation of nintedanib (BIBF-1120) in the treatment of recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. Gynecol Oncol. 2014;135(3):441–445. doi: 10.1016/j.ygyno.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makker V FV, Chen L, Darus C, Kendrick JE, Sutton G, Moxley K, Aghajanian C. Phase II evaluation of dalantercept, a soluble recombinant activin receptor-like kinase 1 (ALK1) receptor-fusion protein, for treatment of recurrent/persistent endometrial cancer: GOG 0229 N. J Clin Oncol. 2014;32(5 s):Abst#5594. [DOI] [PMC free article] [PubMed]
- 8.Powell MA, Sill MW, Goodfellow PJ, Benbrook DM, Lankes HA, Leslie KK, et al. A phase II trial of brivanib in recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. Gynecol Oncol. 2014;135(1):38–43. doi: 10.1016/j.ygyno.2014.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bender DP SM, Lankes H, Darus CJ, Delmore J, Rotmensch J, Gray HJ, Mannel RS, Schilder JM, Leslie KK. A phase II evaluation of cediranib in the treatment of recurrent or persistent endometrial cancer: An NRG Oncology/Gynecologic Oncology Group (GOG) study. Gynecol Oncol. 2015;SGO Annula Meeting 2015:Late Breaking Abstract 3. [DOI] [PMC free article] [PubMed]
- 10.Pectasides D, Pectasides E, Economopoulos T. Systemic therapy in metastatic or recurrent endometrial cancer. Cancer Treat Rev. 2007;33(2):177–190. doi: 10.1016/j.ctrv.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Dellinger TH, Monk BJ. Systemic therapy for recurrent endometrial cancer: a review of North American trials. Expert Rev Anticancer Ther. 2009;9(7):905–916. doi: 10.1586/era.09.54. [DOI] [PubMed] [Google Scholar]
- 12.Dizon DS. Treatment options for advanced endometrial carcinoma. Gynecol Oncol. 2010;117(2):373–381. doi: 10.1016/j.ygyno.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Shekarian T, Valsesia-Wittmann S, Caux C, Marabelle A. Paradigm shift in oncology: targeting the immune system rather than cancer cells. Mutagenesis. 2015;30(2):205–211. doi: 10.1093/mutage/geu073. [DOI] [PubMed] [Google Scholar]
- 14.Longoria TC, Eskander RN. Immune checkpoint inhibition: therapeutic implications in epithelial ovarian cancer. Recent Pat Anticancer Drug Discov. 2015;10(2):133–144. doi: 10.2174/1574892810666150504121000. [DOI] [PubMed] [Google Scholar]
- 15.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12(11):1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. American journal of reproductive immunology (New York, NY : 1989). 2010;63(6):544–65. doi:10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed]
- 18.Vanderstraeten A, Tuyaerts S, Amant F. The immune system in the normal endometrium and implications for endometrial cancer development. J Reprod Immunol. 2015;109:7–16. doi: 10.1016/j.jri.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124(2):192–198. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deligdisch L. Morphologic correlates of host response in endometrial carcinoma. Am J Reproduc Immunol (New York, NY: 1989) 1982;2(1):54–57. doi: 10.1111/j.1600-0897.1982.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 21.Silverberg SG, Sasano N, Yajima A. Endometrial carcinoma in Miyagi Prefecture, Japan: histopathologic analysis of a cancer registry-based series and comparison with cases in American women. Cancer. 1982;49(7):1504–1510. doi: 10.1002/1097-0142(19820401)49:7<1504::AID-CNCR2820490733>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Ambros RA, Kurman RJ. Combined assessment of vascular and myometrial invasion as a model to predict prognosis in stage I endometrioid adenocarcinoma of the uterine corpus. Cancer. 1992;69(6):1424–1431. doi: 10.1002/1097-0142(19920315)69:6<1424::AID-CNCR2820690620>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10(13):4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- 24.de Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AG, Hollema H, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114(1):105–110. doi: 10.1016/j.ygyno.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Yamagami W, Susumu N, Tanaka H, Hirasawa A, Banno K, Suzuki N, et al. Immunofluorescence-detected infiltration of CD4 + FOXP3+ regulatory T cells is relevant to the prognosis of patients with endometrial cancer. Int J Gynecol Cancer. 2011;21(9):1628–1634. doi: 10.1097/IGC.0b013e31822c271f. [DOI] [PubMed] [Google Scholar]
- 26.Giatromanolaki A, Bates GJ, Koukourakis MI, Sivridis E, Gatter KC, Harris AL, et al. The presence of tumor-infiltrating FOXP3+ lymphocytes correlates with intratumoral angiogenesis in endometrial cancer. Gynecol Oncol. 2008;110(2):216–221. doi: 10.1016/j.ygyno.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Kubler K, Ayub TH, Weber SK, Zivanovic O, Abramian A, Keyver-Paik MD, et al. Prognostic significance of tumor-associated macrophages in endometrial adenocarcinoma. Gynecol Oncol. 2014;135(2):176–183. doi: 10.1016/j.ygyno.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Chang WC, Li CH, Huang SC, Chang DY, Chou LY, Sheu BC. Clinical significance of regulatory T cells and CD8+ effector populations in patients with human endometrial carcinoma. Cancer. 2010;116(24):5777–5788. doi: 10.1002/cncr.25371. [DOI] [PubMed] [Google Scholar]
- 29.Vanderstraeten A, Luyten C, Verbist G, Tuyaerts S, Amant F. Mapping the immunosuppressive environment in uterine tumors: implications for immunotherapy. Cancer Immunol Immunother. 2014;63(6):545–557. doi: 10.1007/s00262-014-1537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zsiros E, Odunsi K. Tumor-associated macrophages: co-conspirators and orchestrators of immune suppression in endometrial adenocarcinoma. Gynecol Oncol. 2014;135(2):173–175. doi: 10.1016/j.ygyno.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Dun EC, Hanley K, Wieser F, Bohman S, Yu J, Taylor RN. Infiltration of tumor-associated macrophages is increased in the epithelial and stromal compartments of endometrial carcinomas. Int J Gynecol Pathol. 2013;32(6):576–584. doi: 10.1097/PGP.0b013e318284e198. [DOI] [PubMed] [Google Scholar]
- 32.Jiang XF, Tang QL, Li HG, Shen XM, Luo X, Wang XY, et al. Tumor-associated macrophages correlate with progesterone receptor loss in endometrial endometrioid adenocarcinoma. J Obstet Gynaecol Res. 2013;39(4):855–863. doi: 10.1111/j.1447-0756.2012.02036.x. [DOI] [PubMed] [Google Scholar]
- 33.Salvesen HB, Akslen LA. Significance of tumour-associated macrophages, vascular endothelial growth factor and thrombospondin-1 expression for tumour angiogenesis and prognosis in endometrial carcinomas. Int J Cancer. 1999;84(5):538–543. doi: 10.1002/(SICI)1097-0215(19991022)84:5<538::AID-IJC17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto I, Kodama J, Seki N, Hongo A, Miyagi Y, Yoshinouchi M, et al. Macrophage infiltration and angiogenesis in endometrial cancer. Anticancer Res. 2000;20(6c):4853–4856. [PubMed] [Google Scholar]
- 35.Soeda S, Nakamura N, Ozeki T, Nishiyama H, Hojo H, Yamada H, et al. Tumor-associated macrophages correlate with vascular space invasion and myometrial invasion in endometrial carcinoma. Gynecol Oncol. 2008;109(1):122–128. doi: 10.1016/j.ygyno.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 36.Espinosa I, Jose Carnicer M, Catasus L, Canet B, D'Angelo E, Zannoni GF, et al. Myometrial invasion and lymph node metastasis in endometrioid carcinomas: tumor-associated macrophages, microvessel density, and HIF1A have a crucial role. Am J Surg Pathol. 2010;34(11):1708–1714. doi: 10.1097/PAS.0b013e3181f32168. [DOI] [PubMed] [Google Scholar]
- 37.Ohno S, Ohno Y, Suzuki N, Kamei T, Koike K, Inagawa H, et al. Correlation of histological localization of tumor-associated macrophages with clinicopathological features in endometrial cancer. Anticancer Res. 2004;24(5c):3335–3342. [PubMed] [Google Scholar]
- 38.Fujimoto J, Aoki I, Khatun S, Toyoki H, Tamaya T. Clinical implications of expression of interleukin-8 related to myometrial invasion with angiogenesis in uterine endometrial cancers. Ann Oncol. 2002;13(3):430–434. doi: 10.1093/annonc/mdf078. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka Y, Kobayashi H, Suzuki M, Kanayama N, Suzuki M, Terao T. Thymidine phosphorylase expression in tumor-infiltrating macrophages may be correlated with poor prognosis in uterine endometrial cancer. Hum Pathol. 2002;33(11):1105–1113. doi: 10.1053/hupa.2002.129203. [DOI] [PubMed] [Google Scholar]
- 40.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11(9):509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 41.Coosemans A, Moerman P, Verbist G, Maes W, Neven P, Vergote I, et al. Wilms' tumor gene 1 (WT1) in endometrial carcinoma. Gynecol Oncol. 2008;111(3):502–508. doi: 10.1016/j.ygyno.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 42.Ohno S, Kyo S, Myojo S, Dohi S, Ishizaki J, Miyamoto K, et al. Wilms' tumor 1 (WT1) peptide immunotherapy for gynecological malignancy. Anticancer Res. 2009;29(11):4779–4784. [PubMed] [Google Scholar]
- 43.Coosemans A, Vanderstraeten A, Tuyaerts S, Verschuere T, Moerman P, Berneman ZN, et al. Wilms' Tumor Gene 1 (WT1)--loaded dendritic cell immunotherapy in patients with uterine tumors: a phase I/II clinical trial. Anticancer Res. 2013;33(12):5495–5500. [PubMed] [Google Scholar]
- 44.Coosemans A, Wolfl M, Berneman ZN, Van Tendeloo V, Vergote I, Amant F, et al. Immunological response after therapeutic vaccination with WT1 mRNA-loaded dendritic cells in end-stage endometrial carcinoma. Anticancer Res. 2010;30(9):3709–3714. [PubMed] [Google Scholar]
- 45.Gjerstorff MF, Andersen MH, Ditzel HJ. Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget. 2015;6(18):15772–15787. doi: 10.18632/oncotarget.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Resnick MB, Sabo E, Kondratev S, Kerner H, Spagnoli GC, Yakirevich E. Cancer-testis antigen expression in uterine malignancies with an emphasis on carcinosarcomas and papillary serous carcinomas. Int J Cancer. 2002;101(2):190–195. doi: 10.1002/ijc.10585. [DOI] [PubMed] [Google Scholar]
- 47.Okada T, Akada M, Fujita T, Iwata T, Goto Y, Kido K, et al. A novel cancer testis antigen that is frequently expressed in pancreatic, lung, and endometrial cancers. Clin Cancer Res. 2006;12(1):191–197. doi: 10.1158/1078-0432.CCR-05-1206. [DOI] [PubMed] [Google Scholar]
- 48.Hasegawa K, Koizumi F, Noguchi Y, Hongo A, Mizutani Y, Kodama J, et al. SSX expression in gynecological cancers and antibody response in patients. Cancer Immun. 2004;4:16. [PubMed] [Google Scholar]
- 49.Jager E, Karbach J, Gnjatic S, Neumann A, Bender A, Valmori D, et al. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci U S A. 2006;103(39):14453–14458. doi: 10.1073/pnas.0606512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santin AD, Bellone S, Gokden M, Palmieri M, Dunn D, Agha J, et al. Overexpression of HER-2/neu in uterine serous papillary cancer. Clin Cancer Res. 2002;8(5):1271–1279. [PubMed] [Google Scholar]
- 51.Slomovitz BM, Broaddus RR, Burke TW, Sneige N, Soliman PT, Wu W, et al. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. J Clin Oncol. 2004;22(15):3126–3132. doi: 10.1200/JCO.2004.11.154. [DOI] [PubMed] [Google Scholar]
- 52.Odicino FE, Bignotti E, Rossi E, Pasinetti B, Tassi RA, Donzelli C, et al. HER-2/neu overexpression and amplification in uterine serous papillary carcinoma: comparative analysis of immunohistochemistry, real-time reverse transcription-polymerase chain reaction, and fluorescence in situ hybridization. Int J Gynecol Cancer. 2008;18(1):14–21. doi: 10.1111/j.1525-1438.2007.00946.x. [DOI] [PubMed] [Google Scholar]
- 53.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 54.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 55.Kaumaya PT, Foy KC, Garrett J, Rawale SV, Vicari D, Thurmond JM, et al. Phase I active immunotherapy with combination of two chimeric, human epidermal growth factor receptor 2, B-cell epitopes fused to a promiscuous T-cell epitope in patients with metastatic and/or recurrent solid tumors. J Clin Oncol. 2009;27(31):5270–5277. doi: 10.1200/JCO.2009.22.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inoue M, Shimizu H, Shimizu C, Sasagawa T, Ueda G, Tanizawa O, et al. Antitumor efficacy of recombinant interleukin 2-activated killer cells against endometrial cancers. Nihon Sanka Fujinka Gakkai zasshi. 1987;39(1):143–144. [PubMed] [Google Scholar]
- 57.Shimizu H, Inoue M, Tanizawa O. Adoptive cellular immunotherapy to the endometrial carcinoma cell line xenografts in nude mice. Gynecol Oncol. 1989;34(2):195–199. doi: 10.1016/0090-8258(89)90141-8. [DOI] [PubMed] [Google Scholar]
- 58.Steis RG, Urba WJ, VanderMolen LA, Bookman MA, Smith JW, 2nd, Clark JW, et al. Intraperitoneal lymphokine-activated killer-cell and interleukin-2 therapy for malignancies limited to the peritoneal cavity. J Clin Oncol. 1990;8(10):1618–1629. doi: 10.1200/JCO.1990.8.10.1618. [DOI] [PubMed] [Google Scholar]
- 59.Santin AD, Hermonat PL, Ravaggi A, Bellone S, Cowan C, Coke C, et al. Development and therapeutic effect of adoptively transferred T cells primed by tumor lysate-pulsed autologous dendritic cells in a patient with metastatic endometrial cancer. Gynecol Obstet Invest. 2000;49(3):194–203. doi: 10.1159/000010246. [DOI] [PubMed] [Google Scholar]
- 60.Santin AD, Bellone S, Ravaggi A, Roman JJ, Pecorelli S, Parham GP, et al. Induction of tumour-specific CD8(+) cytotoxic T lymphocytes by tumour lysate-pulsed autologous dendritic cells in patients with uterine serous papillary cancer. Br J Cancer. 2002;86(1):151–157. doi: 10.1038/sj.bjc.6600026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wickramasinghe D. Tumor and T cell engagement by BiTE. Discov Med. 2013;16(88):149–152. [PubMed] [Google Scholar]
- 62.Topp MS, Gokbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134–4140. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 63.Bellone S, Black J, English DP, Schwab CL, Lopez S, Cocco E et al. Solitomab, an EpCAM/CD3 bispecific antibody construct (BiTE(R)), is highly active against primary uterine serous papillary carcinoma cell lines in vitro. American journal of obstetrics and gynecology. 2015. doi:10.1016/j.ajog.2015.08.011. [DOI] [PMC free article] [PubMed]
- 64.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herzog T, Arguello D, Reddy S, Gatalica Z. PD-1 and PD-L1 expression in 1599 gynecological malignancies - implications for immunotherapy. Gynecol Oncol. 2015;137:Suppl. 1. [Google Scholar]
- 66.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]


