Abstract
Background: The Loess Plateau, an ecologically vulnerable region, has long been suffering from serious soil erosion. Revegetation has been implemented to control soil erosion and improve ecosystems in the Loess Plateau region through a series of ecological recovery programs. However, the increasing atmospheric CO2 as a result of human intervention is affecting the climate by global warming, resulting in the greater frequency and intensity of extreme weather events, such as storms that may weaken the effectiveness of revegetation and cause severe soil erosion. Most research to date has evaluated the effectiveness of revegetation on soil properties and soil erosion of different land use or vegetation types. Here, we study the effect of revegetation on soil organic carbon (SOC) storage and erosion-induced carbon loss related to different plant communities, particularly under extreme rainstorm events. Materials and methods: The erosion-pin method was used to quantify soil erosion, and soil samples were taken at soil depths of 0–5 cm, 5–10 cm and 10–20 cm to determine the SOC content for 13 typical hillside revegetation communities in the year of 2013, which had the highest rainfall with broad range, long duration and high intensity since 1945, in the Yanhe watershed. Results and discussion: The SOC concentrations of all plant communities increased with soil depth when compared with slope cropland, and significant increases (p < 0.05) were observed for most shrub and forest communities, particularly for natural ones. Taking the natural secondary forest community as reference (i.e., soil loss and SOC loss were both 1.0), the relative soil loss and SOC loss of the other 12 plant communities in 2013 ranged from 1.5 to 9.4 and 0.30 to 1.73, respectively. Natural shrub and forest communities showed greater resistance to rainstorm erosion than grassland communities. The natural grassland communities with lower SOC content produced lower SOC loss even with higher soil loss, natural secondary forest communities produced higher SOC loss, primarily because of their higher SOC content, and the artificial R. pseudoacacia community with greater soil loss produced higher SOC loss. Conclusions: These results indicate that natural revegetation is more effective in enhancing SOC storage and reducing soil erosion than artificial vegetative recovery on hillsides. However, natural secondary forest communities, with higher SOC content and storage capacity, may also contribute to larger SOC loss under extreme rainstorms.
Keywords: revegetation, plant community, soil erosion, carbon storage, carbon loss, Loess Plateau
1. Introduction
Soil, the largest pool of terrestrial organic carbon in the biosphere and approximately 3.3 times the size of the atmospheric pool and 4.5 times the size of the biotic pool [1,2], plays an important role in the terrestrial carbon cycle. Soil carbon (C) storage is a strategy to achieve food security and mitigate climate change while simultaneously increasing productivity, improving water quality, and restoring degraded soils and ecosystems [2]. Soils act as a carbon sink to store carbon dioxide through afforestation and other land use conversions [3,4,5]. However, soil erosion has significant impacts on soil degradation and the global carbon cycle [6,7]. Lateral C-fluxes are primarily influenced by water erosion, which disturbs the soil surface and transports sediment-bound carbon out of the system [8]. Recent studies suggest that climatic variability will increase as a consequence of global warming and will result in a greater frequency and intensity of extreme weather events, such as storms, leading to a decrease in regional ecosystem carbon stocks, which has the potential to negate a predicted increase in terrestrial carbon uptake [9,10]. As an essential element of soil physical and chemical properties, soil organic carbon (SOC) plays an important role in enhancing crop production and mitigating greenhouse gas emissions [11,12]. Strategies for restorative land use and the adoption of recommended management practices on cropland, grazing lands and forestlands can restore the SOC pool while mitigating climate change [13]. Therefore, it is important for regional ecosystem sustainability to study the effects of land cover conversion on soil carbon storage and erosion-induced carbon loss, particularly under extreme rainstorm events.
The Chinese Loess Plateau has long experienced serious soil erosion, and severe soil and water loss has led to widespread land degradation [14]. Land use and surface cover are the principal determinants of erosion rates [15]. Unsustainable land use and low vegetative cover are the primary reasons for soil erosion and nutrient loss in the Loess Plateau [16,17,18,19]. To implement ecological restoration and soil erosion control, a series of revegetation measures have been conducted since the 1950s in the Loess Plateau, in particular the “Grain for Green” project (GFG) in 1999. This project promoted the conversion of slope arable land to forest or grassland. Revegetation enhanced the storage of SOC in soils by reducing soil erosion and increasing inputs of organic materials [20]. Anthropogenic changes in vegetative cover and natural succession have been shown to be a significant ways of carbon sink [21]. However, on-site SOC losses caused by water erosion under different land uses have been found to have significant effects on lateral carbon fluxes and deserve attention in the context of the global carbon cycle [8,22,23].
Conversion of land use to agriculture has many potential implications for the mobilization and export of SOC [24]. Flooding events in agricultural watersheds are believed to be particularly important because they mobilize large quantities of total organic carbon (TOC) into the stream channel [25,26]. However, land use and land cover (LULC) have a strong influence on the intensity of soil erosion processes and the consequent loss of SOC and nutrients from soils. Among forest, shrubland, pasture and agriculture classes in a Mediterranean catchment, agricultural areas were found to be the most dynamic sites, accounting for 70% of the eroded soil but 45% of the total eroded C, and the remaining percentage of eroded C was derived primarily from relatively C-rich forest soils, which were the dominant LULC class in terms of spatial extent [23].
Revegetation probably enhances the carbon storage capacity of terrestrial ecosystems on the Loess Plateau [27,28]. The different vegetation characteristics and organic carbon content in soils should influence the extent of soil erosion and its associated carbon loss within different plant communities. Following the implementation of a series of vegetation recovery programs in the Loess Plateau region, vegetation community coverage, height, diversity and stability increased as a whole, and thus, vegetation quality and quantity significantly increased accordingly [29]. Tree planting was widely implemented to accelerate community succession because the process of natural succession was very slow in the Loess Plateau region. In addition, planting trees and grasses could increase surface vegetation cover and control soil erosion under certain conditions [17,19,30]. Successful models of afforestation were also found for “ecological forests” (i.e., trees for soil and water conservation, such as Robinia pseudoacacia, Pinus tabuliformis, Caragana intermedia, and Hippophae rhamnoides) and “economic forests” (i.e., horticultural tree crops, such as Malus pumila and Armeniaca vulgaris) [29]. However, low production “small but old tree” forests and tree death in drought years also existed widely, depending on the long-term soil water deficit, soil desiccation and the decrease of streamflow in different scale catchments [31,32,33,34]. Natural vegetation on abandoned land may begin the process of succession after the termination of human disturbance and gradually transform to secondary wild grassland or secondary forest communities [35,36]. In the hill and gully landscape of the Loess Plateau, inter-gullies are the primary location of croplands, and the abandonment of cropland is related to slope gradient, distance from the village, and soil and water conservation policies; as a result, plant communities with different successional stages have developed, such as communities dominated by Artemisia scoparia, Lespedeza davurica, Artemisia gmelinii, Bothriochloa ischaemun, Sophora viciifolia, and Quercus liaotungensis, respectively [37,38]. Although the implementation of a series of ecological recovery programs has achieved remarkable progress in soil erosion control [39,40], it is still likely that soil loss will be extremely severe during heavy rainstorms [41]. Owing to the differential ability of vegetation types to reduce soil loss under extreme rainstorm events [42], corresponding differences in carbon losses also exist. Currently, most related studies in the Loess Plateau region have focused on soil erosion and soil organic carbon distribution under different land uses or vegetation types at the slope scale [43,44] or the watershed scale [45,46,47]. However, little is known about SOC storage and erosion-induced SOC loss related to differences among plant communities, particularly erosion-induced SOC loss under extreme rainstorm events. It happened that Yanhe watershed experienced consecutive heavy rainfalls with broad range, long duration and high intensity in July 2013.
Thus, the objectives of this study were to examine the effects of revegetation on soil organic carbon storage and carbon losses caused by soil erosion on hillsides in response to extreme rainstorm events in the hill and gully area of the Loess Plateau by analyzing: (1) changes in soil organic carbon content and capacity for soil carbon storage; and (2) soil erosion and erosion-induced carbon loss of different plant communities. The results of this study could provide references for strategies towards the optimization of vegetation restoration and climate change mitigation in the Loess Plateau region.
2. Materials and Methods
2.1. Study Area
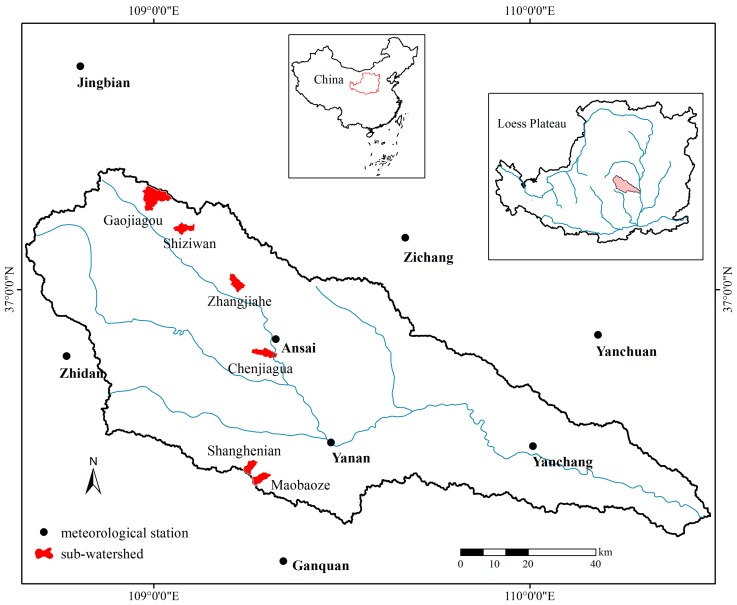
The study area was located in the Yanhe watershed at N 36°23′–37°17′ and E 108°45′–110°28′ (Figure 1). The watershed that occupies 7687 km2 is the primary tributary in the middle reaches of the Yellow River and belongs to the hill-gully region of the Loess Plateau. The climate is semi-arid, with mean annual precipitation of 500 mm (420–540 mm), and mean air temperature of 9.5 °C (8.80 °C to 10.2 °C). The seasonal distribution of precipitation is uneven, characterized by intensive rainfall from July to September that accounts for more than 60% of the annual rainfall. Soils are predominantly loess and have a texture of 64%–73% silt and 17%–20% clay; the loose soils are vulnerable to dispersion and transport [48,49].
Figure 1.
Location and distribution of the six sub-watersheds and meteorological stations in the Yanhe watershed.
The natural vegetation is composed of grassland, shrub and secondary forest. The dominant tree species include Quercus liaotungensis, Pinus tabuliformis, Platycladus orientalis, and Acer buergerianum. The dominant shrub species include Syringa julianae, Sophora viciifolia, Periploca sepium, and Ostryopsis davidiana. And the dominant herb species include Artemisia scoparia, Stipa grandis, Stipa bungeana, Artemisia gmelinii, Artemisia giralaii, Thymus mandshuricus, Bothriochloa ischaemum, Lespedeza davurica, and Carex lanceolata. The primary introduced tree species include Robinia pseudoacacia, Populus simonii, Hippophae rhamnoides, Caragana intermedia, and Salix matsudana. The economic forests are dominated by Malus pumila, Prunus davidiana, and Armeniaca sibirica [29].
2.2. Experimental Design and Soil Sampling
Our research was conducted in six sub-watersheds distributed from south to north in the Yanhe watershed (Figure 1). The survey sites were in inter-gully areas and 13 communities, defined by species dominance, were selected. There were four grassland communities of Artemisia scoparia (A), Stipa bungeana + Lespedeza davurica (SL), Artemisia gmelinii + Stipa bungeana (AS) and Artemisia gmelinii + Artemisia giralaii+Bothriochloa ischaemum(AAB); two natural shrubland communities of Sophora viciifolia − Artemisia gmelinii + Stipa bungeana (SAS) and Syringa julianae − Carex lanceolata (SC); two natural forestland communities of Quercus liaotungensis − Syringa julianae − Carex lanceolata (QSC) and Acer buergerianum − Syringa julianae − Carex lanceolata (ASC), two artificial shrubland communities of Hippophae rhamnoides − Artemisia gmelinii + Stipa bungeana (HAS) and Caragana intermedia − Artemisia gmelinii + Stipa bungeana (CAS); and three artificial forest communities of Armeniaca sibirica − Artemisia gmelinii + Stipa bungeana (AAS), 6–8 years Robinia pseudoacacia − Artemisia gmelinii + Stipa bungeana (RAS1) and 15 years Robinia pseudoacacia − Artemisia gmelinii + Stipa bungeana (RAS2). Each community had three replicate sites distributed in different sub-watersheds, and three replicates of slope croplands (P. miliaceum, Z. mays) were used as the reference (Table 1).
Table 1.
Sites conditions and the cover of different layers of plant communities.
| Vegetation Type | Plant Community a | Aspect b | Slope (°) | Tc c (%) | Sc d (%) | Gc e (%) | Tpc f (%) | Lc g (%) | Bc h (%) |
|---|---|---|---|---|---|---|---|---|---|
| Slope cropland | P | SW | 24 ± 2 | ||||||
| Natural grassland | A | SW | 23 ± 3 | 26 ± 11 | 26 ± 11 | 19 ± 12 | 40 ± 16 | ||
| SL | SW | 22 ± 4 | 15 ± 10 | 15 ± 10 | 16 ± 10 | 44 ± 16 | |||
| AS | SW | 24 ± 1 | 42 ± 13 | 42 ± 13 | 24 ± 10 | 34 ± 14 | |||
| AAB | SW | 25 ± 4 | 28 ± 13 | 28 ± 13 | 21 ± 13 | 44 ± 14 | |||
| Artificial shrubland | HAS | NW | 24 ± 4 | 41 ± 16 | 25 ± 9 | 67 ± 17 | 29 ± 8 | 32 ± 15 | |
| CAS | SW | 28 ± 1 | 41 ± 13 | 37 ± 17 | 78 ± 15 | 24 ± 9 | 19 ± 9 | ||
| Natural shrubland | SAS | SW | 29 ± 1 | 42 ± 5 | 23 ± 12 | 55 ± 17 | 25 ± 7 | 28 ± 9 | |
| SC | NW | 28 ± 2 | 32 ± 2 | 12 ± 3 | 44 ± 6 | 48 ± 17 | 33 ± 2 | ||
| Artificial forestland | AAS | NW | 27 ± 1 | 34 ± 19 | 17 ± 8 | 51 ± 14 | 20 ± 8 | 29 ± 7 | |
| RAS1 | NW | 26 ± 2 | 9 ± 3 | 18 ± 6 | 27 ± 7 | 21 ± 11 | 22 ± 10 | ||
| RAS2 | NW | 25 ± 3 | 28 ± 8 | 25 ± 10 | 53 ± 12 | 31 ± 16 | 23 ± 13 | ||
| Natural forestland | ASC | NW | 27 ± 2 | 45 ± 7 | 25 ± 11 | 15 ± 9 | 85 ± 7 | 57 ± 5 | 5 ± 1 |
| QSC | NW | 26 ± 2 | 60 ± 9 | 35 ± 6 | 10 ± 2 | 105 ± 6 | 87 ± 7 | 5 ± 2 |
a P: slope cropland cultivated with P. miliaceum or Z. mays (20 years); A: Artemisia scoparia (3–5 years); SL: S. bungeana + L. davurica (10–20 years); AS: A. gmelinii + S. bungeana (25–30 years); AAB: A. gmelinii + A. giralaii + B. ischaemum (30 years); HAS: H. rhamnoides − A. gmelinii + S. bungeana (15–20 years); CAS: C. intermedia − A. gmelinii + S. bungeana (15–20 years); SAS: S. viciifolia − A. gmelinii + S. bungeana (30–40 years); SC: S. julianae − C. lanceolata (30–40 years); AAS: A. sibirica − A. gmelinii + S. bungeana (15 years); RAS1: R. pseudoacacia − A. gmelinii + S. bungeana (6–8 years); RAS2: R. pseudoacacia − A. gmelinii + S. bungeana (15 years); ASC: A. buergerianum − S. julianae − C. lanceolata (40–50 years); QSC: Q. liaotungensis − S. julianae − C. lanceolata (60–70 years). b SW: 225°–270°; NW: 270°–315°. c Tree cover. d Shrub cover. e Grass cover. f Total plant cover (sum of tree cover, shrub cover and grass cover). g Litter cover. h Biocrust cover.
The erosion-pin method [50] was used to set up observation plots in May 2013 (before the rainy season). Three 2 m × 2 m plots, each with nine pins at intervals of 50 cm, were laid out at each site in the 13 plant communities. The erosion pins were iron nails 10 cm in length and 5 mm in diameter. The pins were inserted with uniform hammering to minimize soil disturbance. The top of each nail was even with the soil surface. A red stick was inserted 5 cm to the right side of each pin to facilitate locating the pin. Meanwhile, vegetation, litter and biocrust cover of each site were recorded. The cover of the vegetation in plots was estimated visually by two or three observers working together. In late October 2013, the erosion depth (−) or deposition depth (+) of the pins was measured to the nearest 0.1 mm using digital caliper. Fortunately, the largest rainstorms happened in July 2013 since 1945, and the rain was extraordinarily large to differentiate soil erosion among these treatments.
At each site, soil samples were collected with a soil auger (internal diameter 4.8 cm) from nine points in an S-shaped pattern that paralleled the slope at depths of 0–5 cm, 5–10 cm and 10–20 cm. The soil samples from the nine points in each depth were mixed, respectively. Live plant materials (roots and shoots) and pebbles from each sample were separated in the laboratory and discarded. All samples were air-dried, crushed, and passed through a 0.15 mm sieve prior to the SOC analysis. SOC was determined by using the oil bath-K2CrO7 titration method (the Agricultural Chemistry Committee of Chinese Soil Academy, 1984). Soil bulk density of each plot (3 replicates) was measured collecting soil with a bulk sampler (100 cm3) near the soil sampling points, and comparing the original mass of each soil core with the mass after oven-drying at 105 °C for 24 h.
2.3. Data Analysis
As the topsoil was disturbed and transported once water erosion had taken place, the organic carbon percentage content (%) of 0–5 cm soil layer was used as the base value to calculate soil organic carbon loss. The soil erosion and soil organic carbon loss in different plant communities were calculated using the following equations:
| (1) |
| (2) |
| (3) |
where Xj is the average soil erosion of each site, Di is the average depth (mm) of the nine nails in each plot, Bi is the average soil bulk density (g·cm−3) of each plot, 103 is the unit conversion factor, S is the soil erosion (t·km−2·yr−1) of each plant community, SL is the soil organic carbon loss (t·km−2·yr−1) of each plant community, and Pj is the average soil surface (0–5 cm) organic carbon percent content (%) of each site; i is the plot number of each site and m is the total number of plots, j is the site number of each plant community and n is the total number of sites.
As the absolute values were obtained in a year experiencing extraordinary storm events, relative soil loss and soil organic carbon loss values were considered to be more meaningful. Therefore, the natural forestland community Q. liaotungensis − S. julianae − C. lanceolata (QSC), with the lowest soil erosion and the highest soil organic carbon, was used as a reference to calculate relative soil loss and soil organic carbon loss of the other communities. The relative soil loss and soil organic carbon loss in different plant communities were calculated using the following equations:
| RS = Sk/SQSC | (4) |
| RSL = SLk/SLQSC | (5) |
where RS and RSL is the relative soil loss and relative soil organic carbon loss, respectively, Sk and SLk is the soil erosion and soil organic carbon loss in different plant communities, k represents the 13 plant communities, SQSC and SLQSC is the soil erosion and soil organic carbon loss of the natural forestland community Q. liaotungensis − S. julianae − C. lanceolata (QSC), respectively.
A two-way analysis of variance (ANOVA) was used to examine the effects of plant community and soil depth on soil organic carbon content. A one-way ANOVA was used to identify the effects of plant community on relative soil loss and relative soil organic carbon loss. If significant effects were detected with the ANOVA, differences between individual means were tested using the Tukey Honestly Significant Difference (HSD) test at p < 0.05. Statistics were run using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Figures were drawn using Sigmaplot 12.0 (Systat Software Inc., San Jose, CA, USA).
3. Results
3.1. Changes in Soil Organic Carbon Content
The two-way ANOVA indicated that plant community and soil depth had significant effects on SOC content (Table 2). Compared with the slope cropland, the SOC content of the 13 plant communities was increased or significantly increased (p < 0.05) at all soil depths. The SOC content in each soil layer of the natural secondary forest communities was significantly higher (p < 0.05) than in other natural plant communities. In addition, the SOC content of the AS community was significantly higher (p < 0.05) at each soil depth than in other natural grassland communities, and there was no significant difference between the other natural grassland communities. The SOC content of the natural secondary forest and shrub communities was significantly higher (p < 0.05) than the SOC content of artificial forest and shrub communities, respectively. In addition, the SOC content of each soil layer was not significantly different between artificial forest communities (except for the RAS1) or between artificial shrub communities.
Table 2.
Average values and standard deviations of the SOC content of different soil layers under different plant communities.
| Vegetation Type | Plant Community e | SOC (g·kg−1) f | ||
|---|---|---|---|---|
| 0–5 cm | 5–10 cm | 10–20 cm | ||
| Slope cropland | P | 4.86 ± 0.98 a,A | 4.34 ± 0.71 a,A | 2.71 ± 0.52 a,A |
| Natural grassland | A | 6.36 ± 1.11 a,A | 5.54 ± 1.22 a,A | 3.78 ± 0.68 a,A |
| SL | 5.02 ± 0.16 a,A | 3.89 ± 0.67 a,A | 3.51 ± 0.89 a,A | |
| AS | 9.63 ± 0.26 b,A | 8.20 ± 0.33 b,A | 6.28 ± 0.73 b,A | |
| AAB | 5.30 ± 0.78 a,A | 4.27 ± 0.95 a,A | 3.79 ± 0.97 a,A | |
| Artificial shrubland | HAS | 11.52 ± 2.34 b,A | 8.14 ± 2.01 b,A | 5.65 ± 1.02 a,b,B |
| CAS | 11.96 ± 1.79 b,A | 7.05 ± 0.58 b,B | 6.25 ± 0.33 b,B | |
| Natural shrubland | SAS | 18.79 ± 1.98 c,A | 11.61 ± 1.21 c,B | 8.55 ± 1.52 c,C |
| SC | 17.18 ± 2.23 c,A | 12.52 ± 3.28 c,B | 8.45 ± 1.72 c,C | |
| Artificial forestland | AAS | 10.19 ± 1.72 b,A | 5.75 ± 0.60 a,,bB | 5.44 ± 0.68 a,b,B |
| RAS1 | 5.95 ± 0.64 a,A | 4.49 ± 0.49 a,A | 4.01 ± 0.42 a,b,A | |
| RAS2 | 12.60 ± 1.01 b,A | 6.05 ± 0.98 a,b,B | 4.64 ± 0.64 a,b,B | |
| Natural forestland | ASC | 37.25 ± 1.45 d,A | 24.28 ± 1.78 d,B | 15.34 ± 1.26 d,C |
| QSC | 52.89 ± 1.39 d,A | 26.71 ± 4.71 d,B | 18.44 ± 3.07 d,C | |
A,B,C Different letters in the superscript within columns indicate significant different values according to HSD test at p < 0.05. a,b,c,d Different letters in the superscript within rows indicate significant different values according to HSD test at p < 0.05. e P: slope cropland cultivated with P. miliaceum or Z. mays (20 years); A: Artemisia scoparia (3–5 years); SL: S. bungeana + L. davurica (10–20 years); AS: A. gmelinii + S. bungeana (25–30 years); AAB: A. gmelinii + A. giralaii + B. ischaemum (30 years); HAS: H. rhamnoides − A. gmelinii + S. bungeana (15–20 years); CAS: C. intermedia − A. gmelinii + S. bungeana (15–20 years); SAS: S. viciifolia − A. gmelinii + S. bungeana (30–40 years); SC: S. julianae − C. lanceolata (30–40 years); AAS: A. sibirica − A. gmelinii + S. bungeana (15 years); RAS1: R. pseudoacacia − A. gmelinii + S. bungeana (6–8 years); RAS2: R. pseudoacacia − A. gmelinii + S. bungeana (15 years); ASC: A. buergerianum − S. julianae − C. lanceolata (40–50 years); QSC: Q. liaotungensis − S. julianae − C. lanceolata (60–70 years). f Soil organic carbon.
The SOC content of the different plant communities ranged from 4.86 to 52.89 g·kg−1 at 0–5 cm, from 3.89 to 26.71 g·kg−1 at 5–10 cm, and from 2.71 to 18.44 g·kg−1 at 10–20 cm (Table 2). As soil depth increased, the soil organic carbon concentration decreased (p < 0.05), but this change was not significant for the grassland communities. SOC content was significantly higher (p < 0.05) in the 0–5 cm soil layer than in the deeper soil layers of the forest community (except for RAS1) and shrub communities (except for the HAS).
3.2. Extreme Rainfall and Soil Erosion
In July 2013, the Yanhe watershed experienced consecutive heavy rainfalls with broad range, long duration and high intensity. At each meteorological station (Figure 1), the total quantity of rainfall in July ranged from 327 to 793 mm, the frequency of rainstorms ranged from two to eight storms, and this series of storms accounted for 65%–100% of total rainstorms amount (126–678 mm) for the year. The Yan’an station received the highest rainfall in July, which exceeded the average annual rainfall (500 mm). This was the highest rainfall since 1945 in this watershed. The total annual precipitation at each meteorological station ranged from 612 mm to 1357 mm, primarily concentrated between June and September, and represented 92%–96% of the total annual precipitation (Table 3).
Table 3.
Rainfall characteristics of the eight meteorological stations from May to October of 2013 in the Yanhe Watershed.
| Month | Yanan | Ansai | Yanchang | Jingbian | Zhidan | Zichang | Yanchuan | Ganquan | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tr a | Rr b | Tr | Rr | Tr | Rr | Tr | Rr | Tr | Rr | Tr | Rr | Tr | Rr | Tr | Rr | |
| May | 29 | 26 | 3 | 8 | 40 | 19 | 20 | 52 | ||||||||
| June | 141 | 51 (1) c | 150 | 65 | 140 | 156 | 127 | 89 | 109 | 50 (1) | ||||||
| July | 793 | 568 (8) | 524 | 232 (3) | 327 | 213 (3) | 488 | 231 (4) | 545 | 212 (3) | 436 | 126 (2) | 666 | 383 (5) | 488 | 231 (3) |
| August | 262 | 59 (1) | 205 | 120 (2) | 98 | 213 | 54 (2) | 218 | 80 (1) | 143 | 80 | 213 | ||||
| September | 94 | 117 | 100 | 152 | 147 | 138 | 156 | 148 | ||||||||
| October | 38 | 23 | 19 | 37 | 22 | 27 | 24 | 30 | ||||||||
| Total | 1357 | 678 (10) | 1045 | 352 (5) | 612 | 213 (3) | 1038 | 285 (6) | 1128 | 292 (4) | 890 | 126 (2) | 1035 | 383 (5) | 1040 | 281 (4) |
a Total rainfall (mm) at each meteorological station in each month. b Rainfall (mm) from individual rainfall events greater than 50 mm at each meteorological station in each month. c Data in parentheses indicate the number of rainstorm events.
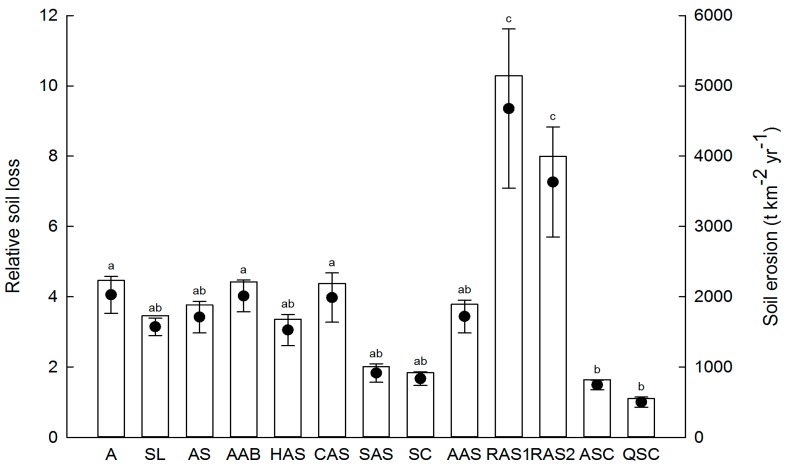
Under the extreme rainfall events of 2013, the soil loss of the 13 communities ranged from 550 t·km−2·yr−1 to 5145 t·km−2·yr−1. The QSC community had the lowest soil loss and was used as a reference to calculate the relative soil loss of the other plant communities. The relative soil loss within the four natural grassland communities ranged from 3.1 to 4.1, and the A community had the lowest resistance to erosion. There was no significant difference in relative soil loss between the two natural shrub communities, which showed a higher resistance to erosion than the grassland communities. Relative soil loss of the secondary forest communities was 1 and 1.5 and showed the greatest efficacy for reducing erosion. In the two artificial shrub communities, relative soil loss was 3 and 3.9. Relative soil loss of the artificial forest communities ranged from 3.4 to 9.4, and the 6–8 years R. pseudoacacia community showed the lowest resistance to erosion among the 13 plant communities (Figure 2).
Figure 2.
Soil erosion and relative soil loss in different plant communities under the extreme rainfall events during 2013. Vertical columns and black circles represent soil erosion and relative soil loss, respectively. Different lowercase letters indicate significance at p < 0.05 for relative soil loss between different plant communities. A: Artemisia scoparia (3–5 years); SL: S. bungeana + L. davurica (10–20 years); AS: A. gmelinii + S. bungeana (25–30 years); AAB: A. gmelinii + A. giralaii + B. ischaemum (30 years); HAS: H. rhamnoides − A. gmelinii + S. bungeana (15–20 years); CAS: C. intermedia − A. gmelinii + S. bungeana (15–20 years); SAS: S. viciifolia − A. gmelinii + S. bungeana (30–40 years); SC: S. julianae − C. lanceolata (30–40 years); AAS: A. sibirica − A. gmelinii + S. bungeana (15 years); RAS1: R. pseudoacacia − A. gmelinii + S. bungeana (6–8 years); RAS2: R. pseudoacacia − A. gmelinii + S. bungeana (15 years); ASC: A. buergerianum − S. julianae − C. lanceolata (40–50 years); QSC: Q. liaotungensis − S. julianae − C. lanceolata (60–70 years).
3.3. Soil Organic Carbon Loss
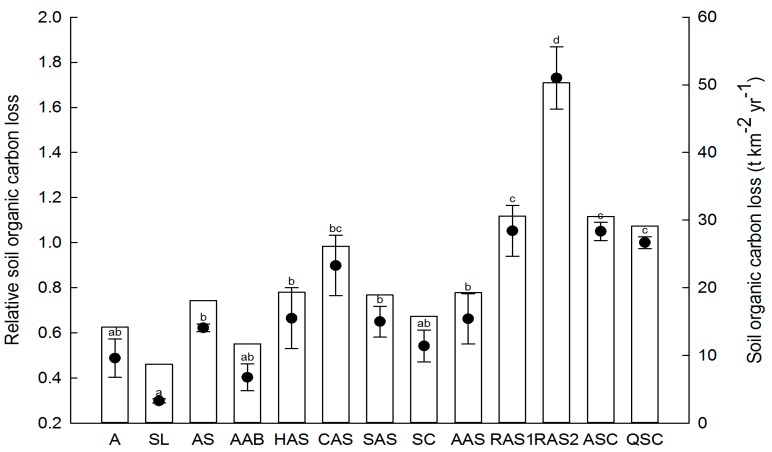
In 2013, with extreme rainstorms, the soil organic carbon loss of the 13 communities ranged from 8.67 t·km−2·yr−1 to 50.34 t·km−2·yr−1. The QSC community experienced the lowest soil erosion and had the highest soil organic carbon content, and was also used as a reference to calculate the relative soil organic carbon loss of the other communities. The relative soil organic carbon loss ranged from 0.30 to 0.62 for the grassland communities, the AS community showed the greatest loss, and the SL community showed the least loss. No significant difference was found between natural and artificial shrub communities, and the relative SOC loss ranged from 0.54 to 0.89. In artificial forest communities, significant differences were found between the R. pseudoacacia communities (1.73 for 15 years and 1.05 for 6–8 years) and the AAS community (0.66). No significant difference was found between the two natural forest communities (1.05 and 1). The 15 years R. pseudoacacia community had the highest relative SOC loss between the 13 plant communities (Figure 3).
Figure 3.
Soil organic carbon loss and relative soil organic carbon loss in different plant communities under extreme rainstorm events during 2013. Vertical columns and black circles represent soil organic carbon loss and relative soil organic carbon loss, respectively. Different lowercase letters indicate significance at p < 0.05 for relative soil organic carbon loss between different plant communities. A: Artemisia scoparia (3–5 years); SL: S. bungeana + L. davurica (10–20 years); AS: A. gmelinii + S. bungeana (25–30 years); AAB: A. gmelinii + A. giralaii + B. ischaemum (30 years); HAS: H. rhamnoides − A. gmelinii + S. bungeana (15–20 years); CAS: C. intermedia − A. gmelinii + S. bungeana (15–20 years); SAS: S. viciifolia − A. gmelinii + S. bungeana (30–40 years); SC: S. julianae − C. lanceolata (30–40 years); AAS: A. sibirica − A. gmelinii + S. bungeana (15 years); RAS1: R. pseudoacacia − A. gmelinii + S. bungeana (6–8 years); RAS2: R. pseudoacacia − A. gmelinii + S. bungeana (15 years); ASC: A. buergerianum − S. julianae − C. lanceolata (40–50 years); QSC: Q. liaotungensis − S. julianae − C. lanceolata (60–70 years).
4. Discussion
4.1. Soil Organic Carbon Sequestration by Vegetation
Land use changes have significant effects on SOC concentrations and the carbon cycle of terrestrial ecosystems. Total SOC concentrations and stocks decreased significantly after grassland was converted to cropland in a semi-arid grassland [51]. In northwestern Spain, Mireia et al. [52] reported approximately 67% of the total carbon loss in the topsoil was a result of the conversion of natural Quercus ilex forest into cropland, and after reforestation with Pinus halepensis, the soil organic carbon increased significantly. In our study, the soil organic carbon (SOC) content of all the vegetation types were higher than slope cropland (Table 2). This corresponds to the results of most studies, which have demonstrated that revegetation increases the soil organic carbon concentrations [5,27,53]. However, differences in SOC content were also found among vegetation types, the rank of average SOC content at each soil depth was as follows: natural secondary forestland > natural shrubland > artificial shrubland > artificial forestland > natural grassland > slope cropland. This order implies that the SOC content was improved through vegetative recovery and that natural secondary forestland had the greatest capacity to store soil organic carbon, particularly in the 0–5 cm soil layer. Compared with slope cropland, the SOC concentration in the surface soil (0–5 cm) increased by 3.29%–98.15%, 253.50%–286.63% and 666.46%–988.27% when it was converted to natural grassland, natural shrubland and natural secondary forestland, respectively. Vegetative cover has fundamental effects on soil properties [54], the lower plant and litter cover of natural grassland communities would have limited the contribution of organic matter to the soil (Table 1). The dense canopy structure and species composition of natural shrubland and forestland, with a higher ability to increase the amount of organic matter in the soil and reduce SOC decomposition rates than natural grassland, have been found to have a positive effect on SOC accumulation [55].
With the conversion of slope cropland to artificial shrubland and forestland, the SOC content of the soil profile (0–20 cm) also showed increased tendency (Table 2). Similar results were found by Wang et al. [21], who showed that the surface (0–20 cm) SOC content increased over eight years of revegetation when cultivated crops were converted to grass (primarily Artemisia Linn, couch grass and small weeds), artificial shrubs (Prunus armniaca) and immature forest plantations (Robinia pseudoacacia). In our study, compared with slope cropland, the SOC content for R. pseudoacacia land was not significantly higher after six to eight years of revegetation, but a significant increase was observed at the soil surface (0–5 cm) after 15 years (Table 2). Lu et al. [56] found that the time required for SOC to transition from source to sink in the Loess Plateau region was three to eight years of afforestation. Qin et al. [57] found that soil organic carbon density (SOCD) in the 0–20 cm soil layer of an immature woodland (<10 a) was lower than that of cultivated land, but the SOCD of mature woodland (>30 a) was 59.54% higher than that of cultivated land. These studies and our data suggest that the time since afforestation should be considered when evaluating the SOC sequestration capacity.
4.2. Soil Organic Carbon Loss through Soil Erosion
With the implementation of “Green for Green” project (GFG), large areas of slope cropland were converted to trees, shrubs, or grasses within the Loess Plateau region [32,58]. Plant structure and cover were the keys to reducing runoff and soil loss by improving water infiltration, reducing the impact of water droplets, intercepting rain and snow and physically stabilizing the soil with roots and leaf litter [59,60]. In the grasslands, biocrust covered most of the soil surface, which played a more important role in soil erosion control than the vascular plant canopy in the hilly Loess Plateau [61]. Pickup et al. [62] found that sediment moves as a series of scour-transport-fill sequences in the arid zone. In our study, soil erosion and deposition coexisted on the hillside, but erosion dominated in all the plant communities in the year of 2013 with extreme rainstorm events in the Yanhe watershed.
Under extreme rainstorm events in 2013, grass communities showed a lower resistance to soil erosion (Figure 2), which may be attributed to the uneven distribution of biocrust and plants on the one hand and the low resilience to physical disturbance and increasing human activity on the other hand; similar results were found in another arid zone by Warren [63]. Shrub communities (except for the CAS), which had a lower soil erosion than grassland communities, were better in protecting the soil (Figure 2). Wei et al. [42] reported that H. rhamnoides, with a mean canopy cover of 92% and a litter depth 30 mm, is a powerful agent of erosion control during extreme hydrological events (maximum 30 min rainfall intensity >0.55 mm/min). However, H. rhamnoides did not achieve a similar effectiveness in controlling soil erosion in our study, which can be accounted for by the significantly lower canopy cover and litter depth (40% and 1.5 mm, respectively). Natural secondary forest communities, especially the QSC, were most effective at protecting the soil from erosion, which can be unequivocally attributed to the dense canopy structure and the higher litter cover (Table 1). Similar results in southeastern Spain suggest that forests with canopies of 60%–85% can effectively reduce rainfall erosivity [59]. It has been shown that afforestation results in soil desiccation and has no additional advantages in terms of species diversity and soil erosion resistance over natural recovery [27]. In our study, the 6–8 years R. pseudoacacia community, with lower tree cover, grass cover and litter cover, had the highest soil erosion (Table 1; Figure 2). This is consistent with the result that young woodland (1–6 years) showed higher soil erosion than ripewood (6–15 years) [48].
The soil carbon pool is tightly linked to soil erosion, and soil erosion can result in net carbon loss from the carbon pool [6,8,22]. However, revegetation reduces soil erosion and enhances soil organic carbon storage capacity [21,64]. In our study, natural secondary forest communities showed higher SOC content, and artificial R. pseudoacacia communities showed higher relative soil loss and carbon loss. More carbon-concentrated plant communities may also produce greater SOC loss when water erosion takes place at the soil surface during extreme rainstorm events, as was found by Nadeu et al. [23] in a Mediterranean catchment. Artificial forests have poor cover, limited understory and compacted soil due to water shortages in the loess hilly area [5], and soil erosion cannot be effectively controlled. Thus, extreme rainfall events may currently play a key role in soil organic carbon loss in the hill and gully areas of the Loess Plateau.
5. Conclusions
These results might have implications for vegetation restoration projects with respect to revegetation pathway selection and global climate change mitigation. We can conclude from the present study that vegetation restoration can increase or significantly increase the SOC content of surface soil (0–20 cm), especially in the 0–5 cm soil layer, and natural vegetative recovery was a more effective pathway for increasing SOC concentration and enhancing SOC storage than artificial vegetative recovery in the Chinese loess hill and gully region.
Soil erosion and erosion-induced carbon loss is tightly linked with vegetation types under extreme rainstorm events. Grassland communities, with a higher soil loss and a lower SOC content, had less SOC loss; shrub communities were intermediate in SOC content and loss; artificial R. pseudoacacia communities, with higher soil loss, produced greater SOC loss; and natural secondary forest communities, with higher carbon-concentrated soils and SOC storage capacity, may produce more SOC loss from water erosion under extreme rainstorm events.
Acknowledgments
This study was supported by a research project form the National Natural Science Foundation of China (reference 41371280) and a public welfare special project of the Ministry of Water resources (reference 201501045). We acknowledged the assistance of An’sai Ecological Experimental Station of Soil and Water Conservation.
Author Contributions
Yujin Li and Juying Jiao carried out the calculation, result analysis and drafted the manuscript, which was revised by all authors. All authors gave their approval of the version submitted for publication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Janzen H.H. Carbon cycling in earth systems—A soil science perspective. Agric. Ecosyst. Environ. 2004;104:399–417. doi: 10.1016/j.agee.2004.01.040. [DOI] [Google Scholar]
- 2.Lal R. Soil carbon sequestration impacts on global climate change and food security. Science. 2004;304:1623–1627. doi: 10.1126/science.1097396. [DOI] [PubMed] [Google Scholar]
- 3.Schimel D.S., House J.I., Hibbard K.A., Bousquet P., Ciais P., Peylin P., Braswell B.H., Apps M.J., Baker D., Bondeau A., et al. Recent patterns and mechanisms of carbon exchange by terrestrial ecosystems. Nature. 2001;414:169–172. doi: 10.1038/35102500. [DOI] [PubMed] [Google Scholar]
- 4.DeGryze S., Six J., Paustian K., Morris S.J., Paul E.A., Merckx R. Soil organic carbon pool changes following land-use conversions. Glob. Chang. Biol. 2004;10:1120–1132. doi: 10.1111/j.1529-8817.2003.00786.x. [DOI] [Google Scholar]
- 5.Chen L., Gong J., Fu B., Huang Z., Huang Y., Gui L. Effect of land use conversion on soil organic carbon sequestration in the loess hilly area, loess plateau of China. Ecol. Res. 2007;22:641–648. doi: 10.1007/s11284-006-0065-1. [DOI] [Google Scholar]
- 6.Lal R. Soil erosion and the global carbon budget. Environ. Int. 2003;29:437–450. doi: 10.1016/S0160-4120(02)00192-7. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z., Govers G., Steegen A., Clymans W., Van den Putte A., Langhans C., Merckx R., Van Oost K. Catchment-scale carbon redistribution and delivery by water erosion in an intensively cultivated area. Geomorphology. 2010;124:65–74. doi: 10.1016/j.geomorph.2010.08.010. [DOI] [Google Scholar]
- 8.Hoffmann T., Mudd S.M., van Oost K., Verstraeten G., Erkens G., Lang A., Middelkoop H., Boyle J., Kaplan J.O., Willenbring J., et al. Short Communication: Humans and the missing C-sink: Erosion and burial of soil carbon through time. Earth Surf. Dyn. 2013;1:45–52. doi: 10.5194/esurf-1-45-2013. [DOI] [Google Scholar]
- 9.Nearing M.A., Jetten V., Baffaut C., Cerdan O., Couturier A., Hernandez M., Le Bissonnais Y., Nichols M.H., Nunes J.P., Renschler C.S., et al. Modeling response of soil erosion and runoff to changes in precipitation and cover. Catena. 2005;61:131–154. doi: 10.1016/j.catena.2005.03.007. [DOI] [Google Scholar]
- 10.Reichstein M., Bahn M., Ciais P., Frank D., Mahecha M.D., Seneviratne S.I., Zscheischler J., Beer C., Buchmann N., Frank D.C., et al. Climate extremes and the carbon cycle. Nature. 2013;500:287–295. doi: 10.1038/nature12350. [DOI] [PubMed] [Google Scholar]
- 11.Post W.M., Kwon K.C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Chang. Biol. 2000;6:317–327. doi: 10.1046/j.1365-2486.2000.00308.x. [DOI] [Google Scholar]
- 12.Schoenholtz S.H., Van Miegroet H., Burger J.A. A review of chemical and physical properties as indicators of forest soil quality: Challenges and opportunities. For. Ecol. Manag. 2000;138:335–356. doi: 10.1016/S0378-1127(00)00423-0. [DOI] [Google Scholar]
- 13.Lal R. Soil carbon management and climate change. Carbon Manag. 2013;4:439–462. doi: 10.4155/cmt.13.31. [DOI] [Google Scholar]
- 14.Chen L., Wei W., Fu B., Lu Y. Soil and water conservation on the Loess Plateau in China: Review and perspective. Prog. Phys. Geog. 2007;31:389–403. doi: 10.1177/0309133307081290. [DOI] [Google Scholar]
- 15.Ruiz-Colmenero M., Bienes R., Eldridge D.J., Marques M.J. Vegetation cover reduces erosion and enhances soil organic carbon in a vineyard in the central Spain. Catena. 2013;104:153–160. doi: 10.1016/j.catena.2012.11.007. [DOI] [Google Scholar]
- 16.Fu B.J., Chen L.D., Ma K.M., Zhou H.F., Wang J. The relationships between land use and soil conditions in the hilly area of the loess plateau in Northern Shaanxi, China. Catena. 2000;39:69–78. doi: 10.1016/S0341-8162(99)00084-3. [DOI] [Google Scholar]
- 17.Zheng F.L. Effect of vegetation changes on soil erosion on the Loess Plateau. Pedosphere. 2006;16:420–427. doi: 10.1016/S1002-0160(06)60071-4. [DOI] [Google Scholar]
- 18.Jiao J.Y., Tzanopoulos J., Xofis P., Bai W.J., Ma X.H., Mitchley J. Can the study of natural vegetation succession assist in the control of soil erosion on abandoned croplands on the Loess Plateau, China? Restor. Ecol. 2007;15:391–399. doi: 10.1111/j.1526-100X.2007.00235.x. [DOI] [Google Scholar]
- 19.Fu B.J., Liu Y., Lu Y.H., He C.S., Zeng Y., Wu B.F. Assessing the soil erosion control service of ecosystems change in the Loess Plateau of China. Ecol. Complex. 2011;8:284–293. doi: 10.1016/j.ecocom.2011.07.003. [DOI] [Google Scholar]
- 20.Jia X., Wei X., Shao M., Li X. Distribution of soil carbon and nitrogen along a revegetational succession on the Loess Plateau of China. Catena. 2012;95:160–168. doi: 10.1016/j.catena.2012.02.018. [DOI] [Google Scholar]
- 21.Wang Y., Fu B., Lü Y., Chen L. Effects of vegetation restoration on soil organic carbon sequestration at multiple scales in semi-arid Loess Plateau, China. Catena. 2011;85:58–66. doi: 10.1016/j.catena.2010.12.003. [DOI] [Google Scholar]
- 22.Zhang J.H., Quine T.A., Ni S.J., Ge F.L. Stocks and dynamics of SOC in relation to soil redistribution by water and tillage erosion. Glob. Chang. Biol. 2006;12:1834–1841. doi: 10.1111/j.1365-2486.2006.01206.x. [DOI] [Google Scholar]
- 23.Nadeu E., Van Oost K., Boix-Fayos C., de Vente J. Importance of land use patterns for erosion-induced carbon fluxes in a Mediterranean catchment. Agric. Ecosyst. Environ. 2014;189:181–189. doi: 10.1016/j.agee.2014.03.040. [DOI] [Google Scholar]
- 24.Gregorich E.G., Greer K.J., Anderson D.W., Liang B.C. Carbon distribution and losses: Erosion and deposition effects. Soil Till. Res. 1998;47:291–302. doi: 10.1016/S0167-1987(98)00117-2. [DOI] [Google Scholar]
- 25.Dalzell B.J., Filley T.R., Harbor J.M. Flood pulse influences on terrestrial organic matter export from an agricultural watershed. Geophys. Res. 2005;110 doi: 10.1029/2005JG000043. [DOI] [Google Scholar]
- 26.Dalzell B.J., Filley T.R., Harbor J.M. The role of hydrology in annual organic carbon loads and terrestrial organic matter export from a midwestern agricultural watershed. Geochim. Cosmochim. Acta. 2007;71:1448–1462. doi: 10.1016/j.gca.2006.12.009. [DOI] [Google Scholar]
- 27.Jiao J., Zhang Z., Bai W., Jia Y., Wang N. Assessing the Ecological Success of Restoration by Afforestation on the Chinese Loess Plateau. Restor. Ecol. 2012;20:240–249. doi: 10.1111/j.1526-100X.2010.00756.x. [DOI] [Google Scholar]
- 28.Deng L., Wang K.-B., Chen M.-L., Shangguan Z.-P., Sweeney S. Soil organic carbon storage capacity positively related to forest succession on the Loess Plateau, China. Catena. 2013;110:1–7. doi: 10.1016/j.catena.2013.06.016. [DOI] [Google Scholar]
- 29.Li R., Yang W.Z., Li B.C. Research and Future Prospects for the Loess Plateau of China. Science Press; Beijing, China: 2008. [Google Scholar]
- 30.Wang Z., Liu G.-B., Xu M.-X., Zhang J., Wang Y., Tang L. Temporal and spatial variations in soil organic carbon sequestration following revegetation in the hilly Loess Plateau, China. Catena. 2012;99:26–33. doi: 10.1016/j.catena.2012.07.003. [DOI] [Google Scholar]
- 31.Cao S., Chen L., Xu C., Liu Z. Impact of three soil types on afforestation in China’s Loess Plateau: Growth and survival of six tree species and their effects on soil properties. Landsc. Urban Plan. 2007;83:208–217. doi: 10.1016/j.landurbplan.2007.04.006. [DOI] [Google Scholar]
- 32.McVicar T.R., Li L., Van Niel T.G., Zhang L., Li R., Yang Q., Zhang X., Mu X., Wen Z., Liu W., et al. Developing a decision support tool for China’s re-vegetation program: Simulating regional impacts of afforestation on average annual streamflow in the Loess Plateau. For. Ecol. Manag. 2007;251:65–81. doi: 10.1016/j.foreco.2007.06.025. [DOI] [Google Scholar]
- 33.Shangguan Z.P. Soil desiccation occurrence an its impact on forest vegetation in the Loess Plateau of China. Int. J. Sustain. Dev. World Ecol. 2007;14:299–306. doi: 10.1080/13504500709469730. [DOI] [Google Scholar]
- 34.Chen H.S., Shao M.G., Li Y.Y. Soil desiccation in the Loess Plateau of China. Geoderma. 2008;143:91–100. doi: 10.1016/j.geoderma.2007.10.013. [DOI] [Google Scholar]
- 35.Novara A., Gristina L., La Mantia T., Ruhl J. Carbon dynamics of soil organic matter in bulk soil and aggregate fraction during secondary succession in a Mediterranean environment. Geoderma. 2013;193:213–221. doi: 10.1016/j.geoderma.2012.08.036. [DOI] [Google Scholar]
- 36.Qin Y., Xin Z., Yu X., Xiao Y. Influence of vegetation restoration on topsoil organic carbon in a small catchment of the loess hilly region, China. PLoS ONE. 2014;9:456. doi: 10.1371/journal.pone.0094489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiao J., Zou H., Jia Y., Wang N. Research progress on the effects of soil erosion on vegetation. Acta Ecol. Sin. 2009;29:85–91. doi: 10.1016/j.chnaes.2009.05.001. [DOI] [Google Scholar]
- 38.Jia Y., Jiao J., Wang N., Zhang Z., Bai W., Wen Z. Soil Thresholds for Classification of Vegetation Types in Abandoned Cropland on the Loess Plateau, China. Arid Land Res. Manag. 2011;25:150–163. doi: 10.1080/15324982.2011.554959. [DOI] [Google Scholar]
- 39.Deng L., Shangguan Z.-P., Li R. Effects of the grain-for-green program on soil erosion in China. Int. J. Sediment. Res. 2012;27:120–127. doi: 10.1016/S1001-6279(12)60021-3. [DOI] [Google Scholar]
- 40.Zhou D., Zhao S., Zhu C. The Grain for Green Project induced land cover change in the Loess Plateau: A case study with Ansai County, Shanxi Province, China. Ecol. Indic. 2012;23:88–94. doi: 10.1016/j.ecolind.2012.03.021. [DOI] [Google Scholar]
- 41.Jiao J., Wang Z., Zhao G., Wang W., Mu X. Changes in sediment discharge in a sediment-rich region of the Yellow River from 1955 to 2010: Implications for further soil erosion control. J. Arid Land. 2014;6:540–549. doi: 10.1007/s40333-014-0006-8. [DOI] [Google Scholar]
- 42.Wei W., Chen L., Fu B., Lü Y., Gong J. Responses of water erosion to rainfall extremes and vegetation types in a loess semiarid hilly area, NW China. Hydrol. Process. 2009;23:1780–1791. doi: 10.1002/hyp.7294. [DOI] [Google Scholar]
- 43.Fu X., Shao M., Wei X., Horton R. Soil organic carbon and total nitrogen as affected by vegetation types in Northern Loess Plateau of China. Geoderma. 2010;155:31–35. doi: 10.1016/j.geoderma.2009.11.020. [DOI] [Google Scholar]
- 44.El Kateb H., Zhang H.F., Zhang P.C., Mosandl R. Soil erosion and surface runoff on different vegetation covers and slope gradients: A field experiment in Southern Shaanxi Province, China. Catena. 2013;105:1–10. doi: 10.1016/j.catena.2012.12.012. [DOI] [Google Scholar]
- 45.Feng X., Wang Y., Chen L., Fu B., Bai G. Modeling soil erosion and its response to land-use change in hilly catchments of the Chinese Loess Plateau. Geomorphology. 2010;118:239–248. doi: 10.1016/j.geomorph.2010.01.004. [DOI] [Google Scholar]
- 46.Han F., Hu W., Zheng J., Du F., Zhang X. Estimating soil organic carbon storage and distribution in a catchment of Loess Plateau, China. Geoderma. 2010;154:261–266. doi: 10.1016/j.geoderma.2009.10.011. [DOI] [Google Scholar]
- 47.Zhang C., Liu G., Xue S., Sun C. Soil organic carbon and total nitrogen storage as affected by land use in a small watershed of the Loess Plateau, China. Eur. J. Soil Biol. 2013;54:16–24. doi: 10.1016/j.ejsobi.2012.10.007. [DOI] [Google Scholar]
- 48.Shi H., Shao M. Soil and water loss from the Loess Plateau in China. J. Arid Environ. 2000;45:9–20. doi: 10.1006/jare.1999.0618. [DOI] [Google Scholar]
- 49.Wang J., Fu B., Qiu Y., Chen L. Analysis on soil nutrient characteristics for sustainable land use in Danangou catchment of the Loess Plateau, China. Catena. 2003;54:17–29. doi: 10.1016/S0341-8162(03)00054-7. [DOI] [Google Scholar]
- 50.Desir G., Marín C. Factors controlling the erosion rates in a semi-arid zone (Bardenas Reales, NE Spain) Catena. 2007;71:31–40. doi: 10.1016/j.catena.2006.10.004. [DOI] [Google Scholar]
- 51.Qiu L., Wei X., Zhang X., Cheng J., Gale W., Guo C., Long T. Soil organic carbon losses due to land use change in a semiarid grassland. Plant Soil. 2012;355:299–309. doi: 10.1007/s11104-011-1099-x. [DOI] [Google Scholar]
- 52.Llorente M., Glaser B., Turrión M.B. Storage of organic carbon and Black carbon in density fractions of calcareous soils under different land uses. Geoderma. 2010;159:31–38. doi: 10.1016/j.geoderma.2010.06.011. [DOI] [Google Scholar]
- 53.Zhang C., Xue S., Liu G.-B., Song Z.-L. A comparison of soil qualities of different revegetation types in the Loess Plateau, China. Plant Soil. 2011;347:163–178. doi: 10.1007/s11104-011-0836-5. [DOI] [Google Scholar]
- 54.Rutigliano F.A., D’Ascoli R., De Santo A.V. Soil microbial metabolism and nutrient status in a Mediterranean area as affected by plant cover. Soil Biol. Biochem. 2004;36:1719–1729. doi: 10.1016/j.soilbio.2004.04.029. [DOI] [Google Scholar]
- 55.Li Y.Y., Shao M.A., Zheng J.Y., Zhang X.C. Spatial-temporal changes of soil organic carbon during vegetation recovery at Ziwuling, China. Pedosphere. 2005;15:601–610. [Google Scholar]
- 56.Lu N., Liski J., Chang R.Y., Akujarvi A., Wu X., Jin T.T., Wang Y.F., Fu B.J. Soil organic carbon dynamics of black locust plantations in the middle Loess Plateau area of China. Biogeosciences. 2013;10:7053–7063. doi: 10.5194/bg-10-7053-2013. [DOI] [Google Scholar]
- 57.Qin Y., Xin Z., Yu X., Xiao Y. Influence of Vegetation Restoration on Topsoil Organic Carbon in a Small Catchment of the Loess Hilly Region, China. PLoS ONE. 2014;9:456. doi: 10.1371/journal.pone.0094489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou H.J., Van Rompaey A., Wang J.A. Detecting the impact of the “Grain for Green” program on the mean annual vegetation cover in the Shaanxi province, China using SPOT-VGT NDVI data. Land Use Policy. 2009;26:954–960. doi: 10.1016/j.landusepol.2008.11.006. [DOI] [Google Scholar]
- 59.Durán Zuazo V.H., Rodríguez Pleguezuelo C.R. Soil-erosion and runoff prevention by plant covers: A review. Agron. Sustain. Dev. 2008;28:65–86. doi: 10.1051/agro:2007062. [DOI] [Google Scholar]
- 60.Durán-Zuazo V.H., Francia-Martínez J.R., García-Tejero I., Tavira S.C. Implications of land-cover types for soil erosion on semiarid mountain slopes: Towards sustainable land use in problematic landscapes. Acta Ecol. Sin. 2013;33:272–281. doi: 10.1016/j.chnaes.2013.07.007. [DOI] [Google Scholar]
- 61.Zhao Y., Xu M. Runoff and Soil Loss from Revegetated Grasslands in the Hilly Loess Plateau Region, China: Influence of Biocrust Patches and Plant Canopies. J. Hydrol. Eng. 2013;18:387–393. doi: 10.1061/(ASCE)HE.1943-5584.0000633. [DOI] [Google Scholar]
- 62.Pickup G. Modelling arid zone soil erosion at the regional scale. In: Warner R.F., editor. Fluvial Geomorphology of Australia. Academic Press; Sydney, Australia: 1988. pp. 105–127. [Google Scholar]
- 63.Warren S.D. Synopsis: Influence of biological soil crusts on arid land hydrology and soil stability. In: Belnap J., Lange O.L., editors. Biological Soil Crusts: Structure, Function, and Management. Springer; Berlin, Germany: 2003. [Google Scholar]
- 64.Fullen M.A., Booth C.A., Brandsma R.T. Long-term effects of grass ley set-aside on erosion rates and soil organic matter on sandy soils in east Shropshire, UK. Soil Till. Res. 2006;89:122–128. doi: 10.1016/j.still.2005.07.003. [DOI] [Google Scholar]